Key Clinical Message
Tetanus is rare and often forgotten in the diagnostic workup. The diagnosis is mainly based on typical clinical symptoms, because of missing sensitive paraclinical test. As described in our case, a missing bilateral blink reflex may occur in severe tetanus, which should not lead to the rejection of the diagnosis.
Keywords: Blink reflex, magnesium sulfate, masseter inhibitory reflex, neurophysiology, silent period, tetanus
Introduction
In developed countries, tetanus is very rare, with an incidence of 1 of 1,000,000/year 1. Eight hours up to weeks after inculcation of C. tetani, nonspecific clinical symptoms start with fever, abnormal fatigue, double vision, and vertigo. Later classical symptoms, such as trismus, dysphagia, and opisthotonus, occur 2, which should lead to the diagnosis. This characteristic muscle hypertonicity results from the missing alpha‐motoneuron inhibition in the brainstem and the spinal cord. The underlying mechanism is that the tetanus toxin cleaves the synaptobrevin 3, a protein necessary for the vesicular release of neurotransmitters. Tetanus toxin specifically acts on inhibitory synapses in Renshaw cells in the spinal cord 4 and a similar cell population in the brainstem.
Tetanus is a disease, which is mainly diagnosed by its typical symptoms. Likely because tetanus is a very rare disease, additional diagnostic testing is recommend to confirm its diagnosis. Recommendations include animal transfer, microbial culture of tissue samples, and serological testing 5. The sensitivity of these tests, however, is limited. For the outcome, immediate active and passive immunization against tetanus toxin as well as supportive treatment is most important. Although the antiserum will have an effect only on circulating and unbound toxin, it is recommended 6.Even in our times, the management of patients with tetanus is challenging, one‐third of the infected die 1.
Here, we describe a case of severe tetanus with successful therapy including prolonged intensive care and high‐dose magnesium sulfate and its diagnostic pitfalls, due to missing sensitive and specific paraclinical tests.
Case Presentation
A 79‐year‐old woman was injured in her right forearm while gardening. Six days later, she developed progressive generalized hypertonicity of muscles, lockjaw, and dysphagia. Four days after the first symptoms, she was admitted to a general hospital and was seen by the consultant neurologist who diagnosed tetanus, undertook both active and passive immunization, and transfer to our neurologic intensive care unit. The patient never had had a tetanus vaccination. Her medical history was remarkable for arterial hypertension and slight cardiac insufficiency.
At admission, she had opisthotonus and neck stiffness, trismus, dysphagia, and generalized, startle inducible hypertonicity of all muscles; muscle tendon reflexes were exaggerated. Muscle spasms were more pronounced in the lower limbs and on the side of the primary wound, which showed no signs of wound infection. Severe dysphagia and reduced coughing strength led to intubation on day 5 after the onset of symptoms.
All laboratory tests for tetanus, including culture of wound probe, serum toxin detection, and mouse test were negative. The diagnosis of tetanus was questioned, and further diagnostic testing was performed. First, a blink reflex was performed for a brainstem lesion was considered. Brainstem lesions can lead to plus symptoms such as spasms of facial muscles seen, for example, in Brissaud–Sicard syndrome 7, caused among others by ischemic stroke 8. Also in inflammatory diseases, such as multiple sclerosis, facial myokymia as a plus symptom for a pontine tegmental lesion occurs 9, 10. Surprisingly, no responses in blink reflex were elicited, although spontaneous eye blinking was seen in our patient (Fig. 1A) and plus symptoms such as trismus were apparent. Similarly, the amplitudes of the compound muscle action potentials (CMAP) were massively reduced or absent in motor neurography of nervus (N.) medianus, N. peroneus, and N. tibialis. A more accurate characterization of the neuropathy was not performed. Further neurophysiologic tests corroborated the diagnosis of tetanus: pathological masseter inhibitory reflex, also known as jaw‐opening reflex, with a missing silent period, as sign of increased motor neuron excitability (Fig. 1C), and a monomorphic late response of the M. flexor digitorum resembling an H‐reflex (Fig. 1E) 11. The bilateral loss of the blink reflex led us to rule out a brainstem lesion by cerebral MRI; cerebrospinal fluid was normal (including total cell count, cytology, total protein level, albumin level and its quotient, lactate concentration, concentration for immunoglobulin G, A, and M as well as its quotient and missing oligoclonal bands).
Figure 1.
