Abstract
chaos1 (for chromosome aberrations occurring spontaneously 1) is a recessive mutation that was originally identified in a phenotype-based screen for chromosome instability mutants in mice. Mutant animals exhibit significantly higher frequencies of spontaneous and radiation- or mitomycin C-induced micronucleated erythrocytes, indicating a potential defect in homologous recombination or interstrand cross-link repair. The chaos1 allele was genetically associated with a missense mutation in Polq, which encodes DNA polymerase θ. We demonstrate here that chaos1 is a mutant allele of Polq by using two genetic approaches: chaos1 mutant phenotype correction by a bacterial artificial chromosome carrying wild-type Polq and a failed complementation test between chaos1 and a Polq-disrupted allele generated by gene targeting. To investigate the potential involvement of Polq in DNA double-strand break repair, we introduced chaos1 into an Atm (for ataxia telangiectasia mutated)-deficient background. The majority (∼90%) of double-homozygous mice died during the neonatal period. Surviving double mutants exhibited synergistic phenotypes such as severe growth retardation and enhanced chromosome instability. However, remarkably, double mutants had delayed onset of thymic lymphoma, significantly increasing life span. These data suggest a unique role of Polq in maintaining genomic integrity, which is probably distinctive from the major homologous recombination pathway regulated by ATM.
Genomic integrity is achieved by concerted functions of genes involved in all aspects of DNA metabolism throughout the cell cycle. Fundamental mechanisms for genome maintenance seem to be conserved throughout eukaryotes (16, 38, 39). However, given the large size and complexity of mammalian genomes, there are likely to be additional genes or pathways involved that remain to be discovered. Since mutations causing chromosome instability increase the risk of developing malignancies (14, 18, 45), exploring genes involved in genome maintenance is a first step to understand fundamental mechanisms of carcinogenesis.
To uncover mammalian genes not previously known to have roles in maintaining genomic integrity, we have taken a forward genetics approach in mice. New chromosome instability mutations, arising from mouse N-ethyl-N-nitrosourea (ENU) mutagenesis (3), have been identified by a phenotype-based screen (37). In this screen, spontaneous micronucleus levels in erythrocytes, measured by a flow cytometric assay, were used to quantitate chromosome damage in vivo (11, 27). Micronuclei are derived from acentromeric chromosome fragments or from a whole chromosome having failed to be incorporated into the nuclei at mitosis, thereby representing chromosome breaks and aneuploidy (29). Spontaneous and radiation-induced chromosome instability in Atm (for ataxia telangiectasia mutated)-deficient mice can be detected as elevated levels of micronuclei in erythrocytes (37).
To induce mutations that potentially cause chromosome instability, C57BL/6J males were mutagenized with the powerful germ line mutagen ENU (3, 13) and were bred in a classical three-generation scheme to produce descendants that are potentially homozygous for induced recessive mutations (37). These mice were subjected to a phenotype screen in which micronuclei in erythrocytes were semiautomatically measured by flow cytometry (6). Mutants were identified as outliers showing significantly higher numbers of spontaneous micronuclei. Using this screen, five independent mutations were identified among 763 pedigrees derived from the mutagenized males.
chaos1 (for chromosome aberrations occurring spontaneously 1) was the first mutation identified in this screen. Treatment with radiation or mitomycin C (MMC) induced significantly higher frequencies of micronuclei in chaos1/chaos1 mice to a level that indicates hypersensitivity to agents inducing double-strand breaks (DSBs) or interstrand cross-links. This recessive mutation was genetically mapped on a 1.3 Mb interval on chromosome 16 (37). Among the genes residing in this region was Polq encoding DNA polymerase θ (theta). POLQ is homologous to Drosophila MUS308 that is believed to be involved in DNA interstrand cross-link repair (5, 10, 21). Orthologs apparently do not exist in single celled organisms such as bacteria and yeast. POLQ is also unique in that it contains helicase and polymerase domains near the N and C termini, respectively (10, 34, 37).
There are at least 15 different DNA polymerases in higher eukaryotes (15, 36), and POLQ belongs to the “A” family. Whereas classical replicative DNA polymerases (α, δ, and ɛ) are essential for chromosomal DNA replication, recently discovered DNA polymerases are specialized for different cellular processes (15, 36). The most characteristic feature of these novel DNA polymerases (such as those of “Y” family) is the ability to bypass DNA lesions that block replicative DNA polymerases, termed translesion synthesis (7, 32, 33). There have been two biochemical studies on the DNA polymerase activity of human POLQ in vitro. Although one suggested a potentially unique translesion synthesis activity (22), it was not clear that the purified activity actually corresponded to full-length POLQ (34). Here, we conducted a genetic analysis of POLQ's involvement in maintenance of genomic integrity, which points to a potential role in recombinational repair.
The Polq coding sequence in chaos1/choas1 mice was found to contain a de novo T-to-C transition, causing a serine-to-proline change (37). However, since this amino acid is not located in a conserved or critical region, it remained uncertain whether or not this mutation actually compromised protein function, to what extent, and if it was responsible for the micronucleus phenotype. Transgenic and knockout experiments reported here confirm the allelism of chaos1 to Polq.
An enigmatic aspect of the chaos1 mice is the absence of overt phenotypes despite the elevated genomic instability in erythroblasts. To explore the suspected role in DSB repair, the chaos1 mutation was placed in a background deficient for ATM, which plays a key role in activating DNA damage signaling and cell cycle checkpoints (1), and in regulating DSB repair by homologous recombination (8, 28, 41-43). This resulted in synthetic semilethality, growth retardation, synergistic elevation in chromosome instability and, surprisingly, significantly delayed development of thymic lymphoma. The role(s) of POLQ in DSB repair and genome maintenance is discussed in light of these data.
MATERIALS AND METHODS
Generation and identification of BAC transgenic mice.
DNA from bacterial artificial chromosome (BAC) clone RPCI24-108G13 was prepared by using a Qiagen large-construct kit (Qiagen, Inc., Valencia, Calif.) and was injected into the pronuclei of C57BL/6J fertilized eggs. Microinjected eggs were then transferred to BALB/cByJ × C57BL/6J recipient mothers. Offspring carrying the BAC transgene were identified by PCR analysis on tail DNA from 5-day-old pups, with primers specific to the T7 (5′-CTT TTA ATT GGG TGC AGA GCT C-3′) or SP6 (5′-CCC ATT CCC TGA ATA AAC TC-3′) ends, in conjunction with standard SP6 and T7 primers.
Creation of a disrupted Polq allele by gene targeting.
