Key Clinical Message
Demineralized dentin matrix block (ABTB: Autogenous Tooth Bone Graft Block) is 3‐D scaffold with same components and geometry with alveolar bone. ABTB is well incorporated and remodelled into cortico‐cancellous bone with dental implant. The shape and volume were maintained with little marginal bone loss after average 44 months of follow‐up.
Keywords: Autogenous tooth bone graft block, demineralized dentin matrix
Introduction
Autogenous tooth bone graft material (AutoBT, Korea Tooth Bank Co., Seoul, Korea), a human demineralized dentin matrix created from extracted human teeth, was first developed in 2008 and has been evaluated for its osteoinductive, osteoconductive and remodeling capacities in implant dentistry. The autogenous tooth bone graft block (ABTB), a block fabricated from root dentin, is a biomimetic of cortical bone that exhibits slow creeping substitution properties with 3 to 5 μm innate micropores (dentinal tubules) and 0.2 to 0.3 mm macropores (Fig. 1A and B) 1, 2, 3.
Figure 1.
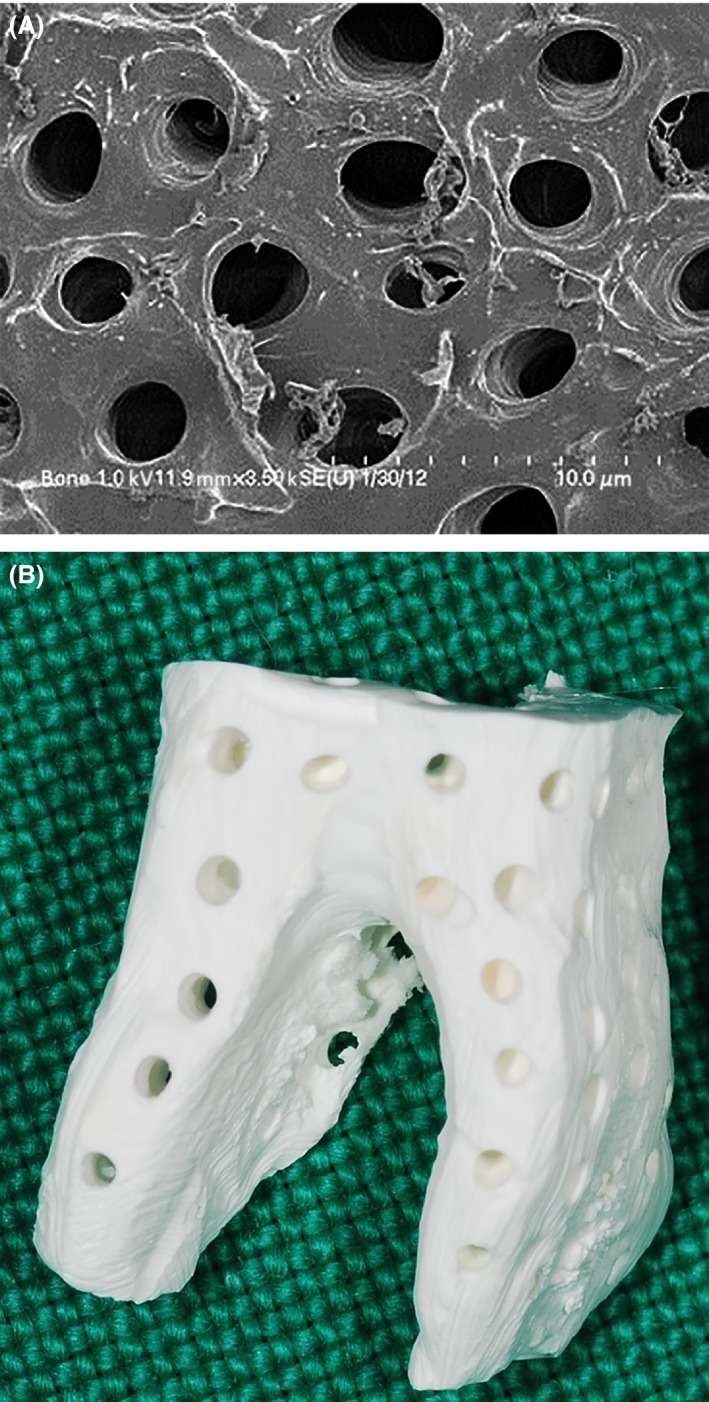
Fabrication of the ABTB. (A) SEM of the processed ABTB surface. A clean surface and 1‐ to 5‐μm dentinal tubules provided space for protein exchange. (B) Lateral view of the ABTB. Macropores (200–300 μm) that penetrated from the surface to the pulp space provided the space for vascular invasion.
Indications for the ABTB include socket preservation and vertical augmentation in cases for which the socket wall is expected to be resorbed or has already been destroyed due to a range of pathological causes. An advantage of the ABTB is that its geometry adapts well to the graft site because the ABTB is prepositioned at the site of the implant and possesses the same type I collagens as alveolar bone 4. Generally, a reduction in alveolar bone volume appears to be caused by progressive resorption, with a loss of 0.34–7.7 mm of ridge width and 0.2–3.25 mm of vertical height during the 6–12 months following extraction 5. In contrast, the augmentation of the extraction sites with graft materials tends to reduce this bone loss, most likely via the maintenance of physical stimulation of the surrounding bone 6. In addition, immediate implant placement has advantages, including the prevention of alveolar bone resorption 7.
The first clinical report addressing the ABTB in socket preservation described procedures performed from March 2009 to June 2010 and indicated that excellent bone formation and a strong union between the ABTB and the recipient bone were achieved in 12 patients. The volume of alveolar bone was well maintained both vertically and horizontally, and the formed bone was not resorbed during early stages 8. These authors also examined the remodeling of the ABTB and reported that based on radiological evaluations, the grafted block was replaced completely by newly formed bone from the host after 14 or 15 months of prosthetic loading 9. However, due to the ABTB's short developmental history, there is currently insufficient evidence regarding the long‐term results of the ABTB, particularly with respect to volumetric changes and remodeling with a dental implant.
