Abstract
Variations in intracellular levels of p53 regulate many cellular functions and determine tumor susceptibility. Major mechanisms modulating p53 levels include phosphorylation and interaction of p53 with specific ubiquitin ligases that promote its degradation. N-terminal phosphorylation regulates the interaction of p53 with several regulatory molecules. Vaccinia-related kinase 1 (VRK1) is the prototype of a new Ser-Thr kinase family in the human kinome. VRK1 is located in the nucleus outside the nucleolus. Overexpression of VRK1 increases the stability of p53 by a posttranslational mechanism leading to its accumulation by a mechanism independent of the Chk2 kinase. Catalytically inactive VRK1 protein (a K179E mutant) does not induce p53 accumulation. VRK1 phosphorylates human p53 in Thr18 and disrupts p53-Mdm2 interaction in vitro, although a significant decrease in p53 ubiquitination by Mdm2 in vivo was not detected. VRK1 kinase does not phosphorylate Mdm2. VRK1-mediated p53 stabilization was also detected in Mdm2−/− cells. VRK1 also has an additive effect with MdmX or p300 to stabilize p53, and p300 coactivation and acetylation of p53 is enhanced by VRK1. The p53 stabilized by VRK1 is transcriptionally active. Suppression of VRK1 expression by specific small interfering RNA provokes several defects in proliferation, situating the protein in the regulation of this process. VRK1 might function as a switch controlling the proteins that interact with p53 and thus modifying its stability and activity. We propose VRK1 as the first step in a new pathway regulating p53 activity during cell proliferation.
Regulation of p53 levels plays a major role in determining the fate of a cell and its susceptibility to tumor development (17, 19). The p53 protein is at a crossroad in the pathways implicated in the cellular response to many different types of stresses, such as genotoxic damage by UV or ionizing irradiation, hypoxia, and heat shock (36), and it has been implicated in different cell cycle checkpoints (7, 48). Several cell protection mechanisms are based on the ability of the p53 protein to regulate the progression of the cell cycle, the induction of apoptosis, or replicative senescence (6, 46, 50). These responses are controlled by the p53 protein level. p53 is a short-lived protein that is maintained at low levels in the cell under normal conditions and increases in response to stress. The levels and stability of the p53 protein in the cell are mainly controlled by its interactions with the negative regulator Mdm2 (hdm2 in humans) (3) or with other E3 ubiquitin ligases, such as COP1 (14) or Pirh2 (24). Mdm2 targets p53 for degradation by the ubiquitination pathway, promotes its nuclear export, and thus allows cell cycle progression (3). The new ubiquitin ligases COP1 and Pirh2 are also negative regulators of p53 function, targeting p53 for degradation by the proteasome in a ubiquitin-dependent fashion, and like Mdm2 are encoded by p53-inducible genes (14, 24). Interactions with stabilizing proteins, such as p300 (53), MdmX (29, 47), or the deubiquitinase HAUSP (25), also play a major role in p53 stabilization and activation.
Phosphorylation of p53 in its transactivation domain in the N-terminal region is one of the mechanisms by which the interaction of p53 with Mdm2 and its transcriptional activity are regulated (37). Several kinases are able to phosphorylate the p53 molecule in its N terminus; each of them is implicated in the response to different types of stress stimulation (31). These phosphorylations have a partial overlap in their effects on either activation of p53 transcriptional activity (15) and coactivator binding (11) or p53 interaction with Mdm2. After stimulation, there is an increase in p53 coactivator response that is followed several hours later by an increase in downregulatory proteins, such as Mdm2.
Thr18 residue phosphorylation, in the transactivation domain of p53, has been implicated in both disruption of p53-Mdm2 interaction and p300 coactivator recruitment, which acetylates the p53 carboxy terminus. This leads to a decrease in p53 degradation and its subsequent stabilization and to an increase in p53-dependent transactivation activity. The p53 activity is also regulated by phosphorylation in its carboxy terminus mediated by several known kinases, such as protein kinase C (35) or casein kinase II, that can modulate its oligomerization status (40, 41) and stabilize p53-DNA interaction.
In the human kinome, the new VRK (vaccinia-related kinase) family of serine-threonine kinases, composed of three members, is an early divergent branch related to casein kinases (30). The conservation of the kinase domain is weak, but in the cases of VRK1 and VRK2, it is catalytically active (5, 27, 44, 45). Originally, these proteins were identified by their homology to the catalytic domain of the B1R vaccinia virus kinase (34). B1R is an early protein essential for viral replication (4, 26). The VRK family has only one member in Drosophila melanogaster, locus 97D3, and one member in Caenorhabditis elegans, gene F28B12.3 on chromosome 2. Knockout of the F28B12.3 gene in C. elegans embryos has lethal effects, which are variable, ranging from arrest at many stages of embryogenesis to arrest soon after hatching, and growth is slow (22). In yeast, there are early precursors of the VRK family. In Schizosaccharomyces pombe, the HHP1 gene is involved in DNA repair in response to gamma irradiation (10); this gene is an ortholog of the HRR25 gene from Saccharomyces cerevisiae. For this reason, it was initially postulated to have possible roles in cell growth regulation and proliferation. VRK1 appears to be expressed ubiquitously, at very different levels, in many tissues (34), and in murine embryos it is implicated in the early phases of hematopoietic development (49). VRK1 in vitro can phosphorylate human p53 in Thr18 (5, 27), a residue that is essential for the interaction between p53 and Mdm2 (21, 43). It also appears to have different expression patterns in specific tumor types (unpublished data).
In this report, we show that VRK1 is a nuclear kinase that stabilizes the intracellular protein levels of p53 by a posttranslational mechanism, and thus, it affects p53-dependent transcription. This VRK1 effect is not only dependent on a direct disruption of the MDM2-p53 interaction, although its overexpression increases endogenous p53 Thr18 phosphorylation. VRK1 also increases p53 carboxy-terminal acetylation directed by p300 and p53-p300 interaction, an effect that might be directed by p53 Thr18 phosphorylation and other interactions. We also show that cells transfected with specific small interfering RNA (siRNA) that suppresses the expression of VRK1 have different defects in cell proliferation, and we propose a model for VRK1 action on p53.
MATERIALS AND METHODS
Reagents, plasmids, and antibodies.
The plasmids containing human p53 wild-type cDNA were obtained from K. Vousden (National Cancer Institute, Frederick, Md.) or J. Pietenpol (Vanderbilt University, Nashville, Tenn.). The human cDNA for VRK1 or the K179E mutant was subcloned in plasmid pcDNA3.1 with the Myc epitope (pcDNA-VRK1myc clone) or in pCEFL-KZ with the hemagglutinin (HA) epitope (pHA-VRK1 clone). The p53-cis reporter system (p53-luc) with the luciferase gene was from Stratagene (La Jolla, Calif.). As a control for transfection, we used the pRenilla-tk (pRL-tk) reporter plasmid from Promega (Madison, Wis.). Also, the reporter constructs p21-luc, Bax-luc, and p14-3-3-Luc, containing the promoter of the p53-responsive genes linked to luciferase (from M. Oren, Rehovot, Israel), were used. The ubiquitin tagged with the His epitope (clone BRR12-his-Ubiquitin) was from S. Lain and D. Lane (University of Dundee, Dundee, Scotland). Plasmid pCDNA-Chk2-Myc was from J. Bartek (Danish Cancer Institute, Copenhagen, Denmark). Plasmids pCMV-FLAG-Chk2 and pCMV-FLAG-Chk2A347 were from T. Halozanetis (University of Pennsylvania, Philadelphia). Plasmid pCOC-Mdm2-X2, coding for the Mdm2 protein, was from Karen Vousden (Beatson Institute, Glasgow, Scotland). Plasmid p3.1MdmXNIF, encoding full-length MdmX, was from S. Berberich (Wright State University, Dayton, Ohio). Plasmid pCMVβ-p300-CHA was from Richard Eckner (University of Zurich, Zurich, Switzerland). All plasmids used for transfection were endotoxin free and purified with the JetStar Maxi kit from Genomed (Bad Oeynhausen, Germany).
The anti-β-actin antibody was from Sigma (St. Louis, Mo.). A polyclonal antibody against the Myc epitope was from Upstate Biotechnology (Lake Placid, N.Y.), and the mouse monoclonal antibody HA probe (F7) against the HA tag was from Santa Cruz (Santa Cruz, Calif.). The monoclonal antibody FTP18 against p53 phosphorylated in Thr18 was a gift of T. Hupp (University of Dundee) (9). The p53 protein was detected with a mix of DO1 antibody (Santa Cruz) and Pab1801 (Santa Cruz) used at 1:500 and 1:1,000, respectively. To detect endogenous p53 Thr18 and Ser15 phosphorylation in vivo, specific rabbit polyclonal antibodies from Santa Cruz were used. A goat anti-mouse-horseradish peroxidase and a goat anti-rabbit-horseradish peroxidase (Amersham Pharmacia Biotech) were used at 1:5,000 in Western blotting. VRK1 was detected by using a rabbit polyclonal antibody against the glutathione S-transferase (GST)-VRK1 fusion protein. The immunoblots were developed by chemiluminescence using ECL reagent (Amersham Biosciences). A rabbit antiserum from Upstate Biotechnology detecting specifically acetylated p53 in Lys373 and Lys382 was used. 4B2 monoclonal antibody (a gift of Sonia Lain) was used for the detection of the Mdm2 protein.
Fusion proteins.
