Key Clinical Message
Dorsal agenesis of the pancreas is a rare congenital anomaly. Fifty‐eight cases were reported from 1913 till 2015, nine of which were associated with tumors. We present the 10th case, the first to be associated with pancreatic mucinous adenocarcinoma and cystic teratoma, successfully managed by Whipple procedure and total pancreatectomy.
Keywords: Agenesis of the dorsal pancreas, pancreatic cystic teratoma, pancreatic mucinous adenocarcinoma, rare pancreatic anomalies
Introduction
Pancreatic development is a complex process. However, pancreatic congenital anomalies are a rare entity. Among these anomalies, dorsal agenesis of the pancreas is the rarest. Even rarer is the association between dorsal agenesis of the pancreas and pancreatic tumors. It can be asymptomatic and diagnosed incidentally during investigations carried out for unrelated causes, or symptoms including abdominal pain, diabetes mellitus, jaundice, and weight loss might present initially. Other anomalies might be present as well, in association with dorsal agenesis of the pancreas such as polysplenia, ectopic spleen, mesenteric malrotation, bicornuate uterus, and cardiac anomalies 25.
To our knowledge, around 58 cases of dorsal agenesis of the pancreas have been reported from 1913 till 2015, and around nine cases only were associated with pancreatic tumor. We present a case of dorsal agenesis of the pancreas associated with pancreatic tumor, along with ectopic splenia, mesenteric malrotation, and vascular malformations.
Case Presentation
This is the case of a 29‐year‐old male patient presenting to the hospital with a 2‐month history of abdominal pain, worsening progressively then localizing in the right upper abdominal quadrant and radiating to the back over the last 2 weeks. Patient admits one episode of abdominal pain a year ago, due to which he underwent an endoscopic gastroduodenoscopy that revealed diffuse mild gastritis and no evidence of Helicobacter pylori infection. Patient was treated with a 3‐month course of proton‐pump inhibitors with clinical improvement in his pain. He denies any gastrointestinal symptoms since then. He has no past medical or surgical history and no allergies, does not consume alcohol and is not a smoker.
No pertinent signs were found on physical examination.
At first, ultrasound of the abdomen was performed showing a right subhepatic complex cystic lesion 12 × 7 × 6 cm containing dense debris inside and a thick wall.
Magnetic resonance cholangiopancreatography (MRCP) was performed showing a loculated mass, 5.6 × 9.7 cm, located behind the head of the pancreas, displacing it anteriorly and to the right. The body and tail of the pancreas were not visualized (Fig. 1).
Figure 1.
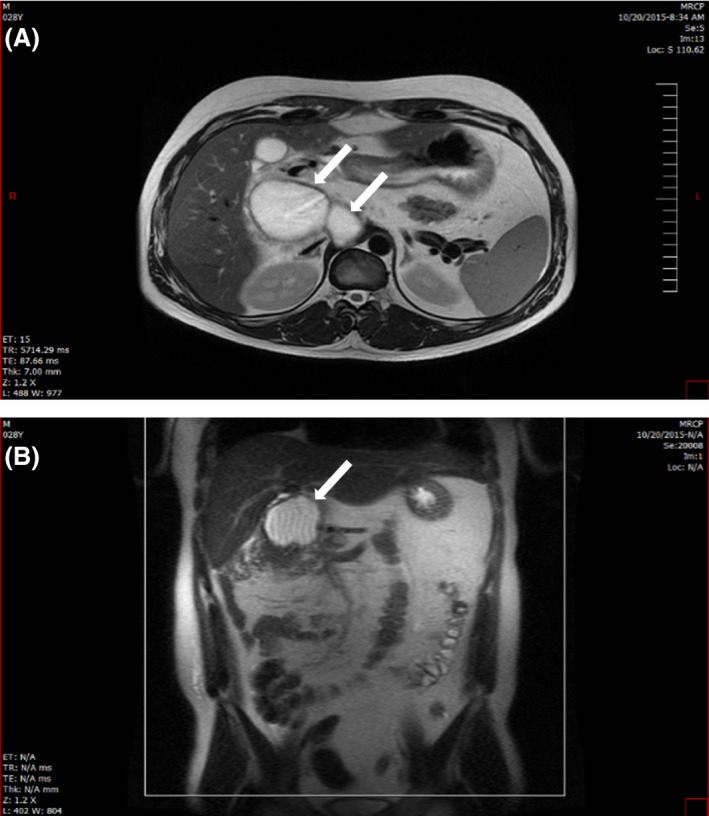
(MRCP): Cuts showing the pancreatic mass (white arrows) as it appeared on MRCP in an axial (A) and coronal fashion (B).