Therefore, the specific aims of this study were to evaluate the fate of the ABTB during long‐term follow‐up by measuring changes in bone area (CBA) and the occurrence of marginal bone resorption (MBR) using cone beam‐computed tomography (CBCT, Vatech, Seoul Korea) and to thereby determine whether the resulting findings are consistent with the short‐term results of other studies 8, 9, 10.
Materials and Methods
This study was conducted after obtaining approval from the Seoul National University Bundang Hospital Institutional Review Board (No. B‐1410‐272‐113).
Study design and patient selection
This case series study was based on twenty‐two patients, who received a single implant with ABTB graft in the posterior area of the maxilla (12 patients) or the mandible (10 patients) between July 2009 and February 2014. The patients were followed up for an average 44 ± 13.2 months, and at least 1 year after the functional loading (FL).
The inclusion criteria of patients were as follows: Patients (i) in need of extraction of a premolar or molar with alveolar ridge augmentation or socket preservation, (ii) with the residual bone height <4 mm to the sinus floor or inferior alveolar canal as a result of extraction, and (iii) who are healthy overall or have controlled systemic disease (ASA I or II).
And the exclusion criteria were as follows: patients (i) who are smokers, (ii) who had received bone graft on the site to be operated, (iii) who had received radiation therapy, (iv) with poor plaque control and untreated chronic periodontitis and (v) who have acute infection.
Fabrication of ABTB
The extracted wisdom tooth was immersed in 75% alcohol. After removing the soft tissues and calculus attached the tooth, crowns were severed at the cementoenamel junctions. Only root dentin part was processed for the ABTB fabrication (European Patent No. 2462899) for its intended use as described in the previous report 2. Additional holes sized in 0.2 mm were made at the surface of the canal area to create macropores for promoting vascular invasion and bone formation. The ABTB went through the same fabrication process with the powder form, but the only difference was not being crushed into pieces so that ABTB maintains the original tooth root shape (Fig. 1B).
Surgical procedure
An ABTB graft with or without an implant was performed ten to fourteen days after extraction due to the time required for ABTB fabrication.
Primarily, if the implant could be placed with the routine preparation (3.8 × 12 mm, Dio®, Busan, Korea) (Fig. 2A), the ABTB was applied over the implant in a hollowed or prepared pulp chamber space. If initial stability could not be achieved, the ABTB was placed before the implant and the patient underwent delayed surgery waiting for average 3 months in the mandible, and 6 months in the maxilla to place implant (Table 1).
Figure 2.
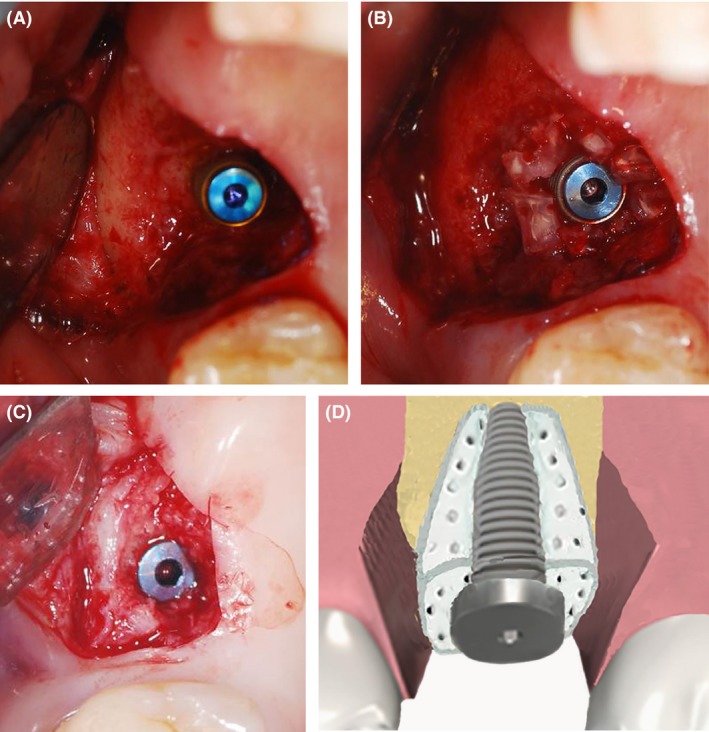
Surgical procedure for socket preservation, case no. 6 in Table 1. (A) The implant was placed into the socket, which had vertical and horizontal defects. (B) The ABTB was grafted for socket preservation. The blood‐soaked appearance of the ABTB fitted to the socket and implant is presented. (C) Removal of tissues over the screw for histological evaluation and the final prosthesis‐related procedure. (D) After getting initial stability of implant, ABTB with multiple macropores was put into the socket to wrap the implant via pulp chamber space.
Table 1.