A targeting construct was designed to modify part of exon 1 and to replace exons 2 to 5 with a neo cassette. The primers Polq5KpnI (5′-GGG GTA CCT GGT TCT TGC TCT GTA G-3′) and Polq5BamHIR2 (5′-CGG GAT CCA TCT CAC GAG AAC GTG TC-3′) were used to amplify a 5′-arm fragment (∼4.3 kb) and to add KpnI and BamHI sites to the ends. PCR was performed with PfuTurbo DNA polymerase (Stratagene) on BAC RP24-108G13 DNA. The amplicon was digested with KpnI and BamHI and ligated into a unique KpnI-BamHI site of the vector ploxPNT. A 4.6-kb 3′ arm was amplified by using primers Polq3XhoI (5′-CCG CTC GAG TTG CAT GCA GCA TG-3′) and Polq3NotR2 (5′-ATA GTT TAG CGG CCG CAC TGC CCA CGC-3′), which include XhoI and NotI sites. After digestion, the fragment was ligated into XhoI- and NotI-digested vector to complete the Polq targeting construct.
Next, 50 μg of Polq targeting vector was linearized and electroporated into 107 v6.4 mouse embryonic stem (ES) cells. Transformants were selected with G418 (250 μg/ml; Gibco-BRL, Rockville, Md.) and 0.2 μM FIAU (2′-fluoro 2′-deoxy-5-iodouracil-β-d-arabinofuranoside; Moravek Biochemicals, Brea, Calif.). Correctly targeted clones were identified by Southern blot analysis with 5′ and 3′ probes as indicated in Fig. 3.
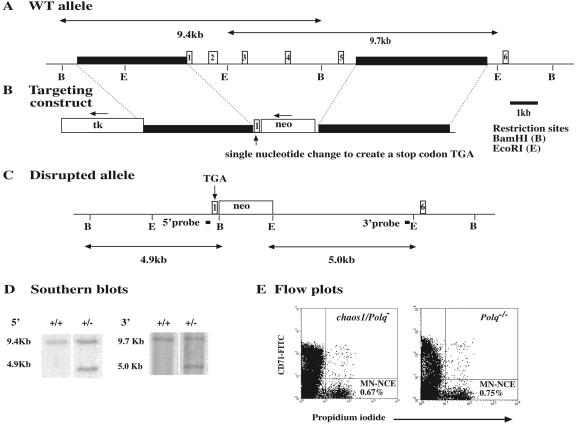
FIG. 3.
Polq gene targeting strategy. (A) Schematic representation of a part of the genomic Polq locus. The first six exons are indicated as boxes with numbers. Locations of selected restriction sites are shown. (B) The targeting construct was designed to replace exons 2 to 5 with a neomycin resistance gene (neo) by homologous recombination. Solid rectangles represent genomic sequences used for each arm, one of which contains a part of exon 1 modified to contain a premature stop codon (TGA). The position of the negatively selectable marker thymidine kinase (tk) is also shown. Small arrows indicate the direction of transcription. (C) The disrupted allele lacks exons 2 to 5 and contains modified exon1 with a stop codon just after the initiation codon. (D) Southern blot analysis of correctly targeted ES cell clones, in which the expected sizes of BamHI (left) and EcoRI (right) fragments were detected by the probes indicated in panel C. (E) Representative flow plots of micronucleus assays on chaos1/Polq− mice and Polq−/− mice. Spontaneous micronuclei in CD71-negative normochromatic erythrocytes were detected by propidium iodide.
The Polq disrupted allele was genotyped by multiplex PCR with a generic neo primer pair plus a pair specific to Polq exon 3 (which is deleted in the disrupted allele), respectively. The primer sequences and PCR conditions are available upon request.
Measurement of micronuclei in erythrocytes.
The flow cytometric peripheral blood micronucleus assay was conducted as described previously (6, 37).
Northern blot analysis.
Total RNA was isolated from various tissues by using Qiagen RNeasy midi kit. Approximately 20 μg of total RNA was electrophoresed on a formaldehyde gel, blotted onto a nylon membrane (MagnaCharge; GE Osmonics, Inc.), probed with a cDNA fragment of Polq (4302 to 5052 bp of Polq CDS; AY074936), and labeled with 32P by random primer extension. As a loading control, the blot was reprobed with a fragment of the Gapdh gene. Sizes were calculated by comparison of mobility to the Gibco-BRL 0.24- to 9.5-kb RNA ladder.
RT-PCR analysis of Polq cDNA.
Whole-blood RNA was extracted by RNAqueous-Blood (Ambion, Inc., Austin, Tex.). Then, 5 μg of total RNA was used for reverse transcription (RT) reactions with Super-Script II (Gibco-BRL), followed by PCR with Polq primer pairs. The primer sequences are available upon request. cDNA was sequenced on an ABI 3700 DNA analyzer (Applied Biosystems, Foster City, Calif.). To examine Polq expression, quantitative PCR was performed on normalized cDNAs (mouse MTC panel I; BD Biosciences/Clontech) from different tissues.
Assays on primary mouse embryonic fibroblasts (MEFs).
Pregnant females were sacrificed to isolate 12.5- to 14.5-day-old embryos. After removal of extraembryonic tissues and red organs (lung, heart, and liver), each embryo was homogenized separately, and the resulting crude cell suspension was seeded in a T-75 flask (passage 1). Cells were cultured in Dulbecco modified Eagle medium (Gibco-BRL) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah) and penicillin-streptomycin (100 U/ml; Gibco-BRL). All assays were conducted with cells at early passages (up to passage 3). Cells were seeded in 60-mm plates (105 cells/plate), grown for various times, and counted for growth curve analyses. To determine sensitivities to MMC or gamma rays, cells (105 cells/60-mm plate) were treated 24 h after seeding with increasing doses of either of these agents (for MMC, 2-h exposure) and cultured for 5 days. The numbers of cells surviving the treatment were counted and compared to those of untreated controls. The relative growth was expressed as a percentage of the control group.
Exponentially growing MEFs were treated with colcemid (0.1 μg/ml) for 3 to 5 h and then treated with trypsin and pelleted. Cells were resuspended in 0.56% KCl solution and incubated for 20 min at 37°C. Cells were then pelleted and fixed with ice-cold 3:1 (vol/vol) methanol-acetic acid for 30 min on ice. Fixed cells were washed with the fixative twice and spread onto glass slides, stained with Giemsa, and observed.
Generation of Atm and chaos1 double homozygotes.
Atm-deficient mice (129S6/SvEvTac-AtmtmAwb) were purchased from The Jackson Laboratory (Bar Harbor, Maine). Progeny from crosses between Atm and C3H.B6-chaos1/chaos1 mice were PCR genotyped at 5 days of age. Genotyping of Atm was performed by using a protocol available at http://jaxmice.jax.org/index.html. For chaos1, 150 bp of Polq genomic sequence was amplified with the primers chaos1typeC (5′-CCT CTG GAC GCA ACA CTA ACT-3′) and chaos1type2 (5′-CTC CAG GGA GAT GCC CCA TG-3′), followed by digestion of the product with NcoI, which cleaves the chaos1 allele but not wild type. These fragments were separated and visualized on 4% MetaPhor agarose gels (Cambrex Bio Science Rockland, Inc., Rockland, Maine). Mice husbandry and all of the procedures were approved by The Jackson Laboratory Animal Care and Use Committee.