The human GST-VRK1 fusion protein has the full-length VRK1 and was described previously (27). The GST-p53 fusion protein FP221 containing the N terminus of murine p53 (residues 1 to 85) (32) was a gift of D. Meek (University of Dundee). A Thr18Ala substitution was introduced using the Quick-Change mutagenesis system from Stratagene (San Diego, Calif.). The GST-Mdm2 protein encoding amino acids 1 to 188 was from A. Levine. p53-GST constructs including the fragments 90 to 290 and 290 to 390 were from T. Kouzarides (Cancer Research UK Institute, Cambridge, United Kingdom). The GST fusion proteins were purified using glutathione-Sepharose resin (Amersham Biosciences, Little Chalfont, United Kingdom) and eluted from the beads with glutathione according to the manufacturer's instructions. Proteins were quantified using a Bradford protein assay kit (Bio-Rad, Hercules, Calif.). To generate an inactive VRK1 kinase, a K179E substitution was introduced using the Quick-Change mutagenesis system in both the GST-VRK1 and eukaryotic expression constructs. The mutant constructs were confirmed by DNA sequencing.
VRK1 protein kinase assay.
The kinase assay was performed in a 25-μl final volume containing 1 or 2 μg of VRK1 protein or VRK1-K179E mutant and 2 μg of protein substrates as appropriate. It was performed in kinase buffer (50 mM Tris-HCl, pH 7.5, 100 μM ATP, 50 mM MgCl2, 1 mM dithiothreitol [DTT]) and 5 μCi of [γ-32P]ATP. The reaction mixture was incubated for 30 min at 30°C. The phosphorylated proteins were analyzed by electrophoresis in a 0.1% sodium dodecyl sulfate (SDS)-10% polyacrylamide gel. After the gel was stained with Coomassie blue (Merck), the radioactivity incorporated was analyzed using a Bio-Rad FX molecular imager system. Quantification was performed with the program Image Quant (Bio-Rad). In all cases, the analysis was performed within the linear response region of the system.
Binding of Mdm2 to GST-p53 fusion proteins.
To determine the abilities of phosphorylated and nonphosphorylated p53 to bind to Mdm2 in vitro, GST pull-down assays were performed using in vitro-translated proteins. For these experiments, glutathione S-transferase-p53 fusion proteins were first phosphorylated by VRK1 using 2 μg of VRK1-His6 recombinant protein and 1 to 2 μg of GST-p53 as a substrate. The reaction was carried out in kinase buffer (50 mM Tris-HCl, pH 7.5, 50 μM MgCl2, 1 mM DTT) at 30°C for 3 h with 0.5 mM Mg-ATP. Following phosphorylation, the GST-p53 proteins were adsorbed onto glutathione-Sepharose 4B beads (Amersham Biosciences), and the beads were washed. Mdm2 protein was translated in the presence of [35S]methionine using a reticulocyte lysate in vitro transcription-translation kit (Promega) according to the manufacturer's instructions. The GST-p53 proteins were incubated at 4°C with 35S-labeled Mdm2 in 200 μl of the binding-washing buffer (50 mM Tris, pH 7.4, 250 mM NaCl, 0.1% Triton X-100, 5 mM EDTA, 2 mM DTT) for 2 h. The complexes were washed with washing buffer, boiled in sample buffer, and analyzed by SDS-polyacrylamide gel electrophoresis. The gels were dried and exposed to X-ray films or analyzed with a Bio-Rad phosphorimager.
Cell lines, transfections, and immunoprecipitation.
The human lung cancer cell lines A549 (p53+/+) and H1299 (p53−/−) were grown in RPMI supplemented with 10% fetal calf serum, glutamine, penicillin, and streptomycin in a humidified 5% CO2 atmosphere. The HCT116 wild-type p53 cell line was grown in McCoy's 5A supplemented medium. For transfection experiments, H1299 cells were plated in 60- or 100-mm-diameter dishes and transfected with the plasmid indicated in the specific experiments, either by the calcium phosphate precipitation method or with JetPI reagent (Polytransfection, Illkirch, France). The cells were lysed 36 h postransfection in lysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100, plus protease and phosphatase inhibitors) or acetylation immunoprecipitation lysis buffer (50 mM HEPES, pH 7.8, 200 mM NaCl, 1% [vol/vol] Triton X-100, 10 mM EDTA, 5 mM DTT, 5 μM trichostatin A, and protease and phosphatase inhibitors), and 40 μg of whole-cell extract was processed for SDS-polyacrylamide gel electrophoresis and subjected to immunoblotting with the appropriate antibodies. A549 cells were transfected with Lipofectamine 2000 reagent (Invitrogen) in 60-mm-diameter dishes. The immortalized fibroblasts from double-knockout p53/Mdm2 mice (33), a gift of G. Lozano (M. D. Anderson Cancer Center, Houston, Tex.), were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum and antibiotics. When indicated, cycloheximide (Sigma) was added to a final concentration of 50 μg/ml, and adriamycin (Sigma) was added to a final concentration of 0.2 μg/ml. The proteasome inhibitor MG132 (Calbiochem) was used at 40 μM for 6 h.
Where indicated, immunoprecipitation of p53 was performed using either a mix of the anti-p53 monoclonal antibodies DO-1 and Pab240 (1 μg each) or the polyclonal anti-p53 antibody CM1 (Novocastra, Newcastle, United Kingdom) and GammaBind G Sepharose (Amersham Biosciences). To immunoprecipitate p300 protein, a mixture of the rabbit polyclonal antibodies p300-1 and p300-2, kindly provided by A. Zantema (Leiden University, Leiden, The Netherlands), was used.
Immunofluorescence and confocal microscopy.
H1299 cells (5 × 105) were plated on 10-cm2 dishes containing 1-cm-diameter sterile glass coverslips and transiently transfected as described above; 36 h postransfection, the slides were transferred to a tissue culture test plate. The cells were washed three times with phosphate-buffered saline (PBS) and then fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. After fixation, the cells were permeabilized in cold PBS containing 0.2% Triton X-100 for 30 min and then treated with 10 mM glycine for 10 min at room temperature. The cells were blocked with a solution containing 1% bovine serum albumin in PBS at room temperature for 30 min and then incubated for 30 min at 37°C or for 1 h at room temperature with a mixture of anti-p53 antibodies DO-1 and Pab1801 or anti-Myc antibody in blocking solution. The cells were washed three times with PBS-0.1% Triton X-100 and incubated with a goat anti-mouse Cy2-labeled antibody (Amersham Biosciences) or a goat anti-rabbit Cy3-labeled antibody (Amersham Biosciences) in blocking solution. The cells were washed three times with PBS-0.1% Triton X-100 and stained with DAPI (4′,6′-diamidino-2-phenylindol) (1:1,000; Sigma) in PBS for 10 min at room temperature. The cells were washed with PBS, and slides were mounted with Gelvatol (Monsanto). Subcellular localization was analyzed with a Zeiss LSM 510 confocal microscope. For detection of the endogenous VRK1 protein, A549 or HCT116 cells were treated as described above, but VC1 polyclonal anti-human VRK1 antibody and goat anti-rabbit Cy3-labeled antibody (Amersham Pharmacia Biotech) were used for detection.
Luciferase assays.
Transcription activity was determined using different reporter constructs with the luciferase gene. H1299 or A549 cells were seeded at 5 × 105 in six-well plates. Twenty-four hours later, the cells were transfected using the JetPI reagent as directed by the manufacturer or with Lipofectamine 2000 reagent, with the indicated construct in each case including the specific reporter vector. As an internal control, the pRL-tk plasmid was used for normalization. Luciferase assays were performed 20 to 36 h after transfection using a dual luciferase reporter kit (Promega). Luminescence was determined with a MiniLumat LB9506 luminometer (Berthold, Bad Wildbad, Germany).
Immune complex kinase assay.
Cells were washed twice with ice-cold PBS, harvested with a rubber policeman, and centrifuged at 4°C. Cell pellets were resuspended in ice-cold lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 10 mM NaF, 10% glycerol, 4 mM EDTA, 1% Triton X-100, 0.1% SDS) and protease inhibitors, incubated on ice for 30 min, and precleared by centrifugation at 16,000 × g for 20 min at 4°C. Extracts were immunoprecipitated with an anti-Myc rabbit polyclonal antibody. Immune complexes were recovered with GammaBind G Sepharose. The beads were sequentially washed with lysis buffer, 100 mM Tris-HCl at pH 7.5, and kinase buffer. The phosphorylation reaction was performed as described above.
RT-PCR.
H1299 cells were cotransfected as described above and washed in ice-cold PBS. Total RNA was extracted using the RNAeasy extraction kit from QIAGEN (Hilden, Germany). The RNA was analyzed and quantified using a Bioanalyzer 2100 nano-lab chip from Agilent Technologies (Waldbronn, Germany). Total RNA (100 ng) was used in a one-step reverse transcription (RT) real-time PCR amplification reaction using the Quantitec SYBR Green RT-PCR kit from QIAGEN in an iCycler (Bio-Rad). The reaction product was analyzed with iCycler software (Bio-Rad), and the PCR products were resolved in a 1.5% agarose ethidium bromide gel. The primers used for p53 amplification detection were 5′-CCCCAGCCAAAGAGAAACC-3′ and 5′-TCCAAGGCCTCATTCAGCTCT-3′, and those for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) amplification detection were 5′-GGTCTTACTCCTTGGAGGCCATGT-3′ and 5′-ACCTAACTACATGGTTTACATGTT-3′.
RNA interference experiments.