Case summary of ABTB in the maxilla and the mandible
| Patient/Mx | Age/Gender Location | FL→BD (M) | BH [change in BH] (mm:% Reduction) | ARW [change in ARW] (mm:% Reduction) | CBA(%) | TFU (M) | MBR (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 57/M#5 | 15 | 6.4 (−0.9): 14.1 | 5.6 (−0.5): 8.9 | 0 | 56 | 0 |
| 2 | 51/M#3 | 5 | 5.7 (−0.7): 12.3 | 10.9 (−3.5): 34 | 35.59 | 45 | 0 |
| 3 | 31/M#15 | 1 | 7.9 (−0.9): 11.4 | 9.1 (−1.7): 18.7 | 22.34 | 57 | 2.5 |
| 4 | 58/M#15 | 19 | 6.0 (−1.5): 25 | 10.6 (−1.1): 10.4. | 16.96 | 53 | 1.0 |
| 5 | 33/M#4 | 5 | 7.7 (−0.6): 7.8 | 7.2 (−4.6): 22.2 | 31.5 | 36 | 0 |
| 6 | 53/M#2 | 0 | 4.4 (−0): 0 | 9.3 (−2.7): 29 | 44.32 | 33 | 0 |
| 7 | 54/M#3 | 7 | 4.1 (−2.0): 48.8 | 6.5 (−2.4): 36.9 | 28.73 | 30 | 0 |
| 8 | 50/F#15 | 1 | 5.5 (−0.6): 10.9 | 9.0 (−0.1): 1.1 | 20.95 | 63 | 0 |
| 9 | 42/F#4 | 0 | 3.1 (−0.5): 16.1 | 6.4 (−1.4): 21.9. | 22.05 | 48 | 0 |
| 10 | 60/F#5 | 4 | 6.6 (−1.0): 15.2 | 5.9 (−1.7): 28.8 | 54.64 | 53 | 0 |
| 11 | 35/F#5 | 9 | 5.9 (−0.8): 13.6 | 6.4 (−2.2): 34.4 | 54.06 | 40 | 0 |
| 12 | 40/F#14 | 0 | 4.8 (−1.4): 29.2 | 7.7 (−0.6): 7.8 | 8.26 | 36 | 0 |
| Average ± SD | 47 ± 10.31 | 5.5 ± 6.2 | 17.03 ± 12.48% | 21.18 ± 11.92% | 28.28 ± 16.89 | 45.83 ± 10.76 | 0.29 ± 0.75 |
| Patient/Mn | Age/Gender Location | FL→BD (M) | BH [change in BH] (mm:% Reduction) | ARW [change in ARW] (mm:% Reduction) | CBA(%) | TFU (M) | MBR (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 58/M#18 | 13 | 5.0 (−1.2): 24 | 9.5 (−2.4): 25.3 | 62.2 | 45 | 10.0 |
| 2 | 44/M#31 | 11 | 1.7 (−0.9): 52.9 | 10.9 (−4.6): 42.2 | 26.7 | 41 | 0 |
| 3 | 51/M#31 | 0 | 2.6 (−1.5): 57.7 | 7.1 (−1.5): 21.1 | 19.97 | 19 | 0 |
| 4 | 24/M#31 | 15 | 4.1 (−1.7): 41.5 | 7.3 (−1.6): 21.9 | 16.89 | 37 | 0 |
| 5 | 57/M#31 | 8 | 2.7 (−1.9): 70.4 | 7.6 (−0.3): 3.9. | 32.53 | 12 | 0 |
| 6 | 47/F#19 | 6 | 2.9 (−1.2): 41.4 | 8.9 (−1.7): 19.1 | 58.79 | 60 | 0 |
| 7 | 60/F#30 | 3 | 2.0 (−0.2): 10 | 3.3 (−1.3): 39.4 | 48.88 | 57 | 0 |
| 8 | 44/F#18 | 11 | 1.4 (−0.7): 50 | 7.3 (−2.0): 27.4 | 59.58 | 53 | 0 |
| 9 | 37/F#18 | 8 | 7.0 (−1.8): 25.7 | 7.9 (−2.4): 30.4 | 48.1 | 54 | 2.0 |
| 10 | 51/F#18 | 0 | 3.2 (−1.1): 34.4 | 7.8 (−1.2): 15.4 | 44.16 | 40 | 0 |
| Average ± SD | 47.3 ± 10.89 | 7.5 ± 5.23 | 40.8 ± 17.93 | 24.61 ± 11.22 | 41.78 ± 16.76 | 41.8 ± 15.9 | 1.2 ± 3.15 |
In the maxilla, MBRs of 2.5 and 1.0 mm were observed at 44 and 26 months after the first surgery, respectively. In the mandible, one patient exhibited 2.0 mm of MBR at #18 at 40 months after the first surgery, which featured a BD of 15 months. In particular, 10 mm of MBR indicated the removal of the 10‐mm implant at #18 due to the complete resorption of bone around the implant fixture (mandibular case no.1). FL, Functional Loading; BD, ABTB Disappearance; BH, Grafted Buccal Height. Buccal Height (reduced buccal height): reduction %; ARW, Grafted Alveolar Ridge Width(reduced ARW):reduction % CBA, Changes in Bone Area %; TFU, Total Follow‐Up; MBR, Marginal Bone Resorption; M, months.
There was no need for a fixation screw or a membrane because the porous nature of the collagenous scaffold of the ABTB allowed adequate results to be achieved via soaking with blood (Fig. 2B) 4. At 3–6 months after the second surgery, a tissue biopsy was performed on the cover screw or using trephine to observe the histological functions of the ABTB during the early stages (Fig. 2C and D).
Measurements and data collection
Cone beam‐computed tomography (Vatech, Seoul, Korea) was performed preoperatively, immediately after the first and second surgeries, and yearly thereafter to evaluate changes that occurred from the first operation to the final follow‐up.
In the cross‐sectional view, CBCT generated 12‐bit gray‐scale images (DICOM‐based datasets) with a resolution of 96 dpi. The CBCT unit was set to 82 kVp and 6 mA with a 24‐sec exposure time. Measurements were obtained using the software program included with the Vatech system (EasyDent Viewer) based on the image standardized in the same spatial orientation, using the implant body as a fixed reference. With the hollow internal screw space in a radiolucent image as a basis, cross‐sectional images were generated parallel to the long axis of the screw space and perpendicular to the occlusal plane.
The durations of ABTB disappearance (block disappearance (BD)) from the graft and FL were examined separately by assessing the disappearance of a hole made in the block (Fig. 1B) and the borders between the host and the ABTB that were relevant to remodeling (Fig. 3A–C).
Figure 3.
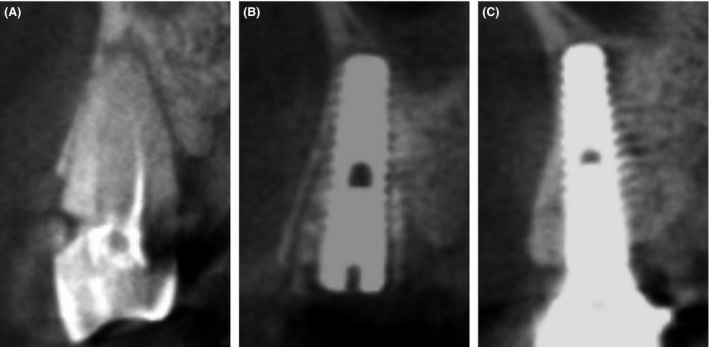
Block disappearance (BD) evaluation of maxillary case no. 1 in Table 1 on CBCT. (A) Before extraction. (B) The ABTB inserted into socket #12 was clearly visible between the buccal wall and the implant fixture. The macropores of the ABTB were clearly distinguishable immediately after surgery. (C) At the final follow‐up at 4 years, the macropores of the ABTB had almost disappeared, and the buccal wall of the socket was fused and replaced with new bone. Traces of the ABTB were difficult to find around the implant fixture, which had been resorbed and replaced with host bone.