RESULTS
Polq BAC transgenes correct the chaos1 mutant phenotype.
Polq resides on central chromosome 16 (coordinates 36.74 to 36.83 Mb of mouse genome assembly 32 presented in Ensembl). The chromosome 16 BAC clone RPCI-24-108G13 contains ∼162 kb of genomic DNA (36.73 to 36.89 Mb), encompassing the entire Polq gene and first three exons of a predicted gene with unknown function, A830015P08Rik. Neither the public databases nor the Celera Discovery System (CDS) annotates other genes on this BAC clone. The presence of Polq genomic sequence on this BAC was confirmed by PCR analysis with primers specific to Polq exons (data not shown).
Of 50 pups derived from microinjected eggs, three founders carrying the BAC transgene (designated as lines 6353, 6354, and 6355) were obtained. Each founder was positive for both BAC ends (see Materials and Methods; also data not shown), indicating that an entire BAC was probably integrated into their genomes. Breeding analyses indicated that the transgenes are unlinked to the Polq locus on chromosome 16.
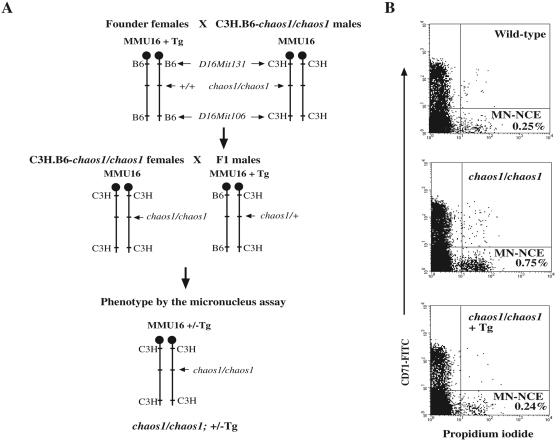
To test for phenotype rescue, the BAC transgenes were introduced into the chaos1/chaos1 background (Fig. 1). Two polymorphic markers on chromosome 16, one proximal (D16Mit131) and the other distal (D16Mit106), were used to identify homozygosity for the chaos1 allele. Four chaos1/chaos1 mice without the transgene had micronucleus frequencies ranging from 0.62 to 0.73%, whereas six chaos1/chaos1 mice with two different transgene insertions (lines 6354 and 6355) had wild-type levels of micronuclei (0.14 to 0.32%), indicating that chaos1 phenotype was corrected. However, one transgenic line (line 6353) failed to correct the chaos1 phenotype (data not shown).
FIG. 1.
Phenotype rescue by BAC transgene (Tg). (A) BAC transgenic founder females (generated in B6 background) were outcrossed to C3H.B6-chaos1/chaos1 males (N10F1) in which the chaos1 allele had been introduced into C3H genome by backcrossing nine generations. This was necessary to identify chromosome 16 that carries the chaos1 allele. Resulting F1 males carrying the BAC transgene were mated with C3H.B6-chaos1/chaos1 females. Among their offspring, chaos1/chaos1 mice were identified as those homozygous for the C3H alleles of the two polymorphic microsatellite markers D16Mit131 (proximal) and D16Mit106 (distal). chaos1/chaos1 mice (+/−Tg) were phenotyped by the micronucleus assay. (B) Spontaneous micronucleus frequencies were measured in CD71-negative normochromatic erythrocytes (NCE; lower quadrants of the plots). Micronucleated erythrocytes (MN-NCE) are in the population positive to propidium iodide (lower right quadrant). Anti-CD71 antibody was used to separate reticulocytes (younger erythrocytes) containing significant amounts of RNA, which potentially interferes with accurate enumeration of micronuclei in NCE. The transgene carriers show a normal range of micronucleus frequencies as comparable to those seen in wild-type mice, whereas chaos1/chaos1 mice exhibited significantly higher micronucleus frequencies, indicating complete phenotype correction. At least 10,000 erythrocytes were collected.
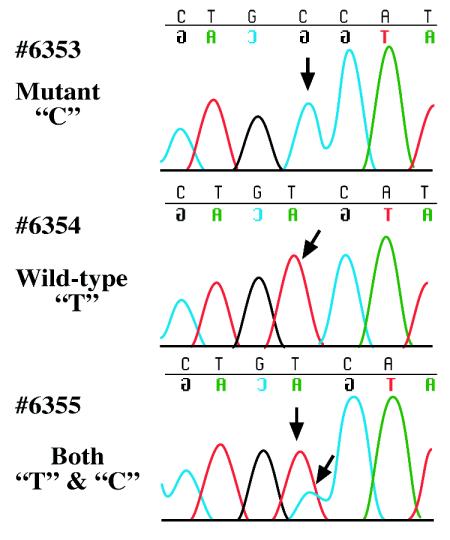
To examine expression of the Polq transgene of all three lines, RT-PCR was performed on peripheral blood RNA, and the cDNAs were sequenced. As shown in Fig. 2, wild-type “T” was observed in samples from the two rescuing lines 6354 and 6355, demonstrating expression of the transgene. In contrast, only mutant “C” was observed in the nonrescuing line 6353, indicating that the transgene is not transcribed. The positive and negative correlations between transgene expression and phenotype rescue supports the conclusion that chaos1 is a mutant allele of Polq.
FIG. 2.
BAC transgene expression is correlated with phenotype rescue. Shown are sequence traces of Polq peripheral blood cDNA from the indicated transgenes in chaos1/chaos1 background. Arrows show the mutated residue in the chaos1 allele. Although cDNA from the rescuing transgene lines 6354 and 6355 contained wild-type “T”; only mutant “C” was observed in the nonrescuing line 6353.
Polq−/− mice are viable, exhibit the micronucleus phenotype, and fail to complement chaos1.
To obtain definitive evidence that chaos1 is an allele of Polq, and to investigate the nature of the allele, Polq was disrupted by gene targeting. A targeting vector was designed to place an in-frame stop codon into exon 1 and to replace exons 2 to 5 with a neomycin resistance gene (neo). Two correctly targeted ES clones were identified by Southern blot analysis (Fig. 3). They were injected into B6 blastocysts, and one produced germ line chimeras that transmitted the disrupted allele to offspring. These germ line chimeras were mated with chaos1/chaos1 females, and resulting progeny were phenotyped by the micronucleus assay. Although progeny carrying only chaos1 or the Polq disrupted allele (Polqtm1Jcs, abbreviated Polq−) exhibited normal spontaneous micronucleus frequencies (data not shown), progeny carrying both chaos1 and the Polq-disrupted alleles exhibited elevated micronucleus frequencies typical of chaos1 homozygotes (Fig. 3). The failure of the disrupted allele to complement chaos1, in conjunction with the BAC rescue data, clearly demonstrate that chaos1 is a mutant allele of Polq. Accordingly, the chaos1 allele is termed Polqm1chaos1Jcs (abbreviated Polqchaos1).
