Abstract
Introduction
Vitamin A and its metabolites (called retinoids) have been thought to play a role in the development of idiopathic intracranial hypertension (IIH). The IIH Treatment Trial (IIHTT) showed the efficacy of acetazolamide (ACZ) in improving visual field function, papilledema grade, quality of life and cerebrospinal fluid (CSF) pressure. We postulated that IIH patients would demonstrate elevated measures of vitamin A metabolites in the serum and CSF.
Methods
Comprehensive measures of serum vitamin A and its metabolites were obtained from 96 IIHTT subjects, randomly assigned to treatment with ACZ or placebo, and 25 controls with similar gender, age and body mass index (BMI). These included retinol, retinol binding protein, all-trans retinoic acid (ATRA), alpha- and beta-carotenes, and beta-cryptoxanthin. The IIHTT subjects also had CSF and serum vitamin A and metabolite measurements obtained at study entry and at six months.
Results
At study entry, of the vitamin A metabolites only serum ATRA was significantly different in IIHTT subjects (median 4.33 nM) and controls (median 5.04 nM, p = 0.02). The BMI of IIHTT subjects showed mild significant negative correlations with serum ATRA, alpha- and beta-carotene, and beta-cryptoxanthin. In contrast, the control subject BMI correlated only with serum ATRA. At six months, the serum retinol, alpha-carotene, beta-carotene, and CSF retinol were increased from baseline in the ACZ treated group, but only increases in alpha-carotene (p = 0.02) and CSF ATRA (p = 0.04) were significantly greater in the ACZ group compared with the placebo group. No other vitamin A measures were significantly altered over the six months in either treatment group. Weight loss correlated with only with the change in serum beta-carotene (r = −0.44, p = 0.006) and the change in CSF retinol (r = −0.61, p = 0.02).
Conclusion
Vitamin A toxicity is unlikely a contributory factor in the causation of IIH. Our findings differ from those of prior reports in part because of our use of more accurate quantitative methods and measuring vitamin A metabolites in both serum and CSF. ACZ may alter retinoid metabolism in IIH patients.
Keywords: vitamin A, retinoids, idiopathic intracranial hypertension, obesity
Introduction
Idiopathic intracranial hypertension (IIH) is a disorder characterized by elevated intracranial pressure with clinical presentation of headaches, pulsatile tinnitus, transient visual obscurations, papilledema and visual loss. The underlying mechanism(s) causing the intracranial hypertension remains unclear with numerous unproven causation theories (1–5). Obesity is a demonstrated strong risk factor for adult IIH, especially for women of child-bearing age (6–9). IIH affects children and adults, with a very strong predilection for women after puberty.
Secondary intracranial hypertension with symptoms and findings similar to IIH can be induced by consumption of large quantities or chronic lower doses of retinoid (vitamin A and its natural and synthetic analogs, referred to as retinoids) (8, 10–12). For example, oral ingestion of preformed vitamin A, present in liver and vitamin A related pharmacological therapies have been reported to elevate CSF pressure in adults (13, 14), and cause acute protrusion of the fontanel in infants (15). This has led to the speculation that IIH may be due to the “toxicity of free retinoids” formed due to abnormal vitamin A metabolism in obesity. However, continuous intake of vitamin A or its metabolite is required to have unremitting intracranial hypertension and, unlike in IIH, CSF pressure readily normalizes after stopping the inciting agent. (12,13)
Vitamin A metabolism is a complicated process and involves multiple enzymes, binding proteins, and receptors (Figure 1).
Figure 1. Generalized scheme for vitamin A (retinoid) metabolism.
Dietary retinyl esters, retinol, and pro-vitamin A carotenoids are ingested. Vitamin A may be esterified into retinyl esters and stored primarily in the liver. In times of dietary retinoid insufficiency, retinyl ester stores are hydrolyzed to retinol for delivery to peripheral tissues through the circulation bound to RBP. Both all-trans-retinol (typically referred to as retinol) and beta-carotene may be converted enzymatically to all-trans-retinal. However, the visual chromophore 11-cis-retinal, owing to energetic considerations, is formed via the coupled enzymatic hydrolysis of all-trans-retinyl ester with the isomerization of the all-trans-retinoid to the 11-cis-isomer. All-trans-retinal either can be enzymatically oxidized to all-trans-retinoic acid, which regulates transcription of over 500 retinoid-responsive genes, or reduced enzymatically to all-trans-retinol. When all-trans-retinoic acid is no longer needed, it is oxidatively metabolized and eliminated from the body.
Retinol, a major form of vitamin A present in humans and other mammals, is derived from either consumption of food containing preformed vitamin A or via bioconversion from provitamin A carotenoids, including beta-carotene, alpha-carotene, and beta-cryptoxanthin, in the diet (16–18). Due to its hydrophobic nature, retinol in the circulation is bound to its carrier protein, retinol-binding-protein (RBP), and together with transthyretin (TTR) forms a complex, with the molar ratio of 1:1:1 (13, 19). Retinol is also found in the CSF bound to the TTR/RBP complex. TTR and RBP are produced abundantly in the choroid plexus, the major site of CSF production and macro-molecule delivery to brain. Retinol uptake into target cells is achieved either by free diffusion (20, 21) or by a receptor-mediated process through a membrane-bound RBP receptor: STRA6 (stimulated-by-retinoic-acid-6) (22, 23). After uptake into the cell, retinol is metabolized into retinaldehyde and then to all-trans-retinoic acid (ATRA). The meninges and choroid plexus are thought to be the primary sites of ATRA production in the adult brain (24). ATRA then plays a critical role in regulating gene expression of more than 500 genes upon its binding to specific nuclear receptors (25–27).