Synthetic selected SMART siRNA duplexes were purchased from Dharmacon RNA Technologies (Lafayette, Colo.). The targeted sequences for VRK1 (accession number NM_003384) were GAAAGAGAGTCCAGAAGTA (duplex siVRK1-1), CAAGGAACCTGGTGTTGAA (duplex siVRK1-2), GGAATGGAAAGTAGGATTA (duplex siVRK1-3), and CAAATCTTCTTCTGAACTA (duplex siVRK1-4). A functional siCONTROL nontargeting siRNA pool from Dharmacon was used as a negative control, and fluorescently labeled siGLO Lamin A/C siRNA was used for silencing and transfection efficiency. Transfections of siRNA duplexes at 100 to 200 nM final concentrations were carried out using Lipofectamine 2000 reagent (Invitrogen) in HCT116 cells following the manufacturer's instructions. After transfection, the cells were processed for Western blotting, immunofluorescence assay, or video microscopy as indicated. Cell proliferation was measured in 96-well plates using a Cell Proliferation Kit II (XTT) (Roche).
RESULTS
Endogenous VRK1 is a nuclear protein excluded from the nucleolus.
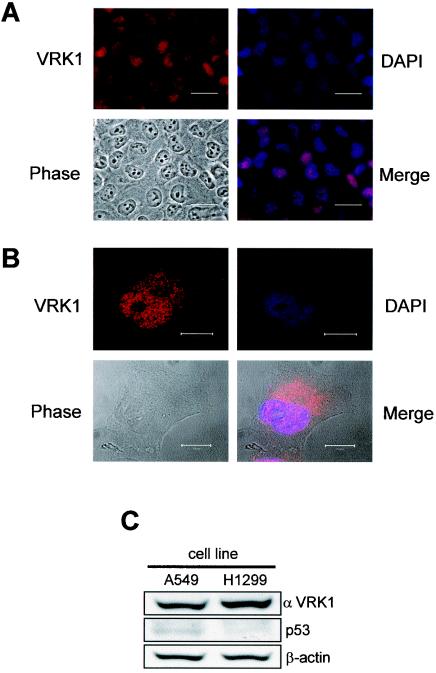
The intracellular location of the human endogenous VRK1 protein in the lung carcinoma A549 (p53+/p53+) cell line was determined by immunofluorescence and confocal microscopy studies. For this aim, a specific polyclonal antibody against the carboxy terminus of human VRK1 was prepared, and its specificity was confirmed by blocking the antibody signal when a VRK1-His protein was included in the incubation (not shown). Analysis of A549 cells by immunofluorescence located the endogenous VRK1 mainly in the nucleus, colocalizing with DAPI staining (Fig. 1A). The analysis of cells in interphase by confocal microscopy revealed that the endogenous VRK1 protein was mainly distributed in the nucleus and excluded from the nucleolus, with some present in the perinuclear area (Fig. 1B). The same location was demonstrated in the H1299 cell line (not shown). The levels of endogenous VRK1 and p53 in the A549 and H1299 cell lines used in this work were determined by Western blotting (Fig. 1C). A549 has a wild-type p53 gene, as in normal cells, and the H1299 cell line has no expression of p53. The two proteins are present in the same subcellular compartment, but they do not form a stable complex that can be immunoprecipitated, as would be expected from a kinase with a casein kinase I type of enzymatic activity. VRK1 protein, monitored by flow cytometry and by Western blotting, was detected at similarly high levels throughout the cell cycle in the different tumor cell lines used (data not shown).
FIG. 1.
Localization of the endogenous human VRK1 protein in human A549 lung cancer cells determined by immunofluorescence (A) and confocal microscopy in interphase (B). The VRK1 protein was detected with the VC1 polyclonal antibody specific for the human protein labeled with Cy3. The nuclei were detected with DAPI staining, and the cells were identified by phase-contrast microscopy. The overlaps of the VRK1 and DAPI signals are shown in the merge panels. The bars represent 30 (A) or 10 (B) μm. (C) Immunoblot to detect endogenous levels of VRK1 and p53 in the two carcinoma cell lines used in the study using a polyclonal antibody specific for human VRK1 or anti-p53 monoclonal antibody.
p53 is stabilized by VRK1 overexpression.
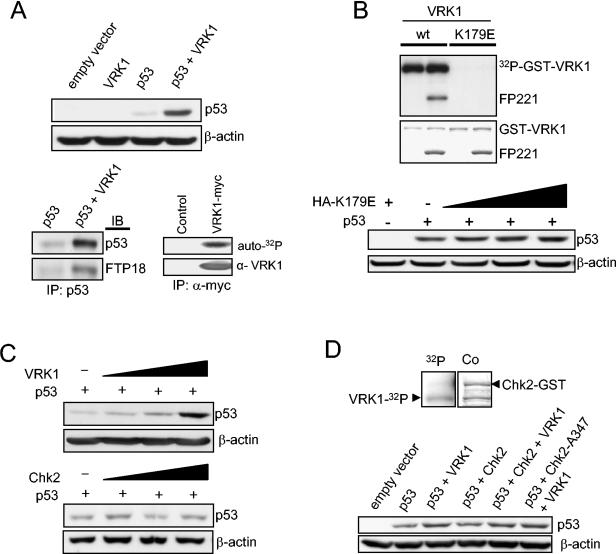
Because VRK1 can phosphorylate p53 in vitro in Thr18 (27), it was decided to study its effects on the levels of p53, which can also be stabilized in the absence of Ser15 or Ser20 phosphorylation (2, 21a). To test this in vivo, the effect of transfected VRK1 on p53 levels was studied by immunoblot analysis, which reflects the overall cell population. H1299 (p53−/−) cells were transfected with plasmids expressing both VRK1 (pcDNA-VRK1myc) and human p53 (pCB6+p53). An increase in the steady-state levels of p53 protein induced by VRK1 was detected by Western blot analysis (Fig. 2A, top). The stabilized p53 protein is extensively phosphorylated in Thr18 (Fig. 2A, bottom left). The activity of tagged VRK1 expressed in cultured cells was confirmed in an in vitro kinase assay with the immunoprecipitated protein (Fig. 2A, bottom right).
FIG. 2.
(A) Overexpression of VRK1 increases the steady-state levels of transiently expressed p53. Human H1299 cells (p53 null) were transfected with 1 μg of plasmid encoding wild-type p53 (pCB6+p53) either alone or together with 8 μg of VRK1 construct (pcDNA-VRK1myc). Cells were also transfected with pCB6+ empty vector or VRK1 alone as controls. Then, p53 was immunoprecipitated, and Thr18 phosphorylation was detected using the FTP18 monoclonal phosphor-specific antibody. An autophosphorylation kinase assay was performed with Myc-tagged inmmunoprecitated protein to show that the exogenously expressed kinase was active. IP, immunoprecipitaton; IB, immunoblotting. (B) The introduction of a substitution, K179E, in the catalytic site of VRK1 generates a kinase that is not active, either on itself (autocatalytic activity) or on other substrates, such as the GST-p53 fusion protein (FP221). The inactive VRK1 protein (K179E) does not induce accumulation of p53. Zero, 3, 5, and 8 μg of the kinase-inactive plasmid were expressed in combination with p53. +, present; −, absent. (C) The accumulation of p53 is dependent on the amount of VRK1. H1299 cells were transfected with a fixed amount of pCB6+p53 (1 μg) and various amounts of pcDNA-VRK1myc (0, 3, 5, and 8 μg). In similar experiments, the cells were transfected with various amounts of pCDNA-Chk2myc (0, 3, 5, and 8 μg) as a control to show its lack of effect on p53 accumulation by itself. (D) In vitro kinase assay to show that VRK1 does not phosphorylate GST-Chk2-A347 kinase-dead fusion protein (top). 32P, autoradiography; Co, Coomassie staining. The stabilization of p53 induced by VRK1 in H1299 cells is not affected if the cotransfection is performed in the presence of an active (pCMV-FLAG-Chk2) or inactive dominant-negative (pCMV-FLAG-Chk2-A347) form of Chk2 protein (bottom). Five micrograms of VRK1 or Chk2 construct was used in combination with 1 μg of p53.
To further demonstrate that the stabilization of p53 requires an active VRK1, a point substitution in the lysine residue essential for phosphotransfer reactions, K179E, was introduced in the catalytic kinase domain of VRK1, both in GST-VRK1 and in a mammalian expression construct. The VRK1 protein with the K179E substitution lacks both autophosphorylation and kinase activity on p53 (Fig. 2B, top). The lack of activity was also confirmed with other substrates (not shown). Therefore, to study the effect in vivo, H1299 cells were transfected with the inactive mutant, VRK1-K179E. Increasing amounts of the inactive kinase do not lead to a significant accumulation of p53 (Fig. 2B, bottom), so we concluded that the effect is dependent on VRK1 kinase activity.
To demonstrate that the accumulation is dependent on VRK1 activity, a dose-response experiment was performed with H1299 cells. The cells were transfected with a fixed amount of p53 (1 μg of pCB6+p53) and various amounts of VRK1 (0, 3, 5, and 8 μg of pcDNA-VRK1myc). The accumulation of p53 is dependent on the amount of transfected VRK1 (Fig. 2C, top). Other kinases, such as Chk2, have been shown to stabilize p53 (28) and phosphorylate the Thr18 residue in response to DNA damage, and an allosteric effect of these molecules has been identified (8). Therefore, a similar stabilization experiment using the construct pCDNA-Chk2-myc instead of VRK1 was performed. The human Chk2 protein does not stabilize p53 by itself in the presence of the endogenous VRK1 protein (Fig. 2C, bottom). To confirm that VRK1 does not phosphorylate and activates Chk2, as is the case with ATM (18, 52), inducing an indirect Thr18 phosphorylation, an in vitro kinase assay was performed with a GST-Chk2 kinase-dead mutant protein as a substrate. There was no phosphorylation of Chk2, but VRK1 was autophosphorylated as expected (Fig. 2D, top). Also, an in vivo experiment was performed with H1299 cells cotransfected with VRK1 and an active (Chk2) or inactive (Chk2-A347) construct. The stabilization of p53 induced by VRK1 is independent of the activity of Chk2 (Fg. 2D, bottom) and is produced even when a dominant-negative form of Chk2 is overexpressed.