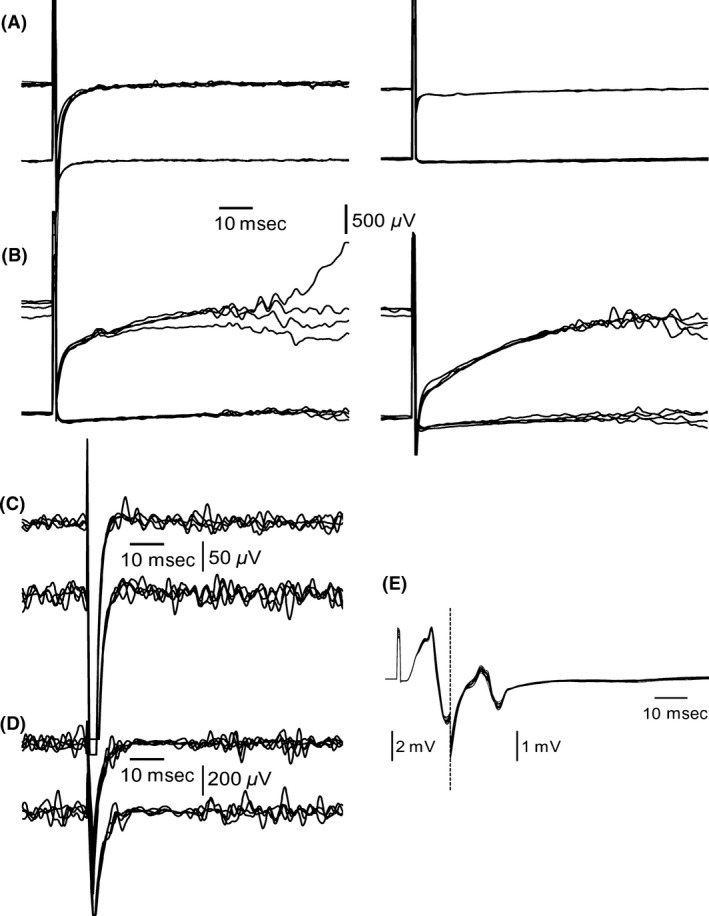
Electrophysiological findings in tetanus. (A) Blink reflex responses were lost bilaterally during the first weeks. (B) Partial recovery of the blink reflex responses occurred 11 weeks after the first symptoms. (C) Typical silent period was absent in masseter inhibitory reflex on day 3. The finding persisted for the following 9 weeks. (D) Normalization of the silent period in masseter inhibitory reflex after recovery. (E) Muscle response of the Musculus flexor digitorum superficialis on day 3 showed abnormal consistency in shape and a marked increase in amplitude.
Initially, the patient was treated with metronidazole for 8 days. After intubation, narcotics (midazolam, diazepam, propofol, esketamine) were used until the hypertonicity of muscles stopped. Rising liver enzymes led to the termination of diazepam and propofol and reduction in midazolam to 15–20 mg/h after 2 weeks. Consecutively, muscle stiffness increased dramatically. Thus, high‐dose magnesium (targeted magnesium level: 3–4 mmol/L), which causes a presynaptic block of the neuromuscular transmission 12, was given for 9 weeks to normalize the muscle tonus successfully. For better evaluation of her neurologic status, midazolam was replaced by propofol (80–100 mg/h). Repeated examination of the masseter inhibitory reflex monitored the stage of recovery: after 10 weeks, the silent period returned (Fig. 1D). Consequently, the magnesium sulfate infusion was stopped. Because her muscle tonus was normal at that time, the sedation with propofol was also stopped. One week later, the R2 components of the blink reflex returned (Fig. 1B). The patient was transferred for neurorehabilitation and further training. From rehabilitation, she was discharged without paresis or muscle contractures, still having mild gait ataxia, being able to walk safely with a walking frame and self‐dependent in most of her daily activities.
Discussion and Conclusion
Misleading in this case was the missing blink reflex, despite of clinical plus symptoms of the brainstem. The toxin inhibits release of inhibiting neurotransmitters 4, so we had expected exaggerated responses in blink reflex. In our case, MRI excluded a visible brainstem lesion. Additionally, cerebrospinal fluid was normal. During the recovery, the blink reflex returned (Fig. 1B). As the first examination was performed before magnesium was given, the magnesium sulfate infusion can be ruled out as the cause for the lost blink reflex. Being vulnerable to anesthesia, the blink reflex was examined after a sedation pause of more than 5 h. During the first 2 weeks, the blink reflex was repeated frequently after pausing sedation and always missing on both sides. One explanation for the lost blink reflex could be a neuropathy, which is known to affect also facial nerves in severe tetanus 13. The results of the motor neurography underline this theory. But because eye blinking was possible, other unknown causes seem to be possible. The combination of clinical plus symptoms of the brainstem and a missing blink reflex could possibly help to confirm the diagnosis of tetanus and rule out other differential diagnosis.