One hypothesis (28) of how retinoid toxicity may lead to IIH is that ATRA increases gene expression of a molecule in the arachnoid granulation cap cells, in ependymal or glial cells, or even in lymphatic pathways, and causes increased resistance to CSF absorption (29)(30). Understanding whether abnormalities in retinoid metabolism may occur in IIH requires measuring retinol levels as well as the transcriptionally active ATRA and its precursors and other retinoid metabolites which can act as antagonists to ATRA. For example, provitamin A carotenoids can be converted to vitamin A through central cleavage by beta-carotene 15,15′-monooxygenase 1 (BCMO1) (31) or can be eccentrically cleaved into beta-apo-carotenoids under the control of beta-carotene-9′,10′-oxygenase (BCO2) (31, 32). Beta-apo-carotenoids can functionally antagonize retinoic acid receptors (33, 34). Therefore, one metabolite of vitamin A can stimulate the retinoic acid receptor and another can antagonize it.
Relationships between vitamin A metabolites, carotenoids and IIH must also be considered in relation to human body mass index (BMI). Vitamin A and its metabolites have been implicated in adipose tissue metabolic pathways and in obesity. In addition, adipose tissue is actively involved in maintaining retinoid homeostasis (35–38). BMI, as a reflection of fat deposition, correlates positively with the risk of IIH (7, 9) and with the plasma levels of apo-RBP (RBP unbound to retinol), but negatively with plasma carotenoids (39) and serum retinol (40, 41).
In this study, we investigated whether plasma vitamin A metabolite and carotenoid levels are elevated in IIH patients enrolled in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) compared with obese controls. We also examined the CSF levels of retinoids and of carotenoids in IIH and changes in the serum and CSF over a six month interval when study subjects were treated with acetazolamide (ACZ) or matching placebo. This is the first study to measure serum and CSF ATRA and the first to offer a comprehensive examination of retinoids and provitamin A carotenoids in both serum and CSF. Additionally, since weight management was one of the therapeutic interventions provided to all study subjects, the IIHTT affords us an opportunity to further understand the complex relationships among vitamin A metabolites, carotenoids, and RBP and obesity and weight loss.
Methods
Subjects
Details of the IIHTT study design and entry criteria and outcome are published. (42, 43) Newly diagnosed IIH patients naïve to treatment with a perimetric mean deviation (PMD) of −2.00 dB to −7.00 dB using the SITA standard 24-2 test pattern on the Humphrey Field Analyzer II perimeter in the worse eye (‘study eye’) were enrolled. All subjects signed informed consent and the study was performed under institutional review board approval and in accordance with the Helsinki Declaration. Participants were randomized to acetazolamide or placebo for six months, with both treatment groups provided a weight management and counseling program. The diet program encouraged limited sodium and calories but did not specifically reduce retinoid or carotenoid intake (42). No study subjects were known to regularly consume a diet high in vitamin A or carotenoids or take vitamin A or carotenoid supplements. Standardized fundus photographs, Frisén grading of photos at the photographic reading center and by clinical examination by site investigators, and high contrast visual acuity and threshold 24-2 perimetry were performed at each visit during the treatment interval of six months. Of the 165 subjects enrolled, 96 participated in this vitamin A study with 25 control subjects without IIH, who had similar age, gender and BMI. Cost considerations prevented the vitamin A study from including all study subjects and a larger number of control subjects as originally planned.
Laboratory Procedures
Retinol, carotenoids (beta-carotene, alpha-carotene, and beta-cryptoxanthin), RBP, and ATRA were measured at baseline (in all subjects) and six months (in IIH subjects). The levels were determined at the Columbia University laboratories using established protocols.
-
Radioimmunoassay (RIA) for RBP levels:
The RIA uses purified human plasma proteins of RBP for iodination by a lactoperoxidase procedure and for standards. A standard displacement curve was established by plotting the percentage of maximal binding of [125I]RBP with known amounts of purified human plasma RBP for a standard dilution of a rabbit polyclonal antihuman RBP antibody. This RIA procedure has been used previously for measures of RBP in human sera and CSF (41, 44–46) and has been previously described in detail (47).
-
High Performance Liquid Chromatography (HPLC) measurements of retinol and carotenoids:
All-trans-retinol and carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin, and lycopene) were extracted from 2 ml of CSF or 1 ml of serum after first denaturing proteins through addition of an equal volume of absolute ethanol containing a known amount of retinyl acetate internal standard. Subsequently, 5 ml of hexane, containing a known amount of echinenone, an internal standard used for carotenoid determination, was added. Following phase separation by centrifugation, the retinoid and carotenoid containing hexane phase was removed, evaporated under nitrogen, and re-dissolved in benzene for HPLC analysis. The extracted vitamin A metabolites and carotenoids were separated on a 5-µm 4.6 × 250-mm Ultrasphere C18 column preceded by a C18 guard column, using 70% acetonitrile, 15% ethanol, 15% methylene chloride as the running solvent flowing at 1.8 ml/min. Retinol and retinyl esters were detected at 325 nm using a Waters 996 Photodiode Array ultraviolet absorbance monitor. Carotenoids were detected simultaneously at 450 nm. Low limits of detection for retinol, retinyl esters, alpha-carotene, beta-carotene, and beta-cryptoxanthin were respectively, 2, 4, 10, 12, and 6 for plasma/CSF. The assay variability for assays performed on the same day was between 3–6%; and between 5–8% for assays performed on different days (48, 49).