To determine whether POLQ-deficient mice have a phenotype different from Polqchaos1 mutants, Polq−/− animals were produced by intercrossing heterozygotes. Homozygotes were born at expected Mendelian ratios (Table 1). They appeared normal through 8 months of age, like Polqchaos1/Polqchaos1 mice. The micronucleus frequencies were essentially indistinguishable from Polqchaos1 homozygotes.
TABLE 1.
Homozygotes at the Polq-disrupted allele (Polq−/−) are viable
| Mice | No. with Polq genotype:
|
Total no. | ||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| Produced | 22 | 47 | 15 | 84 (85)a |
| Expected | 21 | 42 | 21 | |
One mouse with unknown genotype was excluded from analysis.
Polqchaos1 confers no radiation or MMC hypersensitivity in cultured cells or whole animals.
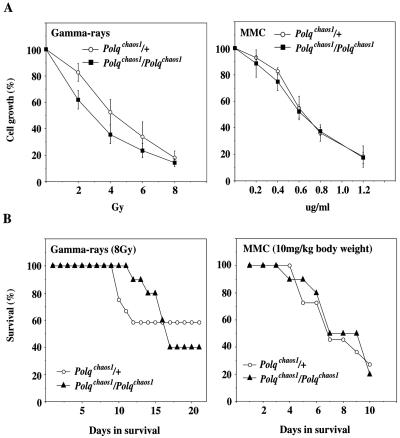
Primary MEFs were generated to examine possible radiation or MMC sensitivity in Polqchaos1/Polqchaos1 cells. Cells were treated with these agents, and 5 days later the relative growth was compared. In contrast to the hypersensitivities of Polqchaos1/Polqchaos1 erythroblasts to these agents as assayed by the micronucleus test, the MEFs did not show significant hypersensitivities to either of these agents (Fig. 4A).
FIG. 4.
Polqchaos1/Polqchaos1 cells and animals exhibit no hypersensitivities to radiation or MMC. (A) Relative growth of MEFs 5 days after gamma-ray or MMC treatment. Each point is shown with the standard deviation. Experiments were replicated at least twice by using independent MEF lines derived from different animals. (B) Survival curves after whole-body exposure to 8 Gy gamma rays (1.35 Gy/min) or a single intraperitoneal injection of MMC (10 mg/kg [body weight]). Mice were monitored daily after the treatment. If mice showed any signs of being moribund, they were immediately euthanized.
To examine possible sensitivities to these agents at the organismal level, young adult mice (8 to 12 weeks old) were exposed to 8 Gy of gamma rays or injected with 10 mg of MMC/kg (body weight), and survival was monitored. The results are shown in Fig. 4B. Polqchaos1/Polqchaos1 mice were no more sensitive to these agents than wild-type mice.
Polq expression is tissue type restricted.
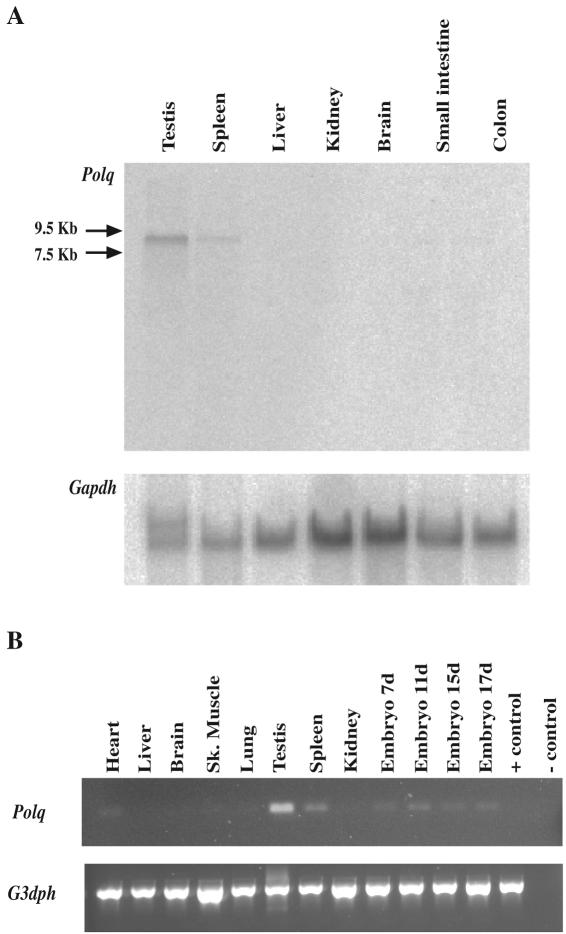
Since Polqchaos1 homozygotes are not hypersensitive to gamma rays or MMC at either the cellular or organismal level, it is possible that Polq expression could be limited to certain tissues. Therefore, Polq expression was examined in a variety of tissues by Northern blotting. An ∼8.5-kb transcript was detectable in the testis and spleen but not in the other tissues examined (Fig. 5). Semiquantitative RT-PCR revealed Polq transcripts in the heart, testis, and spleen of 7-, 11-, 15-, and 17-day-old whole embryos but not in several other organs (Fig. 5). In both experiments, the highest Polq expression was recognized in the testis, and relatively low expression of Polq was observed in embryonic and lymphoid tissues. These data confirmed that Polq expression was limited to certain tissues, and its expression level was relatively low. These data are consistent with previous studies in mice and humans (17, 34).
FIG. 5.
Expression of Polq in mouse tissues. (A) Northern hybridization analysis. Polq expression was detectable only after exposure of a FUJI imaging plate (overnight) to the blot that contains ca. 20 μg of total RNA, whereas the control Gadph expression was clearly detected after only 4 h of exposure. (B) Semiquantitative PCR on normalized cDNA from different tissues. Positive/negative (+/−) controls indicate PCR on control cDNA provided by the manufacturer and on the sample without reverse transcription, respectively. PCR was performed by using Polq or G3dph primers as indicated.
Synthetic semilethality of Polqchaos1 and Atm double mutants.
Aside from having elevated micronuclei in peripheral blood, chaos1/chaos1 mice appear phenotypically normal, which may correlate with the restricted expression profile. Considering the hypersensitivities of chaos1/chaos1 erythroblasts to gamma radiation and cross-linking agents, we hypothesized that POLQ could be involved in a minor pathway of DSB repair potentially by homologous recombination and that such a role could be exposed in a sensitized genetic background. To explore potential involvement of Polq in DSB repair by homologous recombination, the Polqchaos1 mutation was bred into an Atm-deficient background (4). Progeny resulting from the crosses were genotyped at 5 days of age. As shown in Table 2, the number of doubly homozygous mutants was significantly lower than expected, indicating that the combination of these two mutations is semilethal.