Known pathognomonic neurophysiologic findings in tetanus are a missing silent period of the masseter inhibitory reflex and monomorphic late muscle response 11. These tests are easy to apply and helpful to confirm the diagnosis. Additionally, we found the masseter inhibitory reflex useful to monitor the recovery (Fig. 1C and D) and to determine the time point to end magnesium sulfate infusion and sedation.
Finally, we want to outline the positive experience with high‐dose magnesium sulfate in long‐term therapy to reduce muscle tonus. Our positive experience is not confirmed by a meta‐analysis, comparing treatment with magnesium sulfate, diazepam, and placebo in patients with tetanus 14. One explanation is that the functional outcome of the patients was not studied, nor was differentiated by disease severity.
Nevertheless, we strongly recommend magnesium sulfate infusion for treatment of severe tetanus; we think the reduction in muscle contractures to be important for the quality of life and the functional outcome after tetanus infection.
Funding
This work was supported by the German Research Foundation (DFG) within the funding program Open Access Publishing.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Authorship
BN: drafted the manuscript. WSM, KF, SB, and RZ: revised the manuscript critically for important intellectual content. KA: drafted the manuscript. All authors treated the patient described in the manuscript.
Contributor Information
Bernhard Neumann, Email: bernhard.neumann@medbo.de.
Klemens Angstwurm, Email: klemens.angstwurm@medbo.de.
References
- 1. Filia, A. , Bella A., von Hunolstein C., Pinto A., Alfarone G., Declich S., et al. 2014. Tetanus in Italy 2001‐2010: a continuing threat in older adults. Vaccine 32:639–644. [DOI] [PubMed] [Google Scholar]
- 2. Marulappa, V. G. , Manjunath R., Mahesh Babu N., and Maligegowda L.. 2012. A ten year retrospective study on adult tetanus at the Epidemic Disease (ED) Hospital, Mysore in Southern India: a review of 512 cases. J. Clin. Diagn. Res. 6:1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schiavo, G. , Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., et al. 1992. Tetanus and botulinum‐B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359:832–835. [DOI] [PubMed] [Google Scholar]
- 4. Benecke, R. , Takano K., Schmidt J., and Henatsch H. D.. 1977. Tetanus toxin induced actions on spinal Renshaw cells and Ia‐inhibitory interneurones during development of local tetanus in the cat. Exp. Brain Res. 27:271–286. [DOI] [PubMed] [Google Scholar]
- 5. Robert Koch‐Institut . Tetanus: RKI‐Ratgeber für Ärzte. Available at http://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Tetanus.html;jsessionid=C3A19EAA258A767B25AB3F9115B43B6F.2_cid372#doc2398266bodyText9 (accessed April 09, 2016).
- 6. Veronesi, R. 1981. Clinical picture Pp. 183–206 in Veronesi R., ed. Tetanus: Important new concepts. Excerpta Medica, Amsterdam. [Google Scholar]
- 7. Krasnianski, M. , Neudecker S., and Zierz S.. 2004. Classical crossed pontine syndromes. Fortschr. Neurol. Psychiatr. 72:460–468. [DOI] [PubMed] [Google Scholar]
- 8. Vermersch, P. , Petit H., Marion M. H., and Montagne B.. 1991. Hemifacial spasm due to pontine infarction. J. Neurol. Neurosurg. Psychiatry 54:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthews, W. B. 1966. Facial myokymia. J. Neurol. Neurosurg. Psychiatry 29:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobs, L. , Kaba S., and Pullicino P.. 1994. The lesion causing continuous facial myokymia in multiple sclerosis. Arch. Neurol. 51:1115–1119. [DOI] [PubMed] [Google Scholar]
- 11. Risk, W. S. , Bosch E. P., Kimura J., Cancilla P. A., Fischbeck K. H., and Layzer R. B.. 1981. Chronic tetanus: clinical report and histochemistry of muscle. Muscle Nerve 4:363–366. [DOI] [PubMed] [Google Scholar]
- 12. Jenkinson, D. H. 1957. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J. Physiol. 138:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shahani, M. , Dastur F. D., Dastoor D. H., Mondkar V. P., Bharucha E. P., Nair K. G., et al. 1979. Neuropathy in tetanus. J. Neurol. Sci. 43:173–182. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigo, C. , Samarakoon L., Fernando S. D., and Rajapakse S.. 2012. A meta‐analysis of magnesium for tetanus. Anaesthesia 67:1370–1374. [DOI] [PubMed] [Google Scholar]