VRK1 stabilizes p53 by a posttranslational mechanism.
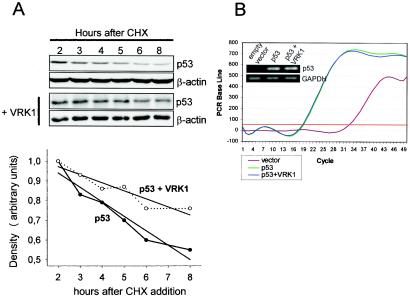
To determine if p53 accumulation is likely to be posttranslational, H1299 cells were transfected with pCB6+p53 alone or together with pcDNA-VRK1myc and incubated in the presence of cycloheximide, which blocks de novo protein synthesis. Cellular extracts were prepared at different times after cycloheximide addition, and the level of p53 protein was determined by immunoblot analysis. As shown in Fig. 3A, in the presence of cycloheximide, the overexpression of VRK1 resulted in a reduced rate of p53 degradation in the cells. Therefore, VRK1 increases the stability of the protein. The half-life of the transfected p53 protein in this cell line increased from 8 to 13.5 h as a consequence of VRK1 overexpression.
FIG. 3.
(A) VRK1 increases the stability of p53 by a posttranslational mechanism. H1299 cells were transfected with p53 with (+) and without VRK1. Twenty-four hours after the transfection, cycloheximide (CHX) was added to the cultures and the levels of p53 protein at different time points were determined by Western blotting. The quantitation of protein levels is shown below the blot. The half-life of p53 increased from 8 to 13.5 h in the presence of VRK1. The straight lines represent the linear regression adjustment of the individual time points. (B) VRK1 overexpression does not affect p53 RNA levels. Real-time quantitative RT-PCR was performed with 100 ng of total RNA obtained from H1299 cells transfected as for Fig. 2A. The amplification curve and a sample of the final product in an agarose gel (inset) are shown.
To exclude any transcriptional effect on the p53 gene, the levels of p53 RNA were also determined by quantitative RT-PCR and agarose gel analysis (Fig. 3B). The levels of p53 RNA were similar in cell lines transfected with only the p53 plasmid or a combination of p53 and VRK1 expression plasmids. Therefore, we concluded that the stabilization effect that VRK1 exerts over exogenously expressed p53 is likely due to a posttranslational event of p53.
VRK1 accumulates transcriptionally active p53.
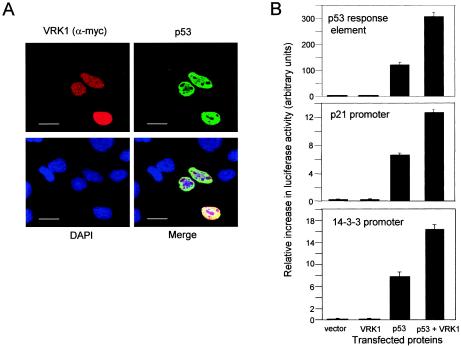
Human H1299 cells (p53−/−) were cotransfected with the plasmid pCB6+p53 (human p53) and the construct pcDNA-VRK1myc for human VRK1 expression. The subcellular localization of both proteins was determined by confocal microscopy. Those cells that expressed only the p53 expression plasmid showed a relatively low level of p53 protein, while cells that expressed both proteins, p53 and VRK1, presented a much higher level of p53 protein (Fig. 4A). Therefore, VRK1 appeared to promote the accumulation of p53 protein within the nuclei.
FIG. 4.
(A) Accumulation and stabilization of nuclear p53 induced by overexpression of VRK1 detected by confocal microscopy. Human H1299 cells (p53−/−) were transfected with pcDNA3.1-VRK1-myc and pCB6+p53. At 36 h postransfection, the cells were analyzed by confocal microscopy with an anti-Myc (labeled with Cy3) or anti-p53 (labeled with Cy2) antibody. The bars represent 20 μm. (B) Activation of p53-dependent transcriptional activity by VRK1 in H1299 cells. Cells were cotransfected with p53 with or without VRK1 and a p53 synthetic reporter plasmid or specific gene promoters, p21-Luc and the 14-3-3-Luc, containing p53 response elements. Reporter luciferase activity was normalized for transfection levels with Renilla luciferase. The error bars represent standard deviations.
A consequence of the accumulation of p53 would be the activation of transcription. Therefore, it was decided to test if the p53 protein stabilized by VRK1 was transcriptionally active. For this purpose, H1299 cells were transfected with pCB6+p53 either with or without pcDNA-VRK1myc. Expression of luciferase activity was determined using three different reporter plasmids, a construct that has several synthetic p53 response sequences (p53-Luc), and two gene-specific promoters, p21-luc and p14-3-3-luc. As controls, empty vector or VRK1 alone were also cotransfected, together with the reporter construct. The transfection of pCB6+p53 alone promoted some transcription due to its overexpression, and the cotransfection of pcDNA3-VRK1myc with pCB6+p53 resulted in a further increase in p53-dependent transcription, which is higher with the synthetic p53 response elements than with the gene-specific promoters (Fig. 4B). Therefore, it was concluded that the p53 molecule stabilized by VRK1 is transcriptionally active, because it activates p53-dependent transcription.
Thr18 phosphorylation interferes with p53 binding to Mdm2 but does not affect its ubiquitination by Mdm2 in vivo.
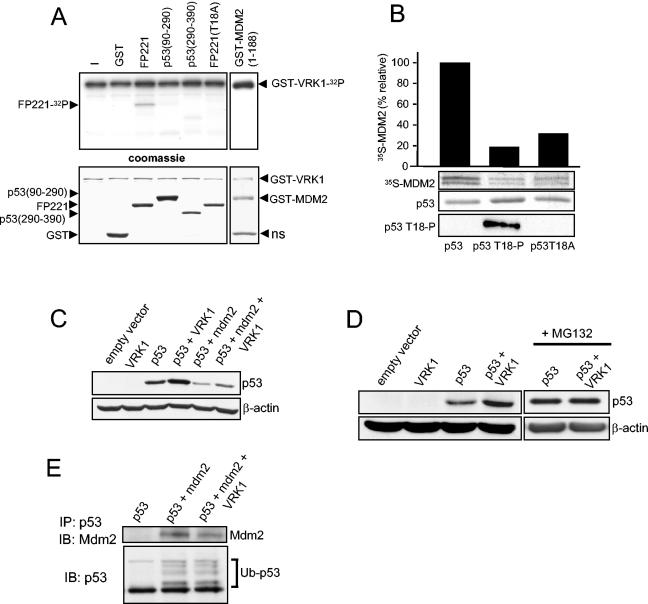
Phosphorylation of Thr18 is likely to be one of the components required for the stabilization of p53 by disruption of the p53-Mdm2 interaction (20, 43). The Thr18 residue forms a hydrogen bond with Asp21 that is essential to maintain the structure of the α-helix in the p53 molecule responsible for the interaction between the two proteins (20, 23, 43). To further characterize the possible effect of VRK1 on this interaction, we determined the phosphorylation of p53 and Mdm2 amino termini by VRK1. A bacterially expressed GST-VRK1 fusion protein was able to phosphorylate p53 in Thr18, but not the p53 mutant T18A, in vitro (Fig. 5A). GST-p53 constructs with the other domains of the p53 molecule did not show a significant phosphorylation in this assay. There was weak detection of p53 (amino acids 290 to 390) fragment phosphorylation compared with that in Thr18, so we excluded a role for VRK1 in phosphorylating this part of the protein in vivo. Furthermore, VRK1 does not appear to phosphorylate Mdm2 in its p53-binding region (Fig. 5A) or in the entire Mdm2 protein (data not shown); thus, any potential effect of VRK1 on the p53-Mdm2 interaction is likely to be a consequence of the phosphorylation on p53.
FIG. 5.
(A) Phosphorylation of p53 in Thr18 in vitro by using GST-VRK1 fusion. As substrates in the kinase reaction, GST-p53wt (FP221 fusion protein), GST-p53T18A (amino acids 1 to 85), GST-p53 (amino acids 90 to 290), GST-p53 (amino acids 290 to 390), GST-Mdm2 (amino acids 1 to 188), and GST proteins were used together with 2 μg of GST-VRK1 in a radioactive kinase assay. At the bottom is shown staining of the gel with Coomassie blue. ns, nonspecific band. (B) Effect of p53 phosphorylation in Thr18 interferes with its binding to Mdm2 analyzed in a pull-down assay. The wild-type GST-p53 and its phosphorylated form with VRK1 were mixed with Mdm2 protein that was synthesized and labeled in an in vitro transcription-translation system with [35S]methionine. The bound Mdm2 was detected by its radioactive signal, which was used for quantitation as represented by the bars. The GST-p53 protein loaded in the gel was detected by Coomassie blue staining. Thr18 phosphorylation was detected with the FTP18 monoclonal antibody. (C) H1299 human tumor cells (p53−/−) cotransfected with VRK1 show stabilization of p53, which is partially reverted but not abolished by overexpression of cotransfected Mdm2. H1299 cells were transfected with 1 μg of pCB6+p53 expression plasmid alone or together with plasmids pCOC-Mdm2 (2 μg) and pCDNA-VRK1 (4 μg) where indicated. One microgram of plasmid encoding ubiquitin was added in all cases. Whole-cell extracts were prepared 36 h after transfection and analyzed by Western blotting with the corresponding antibody. (D) The stabilization of p53 by VRK1 is to some extent independent of the presence of Mdm2. Mouse embryo fibroblasts derived from double-knockout mice (p53−/− Mdm2−/−) were cotransfected with the indicated proteins. The cells were processed as for panel C. MG132 proteasome inhibitor was added for 6 h prior to lysis where indicated on the right. (E) Effect of VRK1 on the ubiquitination of p53 by Mdm2. H1299 cells were transfected with the indicated proteins as for panel C, and 25 μM proteasome inhibitor MG132 was added 30 h after transfection for 6 h. Total p53 was immunoprecipitated with the polyclonal antibody CM1 and detected with the monoclonal antibodies DO-1 and Pab1801. Mdm2 protein coimmunoprecipitated and was detected using the 4B2 antibody. The ubiquitinated form of p53 is indicated. IP, immunoprecipitation; IB, immunoblotting.