Buccal height (BH), alveolar ridge width (ARW), and CBA were determined from measurements obtained immediately postoperation to the final follow‐up; in particular, linear and area rulers in the included Easy Dent Viewer software were utilized to evaluate these parameters on standardized cross‐sectional images. Using a linear ruler, BH was measured from the apex to the top of the ABTB, and the ARW was measured from the buccal end to the lingual end of the ABTB (Fig. 4A). CBA was measured with an area ruler by drawing apparent outlines on the cross‐sectional images; this measurement is closely related to levels of BH and ARW reduction (Fig. 4B and C).
Figure 4.
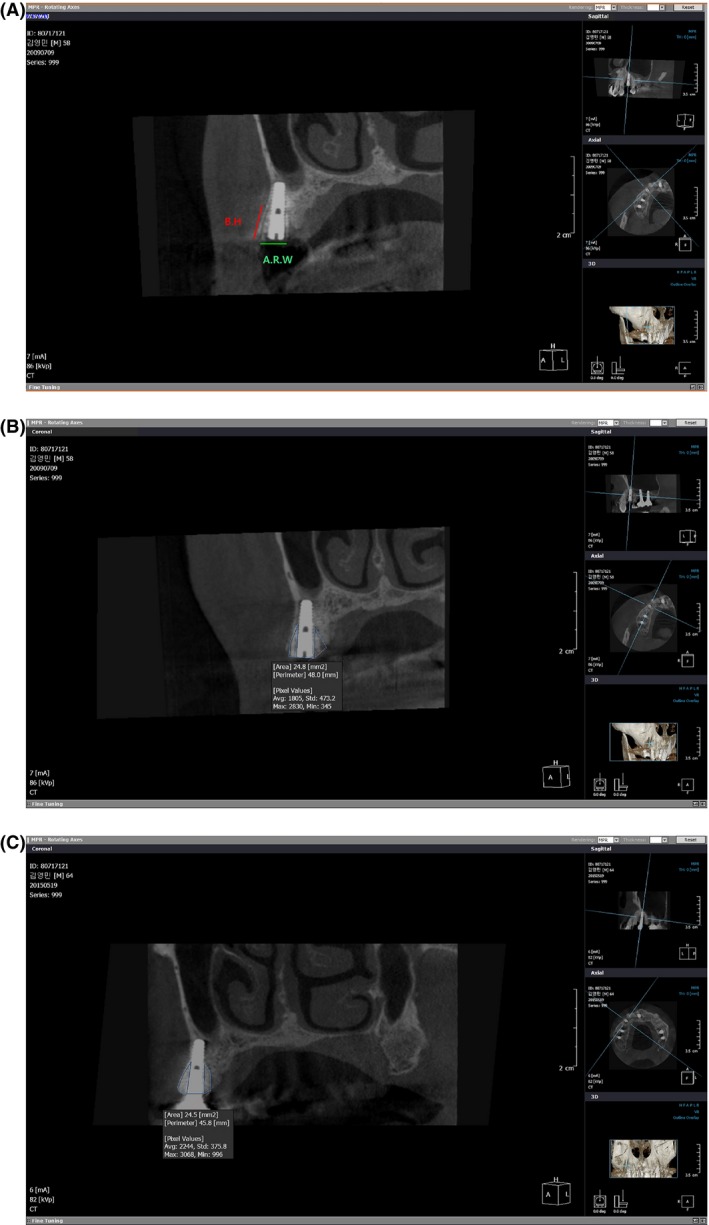
Measurements of parameters in cross‐sectional images from maxillary case no. 1. (A) Method used to measure BH and ARW with a built‐in ruler from Easy Dent. (B) Method used to measure the bone area immediately postoperation with the built‐in ruler from Easy Dent. (C) Method used to measure bone area at the final follow‐up with the built‐in ruler from Easy Dent.
Marginal bone resorption in millimeters was determined during long‐term follow‐up. Because all parameters were measured in terms of changes from immediately after the operation to the final follow‐up, linear and area changes are presented as percentage reductions from the initial parameters for the grafted ABTB.
Data analysis was performed using SPSS/PC software, version 20.0 (SPSS, Inc., Chicago, IL). Means and standard deviations were calculated for all measurements. Maxillary and mandibular parameters were compared using the Mann–Whitney U‐test, with P < 0.05 used as the threshold for significance.
Results
The mean ages of the 22 patients who received maxillary and mandibular ABTB grafts were 47 and 47.3 years, respectively, and these patients’ mean follow‐up periods were 45.83 ± 10.76 and 41.8 ± 15.9 months, respectively. A total of 12 patients received grafts in the maxilla, and 10 patients received grafts in the mandible. Seven maxillary patients received the implant and bone graft simultaneously, and the remaining five patients received the bone graft before the implant was placed during delayed surgery. Among mandibular patients, five patients received the implant and bone graft simultaneously, and the remaining five patients received the bone graft before the implant was placed during delayed surgery. All implants were located in the posterior area. A total of 12 patients were male, and 10 patients were female. There were no postoperative complications due to ABTB graft except the wound dehiscence on two patients (Patient No. 4 and 9 in mandible). However, both of them exhibited favorable secondary healing after 2–4 weeks of the wound dehiscence (Table 1).
The duration of FL to reach BD was examined according to implant location. Times to achieve total BD for maxillary and mandibular grafts did not significantly differ, with mean durations of 13 and 12.4 months, respectively. The average duration from FL to BD was 5.5 months for maxillary grafts and 7.5 months for mandibular grafts; these durations did not significantly differ (Fig. 5).
Figure 5.