Abdomino‐pelvic CT scan was performed showing a multiloculated cystic retroperitoneal lesion at the level of the second portion of the duodenum in continuity with the pancreatic head which is displaced anteriorly and to the right (Fig. 2) associated with a mild malrotation of the mesentery and proximal small bowels (Fig. 3). Vascular variation was noted (Fig. 3), along with ectopic splenia (Fig. 4).
Figure 2.
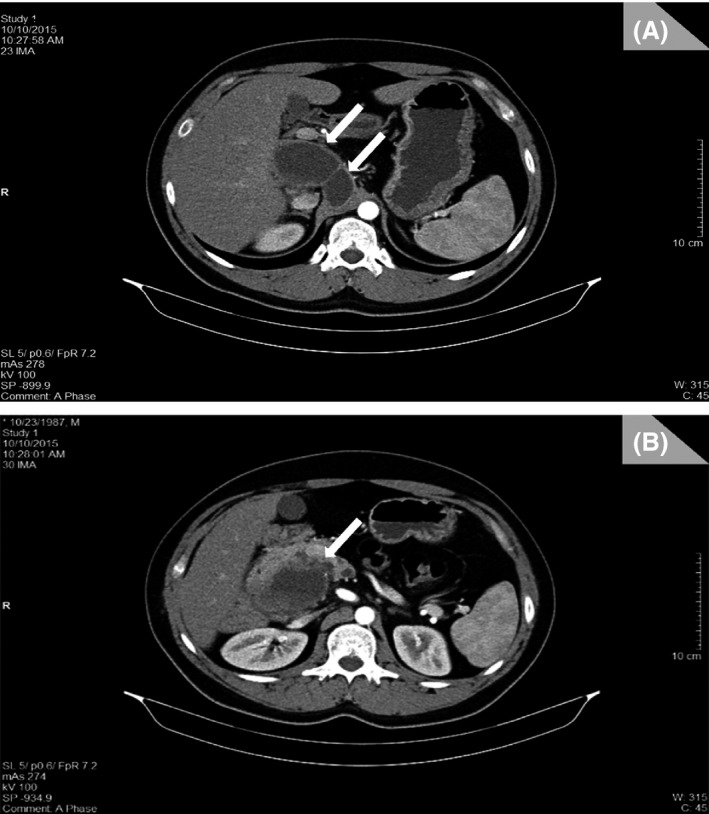
(CT scan): (A and B) The multiloculated cystic retroperitoneal lesion (white arrows), at the level of the second portion of the duodenum in continuity with the pancreatic head which is displaced anteriorly and to the right.
Figure 3.
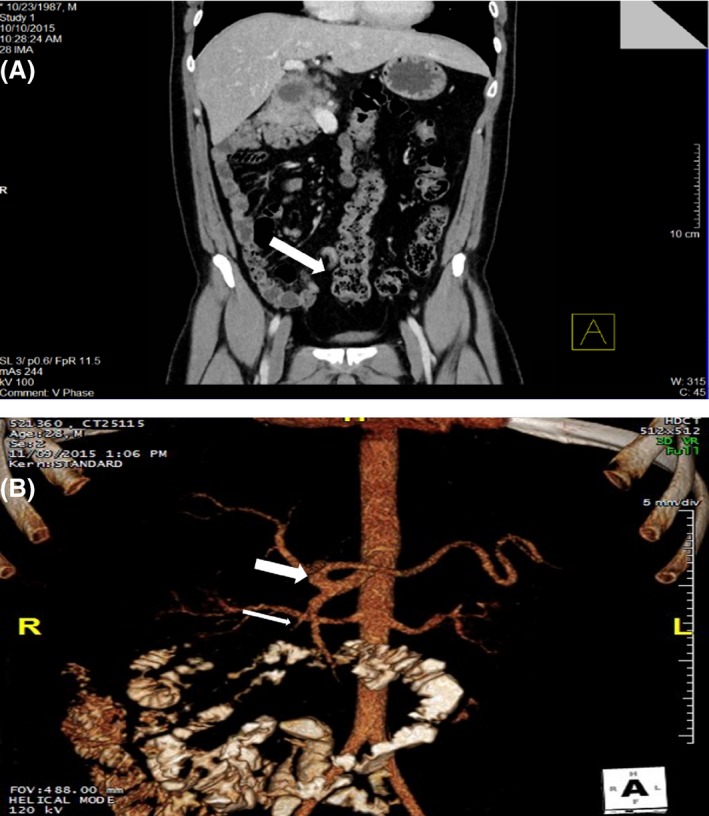
(A) Coronal cut from the abdomino‐pelvic CT scan, showing bowels malrotation and an inferiorly located hepatic flexure (white arrow). (B) 3D reconstruction of the scan performed with IV contrast showing the vascular variation where the SMA branches from the celiac trunk (thick white arrow), giving off the gastroduodenal artery (thin white arrow).