-
Liquid chromatography tandem mass spectrometry (LC/MS/MS) measurements of ATRA:
Owing to the biological and chemical instability of ATRA, plasma and CSF samples were stored in light protected (amber) glass tubes at −80° C prior to analysis. All LC/MS/MS analyses were carried out within 10 days of sample collection and the great preponderance of these were undertaken within seven days after sample collection. Serum and CSF levels of ATRA were determined by ultra-high performance liquid chromatography tandem mass spectrometry (LC/MS/MS) using a Waters Xevo TQ MS ACQUITY UPLC system (Waters, Milford MA). For this analysis, we only employed LC/MS grade acetonitrile and LC/MS grade water purchased from Thermo Fisher (Pittsburgh, PA). All-trans-retinoic acid was purchased from Sigma-Aldrich. Penta-deuterated all-trans-retinoic acid (atRA-d5) was employed as an internal standard and was purchased from Toronto Research Chemicals (North York, Ontario). Retinoid concentrations were verified spectrophotometrically using published ε values (40). Serum and CSF were extracted using the two-step acid-base extraction described by Kane et al. (41). All-trans-retinoic acid was detected and quantified using the multiple reaction monitoring mode (MRM) employing the following transitions: all-trans-retinoic acid, m/z 301.16→123.00; and atRA-d5, m/z 306.15→127.03. The within-day and between-day coefficients of variation for ATRA analysis were respectively 4.9% and 5.8%.
Statistical Analysis
Wilcoxon rank sum tests were used to compare serum and CSF vitamin A and metabolite values between IIH subjects and controls, as well as to compare 6-month changes in these values (as well as changes in weight) between the ACZ and placebo groups among IIH subjects. Associations between vitamin A and metabolite values and clinical measures (BMI, weight, perimetric mean deviation (PMD) and Frisén grade of the study (worse) eye, and CSF pressure were summarized using Spearman rank correlations; associations between 6-month changes in these quantities were similarly analyzed. Box plot figures show median (horizontal line), T-bars that extend from the boxes (whiskers) 1.5 times the height of the box, and outlier points.
Results
IIH and control subjects had similar distributions of age and BMI (Table 1).
Table 1.
Characteristics of IIH and control subjects at baseline
| IIH Subjects (n = 96) | Control Subjects (n = 25) | |
|---|---|---|
| Age (years) | 29.7 (7.7) | 33.4 (8.6) |
| Female Sex (%) | 98% | 100% |
| Weight (kg) | 106.5 (24.8) | 106.8 (20.4) |
| Body Mass Index | 39.7 (7.8) | 40.6 (9.8) |
Values are mean (standard deviation) unless otherwise indicated
RBP and retinol were also in an approximately 1:1 relationship for both controls and IIH subjects (data not shown), which served as an internal control, demonstrating the expected relative values at baseline and validating the measurements. The serum ATRA level was significantly lower in the IIHTT subjects than in obese controls at baseline, but there were no other group differences regarding baseline values of retinoids or carotenoids (pro-vitamin A and non-pro-vitamin A) at study entry (Table 2).
Table 2.
Vitamin A and retinoid levels at baseline in IIH and control subjects
| IIH Subjects (n = 96) | Control Subjects (n = 25) | P-value | |
|---|---|---|---|
| Serum retinol (µM) | 1.56 (1.38, 1.90) | 1.58 (1.32, 1.82) | 0.71 |
| Serum ATRA (nM) | 4.33 (3.59, 5.18) | 5.04 (4.04, 5.84) | 0.02 |
| Serum beta-carotene (µM) | 0.21 (0.14, 0.34) | 0.21 (0.14, 0.34) | 0.96 |
| Serum alpha-carotene (µM) | 0.05 (0.02, 0.07) | 0.04 (0.03, 0.09) | 0.97 |
| Serum beta-cryptoxanthin (µM) |
0.08 (0.05, 0.12) | 0.08 (0.05, 0.10) | 0.59 |
| Serum RBP (µM) | 1.59 (1.32, 2.11) | 1.62 (1.36, 1.89) | 0.98 |
| Molar retinol/RBP ratio | 1.00 (0.85, 1.14) | 0.95 (0.89, 1.12) | 0.73 |
| CSF ATRA (pg/ml) | 3.17 (2.00, 7.45) | NA | |
| CSF RBP (nM) | 3.40 (2.05, 5.21) | NA | |
| CSF retinol (nM) | 2.37 (2.02, 3.14) | NA |
ATRA = all-trans retinoic acid; RBP = Retinol-binding-protein; NA = Not applicable
Values are median (25th percentile, 75th percentile); p-values are based on Wilcoxon rank sum tests.
Note, when the p-values are compared to the adjusted significance for multiple comparisons, significance requires p < 0.007
The BMI of IIHTT subjects showed negative mild to moderate correlations with serum ATRA (r = −0.25, p = 0.01), alpha-carotene (r = −0.35, p = 0.0005), beta-carotene (r = −0.39, p < 0.0001), and beta-crypoxanthin (r = −0.47, p < 0.0001), but not with serum retinol (r = −0.14, p = 0.17) or RBP (r = 0.05, p = 0.61). In control subjects only the serum ATRA negatively correlated with BMI (r = −0.42, p = 0.04). The clinical features of IIH, papilledema grade, mean deviation of the study eye, and CSF pressure, were not associated with any serum retinoid measurements. The CSF pressure was mildly negatively correlated only with the CSF ATRA (r = − 0.27, p = 0.008).