To study the effect of VRK1 phosphorylation on the p53-Mdm2 interaction, pull-down experiments were performed using the GST-p53 fusion protein with residues 1 to 85 (FP221) in combination with the in vitro-transcribed and -translated Mdm2 protein that was labeled with [35S]methionine. The GST-p53 protein binds to Mdm2, but if GST-p53 protein was previously phosphorylated in vitro with VRK1, the in vitro binding to Mdm2 was markedly reduced (Fig. 5B). The in vitro phosphorylation of Thr18 was detected with a specific monoclonal antibody (9); the nonphosphorylatable mutant of p53, p53T18A, did not bind to Mdm2 as expected based on structural information (23), which confirms the importance of this residue for the structure of the p53 interaction loop that is destabilized when the threonine hydroxyl group is lost, either by phosphorylation or substitution by alanine.
The intracellular level of p53 is regulated by proteolytic degradation mediated by interaction with Mdm2 that targets p53 for degradation by the ubiquitin pathway in the proteasome. Therefore, to test whether VRK1 could affect this regulatory process in vivo as well, H1299 (p53−/−) cells were transfected with pcDNA-VRK1myc with and without p53 (pCB6+p53) and with plasmid pCOC-Mdm2 as appropriate. The cotransfection of p53 together with Mdm2 resulted in a very significant reduction of p53 levels, an effect opposite that of VRK1, which increased its levels (Fig. 5C). However, the cotransfection of pCB6+p53 and pCOC-Mdm2 with pcDNA-VRK1myc partially prevented the Mdm2-induced degradation of p53, although p53 accumulation did not reach the levels obtained with VRK1 alone (Fig. 5C). These data could be explained by two different mechanisms, suggesting that at least part of the VRK1 effect is mediated by interference with the Mdm2-induced degradation of p53, presumably through p53 Thr18 phosphorylation, or that VRK1 overexpression induces p53 accumulation independently of the Mdm2 effect. Therefore, to elucidate the real involvement of p53-Mdm2 interaction in this process, similar cotransfection experiments were carried out using a murine fibroblast cell line derived from a double-knockout mouse for p53 and Mdm2. In these cells, the cotransfection of p53 together with VRK1 also resulted in an increase in the intracellular levels of p53 (Fig. 5D). Interestingly, VRK1 was not able to increase the stabilization of p53 induced by the blocking of the proteasome-directed degradation with the inhibitor MG132 in the absence of Mdm2, indicating that VRK1 in this system also increased the stability of p53 protein by interfering with its degradation and not by any other mechanism (Fig. 5D). This result indicates that the VRK1 effect on p53 stabilization is at least partially mediated by an Mdm2-independent process despite the lack of expression of Mdm2 protein in this cell line but is produced in the absence of Mdm2 by interfering with the degradation of p53 in any other way. VRK1 does not appear to significantly affect the p53 ubiquitination directed by Mdm2, although a slight reduction in the amount of Mdm2 bound to p53 in the presence of VRK1 overexpression was detected (Fig. 5E). Therefore, the effect of VRK1 on p53 is not mainly mediated by disruption of the p53-Mdm2 interaction, although this might be an indirect consequence of p53 threonine-18 phosphorylation by VRK1, which contributes to p53 stabilization under some circumstances. p53 is not only targeted for degradation in the proteasome by Mdm2, since a role for other ubiquitin ligases, such as COP1 or Pirh2, has been established (14, 24), although the mechanism of regulation of p53 activity by these other proteins is not so well understood. VRK1 might still affect p53 degradation by interaction with these or other p53 negative regulators.
Effect of VRK1 can cooperate with p300 and MdmX in p53 stabilization.
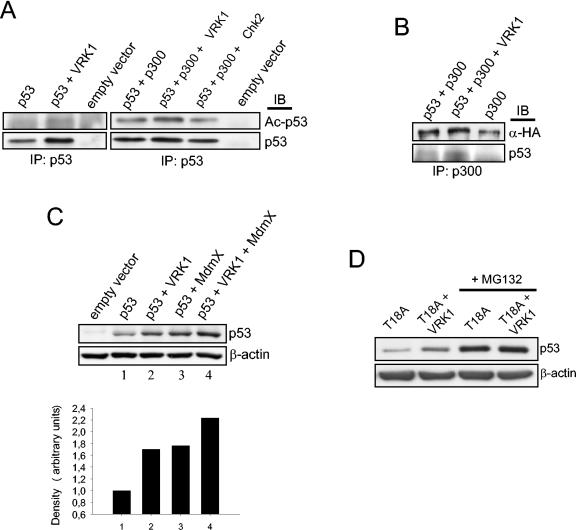
Other molecules that interact with the p53 N terminus, such as the transcriptional coactivator p300 that acetylates p53 (53), and the MdmX protein (29, 47) can also contribute to p53 stabilization. The recruitment of p300 protein to Thr18 and Ser20 phosphorylation and the subsequent p53 acetylation and stabilization have been described (11). MdmX overexpression stabilizes and activates p53, leading to accumulation of nuclear p53, without affecting its Mdm2-mediated ubiquitination (47). Therefore, we decided to test whether the effect exerted by VRK1 on p53 was mediated by p300 or MdmX in our system. H1299 cells were transfected with p53 and the combination of VRK1, or Chk2 as a control, with p300. The total p53 molecule was immunoprecipitated from the transfected cells, and the blot was developed with an antibody recognizing specifically acetylated Lys373 and Lys382 in p53, an indicator of p300 activity (Fig. 6A). VRK1 by itself slightly increases the acetylation of p53, presumably by endogenous p300, but at a level lower than when exogenous p300 is expressed. When both proteins are cotransfected together with p53, an increase in p53 acetylation could be seen compared with that obtained by p300 overexpression alone. As a control, this effect is not exerted by Chk2 (Fig. 6A). To confirm that VRK1 might contribute to increased p53 binding to p300, total p300 was immunoprecipitated and the amount of p53 bound was detected with a specific antibody. A slight increase in p53 binding to p300 could be detected upon VRK1 overexpression, indicating an increase in the formation of the p53-p300 complex (Fig. 6B).
FIG. 6.
(A) H1299 cells were transfected with pCB6+p53, pCDNA-VRK1-myc, pCMV-p300-HA, or pCMV-Chk2 as indicated. The transfected p53 was immunoprecipitated using monoclonal antibodies, and the blot was analyzed for the acetylation of p53 on Lys 373 and Lys382 with a specific antibody. IP, immunoprecipitation; IB, immunoblotting. (B) VRK1 promotes the formation of a p53-p300 complex. H1299 cells were transfected with different combinations of the indicated plasmids (pCMV-p300-HA, pCB6+p53, and pCDNA-VRK1-myc). Extracts from transfected cells were immunoprecipitated with an anti-p300 polyclonal antibody, and the blot was developed with either anti-HA to detect immunoprecipitated transfected p300 or anti-p53 to detect the coimmunoprecipitated p53. (C) H1299 cells were transfected with pCB6+p53, pVRK1-HA, and p3.1MdmX-myc as indicated, and the levels of p53 were determined with a mix of anti-p53 antibodies. Quantification of the amount of p53 is shown below. (D) The H1299 cell line was transfected with plasmid encoding p53 with a substitution of Thr18 for alanine (1 μg) with or without pCDNA-VRK1-myc (8 μg), and 36 h postransfection, whole-cell extract was subjected to immunoblotting to detect p53 total protein. Six hours prior to extract collection, MG132 was added to the cells where indicated.
Next, the effect of MdmX was determined. Both VRK1 and MdmX overexpression by themselves stabilize p53, and when both are cotransfected in H1299 cells, the level of p53 is even higher (Fig. 6C). These data might suggest that MdmX- and VRK1-directed p53 stabilization processes can function independently, although they could cooperate in the cell to reach complete p53 stabilization and might interact in an unknown manner. These data indicate that the effect on p53 stabilization induced by VRK1 might be to promote p53 interactions with stabilizing proteins, such as p300 or MdmX.
To show whether p53 Thr18 phosphorylation was the unique event necessary for VRK1-induced p53 stabilization, we performed a cotransfection experiment using the single-point mutation to alanine of p53 in this phosphorylatable residue. VRK1 overexpression was able to induce an increase in p53 levels even in the absence of possibility of phosphorylation, suggesting the existence of another mechanism distinct from direct p53 phosphorylation that could involve interactions with p53 activators, such as p300. Nevertheless, threonine-to-alanine substitution in position 18 is thought to mimic the phosphorylation at this residue regarding the interaction of the p53 amino terminus with other proteins, as it disrupts the necessary hydrogen bond in the p53 molecule (20, 43). In fact, an increase in the transactivation activity of this mutant has been reported, affecting p53-mdm2 interaction but without affecting or even promoting p53 interaction with transcriptional coactivators, such as TAFII31 (20). The apparent absence of intrinsic p53T18A protein stability indicates the presence of other p53 degradation processes, such as the participation of other p53 ubiquitin ligases. All this makes interpretation of the results with this modified protein difficult. Furthermore, the block of p53 degradation by specific proteasome inhibitors causes a p53T18A accumulation that is not further increased by VRK1 overexpression (Fig. 6D), indicating that the effect of VRK1 on p53T18A is possibly still related to the inhibition of its degradation, although probably not by Mdm2.