Figure 4.
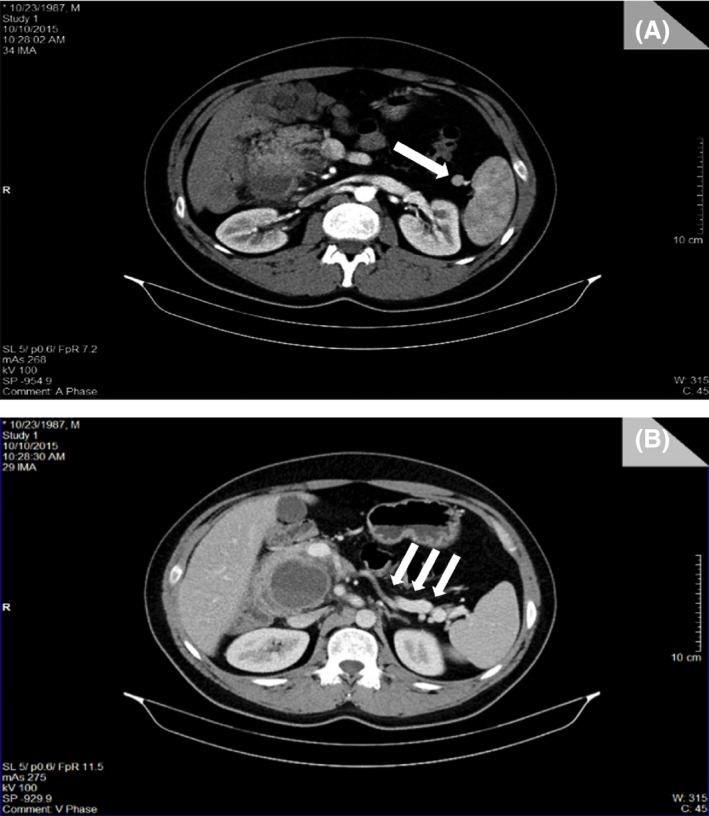
(CT scan): (A) Presence of the ectopic spleen (white arrow); (B) Cut showing the absence of pancreatic tissue anterior to the splenic vein, where it is usually located (white arrows).
Laboratory studies showed the following: hemoglobin 12.8 g/dL, white blood cells 5000/mm3, neutrophils 53%, platelets 345,000/mm3, PT INR 1.1, SGPT (serum glutamic‐pyruvic transaminase) 10 μ/L, SGOT (serum glutamic‐oxaloacetic transaminase) 10 μ/L, direct bilirubin 0.18 mg/dL, total bilirubin 0.45 mg/dL, alkaline phosphatase 101 μ/L, amylase 77 μ/L, lipase 55 μ/L, CEA (carcinoembryonic antigen) 2.09 ng/mL, Ca19‐9 19.95 μ/mL, and GGT (gamma‐glutamyltransferase) 45 μ/L.
Endoscopic ultrasound (EUS) was performed, and cyst aspirate was retrieved. Analysis of the aspirate revealed the following:
Culture: Escherichia coli growth, resistant to ampicillin.
Pathology: Necrotic inflammatory smear with no suspicious content, poorly cellular with no ductal cells, no atypical cells.
Amylase = 982 μ/L; lipase = 4498 μ/L.
CEA = 700 ng/mL; Ca19‐9 = 10.8 μ/mL.
Based on the data collected at that point, the cyst was considered benign and cystojejunostomy was planned.