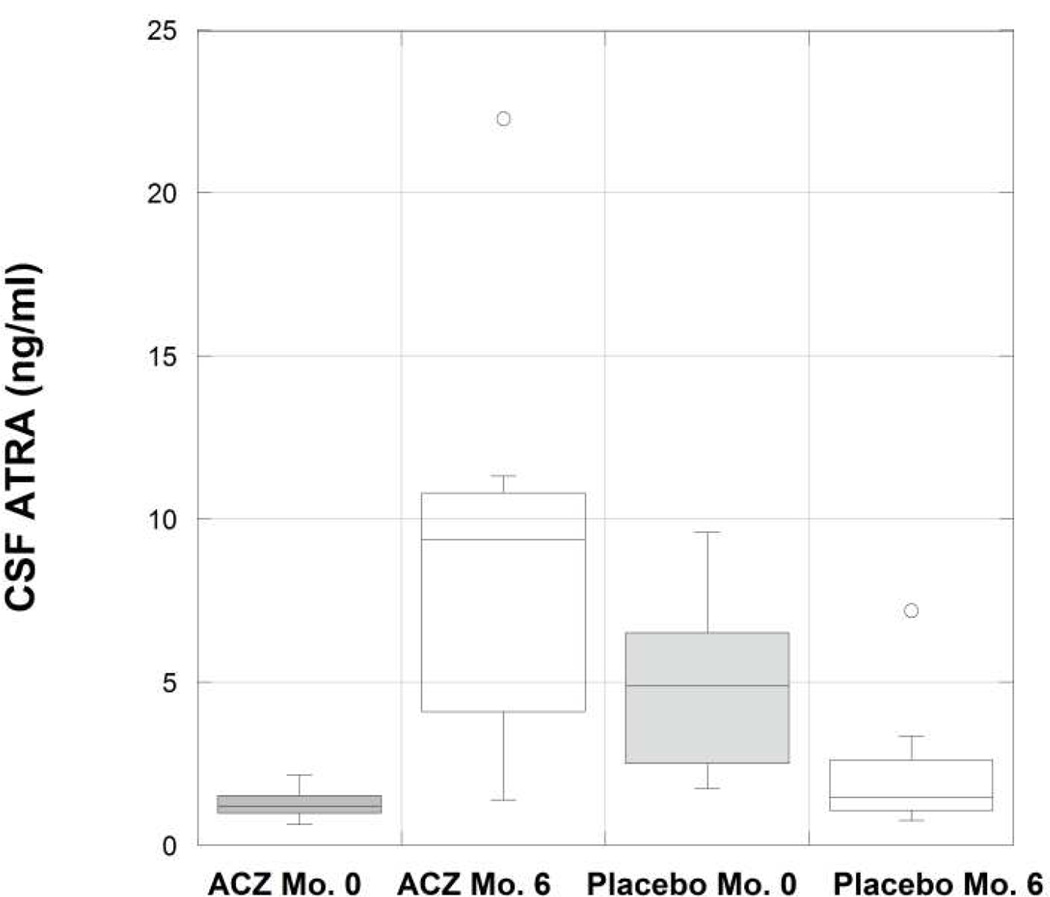
At six months, some vitamin A metabolites increased over baseline in the ACZ treated subjects but not in the placebo treated subjects (Figures 2–6); these included serum retinol, alphacarotene, and beta-carotene, and CSF retinol (Table 3).
Figure 2.
Boxplot (horizontal bar at median value) of serum retinol by treatment group, acetazolamide (ACZ) and placebo, at baseline (Mo. 0) and at six months (Mo. 6). A slight increase in serum retinol level is seen in both groups at six months.
Figure 6.
Boxplot (horizontal bar at median value) of cerebrospinal fluid (CSF) all-trans retinoic acid (ATRA) by treatment group, acetazolamide (ACZ) and placebo, at baseline (Mo. 0) and at six months (Mo. 6). A significant difference is seen between the ACZ and placebo groups with respect to the change in CSF ATRA at six months.
Table 3.
Six-month changes in Vitamin A and retinoid levels by treatment group in IIH subjects
| Acetazolamide (n = 16) | P-value† | Placebo (n = 21) | P-value† | P-value§ | |
|---|---|---|---|---|---|
| Serum retinol (µM) | 0.31 (−0.12, 0.57) | 0.02 | 0.11 (−0.20, 0.36) | 0.19 | 0.28 |
| Serum ATRA (nM) | 0.28 (−1.57, 2.08) | 0.60 | −0.20 (−0.68, 0.99) | 0.91 | 0.75 |
| Serum beta-carotene (µM) |
0.08 (0.01, 0.15) | 0.001 | 0.05 (−0.03, 0.12) | 0.19 | 0.24 |
| Serum alpha-carotene (µM) |
0.02 (0.00, 0.06) | 0.01 | −0.01 (−0.02, 0.01) | 0.40 | 0.01 |
| Serum beta- cryptoxanthin (µM) |
0.02 (−0.01, 0.05) | 0.12 | 0.01 (−0.04, 0.02) | 0.70 | 0.22 |
| Serum RBP (µM) | 0.06 (−0.18, 0.48) | 0.67 | −0.01 (−0.40, 0.30) | 0.54 | 0.37 |
| Molar retinol/RBP ratio |
0.13 (−0.03, 0.28) | 0.12 | 0.25 (−0.08, 0.39) | 0.06 | 0.59 |
| CSF ATRA (pg/ml) | 6.52 (−1.97, 9.20) | 0.11 | −3.07 (−4.89, −0.80) | 0.11 | 0.04 |
| CSF RBP (nM) | 3.29 (2.46, 3.62) | 0.06 | −0.20 (−1.83, 2.33) | 1.00 | 0.08 |
| CSF retinol (nM) | 2.17 (0.28, 2.79) | 0.008 | 0.67 (−0.58, 2.05) | 0.31 | 0.20 |
ATRA = all-trans retinoic acid; RBP = Retinol-binding-protein; NA = Not applicable Values are median (25th percentile, 75th percentile)
P-value for significance of within-group change from baseline based on Wilcoxon signed rank test
P-value for significance of between-group difference at six months based on Wilcoxon rank sum test
Note, when the p-values are compared to the adjusted significance for multiple comparisons, significance requires p < 0.007
The six-month changes in the ACZ group were greater than those in the placebo group only for alpha-carotene (p = 0.01, Figure 3) and CSF ATRA (p = 0.04, Figure 6). In agreement with previously reported findings (43)), ACZ-treated subjects showed greater weight loss (mean −8.6 ± 7.3 kg) than placebo-treated subjects (mean −4.5 ± 6.0 kg, p = 0.007) in this subset of the study cohort. Among all IIHTT subjects, of the serum and CSF retinoid measures, only the change in beta-carotene (r = −0.44, p = 0.006) and the change in CSF retinol (r = −0.61, p = 0.02) were correlated with the change in weight. There were no associations between changes in retinoid and provitamin A carotenoid levels in serum or CSF and changes in CSF pressure, Frisén grade or mean deviation of the study eye.