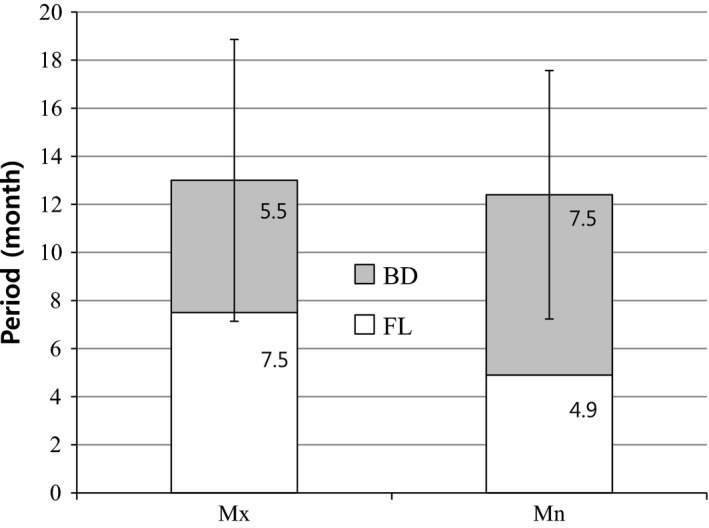
The time needed to reach BD after FL. The average time needed to reach BD after FL was 5.5 months in the maxilla and 7.5 months in the mandible. The achievement of total BD in the maxilla and mandible required, on average, 13 and 12.4 months, respectively; these durations did not significantly differ. TBD, Time to reach BD; Mx, Maxilla; Mn, Mandible; BD, ABTB Disappearance; FL, Functional Loading Time after First Surgery.
Examinations of a macropore of a histological specimen from maxillary patient no. 6 at 8 months after the first surgery revealed chondrocyte‐like cells embedded in an osteoid that were in close contact with the inner wall of the macropore (Fig. 6A). A histological specimen from mandibular patient no. 8 at 3 months after the first surgery indicated that the newly formed osteoid was deposited on the ABTB surface, which had osteocytes and vessels. Cellular fusion without fibrous tissues was observed at the border between the osteoid and the dentin matrix (Fig. 6B).
Figure 6.
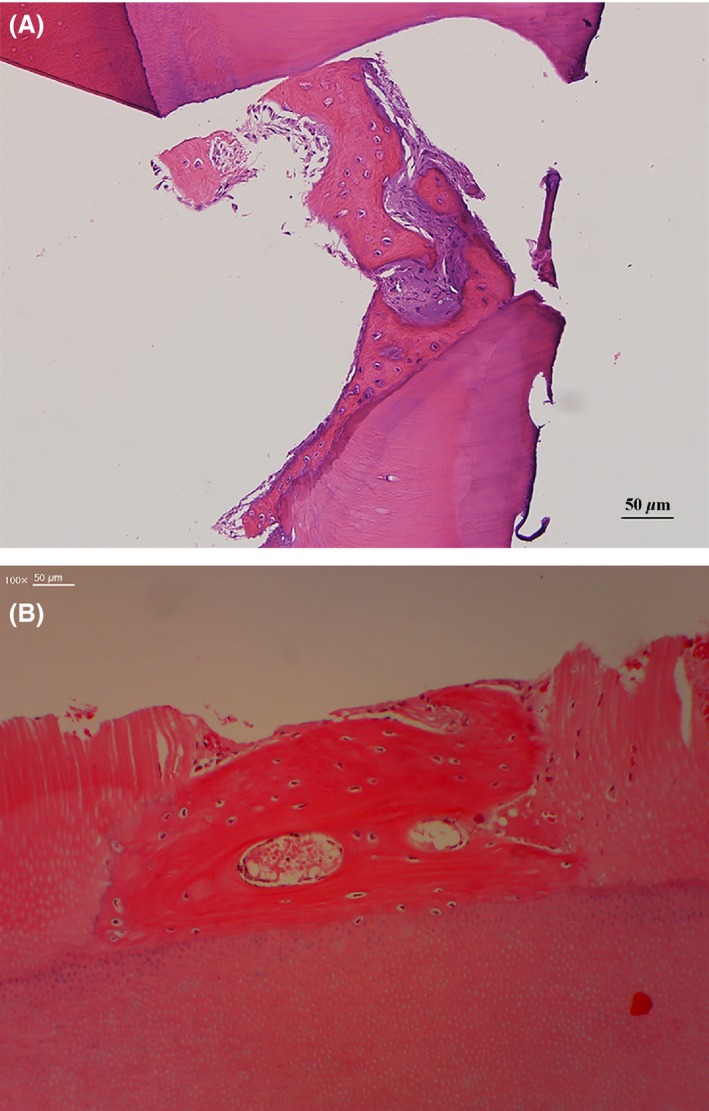
Histological findings at the second surgery. (A) Histological specimen from maxillary case no. 6 in Table 1 at 8 months after the first surgery. A macropore of the ABTB was filled with newly formed osteoids with embedded active chondrocyte‐like cells that closely contacted the inner wall of the macropore. (B) Histological specimen from mandibular case no. 8 in Table 1 at 3 months after the first surgery. A newly formed osteoid, which had osteocytes and vessels, had been deposited on the ABTB surface. Cellular fusion without fibrous tissue invasion was observed on the border between the osteoid and the dentin matrix.
Buccal height reduction from immediately postoperation to the final follow‐up was markedly higher for mandibular implants (40.8%) than for maxillary implants (17.03%); this difference was statistically significant. In contrast, reductions in ARW were similar for mandibular implants (24.6%) and maxillary implants (21.18%). Consequently, CBA was reductions of 41.78% for mandibular implants and 28.30% for maxillary implants (Fig. 7). Among these three parameters, significant differences were only observed for BH.
Figure 7.