TABLE 2.
Combination of Atm and Polqchaos1 mutations results in synthetic semilethality
| Mice | No. with Atm, Polqchaos1 genotypes:
|
Total no. | |||||
|---|---|---|---|---|---|---|---|
| +/+, +/− | +/+, −/− | +/−, +/− | +/−, −/− | −/−, +/− | −/−, −/− | ||
| Produceda | 44 | 38 | 98 | 70 | 29 | 3 | 282 |
| Expected | 30.25 | 30.25 | 70.5 | 70.5 | 30.25 | 30.25 | |
P < 0.001, as determined by χ2 test.
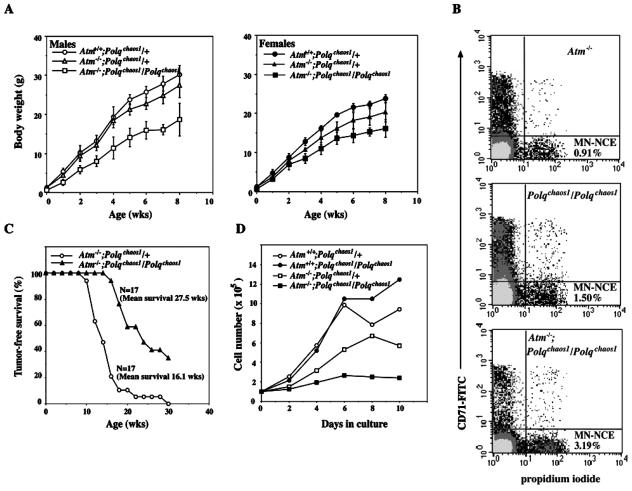
To determine the nature of the lethality, timed matings were conducted to examine 16.5- and 18.5-day-old embryos. As shown in Table 3, compound homozygotes were present at both embryonic stages in Mendelian ratios, suggesting that death was occurring shortly after birth. Therefore, 18.5-day-old embryos were C-section derived, fostered, and observed. The compound homozygotes breathed normally, and some of them had a clear milk spot within a day after birth (data not shown). Histological analyses did not show any signs of developmental abnormalities (data not shown). Therefore, the cause of death remains unknown. However, they were severely growth retarded, having a significantly reduced body weight (ca. 60% of wild-type) at embryonic day 18.5. Therefore, they may simply succumb to an overall lack of fitness. Double mutants that survived the critical neonatal period thrived relatively normally thereafter, despite remaining severely runted relative to littermates. Growth retardation tended to be more severe in males than females (Fig. 6A). The frequencies of spontaneous micronuclei in normochromatic erythrocytes of double homozygotes showed a synergistic increase (>3% of total erythrocytes) over the single mutants (typically 0.5 to 1.5% of total erythrocytes), indicating an enhanced genomic instability (Fig. 6B).
TABLE 3.
Atm/chaos1 double mutants are viable through embryogenesis
| Time (embryonic day) and mice | No. with Atm, Polqchaos1 genotypes:
|
Total no. | ||
|---|---|---|---|---|
| +/+, −/− | +/−, −/− | −/−, −/− | ||
| 16.5 | ||||
| Produced | 6 | 17 | 6 | 29 (31)a |
| Expected | 7.25 | 14.5 | 7.25 | |
| 18.5 | ||||
| Produced | 7 | 16 | 5 | 28 (29)b |
| Expected | 7 | 14 | 7 | |
Two embryos with unknown genotype were excluded from analysis.
One newborn with unknown genotype was excluded from analysis.
FIG. 6.
Synergistic phenotypes observed in Atm/Polqchaos1 double homozygotes. (A) Growth curves of males (left) and females (right) with indicated genotypes. Each point represents at least five animals and is shown with the standard deviation. (B) Enhanced spontaneous micronucleus formation in Atm/Polqchaos1 double homozygotes. Micronuclei in CD71-negative erythrocytes were detected by propidium iodide. (C) The Polqchaos1 mutation significantly delays development of thymic lymphoma in Atm-deficient mice (P < 0.0005, t test). (D) Cell growth of MEFs. Atm/Polqchaos1 double homozygous cell show severely impaired proliferation. Each point is shown with the standard deviation. Experiments were replicated at least once by using two independent MEF lines.
Delayed onset of thymic lymphoma in Atm/Polqchaos1 double mutants.
The majority of ATM-deficient mice succumb to thymic lymphoma at around 3 to 4 months of age (4). To determine whether disruption of POLQ function affects tumor latency or progression, cohorts of surviving Atm−/−; Polqchaos1/Polqchaos1 mice were monitored for development of the malignancy (Fig. 6C). Atm single mutants started dying as early as 10 weeks of age and, by the age of 30 weeks, all 17 Atm−/− mice developed thymic lymphoma. The average tumor-free survival was 16.1 weeks. Whereas double mutants also began developing thymic lymphoma at 17 weeks of age, most outlived the Atm single mutants (Fig. 6). Although 12 of 17 double mutants died by the age of 30 weeks, one double mutant survived nearly a year (data not shown), significantly increasing the average latency at least 27.5 weeks (P < 0.0005 by t test). Therefore, inactivation of Polq partially rescues lymphomagenesis in ATM-deficient mice.
Severe proliferation defects and enhanced chromosome instability in cells mutant for both Atm and Polq.
To characterize cellular phenotypes, primary MEFs were generated from 12.5- to 14.5-day-old embryos and placed in culture to evaluate growth rates (Fig. 6D). Wild-type and Polqchaos1/Polqchaos1 single mutant cells grew normally to confluence. As reported previously (35, 44), Atm homozygous mutant cells exhibited slower growth. Atm−/−; Polqchaos1/Polqchaos1 cells had severe growth defects. They divided once and did not proliferate further.
Chromosome integrity was also characterized in these cells (Table 4). Although chromosome aberrations were rarely observed in wild-type cells (7.7% of metaphases observed), 37.0% of metaphases in Polqchaos1 single mutant cells had abnormalities, most of which were classified as chromatid breaks. More than 80% of metaphases had abnormalities in both Atm single and Atm/Polqchaos1 double mutants. However, double mutants cells tended to have multiple aberrations and more chromosome breaks. Along with the results showing a synergistic increase in micronucleated erythrocytes (Fig. 6B), these data suggest that the absence of functional POLQ enhances chromosome instability in ATM-deficient cells.
TABLE 4.