Figure 3.
Boxplot (horizontal bar at median value) of serum alpha-carotene by treatment group, acetazolamide (ACZ) and placebo, at baseline (Mo. 0) and at six months (Mo. 6). A significant difference is seen between the ACZ and placebo groups with respect to the change in serum alpha-carotenes at six months.
Discussion
The data in this study raise doubts about a theory of “toxicity of free retinoids” as a principal pathophysiologic mechanism for IIH. We investigated the baseline and the six month response of retinoids and provitamin A carotenoids in serum and CSF in IIH subjects in a prospective randomized clinical trial and unexpectedly found low serum levels of the transcriptionally active ATRA in IIH subjects at baseline and evidence of a treatment effect of ACZ on retinoid metabolism (in CSF) in IIH subjects. We had hypothesized that we would see elevated levels of vitamin A metabolites either in serum or CSF, with IIH mimicking the intracranial hypertension seen in association with vitamin A toxicity, but we observed no increased measures prior to treatment. Further, there were no differences between IIH subjects and obese controls regarding the intake of the non-provitamin A carotenoids that are abundant in regular diets. The ratio of retinol:RBP in blood, proposed as a measure of the availability of free retinol (50), did not differ significantly at baseline in our study between the IIH patients and the controls. Additionally, the data are only suggestive that ACZ treatment has an impact on vitamin A metabolism in IIH. We could not address whether a weight management program had any effect on vitamin A metabolism since we had no control group without weight management. However, given the known association of exogenous retinoids with secondary intracranial hypertension, reporting the findings of our prospective study and putting them into context of the current knowledge of human vitamin A metabolism is warranted to apply to future investigations.
Our review of the literature found little agreement among previous studies examining retinol levels or RBP levels in serum or CSF in IIH patients. Some previous clinical studies showed that the level of serum retinol is elevated in IIH patients (50, 51) while other studies found no difference in serum retinol in those with and without IIH (52–54). One study reported an elevated level of CSF retinol in an individual with IIH (54). Only one prior study (55), reported as an abstract, measured retinoic acid (total RA and 13-cis-retinoic acid) in the CSF but did not report ATRA in the serum. In a prior study of RBP in IIH, serum RBP was elevated in seven of the 30 IIH patients, but not in any of the 17 control subjects (53).
The variable results across studies may be due to differing methodology with some approaches being less quantitative than the HPLC and LC/MS/MS used in our study. The volume of CSF and serum used for extraction and whether or not the control samples were protected from light also likely contributed to the differences between studies. Furthermore, patients and controls did not have comparable BMI in all studies. For example, the IIH patients had significantly greater BMI than controls in the Jacobson report (51). The dietary intake of vitamin A and of provitamin A carotenoids was not accounted for in most studies and there may have been group differences and differences between studies. Our study had a larger enrollment and measured carotenoids and retinoids individually, which may also explain some of the differences in our findings compared with prior reports. Carotenoids levels were the same in IIH patients and control subjects at study entry, suggesting that vitamin A dietary intake was the same between groups.
We also compared retinoids and provitamin A carotenoids in serum and CSF in IIH patients treated with ACZ and compared to study entry and to an IIH patient placebo group. Although serum retinol, beta-carotene, alpha-carotene and CSF retinol were all increased at six months after treatment with ACZ compared with values at study entry, the difference between the ACZ and placebo groups was significant only for the 6-month change in alpha-carotene. Both the ACZ group and the placebo group were placed on weight loss diets and the ACZ group experienced greater weight loss (43). There is evidence in the literature for a negative correlation between serum carotenoids and human body weight (39). We cannot determine whether ACZ has an independent impact on carotenoid metabolism given that among all of the IIHTT subjects, the increase in serum beta-carotene modestly correlated (r = −0.44, p = 0.006) with the weight reduction. In the CSF, there was an unexpected increase in CSF ATRA and retinol levels in the ACZ group at six months, not seen in the placebo group, although the group differences were not statistically significant. The number of subjects in each treatment group was too small to determine whether any effects of ACZ on retinoid levels in serum or CSF were independent of its effect on weight loss. 6-month changes in CSF pressure and papilledema did not appear to be associated with corresponding changes in vitamin A metabolism.
We found the baseline levels of serum ATRA in IIH subjects were low compared to the levels in obese controls. Others have suggested (without supportive data) that serum ATRA would be elevated in IIH patients. This was hypothesized based on the observation of elevated intracranial pressure with vitamin A-related pharmacological therapies such as ATRA used in the treatment of acute promyelocytic leukemia, as well as the previous reports describing elevated serum retinol in IIH in some (50, 51) reports. The low serum ATRA in IIH patients, but not in obese control subjects, is suggestive of a perturbation in the retinoid metabolic pathway. Our study is the first study to measure ATRA, which outside of the retina is the major biologically active vitamin A metabolite (see (13) for review). It is of note that, while ACZ treatment plus diet was associated with an increase in the ATRA, there was no similar change with placebo plus diet.