VRK1 overexpression increases endogenous p53 Thr18 phosphorylation and acetylation.
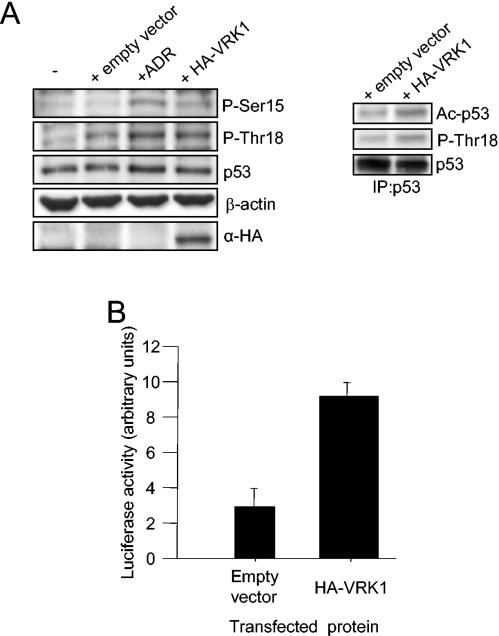
We overexpressed VRK1 in the lung carcinoma cell line A549, which expresses wild-type p53 at significantly high levels, to study the effect of VRK1 on the endogenously expressed p53 (Fig. 7A, left). We were not able to see an accumulation of p53 at the levels obtained for the exogenously expressed protein. Probably, the endogenous p53 status did not make it possible to detect the effect of VRK1-induced accumulation observed with the transfected protein, which is expressed de novo and at levels that resemble an initial stress and localized directly in the nucleus without the requirement for any shuttle from the cytoplasm. It was possible that this status would be necessary for the VRK1 effect on p53 or that the high levels of p53 in this cell line made it difficult to measure this activation by Western blotting in an overall population where every cell expresses p53 but not the transfected VRK1. We decided to analyze the p53 phosphorylation status under these conditions, and we observed that there was an increase in p53 phosphorylation at Thr18 greater than the one observed upon transfection itself, as Lipofectamine elicits a “stress-like” response in human cells (38). This phosphorylation reached a level similar to that induced by treatment of these cells with adriamycin, a DNA damage-inducing agent that increases p53 Thr18 phosphorylation (38). We have demonstrated that this DNA-damaging agent does not produce any effect on VRK1 expression or activity (data not shown). An increase in the p53 acetylated form was also measured after VRK1 overexpression (Fig. 7A, right). A smaller increase in phosphorylation could also be seen in Ser15, suggesting the idea of collaboration of VRK1 with other kinases, as VRK1 does not phosphorylate the Ser15 residue by itself. This increase in Thr18 phosphorylation is not sufficient to accumulate p53 at levels detectable by Western blotting, but it has been reported that this phosphorylation increases p53 transcriptional activity (15, 20). To detect in an indirect way the expected accumulation of p53 induced by VRK1, we measured p53-dependent transcription in the A549 cell line using the Bax gene promoter with luciferase as a reporter system containing a p53 response element (Fig. 7B). We observed an increase in p53-dependent transcription in these cells upon VRK1 overexpression even without a detectable increase in p53 protein levels. Therefore, we concluded that VRK1 overexpression in the A549 cell line activates p53-dependent transcription and increases endogenous p53 amino-terminal phosphorylation. Similar results were obtained with other p53 wild-type cancer cell lines.
FIG. 7.
(A) VRK1 induces endogenous p53 phosphorylation and acetylation. A549 cells were transfected either with 4 μg of pCEFL-KZ plasmid alone or with HA-VRK1 construct and subjected to Western blotting with specific antibodies to detect p53 phosphorylated in Thr18, Ser15, or total protein. Untransfected cells and adriamycin (ADR)-treated cells are shown as controls (left). Total p53 protein was then immunoprecipitated (IP) and subjected to Western blotting to detect the acetylated form of the protein (right). (B) VRK1 overexpression enhances p53-dependent transcription. Cells were cotransfected with HA-VRK1 and a Bax reporter plasmid containing p53 response elements. Reporter luciferase activity was normalized for transfection levels with Renilla luciferase. The error bars represent standard deviations.
VRK1 protein expression suppression by specific siRNA provokes defects in cell proliferation.
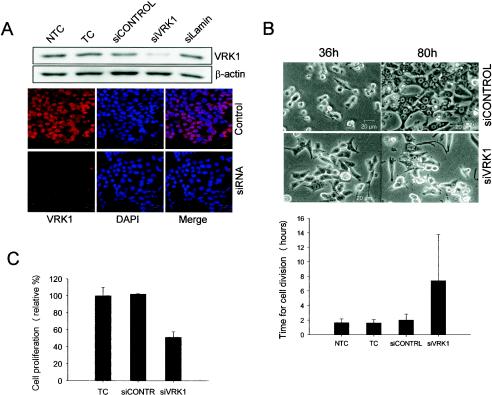
VRK1 does not seem to be activated by DNA damage or any other classical p53-activating stress. All the previous data point to an involvement of VRK1 in cell growth and proliferation regulation (34, 49). Suppression of VRK1 homologous expression by siRNA in C. elegans has a phenotype consistent with this role (22). We proposed that VRK1 might have a role in p53 initial activation during normal cell proliferation rather than during severe stress, and to test whether in fact VRK1 plays a role in this property, we decided to perform transient siRNA transfection targeting VRK1 expression. We used the HCT116 cell line, with wild-type p53, a high proliferation rate, and easy transfection of siRNA duplexes, for these experiments. We tested four different siRNA duplexes by Western blotting and immunofluorescence assays, first as a pool and then as individual duplexes, for the ability to specifically suppress VRK1 expression in these cells (Fig. 8A). A reduction to ∼20% of the initial amount of VRK1 protein was obtained. All the experiments were finally carried out with two independent duplexes (siVRK1-1 and -2). Cells were analyzed by video microscopy 36 to 80 h after transfection (Fig. 8B). Cells transfected with the control nontargeting siRNA divided normally and reached near confluence on the plate after 80 h. The cells completed their divisions in 1.5 to 2 h on average, the same time as nontransfected control cells. However, cells transfected with the specific siVRK1 failed to divide normally and as a consequence did not reach 50% confluence at the same time. The average time spent in cell division was abnormal. Some cells completed cell division normally, while others remained detached from the plate surface for 7 to 10 h before cytokinesis and attachment of the daughter cells to the plate occurred. Almost 50% of the cells analyzed collapsed before completion of cell division and died, or they divided and at least one of the daughter cells died. We also quantified cell proliferation in an indirect form by measuring metabolically active cells, using the XTT reagent, 60 h after transfection with siRNA (Fig. 8C). A decrease in cell proliferation after VRK1 suppression was detected.
FIG. 8.
VRK1 suppression by specific siRNA provokes abnormalities in proliferation. (A) HCT116 cells were transfected with the specific siRNA for VRK1 (siVRK1), nontargeting siRNA (siCONTROL), or lamin-targeting siRNA (siLamin). Then the cells were processed for Western blotting or immunofluorescence assay as previously described with a specific antibody against endogenous VRK1 (red) or nuclear staining (DAPI). NTC, nontransfected cell control; TC, transfected cell control without siRNA. (B) After transfection as for panel A, cells were submitted to in vivo video microscopy. Representative images of cells 36 and 80 h after transfection are shown. The durations of cell division (plus standard deviations) were determined for 20 to 40 cells from several different experiments (below). (C) Quantification of cell proliferation and viability determined 60 h after transfection by colorimetric XTT-based assay.
DISCUSSION
In this report, it was shown that a novel kinase, VRK1, a member of a new Ser-Thr kinase family, is able to contribute to the stability and nuclear accumulation of a transcriptionally active p53 molecule. This accumulation is clearly due to a posttranslational effect on p53. Due to the activity of the VRK1 protein and the change in the phosphorylation status of p53 after its stabilization, this effect was likely to be a consequence of the kinase activity of VRK1. Recently, it has been shown that p53 stabilization can occur in the absence of Ser15 or Ser20 phosphorylation (2, 21a), suggesting that other residues, such as Thr18, might play a more prominent role under these circumstances. Phosphorylation of p53 in Thr18 by VRK1 abolishes the interaction between it and Mdm2 in vitro and can partially prevent the downregulation of p53 by Mdm2. However, VRK1 has no significant effect on Mdm2-dependent p53 ubiquitination, although it could impair the formation of the complex, as seen in coimmunoprecipitation experiments. Moreover, the cumulative effect induced by VRK1 is also detected in cells with no expression of Mdm2, as in murine p53-Mdm2 double-null cells. This observation indicates that, although the interruption of the p53-Mdm2 interaction might play a role in promoting p53 transcriptional activity, VRK1 can also stabilize p53 by an alternative mechanism different from its effect on the interaction with Mdm2. This mechanism might also be related to inhibition of the degradation of p53, since it does not have an additive effect with the stabilization induced by the proteasome-specific inhibitor MG132 (Fig. 5D).
MdmX has been shown to inhibit p53 transactivation activity and to stabilize p53 by an antagonist effect of Mdm2-dependent degradation. The latter effect is exerted without affecting the p53 ubiquitination pattern (47). MdmX overexpression leads to p53 stabilization, and the view of this protein as a positive regulator of p53 under stress conditions is gaining relevance (29). In H1299, overexpression of MdmX stabilized the exogenously expressed p53 similarly to VRK1. Furthermore, the overexpression of both VRK1 and MdmX at the same time induces a greater increase in p53 levels (Fig. 6C). This could mean that VRK1 does not act on p53 by the same mechanism as MdmX, since their effects are additive, although we cannot exclude collaborative effects on p53 activation. Further studies are needed to clarify this point.