The Polqchaos1 allele enhances chromosome instability in MEFs
| Parameter | Result with Atm, Polqchaos1 genotypes:
|
|||
|---|---|---|---|---|
| +/+, +/− | +/+, −/− | −/−, +/− | −/−, −/− | |
| No. of metaphases | 26 | 27 | 26 | 25 |
| No. of chromatid gaps | 1 | 8 | 15 | 21 |
| No. of chromatid breaks | 1 | 3 | 30 | 46 |
| No. of chromosome breaks | 0 | 1 | 4 | 12 |
| No. of other abnormalities (including translocations) | 0 | 0 | 3 | 4 |
| Total no. of abnormalities | 2 | 12 | 52 | 83 |
| % Cells with abnormalities | 7.7 | 37.0 | 88.5 | 84.0 |
| No. of abnormalities per cell | 0.08 | 0.44 | 2.0 | 3.32 |
DISCUSSION
chaos1 is a mutant allele of Polq.
chaos1 was originally identified as an autosomal-recessive mutation that caused an elevation in spontaneous and radiation-induced micronuclei in erythrocytes. chaos1 was mapped on a 1.3-Mb interval of chromosome 16 that contains Polq. Mutation analysis revealed the presence of a T-to-C transition resulting in a Ser1932Pro change (37). In the present study, we confirmed that chaos1 is a mutant allele of Polq by two complementary approaches. First, expression of wild-type Polq from a BAC transgene corrected the chaos1 mutant phenotype. Second, a Polq-disrupted allele generated by gene targeting failed to complement chaos1.
From the present data, it is not clear if the Ser1932Pro mutation in the chaos1 allele completely abolishes POLQ biochemical function(s). The targeted allele is likely a null, since a stop codon was inserted into exon 1 and exons 2 and 3 were deleted. With respect to the micronucleus assay, there were no significant differences between Polqchaos1/Polqchaos1, Polqchaos1/Polq−, and Polq−/Polq− mice. However, unlike Polqchaos1 MEFs, Polq−/− ES cells show modest hypersensitivity to radiation and cross-linking agents (N. Shima and J. C. Schimenti, unpublished data). Further experimentation is needed to fully evaluate any potential activity associated with the chaos1 allele.
Polq is preferentially expressed in testis and lymphoid tissues.
By Northern blot and semiquantitative PCR analyses, Polq expression seemed to be very low and limited to the testis, embryonic tissues, and lymphoid tissues such as the spleen. This could partially explain why Polqchaos1 mutant mice exhibit such a mild phenotype. Although the highest expression is in testis, Polqchaos1 and Polq−/− mutant males are fertile. Thus, it is not certain whether POLQ has a role in premeiotic replication or recombination.
Recently, two new POLQ homologs, HEL308 and POLN (pol υ), were discovered. Interestingly, each contains only a helicase or polymerase domain (23, 24). Although helicase activity has not been detected in POLQ (22, 34), HEL308 has active 3′-5′ helicase activity in vitro (24). The modest phenotypes observed in Polqchaos1 or Polq−/− mice could be due to potential redundant functions performed by these paralogs. All of these three genes are highly expressed in testis; however, Hel308 and Poln are also preferentially expressed in other tissues such as the heart and skeletal muscle (23, 24). Because of such differences in expression profiles, it is also possible that HEL308 and POLN have distinct biological roles.
In humans, it was reported that POLQ expression was limited to the testis, colon, primary lymphoid tissues (bone marrow, thymus, and fetal liver), and fetal brain (17, 34). The NCBI Gene Expression Omnibus (GEO) site (http://www.ncbi.nlm.nih.gov/geo/) contains similar data, indicating preferential expression of POLQ in bone marrow and thymus (GEO accession no. GPL95 and sequence accession no. AA767021). Weak Polq expression was detected in mouse spleen but not in humans. Notably, the spleen is an active hematopoietic organ in mice. Interestingly, POLQ expression (clone = 1335954) was found to be upregulated in a subset of diffuse large B-cell lymphomas (2), the most common subtype of non-Hodgkin's lymphoma. Kawamura et al. (17) further explored POLQ expression in a variety of lymphoid tissues and identified that its expression was strongly upregulated in germinal center B cells, where class switch recombination and somatic hypermutation of the immunoglobulin genes occurs. These data suggest the potential involvement of POLQ in such biological processes, and Polq mutant mice will be useful for testing this hypothesis.
Potential function(s) of Polq in genome maintenance.
Since Polqchaos1 mutant phenotypes are so subtle (except the micronucleus phenotype originally used to identify this mutation), and its expression is apparently restricted to certain tissues, it could be hypothesized that this novel polymerase might have a specialized function only in certain tissues or cells. However, the synergistic phenotypes observed in Atm and Polqchaos1 double mutants may suggest a more general role of Polq in genome maintenance. For example, because Polqchaos1/Atm− compound homozygotes exhibit neonatal semilethality and severe growth retardation, POLQ may actively participate in genome maintenance during embryonic development. Its preferential expression during embryogenesis could support this hypothesis.
In early embryogenesis, undifferentiated cells proliferate very rapidly. DNA damage and modifications associated with rapid DNA replication and cell proliferation could occur more often during this period, and these could potentially interfere with proper development and survival if left unrepaired. Therefore, cells must preserve genomic integrity by exerting defense systems such as DNA repair, cell cycle arrest, and apoptosis. It has been reported that cells in gastrulating embryos have a very low threshold for DNA damage and undergo ATM- and p53-dependent apoptosis without cell cycle arrest (12). Therefore, any compromise of genome maintenance pathways may increase unrepaired lesions, potentially triggering apoptosis and/or embryonic death. Disruption of recA-related recombinational repair genes result in early embryonic lethality (reviewed in reference 39), indicating essential roles of such genes in genome maintenance during embryogenesis. It is likely that the absence of both functional ATM and POLQ could place developing embryos under tremendous pressure of DNA damage. Nevertheless, since the double mutants survive until birth, and sometimes longer, potential role(s) of POLQ in genome maintenance may therefore be supplementary. It is not clear why a small subset of the double mutants manage to survive and actually outlive Atm single mutants. It is possible that modifier gene(s) segregating in the 129 and/or C3H genetic backgrounds are responsible, or that some type(s) of rare developmental compensation or epigenetic modification is responsible.
Interestingly, embryos deficient for both ATM and poly(ADP-ribose) polymerase-1 (Parp-1) undergo apoptosis and die at an early embryonic stage (26). PARP-1 participates in different forms of genome maintenance, including DSB and base excision repair (reviewed in reference 25). Moreover, deficiencies at both Atm and Prkdc also cause early embryonic lethality (9, 35). Prkdc encodes DNA-PKcs (DNA-dependent protein kinase, catalytic subunit) that is required for nonhomologous end joining, another major pathway for DSB repair and responsible for V(D)J recombination in lymphocytes (reviewed in reference 20). However, double mutants at Atm and Rad52, the latter encoding a protein involved in homologous recombination, were viable and indistinguishable from Atm single mutants except for tumor latency (see below and reference 40). Considering these data, we therefore hypothesize that POLQ has a unique role in DSB repair that complements the recombination machinery regulated by ATM. Since inhibition of ATM does not completely abolish homologous recombination (8), there might exist a minor, alternative and ATM-independent pathway. Alternatively, POLQ could be involved in nonhomologous end joining despite the apparently normal immune systems of Polqchaos1/Polqchaos1 mice (37).