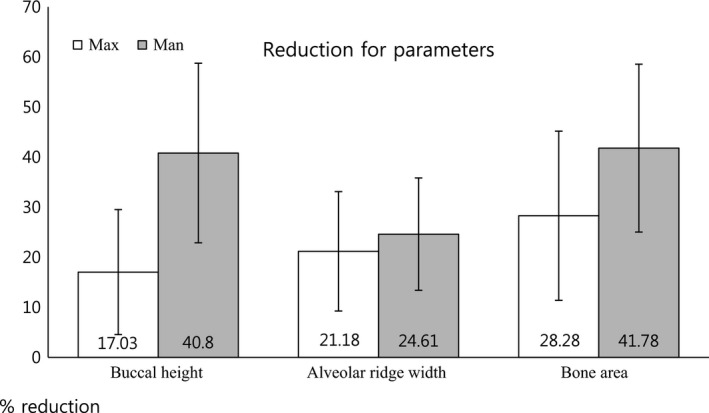
Total % reductions for BH, ARW, and CBA. BH reduction from immediately postoperation to the final follow‐up was markedly greater in the mandible (40.8%) than in the maxilla (17.03%). In contrast, similar reductions in ARW were observed in the mandible (24.6%) and the maxilla (21.18%). Consequently, reductions in bone area according to cross‐sectional images were 41.78% in the mandible and 28.30% in the maxilla. Among these three parameters, BH was the only parameter with statistically significant differences between the mandible and the maxilla. Max, maxilla; Man, mandible.
Marginal bone resorption values of 2.5 and 1.0 mm were observed at 44 and 26 months after the first surgery, respectively, for maxillary implants. In the mandible, one patient presented with 2.0 mm of MBR at #18 40 months after the first surgery, which featured a BD of 15 months. In particular, 10 mm of MBR in the mandible was indicative of the removal of an implant with an MBR of the same length at #18 due to the complete resorption of bone around the implant fixture (mandibular case no. 1). This failure had the following characteristics: the longest duration to reach BD and the earliest start of MBR among the four MBR cases. In addition, the overall CBA was a reduction of 62.2% over an 18‐month follow‐up; this change was the greatest observed reduction in the mandible (Fig. 8, Table 1).
Figure 8.
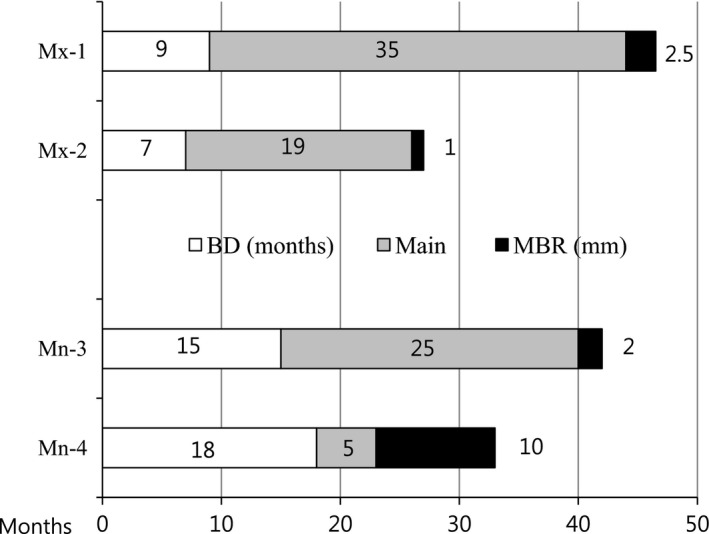
MBR in the maxilla and the mandible. In the maxilla, MBRs of 2.5 and 1.0 mm were observed at 44 and 26 months after the first surgery, respectively. In the mandible, one patient exhibited 2.0 mm of MBR at #18 at 40 months after the first surgery, which featured a BD of 15 months. In particular, 10 mm of MBR indicated the removal of the 10‐mm implant at #18 due to the complete resorption of bone around the implant fixture (mandibular case no. 1). Mx, Maxilla; Mn, Mandible; Ma, Male; Fe, Female; BD, ABTB Disappearance; Main, maintenance; MBR, Marginal Bone Resorption.
Discussion
The aim of this study was to evaluate long‐term results of the ABTB when grafted for socket preservation by measuring BD, BH, ARW, CBA, and the occurrence of MBR during long‐term follow‐up. Given the hypothesis that all examined parameters would be no worse after an ABTB graft and implant placement than after an alternative procedure, the ABTB graft was expected to promote the favorable incorporation and remodeling of implants under an occlusal force based on findings from clinical studies with a short follow‐up period. Therefore, it is necessary to confirm whether the bone maintains its volume and quality during a long‐term follow‐up period 8, 9, 10, 11.
Because the ABTB is a biomimetic of a cortical bone block, the initial remodeling response to the ABTB is resorptive. This response predominantly promotes osteoclastic activity in which the dentin matrix around the macropores is slowly resorbed and simultaneously replaced with new viable bone throughout the early inflammatory and repair stages. The macropores also provide space for vascular invasion, during which newly deposited bone replaces the scaffold and eventually contributes to creeping substitution (Figs 3B, 3C, 6A, 6B) 8, 9, 10.
Therefore, a parameter for assessing in corporation and remodeling via radiological evaluation is BD, which is measured by the complete disappearance of holes in the ABTB and the borders between the host and the ABTB. The time periods to reach BD after the ABTB graft in the maxilla and the mandible were similar, with averages of approximately 13.4 and 12.5 months, respectively. In contrast, the time period from FL to BD was 2 months faster in the maxilla than in the mandible, although this difference was not significant. The ABTB's response to FL was assumed to be more favorable in the maxilla than in the mandible. Although implant restoration results in significant growth of the mandible by pushing and pulling stimuli according to Wolff's law 12, resorption of alveolar bone after the loss of teeth is four times higher in the mandible than in the maxilla (Fig. 5) 13.
From the histological perspective, the ABTB exhibited its osteoinductive and osteoconductive capacities. The previous study indicated that osteoconductive properties of ABTB could be dependent on the accessibility and quantity of blood supply from the surrounding host tissues 14. In this study, macropores of the ABTB were filled with woven bone by blood vessel development, which shows the osteoconduction by the ABTB (Fig. 6A). Moreover, a newly formed osteoid containing osteocytes and blood vessels was deposited on the surface of the ABTB, and the border between the osteoid and the dentin matrix featured cellular fusion without fibrous tissues 15 that shows osteoinductive property of ABTB (Fig. 6B).