Another candidate for VRK1 action is the p300 coactivator. It has been reported that p300 binding to the amino-terminal domain of p53 is regulated by phosphorylation in residues such as Ser20 and Thr18 that promotes its union and impairs the Mdm2 interaction in the same region (11, 12, 13). We show in this report that VRK1-induced stabilized p53 is extensively phosphorylated in Thr18, as is endogenous p53 upon VRK1 overexpression. As we describe, VRK1 overexpression induces p53 acetylation in residues Lys373 and Lys382 (Fig. 6A), typically acetylated by p300, and enhances the formation of stable p53-p300 complexes (Fig. 6B). These data suggest a mechanism by which VRK1 can stabilize and induce p53 transcriptional activity. However, VRK1 is able to induce the accumulation of the nonphosphorylatable p53 mutant T18A, a mutant that has been shown to have some of the properties of the phosphorylated protein. This observation leaves the door open to another possible mechanism, in addition to the direct phosphorylation of p53. This mechanism might involve phosphorylation or interaction with other activators of p53, such as p300, linking the two effects. Phosphorylation might favor the binding of p53 to other stabilizing molecules, perhaps by a docking mechanism in which VRK1-p53 transient interaction might favor the activation of p53 by promoting binding to other effectors, with p53 phosphorylation by VRK1 being an indispensable mechanism (8). VRK1 could also affect the action of other p53 ubiquitin ligases, such as COP1 and Pirh2 (14, 24), or another that has not yet been found, leading to p53 stabilization by interfering with proteasome-dependent p53 degradation. These proteins exert their effects independently of Mdm2 and bind to p53 in a different region, explaining why VRK1 is still capable of stabilizing p53 in the absence of Mdm2 or with the T18A mutation. The abolition of the effect of VRK1 on MG132-stabilized p53 even in the absence of Mdm2 might indicate that VRK1 is still acting by disruption of p53 degradation. VRK1 activation of p53 might also involve a cooperative effect with other kinases. Indeed, an increase in Ser15 phosphorylation can be observed after VRK1 overexpression (Fig. 7A). Nevertheless, the effect of VRK1 on p53 could still be seen in the presence of ATM kinase inhibitors (unpublished data), such as caffeine or wortmannin (42), indicating that prior phosphorylation of these residues by this kinase is not necessary. This alternative mechanism remains to be elucidated and is under investigation.
The phosphorylation of p53 is probably conditioned by the particular set of kinases activated in response to a specific stimulus. Thr18 phosphorylation is a component of the cell response to genotoxic damage, in which a complex web of signaling pathways converge in several p53 residues, such as Ser15, Thr18, and Ser20 (38). Thr18 phosphorylation has been reported to be carried out specifically by Chk2 (8), although the role of this kinase in the p53 pathway is controversial (1, 51), and by CK1, with the prior phosphorylation of Ser 15 (16, 39). A docking mechanism between p53 and Chk2 has been proposed that leads to p53 phosphorylation in Thr18 and Ser20 by this kinase (8). VRK1 phosphorylation of p53 is independent of the Chk2 role, as can be seen, for example, when a dominant-negative form of the protein is expressed together with VRK1 (Fig. 2C and 6A).
The consequence of the p53 accumulation induced by VRK1 is the activation of transcription of p53-dependent promoters, such as a synthetic p53 response element, or some specific promoters, such as the p21 promoter. This observation is consistent with the parallel accumulation of p21 protein when there is phosphorylation of Thr18 in response to irradiation and with the promotion of binding to general transcription factors, such as TAFII31 (20).
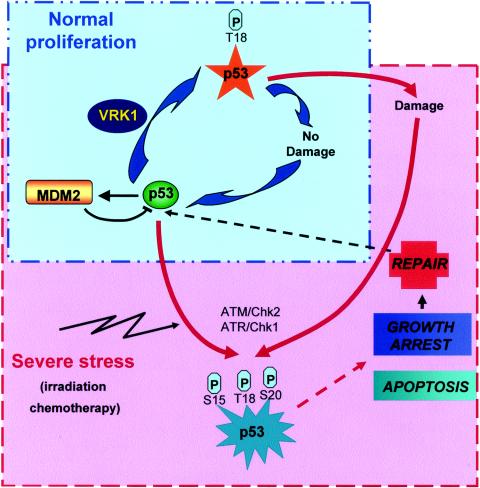
Nothing is known about the regulation and stimulation of VRK1 activity. VRK1 activity by itself is not affected by genotoxic treatment with adriamycin, etoposide, or hydroxyurea, thus excluding response to these stresses as the physiological connection between VRK1 and p53. VRK1 is ubiquitously expressed, and its expression is high in all cell types tested. The highest levels are detected in proliferating cells, such as the expansion of the murine hematopoietic system (49), proliferating tumor cell lines (34), or the proliferation compartment in tumor biopsies (unpublished). The data obtained from the homologous C. elegans protein also point to a role in the regulation of cell proliferation (22). Using specific siRNA experiments to transiently suppress VRK1 protein expression, we have shown that human VRK1 is also important for normal proliferation. Cells without VRK1 have different defects in proliferation, being unable to complete normal cell division and inducing cell death in some cases (Fig. 8). The exact mechanism of this effect needs to be further characterized. We propose a model for VRK1 action on p53 (Fig. 9). Under physiological nonstress conditions, the VRK1-p53 connection might function as a basic mechanism that maintains a basal level of p53 protein in a ready status. VRK1 might induce an initial activation of p53 that is not sufficient to trigger a full p53 response but is enough to respond to minor and suboptimal damage, such as an occasional individual replication block, during normal proliferation, and it possibly induces the full activation of p53 in case of severe damage during cell division (Fig. 9). This minor activation of p53 by VRK1 could help maintain a basal level of expression of p53. When severe or catastrophic damage occurs, such as exposure to irradiation or chemotherapeutic drugs, additional mechanisms that promote additional effects in addition to repair, such as growth arrest or apoptosis, become active (Fig. 9). These secondary mechanisms are very well characterized and implicate well-known kinases, as the ATM-Chk2 or ATR-Chk1 route.
FIG. 9.
Diagram illustrating proposed VRK1 action on p53. Two types of cycles can regulate p53 functions. One of the cycles is operational under normal proliferation conditions (blue box), where the damage is occasional and minimal and not enough to trigger a full p53 stress response. VRK1 somehow regulates proliferation and might be involved in p53 basal stabilization during normal proliferation. In response to severe or catastrophic damage (red box), like that induced by radiation, chemotherapy, or severe failure of DNA replication, p53 is fully activated and triggers a complete p53 response. Well-known kinases, such as ATM and ATR, are implicated in this p53 activation.
We conclude that VRK1 represents a new regulator of cell proliferation and could also be the first step in a new pathway regulating p53 in normal proliferating cells that might function as a switch controlling the proteins that interact with p53 and thus modify its stability and activity.
Acknowledgments
We thank D. Lane, S. Lain, T. Hupp, D. Meek, K. J. Pietenpol, G. Lozano, J. Bartek, S. Berberich, M. Scheffner, A. Zantema, T. Halozanetis, and M. Oren for providing us with reagents used in this work.
F.M.V. and A. S. were supported by fellowships from Fundación Ramón Areces and the I3P program of the Consejo Superior de Investigaciones Científicas. This work was funded by grants from the Ministerio de Educación y Ciencia (SAF2000-0169 and SAF2004-2900), the Fondo de Investigación Sanitaria (FIS-PI02-0585), the Junta de Castilla y León (CSI18-03), the Fundación de Investigación Médica MMA, and the Fundación Memoria Samuel Solórzano Barruso.