Involvement of Polq in carcinogenesis.
In contrast to the synergistic increase in chromosome instability, Atm/chaos1 double mutant mice exhibited delayed onset of thymic lymphoma, which significantly increased their life span. A simple explanation is that the severely impaired proliferation of doubly mutant cells could contribute to this phenomenon, which would be consistent with their smaller overall size. Alternatively, Atm/chaos1 double mutant tumor or pretumor cells, in the absence of a DSB-handling pathway involving both ATM and POLQ coupled with elevated chromosome instability, might be more prone to apoptosis. However, other explanations are suggested by studies reporting similar phenomena. The majority of thymic lymphomas in Atm-deficient mice consist of immature T cells (4), associated with translocations involving the Tcrα/δ locus (4, 30, 31). Thus, by inactivating Rag-1 or Rag-2, which induces specific DSBs initiating V(D)J recombination, the development of thymic lymphoma could be significantly suppressed (30, 31). Lymphomas developed in Atm/Rag-1 or Atm/Rag-2 double mutant mice, however, did contain different types of translocations, leading to the hypothesis that translocations and other chromosome aberrations derived from aberrant responses to DSBs are the major mechanisms of ATM deficiency-associated lymphomagenesis (30, 31). Inactivation of Rad52 also increased lymphoma latency in Atm-deficient mice, suggesting the involvement of homologous recombination in creating such translocations (40). The recent identification of the Drosophila ortholog of the human HEL308 (a POLQ paralog) as mus301/spn-C, which is involved in repair of meiotic DSBs and meiotic checkpoint activation (19), suggests potential involvement of POLQ in homologous recombination. Thus, the absence of functional POLQ might also decrease the occurrence of such translocations.
It was recently reported that POLQ expression was upregulated in a wide range of human cancers accompanied with poor clinical outcome (17). Considering a potential role of POLQ in cross-link repair, it is possible that elevated POLQ expression could confer increased resistance to anticancer drugs, many of which are cross-linkers. Future studies with the mutant Polq mice plus BAC transgenic overexpressors will be useful in addressing these possibilities.
Acknowledgments
We thank Ellen Akeson and Peter Reifsnyder for technical support and Laura Bannister and David Bergstrom for useful comments on the manuscript.
This study was supported by grant GM45415 from the National Institutes of Health. N.S. was a recipient of Department of Defense Breast Cancer Research fellowship DAMD 17-01-1-0277.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh, A. A., M. B. Eisen, R. E. Davis, C. Ma, I. S. Lossos, A. Rosenwald, J. C. Boldrick, H. Sabet, T. Tran, X. Yu, J. I. Powell, L. Yang, G. E. Marti, T. Moore, J. J. Hudson, L. Lu, D. B. Lewis, R. Tibshirani, G. Sherlock, W. C. Chan, T. C. Greiner, D. D. Weisenburger, J. O. Armitage, R. Warnke, R. Levy, W. Wilson, M. R. Grever, J. C. Byrd, D. Botstein, P. O. Brown, and L. M. Staudt. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511. [DOI] [PubMed] [Google Scholar]
- 3.Balling, R. 2001. ENU mutagenesis: analyzing gene function in mice. Annu. Rev. Genomics Hum. Genet. 2:463-492. [DOI] [PubMed] [Google Scholar]
- 4.Barlow, C., S. Hirotsune, R. Paylor, M. Liyanage, M. Eckhaus, F. Collins, Y. Shiloh, J. N. Crawley, T. Ried, D. Tagle, and A. Wynshaw-Boris. 1996. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159-171. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, J. B., K. Sakaguchi, and P. V. Harris. 1990. mus308 mutants of Drosophila exhibit hypersensitivity to DNA cross-linking agents and are defective in a deoxyribonuclease. Genetics 125:813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dertinger, S. D., D. K. Torous, and K. R. Tometsko. 1996. Simple and reliable enumeration of micronucleated reticulocytes with a single-laser flow cytometer. Mutat. Res. 371:283-292. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg, E. C., R. Wagner, and M. Radman. 2002. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science 296:1627-1630. [DOI] [PubMed] [Google Scholar]
- 8.Golding, S. E., E. Rosenberg, A. Khalil, A. McEwen, M. Holmes, S. Neill, L. F. Povirk, and K. Valerie. 2004. Double strand break repair by homologous recombination is regulated by cell cycle-independent signaling via ATM in human glioma cells. J. Biol. Chem. 279:15402-15410. [DOI] [PubMed] [Google Scholar]
- 9.Gurley, K. E., and C. J. Kemp. 2001. Synthetic lethality between mutation in Atm and DNA-PK(cs) during murine embryogenesis. Curr. Biol. 11:191-194. [DOI] [PubMed] [Google Scholar]
- 10.Harris, P. V., O. M. Mazina, E. A. Leonhardt, R. B. Case, J. B. Boyd, and K. C. Burtis. 1996. Molecular cloning of Drosophila mus308, a gene involved in DNA cross-link repair with homology to prokaryotic DNA polymerase I genes. Mol. Cell. Biol. 16:5764-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heddle, J. A. 1973. A rapid in vivo test for chromosomal damage. Mutat. Res. 18:187-190. [DOI] [PubMed] [Google Scholar]
- 12.Heyer, B. S., A. MacAuley, O. Behrendtsen, and Z. Werb. 2000. Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Genes Dev. 14:2072-2084. [PMC free article] [PubMed] [Google Scholar]
- 13.Hitotsumachi, S., D. A. Carpenter, and W. L. Russell. 1985. Dose-repetition increases the mutagenic effectiveness of N-ethyl-N-nitrosourea in mouse spermatogonia. Proc. Natl. Acad. Sci. USA 82:6619-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeijmakers, J. H. 2001. Genome maintenance mechanisms for preventing cancer. Nature 411:366-374. [DOI] [PubMed] [Google Scholar]
- 15.Hubscher, U., G. Maga, and S. Spadari. 2002. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 71:133-163. [DOI] [PubMed] [Google Scholar]
- 16.Kanaar, R., J. H. Hoeijmakers, and D. C. van Gent. 1998. Molecular mechanisms of DNA double strand break repair. Trends Cell Biol. 8:483-489. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura, K., R. Bahar, M. Seimiya, M. Chiyo, A. Wada, S. Okada, M. Hatano, T. Tokuhisa, H. Kimura, S. Watanabe, I. Honda, S. Sakiyama, M. Tagawa, and J. O. Wang. 2004. DNA polymerase theta is preferentially expressed in lymphoid tissues and upregulated in human cancers. Int. J. Cancer 109:9-16. [DOI] [PubMed] [Google Scholar]