Of all the channels and receptors found in choroid plexus epithelia, aquaporin-1 has raised particular interest in IIH research (56). Notably, the expression of aquaporin-1 in vitro is increased by ATRA (57) and specific deletion of aquaporin-1 in mice reduces CSF production and intracranial pressure (58). It is possible that if vitamin A metabolites play a role in IIH pathophysiology or treatment, it is through the transcriptional regulatory actions of ATRA and production of CSF in the choroid plexus (13). However, there is evidence that retinoic acid may also induce expression of aquaporin-4 in vitro and aquaporin-4 null mice have raised intracranial pressure and ventricular dilation (59). Aquaporin-4 may be found in ependyma and astrocytes, and there is evidence that these cells take up CSF, possibly balancing the role of aquaporin-1 in the production of CSF in the choroid plexus. Yet another plausible explanation for the effectiveness of ACZ lowering CSF pressure might be an interaction between vitamin A metabolites and aquaporin control of fluid movements (60). ACZ decreases expression of the AQP1 protein that ordinarily increases CSF production (61). ACZ also inhibits the hydration of acetaldehyde (62), which is an inhibitor of ATRA production from all trans-retinol (63). Given our finding that ACZ raises CSF ATRA levels, the exact mechanism for ACZ effectiveness remains unanswered.
Our study had limitations. Although the distributions of gender and BMI were comparable in IIH subjects and controls, there were fewer controls than IIH subjects. The small size of the control sample in particular resulted in limited power to detect potentially meaningful differences between the IIH and control groups. The controls had no CSF studies and not all IIH subjects had follow-up serum or CSF studies. Controls did not have follow up serum studies. Furthermore, we did not control for ingestion of vitamins or supplements that might have altered the retinoid and carotenoid values. Finally, interpretation of the findings of this exploratory study needs to account for the multiple statistical tests performed.
Our results further understanding of the roles of vitamin A metabolites and carotenoids in IIH and document lower ATRA in the blood of IIH patients than in the blood of obese controls. The increase in retinoids and pro-vitamin A carotenoids associated with the treatment of IIH with ACZ is suggestive of ACZ having a direct effect on retinoid and pro-vitamin A carotenoid metabolism. Importantly, our data do not support the hypothesis that a “toxic” effect of vitamin A due to increased levels of free retinol or ATRA in blood or CSF is required to develop IIH.
Figure 4.
Boxplot (horizontal bar at median value) of serum beta-carotene by treatment group, acetazolamide (ACZ) and placebo, at baseline (Mo. 0) and at six months (Mo. 6). An increase in beta-carotene level was seen in both groups, which was greater in the ACZ-treated group, at six months.
Figure 5.
Boxplot (horizontal bar at median value) of cerebrospinal fluid (CSF) retinol by treatment group, acetazolamide (ACZ) and placebo, at baseline (Mo. 0) and at six months (Mo. 6). An increase in CSF retinol is seen in the ACZ-treated group at six months.
Highlights for Vitamin A Substudy of IIHTT.
Idiopathic intracranial hypertension (IIH) is not due to vitamin A metabolism toxicity.
Some vitamin A metabolite levels in IIH patients differ from obese normal subjects.
Acetazolamide treatment, a proven therapy of IIH, appears to also alter vitamin A metabolism.
Acknowledgments
Supported by U10 EY017281-01A1, U10 EY017387-01A1, 3U10EY017281-01A1S1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: clinicaltrials.gov identifier: NCT01003639
References
- 1.Ireland B, Corbett JJ, Wallace RB. The search for causes of idiopathic intracranial hypertension. A preliminary case-control study. Archives of neurology. 1990;47(3):315–320. doi: 10.1001/archneur.1990.00530030091021. [DOI] [PubMed] [Google Scholar]
- 2.Wall M. Idiopathic intracranial hypertension. Neurologic clinics. 2010;28(3):593–617. doi: 10.1016/j.ncl.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salpietro V, Polizzi A, Berte LF, Chimenz R, Chirico V, Manti S, Ferrau V, Salpietro A, Arrigo T, Ruggieri M. Idiopathic intracranial hypertension: a unifying neuroendocrine hypothesis through the adrenal-brain axis. Neuro endocrinology letters. 2012;33(6):569–573. [PubMed] [Google Scholar]
- 4.Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. The Lancet Neurology. 2016;15(1):78–91. doi: 10.1016/S1474-4422(15)00298-7. [DOI] [PubMed] [Google Scholar]
- 5.Mollan SP, Ali F, Hassan-Smith G, Botfield H, Friedman DI, Sinclair AJ. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. Journal of neurology, neurosurgery, and psychiatry. 2016;87(9):982–992. doi: 10.1136/jnnp-2015-311302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greer M. Benign Intracranial Hypertension. Vi. Obesity. Neurology. 1965;15:382–388. doi: 10.1212/wnl.15.4.382. [DOI] [PubMed] [Google Scholar]
- 7.Daniels AB, Liu GT, Volpe NJ, Galetta SL, Moster ML, Newman NJ, Biousse V, Lee AG, Wall M, Kardon R, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri) Am J Ophthalmol. 2007;143(4):635–641. doi: 10.1016/j.ajo.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Wall M. Epidemiology and risk factors for idiopathic intracranial hypertension. International ophthalmology clinics. 2014;54(1):1–11. doi: 10.1097/IIO.0b013e3182aabf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2013;33(1):4–8. doi: 10.1097/WNO.0b013e31823f852d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber A, Raab AP, Sobel AE. Vitamin A poisoning in adults; with description of a case. The American journal of medicine. 1954;16(5):729–745. doi: 10.1016/0002-9343(54)90281-8. [DOI] [PubMed] [Google Scholar]