In the operating room, a right subcostal incision was made, and inspection and palpation of the abdominal organs revealed no evidence of metastatic lesions or carcinomatosis. Chevron incision was then performed, and the transverse colon was not in its anatomical position, with the hepatic flexure located inferiorly around the right gutter. Kocher maneuver was performed, and the cyst was identified in the pancreatic head. Cyst fluid was suctioned with care taken to avoid any spillage; then, intraoperative wedge biopsy was performed and sent to the pathology laboratory as frozen. Results came back positive for mucinous cystadenocarcinoma, moderately to poorly differentiated cells, ductal in origin.
At this point, Whipple procedure and total pancreatectomy were decided.
Dissection was started at the hepatic pedicle, identification of the common hepatic duct, portal vein, and right hepatic artery. The anatomical variation at this level was as follows: superior mesenteric artery branching off the celiac trunk, superior mesenteric vein superficially located with a malrotation of the mesentery, and a right‐sided ligament of Treitz. Furthermore, a total absence of the pancreatic body and tail was noted. Cholecystectomy was performed. Common hepatic duct was divided and enterectomy was performed, and retroportal pancreatic dissection with ligation of venous tributaries and total pancreatectomy were performed. Lymph node dissection around the hepatic artery and celiac trunk was performed, and hepaticojejunostomy and Roux‐en‐Y gastrojejunostomy were performed as well. Specimen was removed en bloc and sent to the pathology laboratory for analysis.
Macroscopically, the pancreatic piece was 14 × 7.5× 7.5 cm showing on cut section a partly necrotic partly cystic surface, and the cyst measures 6.5 × 4 cm and is surrounded by a necrotic parenchyma. Another cystic pouch is seen in the lateral aspect of the pancreas.
Microscopically, the cyst located in the lateral peripancreatic region is composed of dense mesenchymal tissues (muscle, fat, cartilage) admixed with neural elements on a background of dense lymphoid tissue with lymphoid follicles, all lined by mature squamous epithelium. All elements appear mature. Pancreatic head shows numerous cystic dilated ducts lined by flattened epithelium alternating with small cystic spaces lined by tall columnar epithelium exhibiting dysplasia. Common bile duct lining epithelium shows marked atypia and is surrounded by numerous clusters and neoplastic glands invading the underlying pancreas (Figs 5, 6, 7, 8).
Figure 5.
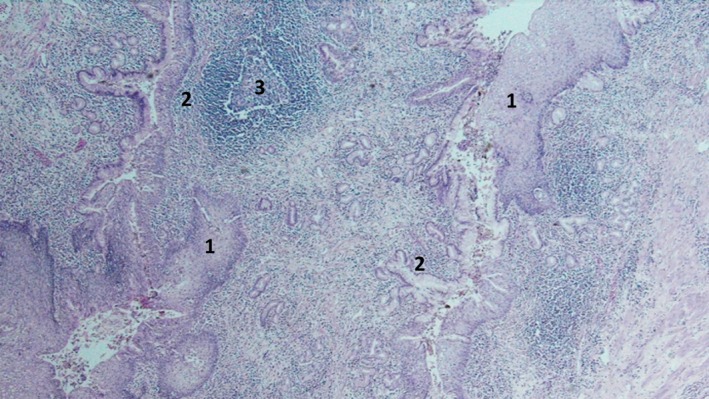
Cyst lined by mature squamous epithelium (1) continued by either columnar (2) or at places by cuboidal epithelium. Background shows diffuse inflammation with lymphoid follicles (3) surrounding adnexal‐type glands.
Figure 6.
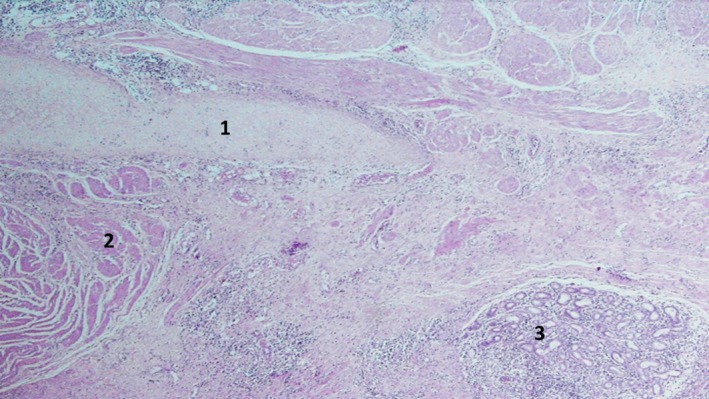
Cyst wall composed of mature mesenchymal tissue: cartilage (1), muscle (2), and clusters of benign salivary‐type glands (3).