- 18.Khanna, K. K., and S. P. Jackson. 2001. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 27:247-254. [DOI] [PubMed] [Google Scholar]
- 19.Laurencon, A., C. M. Orme, H. K. Peters, C. L. Boulton, E. K. Vladar, S. A. Langley, E. P. Bakis, D. T. Harris, N. J. Harris, S. M. Wayson, R. S. Hawley, and K. C. Burtis. 2004. A large-scale screen for mutagen-sensitive Loci in Drosophila. Genetics 167:217-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lees-Miller, S. P., and K. Meek. 2003. Repair of DNA double strand breaks by non-homologous end joining. Biochimie 85:1161-1173. [DOI] [PubMed] [Google Scholar]
- 21.Leonhardt, E. A., D. S. Henderson, J. E. Rinehart, and J. B. Boyd. 1993. Characterization of the mus308 gene in Drosophila melanogaster. Genetics 133:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maga, G., I. Shevelev, K. Ramadan, S. Spadari, and U. Hubscher. 2002. DNA polymerase theta purified from human cells is a high-fidelity enzyme. J. Mol. Biol. 319:359-369. [DOI] [PubMed] [Google Scholar]
- 23.Marini, F., N. Kim, A. Schuffert, and R. D. Wood. 2003. POLN, a nuclear PolA family DNA polymerase homologous to the DNA cross-link sensitivity protein Mus308. J. Biol. Chem. 278:32014-32019. [DOI] [PubMed] [Google Scholar]
- 24.Marini, F., and R. D. Wood. 2002. A human DNA helicase homologous to the DNA cross-link sensitivity protein Mus308. J. Biol. Chem. 277:8716-8723. [DOI] [PubMed] [Google Scholar]
- 25.Masutani, M., H. Nakagama, and T. Sugimura. 2003. Poly(ADP-ribose) and carcinogenesis. Genes Chromosomes Cancer 38:339-348. [DOI] [PubMed] [Google Scholar]
- 26.Menisser-de Murcia, J., M. Mark, O. Wendling, A. Wynshaw-Boris, and G. de Murcia. 2001. Early embryonic lethality in PARP-1 Atm double-mutant mice suggests a functional synergy in cell proliferation during development. Mol. Cell. Biol. 21:1828-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita, T., N. Asano, T. Awogi, Y. F. Sasaki, S. Sato, H. Shimada, S. Sutou, T. Suzuki, A. Wakata, T. Sofuni, M. Hayashi, et al. 1997. Evaluation of the rodent micronucleus assay in the screening of IARC carcinogens (groups 1, 2A and 2B): the summary report of the 6th collaborative study by CSGMT/JEMS MMS. Mutat. Res. 389:3-122. [DOI] [PubMed] [Google Scholar]
- 28.Morrison, C., E. Sonoda, N. Takao, A. Shinohara, K. Yamamoto, and S. Takeda. 2000. The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J. 19:463-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nusse, M., B. M. Miller, S. Viaggi, and J. Grawe. 1996. Analysis of the DNA content distribution of micronuclei using flow sorting and fluorescent in situ hybridization with a centromeric DNA probe. Mutagenesis 11:405-413. [DOI] [PubMed] [Google Scholar]
- 30.Petiniot, L. K., Z. Weaver, C. Barlow, R. Shen, M. Eckhaus, S. M. Steinberg, T. Ried, A. Wynshaw-Boris, and R. J. Hodes. 2000. Recombinase-activating gene (RAG) 2-mediated V(D)J recombination is not essential for tumorigenesis in Atm-deficient mice. Proc. Natl. Acad. Sci. USA 97:6664-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petiniot, L. K., Z. Weaver, M. Vacchio, R. Shen, D. Wangsa, C. Barlow, M. Eckhaus, S. M. Steinberg, A. Wynshaw-Boris, T. Ried, and R. J. Hodes. 2002. RAG-mediated V(D)J. recombination is not essential for tumorigenesis in Atm-deficient mice. Mol. Cell. Biol. 22:3174-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash, S., and L. Prakash. 2002. Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev. 16:1872-1883. [DOI] [PubMed] [Google Scholar]
- 33.Rattray, A. J., and J. N. Strathern. 2003. Error-prone DNA polymerases: when making a mistake is the only way to get ahead. Annu. Rev. Genet. 37:31-66. [DOI] [PubMed] [Google Scholar]
- 34.Seki, M., F. Marini, and R. D. Wood. 2003. POLQ (Pol theta), a DNA polymerase and DNA-dependent ATPase in human cells. Nucleic Acids Res. 31:6117-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekiguchi, J., D. O. Ferguson, H. T. Chen, E. M. Yang, J. Earle, K. Frank, S. Whitlow, Y. Gu, X. Y., A. Nussenzweig, and F. W. Alt. 2001. Genetic interactions between ATM and the nonhomologous end-joining factors in genomic stability and development. Proc. Natl. Acad. Sci. USA 98:3243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shcherbakova, P. V., K. Bebenek, and T. A. Kunkel. 2003. Functions of eukaryotic DNA polymerases. Sci. Aging Knowledge Environ. 2003:RE3. [DOI] [PubMed]
- 37.Shima, N., S. A. Hartford, T. Duffy, L. A. Wilson, K. J. Schimenti, and J. C. Schimenti. 2003. Phenotype-based identification of mouse chromosome instability mutants. Genetics 163:1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson, L. H., and D. Schild. 1999. The contribution of homologous recombination in preserving genome integrity in mammalian cells. Biochimie 81:87-105. [DOI] [PubMed] [Google Scholar]
- 39.Thompson, L. H., and D. Schild. 2001. Homologous recombinational repair of DNA ensures mammalian chromosome stability. Mutat. Res. 477:131-153. [DOI] [PubMed] [Google Scholar]
- 40.Treuner, K., R. Helton, and C. Barlow. 2004. Loss of Rad52 partially rescues tumorigenesis and T-cell maturation in Atm-deficient mice. Oncogene 23:4655-4661. [DOI] [PubMed] [Google Scholar]
- 41.Valerie, K., and L. F. Povirk. 2003. Regulation and mechanisms of mammalian double-strand break repair. Oncogene 22:5792-5812. [DOI] [PubMed] [Google Scholar]
- 42.Wang, H., X. Wang, G. Iliakis, and Y. Wang. 2003. Caffeine could not efficiently sensitize homologous recombination repair-deficient cells to ionizing radiation-induced killing. Radiat. Res. 159:420-425. [DOI] [PubMed] [Google Scholar]
- 43.Wang, X., H. Wang, G. Iliakis, and Y. Wang. 2003. Caffeine-induced radiosensitization is independent of nonhomologous end joining of DNA double-strand breaks. Radiat. Res. 159:426-432. [DOI] [PubMed] [Google Scholar]
- 44.Wang, Y. A., A. Elson, and P. Leder. 1997. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc. Natl. Acad. Sci. USA 94:14590-14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo, R. A., and R. Y. Poon. 2004. Activated oncogenes promote and cooperate with chromosomal instability for neoplastic transformation. Genes Dev. 18:1317-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]