Decreases in BH were markedly higher for the mandible than for the maxilla, a finding that is consistent with the reported resorption rate for the mandible 13. However, mandibular and maxillary implants featured similar reductions in ARW (Fig. 7). The observed reductions in BH and ARW, which were 17.03% and 21.18%, respectively, in the maxilla and 40.8% and 24.6%, respectively, in the mandible, were no lower than the reductions calculated in other studies. These studies revealed estimated bone width losses to the resorptive process of 31.6% after 3 months, 42.4% after 6 months, and 50.73% after 12 months. This loss is attributable to the progressive resorption of 0.2–3.25 mm of BH and 0.34–7.7 mm of ARW during the 6–12 months after extraction 5. In addition, the height loss during the first 5 years is more than twice the height loss during the subsequent 20 years (7.6 mm/3.1 mm) 16.
In cases involving an immediate implant after extraction, Chen et al. reported that the use of membranes and/or bone grafts produced no additive effect on bone regeneration for the reduction of vertical defects, with a mean height reduction of 73.6 ± 7% from the 9.7 ± 0.6 mm mean height of the initial vertical defect 17. Therefore, the effectiveness of materials used to prevent vertical resorption remains unclear.
When demineralized freeze‐dried bone allograft (DFDBA) was used in conjunction with a collagen membrane, the ARW decreased from 9.2 to 8.0 mm, whereas the average width of socket sites that healed naturally decreased from 9.1 to 6.4 mm 6.
Changes in Bone Area, which was 41.78% in the mandible and 28.28% in the maxilla, appear to be closely related to decreases in BH because this phenomenon was mainly caused by a reduction in BH (17.03% in the maxilla and 40.8% in the mandible) rather than ARW (21.18% in the maxilla and 24.61% in the mandible) (Fig. 7). These findings from long‐term follow‐up are also promising relative to other results, which indicated that the mean resorption volumes for 14 patients with 32 iliac and chin grafts at one year after implant positioning were 35–51% 18. In contrast, mean volume reductions of 16.2% at 6 months (15 patients) and 19.2% at 12 months (five patients) were observed in other studies 19. Verhoeven et al. reported a 36% mean resorption rate for the graft, with resorption mainly occurring during the first year after surgery 20.
Marginal bone resorption was observed in four patients. Two of these patients who received maxillary implants at #15 did not experience suppuration, bleeding, increases in probing pocket depth, or other signs and symptoms (Fig. 8, Mx‐1 and Mx‐2). In contrast, one mandibular patient underwent a local flap operation for symptom relief at #18 (Fig. 8, Mx‐1). The remaining patient, who experienced implant failure at #18, exhibited not only a longer BD duration than the other three MBR patients but also an earlier start to MBR (Fig. 8, Mx‐2). With respect to CBA, this patient presented with 62.2% reduction 18 months after the graft, with anticipated failure due to excessive reduction(Fig. 8). Another study indicated that with an autogenous bone graft, marginal bone loss was 0.74 mm at 3 months, with up to 50% cumulative resorption (1.67 mm) at 12 months 21. A case series from 2014 examining AutoBT on 10 implant sites found that only two implants produced 0.2 and 0.3 mm of crestal bone loss during the 12–25 months after FL 10. Another investigation focusing on AutoBT indicated that the use of a membrane did not affect bone‐level changes during the period between the first surgery and the second surgery (1–7 months). This result is also consistent with the findings of a study that concluded that AutoBT is resistant to MBR 22.
These results suggest that the MBR result using ABTB was more favorable in maxilla than in mandible. Additionally, in three cases, the amount of MBR satisfies the success criteria of a dental implant; in particular, MBR and peri‐implant bone remodeling, for which was up to 2 mm of resorption during the first year of function followed by a maximum of 0.2 mm annually is universally accepted, could be reliable success criteria for dental implants 23.
There was another statistical analysis without the case no.1 that the patient lost the implant in mandible due to 10 mm MBR. As a result, the BH, ARW, and CBA have been changed from 40.8%, 24.61%, and 41.78% to 42.67%, 24.53%, and 39.51%, respectively. Even this statistical analysis could not make the differences between maxilla and mandible. This result could be by the marginal bone resorption pattern of case no.1, which did not show saucerization, but periodontal ligament widening. Consequently, the parameters of BH and ARW were not reduced as much as that of CBA indicated in Table I.
The findings from the examined 22 cases were consistent with other short‐term studies indicating that the ABTB has a capacity for continuous remodeling under a functional load with appropriate volume maintenance. Moreover, the CB and MBR, which are relevant to bone resorption, showed that ABTB is more favorable in the maxilla than in the mandible.
In addition, it was hard to reason why CBA was larger and the period between FL and BD was longer in the mandible compared to maxilla. But, one suggestion is that postoperative dehiscence developed only in mandible and that could affect the initial healing stage. However, in case no.4, BH is very similar to average and ARW and CBA are lower than average. On the other side, the BH is lower than the average and ARW and CBA are slightly higher than average, but within standard deviation in case no.9.
Another suggestion is the nature of ABTB and the different amount of blood supply between maxilla and mandible. Due to the highly porous mineralized collagenous nature of ABTB, highly vascularized spongiosa bone of maxilla might be more favorable for remodeling than the less vascularized cortical bone of mandible.
Within this limited case series, there exist no block‐type graft materials similar to the ABTB that could act as a control group, weakening the reliability of ABTB studies. The longer case series or prospective studies are needed to validate the ABTB as a preferred method for socket preservation or ridge augmentation compared to current therapies that demonstrate consistent success in the long term.
Conclusion
Examinations of remodeling capacity and CBA as indicated by changes in BH and ARW, and the occurrence of MBR for the ABTB during long‐term follow‐up, produced results that were consistent with the findings of other ABTB studies with short‐term follow‐up and not inferior to findings from studies of other materials.
Authorship
YKK: performed concept/design, and approval of article. KMP: performed data collection and writing the manuscript. PYY: performed data collection and statistics. DHL: performed data analysis/interpretation. IWU: funding secured by Korea Health Industry Development Institution, drafting article and critical revision of article.
Conflict of Interest
Authors have no conflict of interest relevant to the content of this submission.
Acknowledgments
This study was supported by a grant from the Korean Health Technology Development R&D Project, Korea Health Industry Development Institution, Republic of Korea (HI15C3136). I appreciate Dr. Masaharu Mitsugi for serving as scientific advisor of this article and also special thanks to my research assistant, Ms. Hae‐Jin Jang and Ms. Yu‐Mi Kim, for participating in writing or technical editing of the manuscript.