Figure 7.
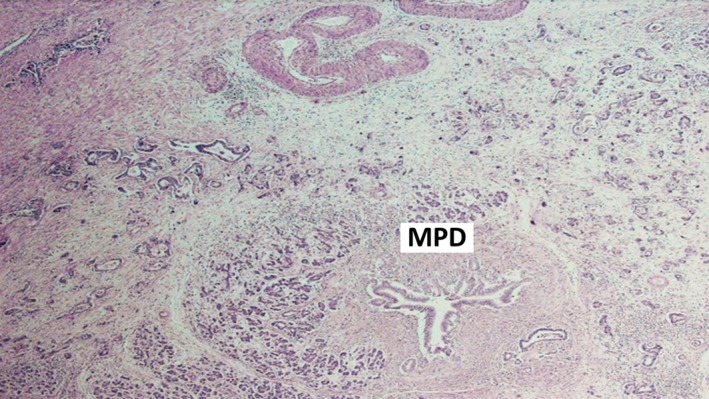
Major pancreatic duct (MPD) surrounded by small irregular neoplastic glands (adenocarcinoma).
Figure 8.
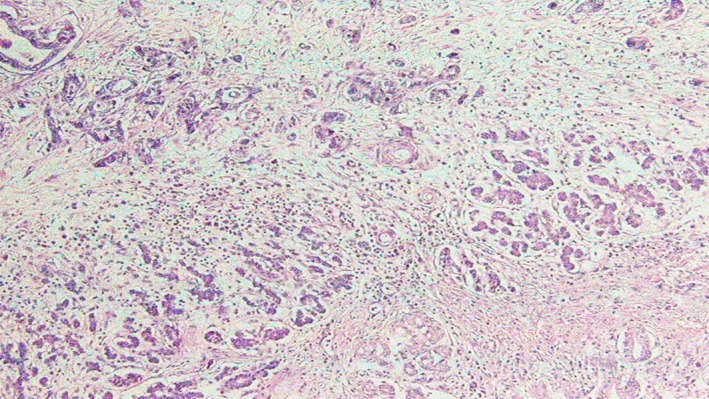
Higher magnification of invasive adenocarcinoma.
Diagnosis: pancreatic adenocarcinoma, moderately differentiated mucin secreting arising in common bile duct region, infiltrating pancreatic head with absence of body and tail, measuring 6 × 4 × 4 cm with free surgical margins. Metastasis was noted in 2/24 peripancreatic lymph nodes. The presence of mature cystic teratoma was observed in the lateral peripancreatic region.
Patient was transferred to the surgical ICU; one night's monitoring was uneventful. He was then transferred to the floor on continuous insulin IV. CT scan (computed tomography scan) was then performed day 7 postoperation showing no intra‐abdominal collections, drains were removed, patient was started on liquid diet, and no complications were encountered. Patient's diet was progressed and he was discharged home on insulin replacement therapy and pancreatic enzyme supplementation.
Discussion
Being part of the gastrointestinal organs system, the pancreas develops from the endoderm. Between the sixth and eighth week of embryonic life, two evaginations, dorsal and ventral, emerge from the primitive duodenum. The ventral evagination arises from a liver diverticulum and will eventually form the posterior part of the pancreatic head and the uncinate process. On the opposite site of the foregut, the dorsal evagination develops and will eventually form the tail and body of the pancreas. Later on, ducts begin to develop in a treelike fashion, endocrine cells appear, and the islets of Langerhans emerge with time 1, 2. The pancreas is a retroperitoneal organ, extending from the duodenal arc to the spleen. It harbors two ducts: the main duct of Wirsung and the duct of Santorini 3, 4.
With the advancement of imaging techniques, and increasing diagnostic workups made, pancreatic malformations are more described.
Total pancreatic agenesis has been described in the literature but is an extremely rare entity, usually incompatible with life, and associated mainly with sever intrauterine growth retardation 5, 6. Other types of congenital anomalies of the pancreas are more frequently encountered such as pancreas divisum, common bile duct syndrome, ectopic pancreas, accessory pancreatic lobe, annular pancreas, and agenesis of the dorsal pancreas (Table 1).