REFERENCES
- 1.Ahn, J., M. Urist, and C. Prives. 2003. Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J. Biol. Chem. 278:20480-20489. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft, M., Y. Taya, and K. H. Vousden. 2000. Stress signals utilize multiple pathways to stabilize p53. Mol. Cell. Biol. 20:3224-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft, M., and K. H. Vousden. 1999. Regulation of p53 stability. Oncogene 18:7637-7643. [DOI] [PubMed] [Google Scholar]
- 4.Banham, A. H., and G. L. Smith. 1992. Vaccinia virus B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology 191:803-812. [DOI] [PubMed] [Google Scholar]
- 5.Barcia, R., S. Lopez-Borges, F. M. Vega, and P. A. Lazo. 2002. Kinetic properties of p53 phosphorylation by the human vaccinia-related kinase 1. Arch. Biochem. Biophys. 399:1-5. [DOI] [PubMed] [Google Scholar]
- 6.Bargonetti, J., and J. J. Manfredi. 2002. Multiple roles of the tumor suppressor p53. Curr. Opin. Oncol. 14:86-91. [DOI] [PubMed] [Google Scholar]
- 7.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 8.Craig, A., M. Scott, L. Burch, G. Smith, K. Ball, and T. Hupp. 2003. Allosteric effects mediate CHK2 phosphorylation of the p53 transactivation domain. EMBO Rep. 4:787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig, A. L., L. Burch, B. Vojtesek, J. Mikutowska, A. Thompson, and T. R. Hupp. 1999. Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem. J. 342:133-141. [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon, N., and M. F. Hoekstra. 1994. Characterization of two protein kinases from Schizosaccharomyces pombe involved in the regulation of DNA repair. EMBO J. 13:2777-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dornan, D., and T. R. Hupp. 2001. Inhibition of p53-dependent transcription by BOX-I phospho-peptide mimetics that bind to p300. EMBO Rep. 2:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dornan, D., H. Shimizu, L. Burch, A. J. Smith, and T. R. Hupp. 2003. The proline repeat domain of p53 binds directly to the transcriptional coactivator p300 and allosterically controls DNA-dependent acetylation of p53. Mol. Cell. Biol. 23:8846-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dornan, D., H. Shimizu, N. D. Perkins, and T. R. Hupp. 2003. DNA-dependent acetylation of p53 by the transcription coactivator p300. J. Biol. Chem. 278:13431-13441. [DOI] [PubMed] [Google Scholar]
- 14.Dornan, D., I. Wertz, H. Shimizu, D. Arnott, G. D. Frantz, P. Dowd, K. O'Rourke, H. Koeppen, and V. M. Dixit. 2004. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429:86-92. [DOI] [PubMed] [Google Scholar]
- 15.Dumaz, N., and D. W. Meek. 1999. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J. 18:7002-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumaz, N., D. M. Milne, and D. W. Meek. 1999. Protein kinase CK1 is a p53-threonine 18 kinase which requires prior phosphorylation of serine 15. FEBS Lett. 463:312-316. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Cao, I., M. Garcia-Cao, J. Martin-Caballero, L. M. Criado, P. Klatt, J. M. Flores, J. C. Weill, M. A. Blasco, and M. Serrano. 2002. Super p53′ mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 21:6225-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatei, M., K. Sloper, C. Sorensen, R. Syljuasen, J. Falck, K. Hobson, K. Savage, J. Lukas, B. B. Zhou, J. Bartek, and K. K. Khanna. 2003. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem. 278:14806-14811. [DOI] [PubMed] [Google Scholar]
- 19.Hemann, M. T., J. S. Fridman, J. T. Zilfou, E. Hernando, P. J. Paddison, C. Cordon-Cardo, G. J. Hannon, and S. W. Lowe. 2003. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat. Genet. 33:396-400. [DOI] [PubMed] [Google Scholar]
- 20.Jabbur, J. R., A. D. Tabor, X. Cheng, H. Wang, M. Uesugi, G. Lozano, and W. Zhang. 2002. Mdm-2 binding and TAF(II)31 recruitment is regulated by hydrogen bond disruption between the p53 residues Thr18 and Asp21. Oncogene 21:7100-7113. [DOI] [PubMed] [Google Scholar]
- 21.Jabbur, J. R., and W. Zhang. 2002. p53 Antiproliferative function is enhanced by aspartate substitution at threonine 18 and serine 20. Cancer Biol. Ther. 1:277-283. [DOI] [PubMed] [Google Scholar]
- 21a.Jackson, M. W., M. K. Agarwal, M. L. Agarwal, A. Agarwal, P. Stanhope-Baker, B. R. Williams, and G. R. Stark. 2004. Limited role of N-terminal phosphoserine residues in the activation of transcription by p53. Oncogene 23:4477-4487. [DOI] [PubMed] [Google Scholar]
- 22.Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin, M. Gotta, A. Kanapin, N. Le Bot, S. Moreno, M. Sohrmann, D. P. Welchman, P. Zipperlen, and J. Ahringer. 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:231-237. [DOI] [PubMed] [Google Scholar]
- 23.Kussie, P. H., S. Gorina, V. Marechal, B. Elenbass, J. Moreau, A. J. Levine, and N. P. Pavletich. 1996. Structure of the mdm2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274:948-953. [DOI] [PubMed] [Google Scholar]
- 24.Leng, R. P., Y. Lin, W. Ma, H. Wu, B. Lemmers, S. Chung, J. M. Parant, G. Lozano, R. Hakem, and S. Benchimol. 2003. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112:779-791. [DOI] [PubMed] [Google Scholar]
- 25.Li, M., C. L. Brooks, N. Kon, and W. Gu. 2004. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 13:879-886. [DOI] [PubMed] [Google Scholar]
- 26.Lin, S., W. Chen, and S. S. Broyles. 1992. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J. Virol. 66:2717-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Borges, S., and P. A. Lazo. 2000. The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene 19:3656-3664. [DOI] [PubMed] [Google Scholar]
- 28.Lukas, C., J. Falck, J. Bartkova, J. Bartek, and J. Lukas. 2003. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5:255-260. [DOI] [PubMed] [Google Scholar]
- 29.Mancini, F., F. Gentiletti, M. D'Angelo, S. Giglio, S. Nanni, C. D'Angelo, A. Farsetti, G. Citro, A. Sacchi, A. Pontecorvi, and F. Moretti. 2004. MDM4 (MDMX) overexpression enhances stabilization of stress-induced p53 and promotes apoptosis. J. Biol. Chem. 279:8169-8180. [DOI] [PubMed] [Google Scholar]
- 30.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 298:1912-1934. [DOI] [PubMed] [Google Scholar]
- 31.Meek, D. W. 1998. Multisite phosphorylation and the integration of stress signals at p53. Cell Signal. 10:159-166. [DOI] [PubMed] [Google Scholar]
- 32.Milne, D. M., D. G. Campbell, F. B. Caudwell, and D. W. Meek. 1994. Phosphorylation of tumor suppressor protein p53 by mitogen-activated protein kinase. J. Biol. Chem. 269:9253-9260. [PubMed] [Google Scholar]
- 33.Montes de Oca Luna, R., D. S. Wagner, and G. Lozano. 1995. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378:203-206. [DOI] [PubMed] [Google Scholar]
- 34.Nezu, J., A. Oku, M. H. Jones, and M. Shimane. 1997. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics 45:327-331. [DOI] [PubMed] [Google Scholar]
- 35.Pospisilova, S., V. Brazda, K. Kucharikova, M. G. Luciani, T. R. Hupp, P. Skladal, E. Palecek, and B. Vojtesek. 2004. Activation of the DNA-binding ability of latent p53 protein by protein kinase C is abolished by protein kinase CK2. Biochem. J. 378:939-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prives, C., and P. A. Hall. 1999. The p53 pathway. J. Pathol. 187:112-126. [DOI] [PubMed] [Google Scholar]
- 37.Ryan, K. M., A. C. Phillips, and K. H. Vousden. 2001. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13:332-337. [DOI] [PubMed] [Google Scholar]
- 38.Saito, S., H. Yamaguchi, Y. Higashimoto, C. Chao, Y. Xu, A. J. Fornace, Jr., E. Appella, and C. W. Anderson. 2003. Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J. Biol. Chem. 278:37536-37544. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi, K., S. Saito, Y. Higashimoto, S. Roy, C. W. Anderson, and E. Appella. 2000. Damage-mediated phosphorylation of human p53 threonine 18 through a cascade mediated by a casein 1-like kinase. Effect on mdm2 binding. J. Biol. Chem. 275:9278-9283. [DOI] [PubMed] [Google Scholar]
- 40.Sakaguchi, K., H. Sakamoto, M. S. Lewis, C. W. Anderson, J. W. Erickson, E. Appella, and D. Xie. 1997. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry 36:10117-10124. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi, K., H. Sakamoto, D. Xie, J. W. Erickson, M. S. Lewis, C. W. Anderson, and E. Appella. 1997. Effect of phosphorylation on tetramerization of the tumor suppressor protein p53. J. Protein Chem. 16:553-556. [DOI] [PubMed] [Google Scholar]
- 42.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 43.Schon, O., A. Friedler, M. Bycroft, S. Freund, and A. Fersht. 2002. Molecular mechanism of the interaction between MDM2 and p53. J. Mol. Biol. 323:491-501. [DOI] [PubMed] [Google Scholar]
- 44.Sevilla, A., C. R. Santos, R. Barcia, F. M. Vega, and P. A. Lazo. c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK). Oncogene, in press. [DOI] [PubMed]
- 45.Sevilla, A., C. R. Santos, F. M. Vega, and P. A. Lazo. 2004. Human vaccinia-related kinase 1 (VRK1) activates the ATF2 transcriptional activity by novel phosphorylation on Thr-73 and Ser-62 and cooperates with JNK. J. Biol. Chem. 279:27458-27465. [DOI] [PubMed] [Google Scholar]
- 46.Sherr, C., and F. McCormick. 2002. The RB and p53 pathways in cancer. Cancer Cell 2:103-112. [DOI] [PubMed] [Google Scholar]
- 47.Stad, R., N. A. Little, D. P. Xirodimas, R. Frenk, A. J. van der Eb, D. P. Lane, M. K. Saville, and A. G. Jochemsen. 2001. Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms. EMBO Rep. 2:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor, W. R., and G. R. Stark. 2001. Regulation of the G2/M transition by p53. Oncogene 20:1803-1815. [DOI] [PubMed] [Google Scholar]
- 49.Vega, F. M., P. Gonzalo, M. L. Gaspar, and P. A. Lazo. 2003. Expression of the VRK (vaccinia-related kinase) gene family of p53 regulators in murine hematopoietic development. FEBS Lett. 544:176-180. [DOI] [PubMed] [Google Scholar]
- 50.Vousden, K. H., and X. Lu. 2002. Live or let die: the cell's response to p53. Nat. Rev. Cancer 2:594-604. [DOI] [PubMed] [Google Scholar]
- 51.Wu, Z., J. Earle, S. Saito, C. W. Anderson, E. Appella, and Y. Xu. 2002. Mutation of mouse p53 Ser23 and the response to DNA damage. Mol. Cell. Biol. 22:2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, X., L. M. Tsvetkov, and D. F. Stern. 2002. Chk2 activation and phosphorylation-dependent oligomerization. Mol. Cell. Biol. 22:4419-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan, Z. M., Y. Huang, T. Ishiko, S. Nakada, T. Utsugisawa, H. Shioya, Y. Utsugisawa, K. Yokoyama, R. Weichselbaum, Y. Shi, and D. Kufe. 1999. Role for p300 in stabilization of p53 in the response to DNA damage. J. Biol. Chem. 274:1883-1886. [DOI] [PubMed] [Google Scholar]