References
- 1. Kim, Y. K. , Kim S. G., Byeon J. H., Lee H. J., Um I. U., Lim S. C., et al. 2010. Development of a novelbone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109:496–503. [DOI] [PubMed] [Google Scholar]
- 2. Kim, Y. K. , Um I. W., and Murata M.. 2014. Tooth bank system for bone regeneration ‐ safety report. J. Hard Tissue Biol. 23:371–376. [Google Scholar]
- 3. Murata, M. 2003. Autogenous demineralized dentin matrix for maxillary sinus augmentation in humans: the first clinical report. J. Dent. Res. 82:B‐243. [Google Scholar]
- 4. Murata, M. 2012. Collagen biology for bone regenerative surgery. J. Korean Assoc. Oral Maxillofac. Surg. 38:321–325. [Google Scholar]
- 5. Schropp, L. , Wenzel A., Kostopoulos L., and Karring T.. 2003. Bone healing and soft tissue contour changes following single‐tooth extraction: a clinical and radiographic 12‐month prospective study. Int. J. Periodontics Restorative Dent. 23:313–323. [PubMed] [Google Scholar]
- 6. Iasella, J. M. , Greenwell H., Miller R. L., Hill M., Drisko C., Bohra A. A., et al. 2003. Ridge preservation with freeze‐dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J. Periodontol. 74:990–999. [DOI] [PubMed] [Google Scholar]
- 7. Becker, W. 2005. Immediate implant placement: diagnosis, treatment planning and treatment steps/or successful outcomes. J. Calif. Dent. Assoc. 33:303–310. [PubMed] [Google Scholar]
- 8. Kim, Y. K. , Kim S. G., Um I. W., and Kim K. W.. 2013. Bone grafts using autogenous tooth blocks: a case series. Implant Dent. 22:584–589. [DOI] [PubMed] [Google Scholar]
- 9. Lee, E. G. , Lee J. Y., Kim Y. K., Um I. W., and Choi J. H.. 2013. Delayed implant placement after extraction socket reconstruction and ridge augmentation using autogenous tooth bone graft material. Case Rep. Dent. 3:174. [Google Scholar]
- 10. Kim, Y. K. , Yun P. Y., Um I. W., Lee H. J., Yi Y. J., Bae J. H., et al. 2014. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: prospective case series. J. Adv. Prosthodont. 6:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim, Y. K. , Lee J. K., Kim K. W., Um I. W., and Murata M.. 2013. Healing mechanism and clinical application of autogenous tooth bone graft material Pp. 405–435 in Pignatello A., ed. Advances in biomaterials science and biomedical applications. InTech Open Publishers, Croatia. doi:10.5772/53200. [Google Scholar]
- 12. Reddy, M. S. I. , Geurs N. C., Wang I. C., Liu P. R., Hsu Y. T., Jeffcoat R. L., et al. 2002. Mandibular growth following implant restoration: does Wolff's law apply to residual ridge resorption. Int. J. Periodontics Restorative Dent. 22:315–321. [PubMed] [Google Scholar]
- 13. Bodic, F. , Hamel L., Lerouxel E., Baslé M. F., and Chappard D.. 2005. Bone loss and teeth. Joint Bone Spine 72:215–221. [DOI] [PubMed] [Google Scholar]
- 14. Reddi, A. H. , and Huggins C. B.. 1973. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc. Soc. Exp. Biol. Med. 143:634–637. [DOI] [PubMed] [Google Scholar]
- 15. Urist, M. R. , Dowell T. A., Hay P. H., and Startes B. S.. 1968. Inductive substrates for bone formation. Clin. Orthop. Relat. Res. 59:59–96. [PubMed] [Google Scholar]
- 16. Veldhuis, H. , Driessen T., Denissen H., and de Groot K.. 1984. A 5‐year evaluation of apatite tooth roots as means to reduce residual ridge resorption. Clin. Prev. Dent. 6:5–8. [PubMed] [Google Scholar]
- 17. Chen, S. T. , Darby I. B., Adams G. G., and Reynolds E. C.. 2005. A prospective clinical study of bone augmentation techniques at immediate implants. J. Clin. Oral Implants Res. 16:176–184 [DOI] [PubMed] [Google Scholar]
- 18. Sbordone, L. , Toti P., Menchini‐Fabris G. B., Sbordone C., Piombino P., and Guidetti F.. 2009. Volume changes of autogenous bone grafts after alveolar ridge augmentation of atrophic maxillae and mandibles. Int. J. Oral Maxillofac. Surg. 38:1059–1065. [DOI] [PubMed] [Google Scholar]
- 19. Smolka, W. , Eggensperger N., Carollo V., Ozdoba C., and Iizuka T.. 2006. Changes in the volume and density of calvarial split bone grafts after alveolar ridge augmentation. J. Clin. Oral Implants Res. 17:149–155. [DOI] [PubMed] [Google Scholar]
- 20. Verhoeven, J. W. , Cune M. S., Terlou M., Zoon M. A. O. W., and De Putter C.. 1997. The combined use of endosteal implants and iliac crest onlay grafts in the severely atrophic mandible: a longitudinal study. Int. J. Oral Maxillofac. Surg. 26:351–357. [DOI] [PubMed] [Google Scholar]
- 21. Kim, T. Y. , Kim Y. M., Kim J. Y., Kim M. R., and Kim S. J.. 2011. The retrospective study of marginal bone loss around dental implants according to different autogenous bone grafts. J. Korean Assoc. Oral Maxillofac. Surg. 37:483–489. [Google Scholar]
- 22. Lee, J. Y. , Lee J. H., and Kim Y. K.. 2013. Comparative analysis of guided bone regeneration using autogenous tooth bone graft material with and without resorbable membrane. J. Dent. Sci. 8:281–286. [Google Scholar]
- 23. Papaspyridakos, P. , Chen C.‐J., Singh M., Weber H.‐P., and Gallucci G. O.. 2012. Success criteria in implant dentistry: a systematic review. J. Dent. Res. 91:242–248. [DOI] [PubMed] [Google Scholar]