Table 1.
Pancreatic malformations 7
| Anomalies | Anatomical disorder | Frequency |
|---|---|---|
| Pancreas divisum | Fusion failure between dorsal and ventral buds, mainly ducts: Wirsung and Santorini with two distinct draining orifices | 4–14% |
| Annular pancreas | Rotation failure of ventral bud: pancreatic tissue enveloping the second part of the duodenum | 1/20,000 |
| Ectopic pancreas | Pancreatic tissue arising elsewhere in the GI tract (gastric antrum, jejunum, duodenum, appendix, Meckel's diverticulum, etc.) with no vascular or anatomical continuity with the pancreas | 1–15% |
| Accessory pancreatic lobe | Pancreatic tissue, arising from the pancreas, containing an aberrant pancreatic duct, in continuity with the main pancreatic duct and in most often with a gastric duplication cyst | Extremely rare |
| Agenesis of the dorsal pancreas |
Complete: Anterior head, body, tail, Santorini's duct, and minor papilla are absent Partial: Some pancreatic tissue is present, and Santorini's duct and minor papilla remnants are present |
Extremely rare |
Over the last 100 years, 58 cases were reported with agenesis of the dorsal pancreas, whether partial or complete, with the latter being less frequent, and around nine cases only were associated with pancreatic tumor (Table 2). The first case was published in 1911 8. It has been associated with pancreatic bud primary dysgenesis, ischemic insult to the organ during its development, and an autosomal dominant genetic mode of transmission 1, 9, 10.
Table 2.
Cases of agenesis of the dorsal pancreas with associated pancreatic tumors found in the literature
| Case | Age/gender | Presentation | Tumor | Management | Outcome |
|---|---|---|---|---|---|
| Matsusue et al. 11 | 53/F | Abdominal pain, weight loss, hyperglycemia | Adenocarcinoma | Total pancreatectomy, lymph node dissection, Roux‐en‐Y gastrojejunostomy, hepaticojejunostomy | No recurrence |
| Ulusan et al. 12 | 72/M | Abdominal pain, jaundice, hyperglycemia | Adenocarcinoma | Hepaticojejunostomy, cholecystectomy + chemotherapy | Unknown |
| Ulusan et al. 13 | 49/F | Abdominal pain, hyperglycemia | Solid pseudopapillary tumor | Whipple procedure | No recurrence |
| Nakamura et al. 14 | 28/F | Asymptomatic | Solid papillary tumor | Partial pancreatic head resection | No recurrence |
| Rittenhouse et al. 15 | 37/F | Abdominal pain, hyperglycemia | Ductal adenocarcinoma | Pancreatic head and uncinate process resection, hepaticojejunostomy, duodenojejunostomy + chemotherapy | Death (at 17 month) |
| Rittenhouse et al. 15 | 59/F | Weight loss | Ductal adenocarcinoma | Pancreatic head and uncinate process resection, hepaticojejunostomy, duodenojejunostomy + chemotherapy + radiation | No recurrence (at 38 month) |
| Rittenhouse et al. 15 | 68/M | Elevated LFTs | Ductal adenocarcinoma | Pancreatic head and uncinate process resection, hepaticojejunostomy, duodenojejunostomy + chemotherapy + radiation | No recurrence (at 38 month) |
| Sakpal et al. 16 | 49/M | Fatigue, diarrhea, weight loss | IPMN with well differentiated, invasive, mucinous adenocarcinoma | Whipple procedure with total pancreatectomy, lymph node dissection | No recurrence (at 6 week) |
| Sannappa et al. 17 | 51/F | Painless jaundice | Adenocarcinoma | Whipple procedure with total pancreatectomy, lymph node dissection + chemotherapy | Unknown |
| Our case | 29/M | Abdominal pain | Infiltrating, moderately differentiated mucinous adenocarcinoma + cystic teratoma | Whipple procedure (pyloric preserving) with total pancreatectomy, lymph node dissection + chemotherapy | No recurrence (at 3 month) |
Agenesis of the dorsal pancreas is a rare congenital malformation, and its association with tumors makes it a hard condition to deal with. Many studies are trying to identify the reason behind this anomaly, with findings suggestive of genetic inheritance in an autosomal dominant mode or X‐linked mode 11, 19. Other hypotheses state a genomic activation process, notably the Sonic and Indian hedgehog genes 20, HNF1β gene 18, and others. As demonstrated by the literature review done, the cornerstone for curing tumors in association with dorsal agenesis of the pancreas is surgery. Most of the time, the lack of sufficient pancreatic tissue makes it hard to achieve safe margins without undergoing a total pancreatectomy. Our patient presented with an additional associated cystic teratoma. Adenocarcinoma associated or arising from previous teratoma tumors is by itself a rare entity 21, 22, 23, 24. This constellation of presenting tumors with a rare congenital anomaly makes the genetic hypothesis in the frontline of investigations.