- 11.Fraunfelder FW, Fraunfelder FT. Evidence for a probable causal relationship between tretinoin, acitretin, and etretinate and intracranial hypertension. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2004;24(3):214–216. doi: 10.1097/00041327-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fraunfelder FW, Fraunfelder FT, Corbett JJ. Isotretinoin-associated intracranial hypertension. Ophthalmology. 2004;111(6):1248–1250. doi: 10.1016/j.ophtha.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 13.Libien J, Blaner WS. Retinol and retinol-binding protein in cerebrospinal fluid: can vitamin A take the "idiopathic" out of idiopathic intracranial hypertension? Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2007;27(4):253–257. doi: 10.1097/WNO.0b013e31815c44bc. [DOI] [PubMed] [Google Scholar]
- 14.Morrice G, Jr, Havener WH, Kapetansky F. Vitamin A intoxication as a cause of pseudotumor cerebri. Jama. 1960;173:1802–1805. doi: 10.1001/jama.1960.03020340020005. [DOI] [PubMed] [Google Scholar]
- 15.Lombaert A, Carton H. Benign intracranial hypertension due to A-hypervitaminosis in adults and adolescents. European neurology. 1976;14(5):340–350. doi: 10.1159/000114758. [DOI] [PubMed] [Google Scholar]
- 16.D'Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3(1):63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK. Beta-carotene is an important vitamin A source for humans. The Journal of nutrition. 2010;140(12):2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickenbottom SJ, Lemke SL, Dueker SR, Lin Y, Follett JR, Carkeet C, Buchholz BA, Vogel JS, Clifford AJ. Dual isotope test for assessing beta-carotene cleavage to vitamin A in humans. Eur J Nutr. 2002;41(4):141–147. doi: 10.1007/s00394-002-0368-0. [DOI] [PubMed] [Google Scholar]
- 19.Raghu P, Sivakumar B. Interactions amongst plasma retinol-binding protein, transthyretin and their ligands: implications in vitamin A homeostasis and transthyretin amyloidosis. Biochim Biophys Acta. 2004;1703(1):1–9. doi: 10.1016/j.bbapap.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Fex G, Johannesson G. Retinol transfer across and between phospholipid bilayer membranes. Biochim Biophys Acta. 1988;944(2):249–255. doi: 10.1016/0005-2736(88)90438-5. [DOI] [PubMed] [Google Scholar]
- 21.Noy N, Xu ZJ. Interactions of retinol with binding proteins: implications for the mechanism of uptake by cells. Biochemistry-Us. 1990;29(16):3878–3883. doi: 10.1021/bi00468a012. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315(5813):820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 23.Sun H. Membrane receptors and transporters involved in the function and transport of vitamin A and its derivatives. Biochim Biophys Acta. 2012;1821(1):99–112. doi: 10.1016/j.bbalip.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman T, Crandall JE, Nanescu SE, Quadro L, Shearer K, Ross A, McCaffery P. Patterning of retinoic acid signaling and cell proliferation in the hippocampus. Hippocampus. 2012;22(11):2171–2183. doi: 10.1002/hipo.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee EJ, Plutzky J. Retinoid metabolism and diabetes mellitus. Diabetes Metab J. 2012;36(3):167–180. doi: 10.4093/dmj.2012.36.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shulman AI, Mangelsdorf DJ. Retinoid x receptor heterodimers in the metabolic syndrome. The New England journal of medicine. 2005;353(6):604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 27.Duong V, Rochette-Egly C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim Biophys Acta. 2011;1812(8):1023–1031. doi: 10.1016/j.bbadis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen B, Malm J, Markgren P, Ekstedt J. CSF hydrodynamics in superior sagittal sinus thrombosis. Journal of neurology, neurosurgery, and psychiatry. 1992;55(4):287–293. doi: 10.1136/jnnp.55.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bercaw BL, Greer M. Transport of intrathecal 131-I risa in benign intracranial hypertension. Neurology. 1970;20(8):787–790. doi: 10.1212/wnl.20.8.787. [DOI] [PubMed] [Google Scholar]
- 30.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids and barriers of the CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J Lipid Res. 2013;54(7):1719–1730. doi: 10.1194/jlr.R039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Lintig J. Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr. 2012;96(5):1234S–1244S. doi: 10.3945/ajcn.112.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW, Jr, Harrison EH. Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. The Journal of biological chemistry. 2012;287(19):15886–15895. doi: 10.1074/jbc.M111.325142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Narayanasamy S, Curley RW, Jr, Harrison EH. beta-Apo-13-carotenone regulates retinoid X receptor transcriptional activity through tetramerization of the receptor. The Journal of biological chemistry. 2014;289(48):33118–33124. doi: 10.1074/jbc.M114.610501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61(5):1112–1121. doi: 10.2337/db11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercader J, Ribot J, Murano I, Felipe F, Cinti S, Bonet ML, Palou A. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology. 2006;147(11):5325–5332. doi: 10.1210/en.2006-0760. [DOI] [PubMed] [Google Scholar]
- 37.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 38.Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Archives of biochemistry and biophysics. 2015;572:112–125. doi: 10.1016/j.abb.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Andersen LF, Jacobs DR, Jr, Gross MD, Schreiner PJ, Dale Williams O, Lee DH. Longitudinal associations between body mass index and serum carotenoids: the CARDIA study. Br J Nutr. 2006;95(2):358–365. doi: 10.1079/bjn20051638. [DOI] [PubMed] [Google Scholar]
- 40.Viroonudomphol D, Pongpaew P, Tungtrongchitr R, Changbumrung S, Tungtrongchitr A, Phonrat B, Vudhivai N, Schelp FP. The relationships between anthropometric measurements, serum vitamin A and E concentrations and lipid profiles in overweight and obese subjects. Asia Pac J Clin Nutr. 2003;12(1):73–79. [PubMed] [Google Scholar]
- 41.Guenegou A, Leynaert B, Pin I, Le Moel G, Zureik M, Neukirch F. Serum carotenoids, vitamins A and E, 8 year lung function decline in a general population. Thorax. 2006;61(4):320–326. doi: 10.1136/thx.2005.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman DI, McDermott MP, Kieburtz K, Kupersmith M, Stoutenburg A, Keltner JL, Feldon SE, Schron E, Corbett JJ, Wall M, et al. The idiopathic intracranial hypertension treatment trial: design considerations and methods. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2014;34(2):107–117. doi: 10.1097/WNO.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 43.Committee NIIHSGW. Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. Jama. 2014;311(16):1641–1651. doi: 10.1001/jama.2014.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng W, Lu YM, Lu GY, Zhao Q, Cheung O, Blaner WS. Transthyretin, thyroxine, and retinol-binding protein in human cerebrospinal fluid: effect of lead exposure. Toxicological sciences : an official journal of the Society of Toxicology. 2001;61(1):107–114. doi: 10.1093/toxsci/61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng W, Shen H, Blaner WS, Zhao Q, Ren X, Graziano JH. Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: the role of the choroid plexus. Toxicology and applied pharmacology. 1996;139(2):445–450. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li MD, Kane JK, Matta SG, Blaner WS, Sharp BM. Nicotine enhances the biosynthesis and secretion of transthyretin from the choroid plexus in rats: implications for beta-amyloid formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(4):1318–1323. doi: 10.1523/JNEUROSCI.20-04-01318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blaner WS. Radioimmunoassays for retinol-binding protein, cellular retinol-binding protein, and cellular retinoic acid-binding protein. Methods in enzymology. 1990;189:270–281. doi: 10.1016/0076-6879(90)89298-v. [DOI] [PubMed] [Google Scholar]
- 48.Redlich CA, Blaner WS, Van Bennekum AM, Chung JS, Clever SL, Holm CT, Cullen MR. Effect of supplementation with beta-carotene and vitamin A on lung nutrient levels. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7(3):211–214. [PubMed] [Google Scholar]
- 49.Burger H, Kovacs A, Weiser B, Grimson R, Nachman S, Tropper P, van Bennekum AM, Elie MC, Blaner WS. Maternal serum vitamin A levels are not associated with mother-to-child transmission of HIV-1 in the United States. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;14(4):321–326. doi: 10.1097/00042560-199704010-00003. [DOI] [PubMed] [Google Scholar]
- 50.Warner JE, Larson AJ, Bhosale P, Digre KB, Henley C, Alder SC, Katz BJ, Bernstein PS. Retinol-binding protein and retinol analysis in cerebrospinal fluid and serum of patients with and without idiopathic intracranial hypertension. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2007;27(4):258–262. doi: 10.1097/WNO.0b013e31815b9af0. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson DM, Berg R, Wall M, Digre KB, Corbett JJ, Ellefson RD. Serum vitamin A concentration is elevated in idiopathic intracranial hypertension. Neurology. 1999;53(5):1114–1118. doi: 10.1212/wnl.53.5.1114. [DOI] [PubMed] [Google Scholar]
- 52.Warner JE, Bernstein PS, Yemelyanov A, Alder SC, Farnsworth ST, Digre KB. Vitamin A in the cerebrospinal fluid of patients with and without idiopathic intracranial hypertension. Annals of neurology. 2002;52(5):647–650. doi: 10.1002/ana.10377. [DOI] [PubMed] [Google Scholar]
- 53.Selhorst JB, Kulkantrakorn K, Corbett JJ, Leira EC, Chung SM. Retinol-binding protein in idiopathic intracranial hypertension (IIH) Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2000;20(4):250–252. [PubMed] [Google Scholar]
- 54.Tabassi A, Salmasi AH, Jalali M. Serum and CSF vitamin A concentrations in idiopathic intracranial hypertension. Neurology. 2005;64(11):1893–1896. doi: 10.1212/01.WNL.0000163556.31080.98. [DOI] [PubMed] [Google Scholar]
- 55.Grzybowski DM, Katz SE, Criden MR, Mouser JG. The role of vitamin A and its CSF metabolites in supporting a novel mechanism of idiopathic intracranial hypertension. Cerebrospinal Fluid Research. 2007;4(Suppl 1):S44. [Google Scholar]
- 56.Stiebel-Kalish H, Eyal S, Steiner I. The role of aquaporin-1 in idiopathic and drug-induced intracranial hypertension. Med Hypotheses. 2013;81(6):1059–1062. doi: 10.1016/j.mehy.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Umenishi F, Schrier RW. Induction of human aquaporin-1 gene by retinoic acid in human erythroleukemia HEL cells. Biochem Biophys Res Commun. 2002;293(3):913–917. doi: 10.1016/S0006-291X(02)00316-9. [DOI] [PubMed] [Google Scholar]
- 58.Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(1):76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 59.Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26(12):1527–1537. doi: 10.1038/sj.jcbfm.9600306. [DOI] [PubMed] [Google Scholar]
- 60.Fishman RA. Polar bear liver, vitamin A, aquaporins, and pseudotumor cerebri. Annals of neurology. 2002;52(5):531–533. doi: 10.1002/ana.10389. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, An Y, Gao J, Han J, Pan X, Pan Y, Tie L, Li X. Aquaporin-1 translocation and degradation mediates the water transportation mechanism of acetazolamide. PloS one. 2012;7(9):e45976. doi: 10.1371/journal.pone.0045976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ameli PA, Madan M, Chigurupati S, Yu A, Chan SL, Pattisapu JV. Effect of acetazolamide on aquaporin-1 and fluid flow in cultured choroid plexus. Acta neurochirurgica Supplement. 2012;113:59–64. doi: 10.1007/978-3-7091-0923-6_13. [DOI] [PubMed] [Google Scholar]
- 63.Shiraishi-Yokoyama H, Yokoyama H, Matsumoto M, Imaeda H, Hibi T. Acetaldehyde inhibits the formation of retinoic acid from retinal in the rat esophagus. Scand J Gastroentero. 2006;41(1):80–86. doi: 10.1080/00365520510023936. [DOI] [PubMed] [Google Scholar]