Until today, the proper management of tumors associated with agenesis of the dorsal pancreas consists of surgical intervention for malignant cases and/or symptomatic benign cysts, with or without chemotherapy regimens with respect to the pathology finding. The additional challenge comes afterward involving pancreatic endocrine and exocrine replacement therapies. Such cases should be handled in referral center where patient's monitoring can be achieved optimally on the surgical and medical level.
Furthermore, associated genes should be evaluated in such patients and on a larger scale as well.
Ethics Approval and Consent to Participate
The approval of “The University of Balamand Faculty of Medicine IRB” was obtained (IRB/O/031‐16).
Consent for Publication
A written consent was obtained from the patient to publish this case report.
Availability of Data and Materials Section
The datasets supporting the conclusions of this article are included within the article and its references.
Authorship
ES: did the literature review and corrected the article. AEA: wrote the article and organized the figures, tables, and legends. FAF: did the data collection and obtained the patient's consent. MA: followed up on all pathology data, figures, and annotations. ZER: performed the operation and reviewed the written article.
Conflict of Interest
None of the authors has any competing interest.
Acknowledgments
This research was supported by Michelle Arnaout, MD, and Fatmeh Ghandour, MD. We thank our colleagues from the pathology department who provided insight and expertise that greatly assisted this manuscript.
References
- 1. Cano, D. A. , Hebrok M., and Zenker M.. 2007. Pancreatic development and disease. Gastroenterology 132:745–762. [DOI] [PubMed] [Google Scholar]
- 2. Spagnoli, F. M. 2007. From endoderm to pancreas: a multistep journey. Cell. Mol. Life Sci. 64:2378–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brunicardi, F. C. , Andersen D. K., Billiar T. R., Dunn D. L., Hunter J. G., Matthews J. B., and Pollock R. E.. 2014. Schwartz's principles of surgery. 10th ed. McGraw‐Hill Education, New York. [Google Scholar]
- 4. Skandalakis, L. J. , Skandalakis J. E., and Skandalakis P. N.. 2008. Surgical anatomy and technique. Springer, New York. [Google Scholar]
- 5. Ashraf, A. , Abdullatif H., Hardin W., and Moates J. M.. 2005. Unusual case of neonatal diabetes mellitus due to congenital pancreas agenesis. Pediatr. Diabetes 6:239–243. [DOI] [PubMed] [Google Scholar]
- 6. Baumeister, F. A. , Engelsberger I., and Schulze A.. 2005. Pancreatic agenesis as cause for neonatal diabetes mellitus. Klin. Pädiatr. 217:76–81. [DOI] [PubMed] [Google Scholar]
- 7. Türkvatan, A. , Erden A., Türkoğlu M. A., and Yener Ö.. 2013. Congenital variants and anomalies of the pancreas and pancreatic duct: imaging by magnetic resonance cholangiopancreaticography and multidetector computed tomography. Korean J. Radiol. 14:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schnedl, W. J. , Piswanger‐Soelkner C., Wallner S. J., Reittner P., Krause R., Lipp R. W., et al. 2009. Agenesis of the dorsal pancreas and associated diseases. Dig. Dis. Sci. 54:481–487. [DOI] [PubMed] [Google Scholar]
- 9. Macari, M. , Giovanniello G., Blair L., and Krinsky G.. 1998. Diagnosis of agenesis of the dorsal pancreas with MR pancreatography. AJR Am. J. Roentgenol. 170:144–146. [DOI] [PubMed] [Google Scholar]
- 10. Schnedl, W. J. , Reisinger E. C., Schreiber F., Pieber T. R., Lipp R. W., and Krejs G. J.. 1995. Complete and partial agenesis of the dorsal pancreas within one family. Gastrointest. Endosc. 42:485–487. [DOI] [PubMed] [Google Scholar]
- 11. Matsusue, S. , Kashihara S., and Koizumi S.. 1984. Pancreatectomy for carcinoma of the head of the pancreas associated with multiple anomalies including the preduodenal portal vein. Surg. Today 14:394–398. [DOI] [PubMed] [Google Scholar]
- 12. Ulusan, S. , Yakar T., Koc Z., Kayaselcuk F., and Torer N.. 2006. Adenocarcinoma of the pancreas associated with dorsal agenesis. Pancreas 33:437–439. [DOI] [PubMed] [Google Scholar]
- 13. Ulusan, S. , Bal N., Kizilkilic O., Bolat F., Yildirim S., Yildirim T., et al. 2014. Solid‐pseudopapillary tumour of the pancreas associated with dorsal agenesis. Br. J. Radiol. 78:441–443. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura, Y. , Egami K., Maeda S., Hosone M., and Onda M.. 2001. Solid and papillary tumor of the pancreas complicating agenesis of the dorsal pancreas. J. Hepatobiliary Pancreat. Surg. 8:485–489. [DOI] [PubMed] [Google Scholar]
- 15. Rittenhouse, D. W. , Kennedy E. P., Mascaro A. A., Brumbaugh J. L., Stein L. H., Rosenberger L. H., et al. 2011. The novel triad of dorsal agenesis of the pancreas with concurrent pancreatic ductal adenocarcinoma and nonalcoholic chronic calcific pancreatitis: a case series and review of the literature. J. Gastrointest. Surg. 15:1643–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakpal, S. V. , Sexcius L., Babel N., and Chamberlain R. S.. 2009. Agenesis of the dorsal pancreas and its association with pancreatic tumors. Pancreas 38:367–373. [DOI] [PubMed] [Google Scholar]
- 17. Sannappa, R. M. , Buragohain J., Sarma D., Saikia U. K., and Choudhury B. K.. 2014. Agenesis of dorsal pancreas associated with periampullary pancreaticobiliary type adenocarcinoma. JOP 15:489–492. [DOI] [PubMed] [Google Scholar]
- 18. Rastogi, R. , Kumar R., Bhargava S., and Rastogi V.. 2009. Isolated pancreatic hypoplasia: a rare but significant radiological finding. Saudi J. Gastroenterol. 15:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schnedl, W. J. , Schreiber F., and Krejs G.. 1993. Agenesis of the dorsal pancreas in a woman with diabetes mellitus and in both of her sons. Gastroenterology 104:1182–1186. [DOI] [PubMed] [Google Scholar]
- 20. Balakrishnan, V. , Narayanan V. A., Siyad I., Radhakrishnan L., and Nair P.. 2006. Agenesis of the dorsal pancreas with chronic calcific pancreatitis. Case report, review of the literature and genetic basis. JOP 7:651–659. [PubMed] [Google Scholar]
- 21. Li, H. , Arber S., Jessell T. M., and Edlund H.. 1999. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat. Genet. 23:67–70. [DOI] [PubMed] [Google Scholar]
- 22. Martín, M. , Gallego‐Llamas J., Ribes V., Kedinger M., Niederreither K., Chambon P., et al. 2005. Dorsal pancreas agenesis in retinoic acid‐deficient Raldh2 mutant mice. Dev. Biol. 284:399–411. [DOI] [PubMed] [Google Scholar]
- 23. Cheung, W. L. , and Cao D.. 2008. Colonic‐type adenocarcinoma arising in a primary retroperitoneal mature cystic teratoma. Pathol. Int. 58:792–796. [DOI] [PubMed] [Google Scholar]
- 24. Chang, Y. L. , Wu C. T., and Lee Y. C.. 2006. Mediastinal and retroperitoneal teratoma with focal gastrointestinal adenocarcinoma. J. Thorac. Oncol. 1:729–731. [PubMed] [Google Scholar]
- 25. Soler, R. , Rodríguez E., Comesaña M. L., Pombo F., and Marini M.. 1992. Agenesis of the dorsal pancreas with polysplenia syndrome: CT features. J. Comput. Assist. Tomogr. 16:921–923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its references.


