Abstract
Introduction:
Extracorporeal membrane oxygenation (ECMO) has been extensively used for potentially reversible acute respiratory failure associated with severe influenza A (H1N1) pneumonia; however, it remains an expensive, resource-intensive therapy, with a high associated mortality. This systematic review and meta-analysis aims to summarize and pool outcomes data available in the published literature to guide clinical decision-making and further research.
Methods:
We conducted a systematic search of MEDLINE (1966 to April 15, 2015), EMBASE (1980 to April 15, 2015), CENTRAL, and Google Scholar for patients with severe H1N1 pneumonia and respiratory failure who received ECMO. The study validity was appraised by Newcastle–Ottawa Scale. The primary outcome was all-cause mortality. The secondary outcomes were duration of ECMO therapy, mechanical ventilation, and Intensive Care Unit (ICU) length of stay.
Results:
Of 698 abstracts screened and 142 full-text articles reviewed, we included 13 studies with a total of 494 patients receiving ECMO in our final review and meta-analysis. The study validity was satisfactory. The overall mortality was 37.1% (95% confidence interval: 30–45%) limited by underlying heterogeneity (I2 = 65%, P value of Q statistic = 0.006). The median duration for ECMO was 10 days, mechanical ventilation was 19 days, and ICU length of stay was 33 days. Exploratory meta-regression did not identify any statistically significant moderator of mortality (P < 0.05), except for the duration of pre-ECMO mechanical ventilation in days (coefficient 0.19, standard error: 0.09, Z = 2.01, P < 0.04, R2 = 0.16). The visual inspection of funnel plots did not suggest the presence of publication bias.
Conclusions:
ECMO therapy may be used as an adjunct or salvage therapy for severe H1N1 pneumonia with respiratory failure. It is associated with a prolonged duration of ventilator support, ICU length of stay, and high mortality. Initiating ECMO early once the patient has been instituted on mechanical ventilation may result in improved survival.
Keywords: Acute respiratory distress syndrome, extracorporeal membrane oxygenation, influenza A pneumonia, meta-analysis, outcomes
Introduction
Severe acute respiratory failure has a high mortality despite the recent advances in intensive care.[1] Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to support gas exchange independent of mechanical ventilation in severe acute respiratory failure. The global experience of the 2009 novel influenza A (H1N1) pandemic witnessed large-scale use of rescue ECMO therapy in patients with severe acute respiratory distress syndrome (ARDS), since these were younger patients with fewer comorbidities, and had a higher likelihood of reversible respiratory failure.[2] In the postpandemic phase, H1N1 pdm09 remains the predominant seasonal strain, and the percentage of deaths attributed to severe influenza and pneumonia has crossed the epidemic threshold every year in the last 4 years.[3] However, the implementation of a successful ECMO program requires significant institutional investment in terms of resources, staffing, and training in addition to the high costs of the infrastructure required, leading some to question the cost-effectiveness of the widespread implementation of such programs.[4]
The aim of this review is to provide an updated review on the global experience of ECMO in acute respiratory failure due to H1N1 pneumonia in the postpandemic phase, to guide clinical decisions in the implementation of ECMO and future research efforts. We developed a protocol for this systematic review and followed reporting recommendations from Preferred Reporting Items for Systematic Reviews and Meta-analyses.[5]
Methods
Data sources
Using relevant keywords, MeSH terms, and text with the following search strategy: (influenza OR H1N1) AND (ALI OR (“acute lung injury”) OR ARDS OR ("acute respiratory distress syndrome") OR ("acute respiratory failure")) AND ECMO OR ("extracorporeal membrane oxygenation"), we performed a systematic search of MEDLINE (1946 to April 15, 2015) via Ovid, EMBASE (1980 to April 15, 2015) via Scopus, CENTRAL, and Google Scholar for all English language abstracts. We also examined bibliography of included articles to identify additional references.
Study selection
We screened citations by title and abstract for patients with H1N1 influenza infection on ECMO. Full-text review of pertinent citations was done with the following selection criteria for study inclusion: (a) Patients with suspected or confirmed H1N1 influenza infection; (b) with respiratory failure; and (c) receiving ECMO. We excluded (a) case reports; (b) case series describing less than ten patients; and (c) studies where outcomes of interest were not available. In cases of multiple publications describing the same patient cohort, the one with more patients describing relevant outcomes was used.
Study outcomes
The primary outcome of interest was all-cause mortality presented as the longest time to follow-up available. The secondary outcomes were duration of ECMO therapy, mechanical ventilation, and Intensive Care Unit (ICU) length of stay.
Data extraction and synthesis
Two authors (Shashvat Sukhal and Jaskaran Sethi) independently reviewed titles and abstracts of the identified resources. They obtained the full text of all studies of possible relevance for independent assessment. All the authors decided which trials fit the inclusion criteria. The authors resolved any disagreement by consensus. Two authors (Shashvat Sukhal and Malini Ganesh) performed data extraction independently with specific data extraction forms, and the third author (Jaskaran Sethi) confirmed the accuracy. Outcome variables and 95% confidence intervals (CIs) were derived from each study and summary statistics were applied as appropriate. Two reviewers (Shashvat Sukhal and Jaskaran Sethi) independently assessed the risk of bias using standard criteria defined in the Cochrane Handbook for Systematic Reviews of Interventions[6] and the Newcastle–Ottawa Scale for observational studies.[7]
Statistical analysis
Data were summarized using the generic inverse variance method with random effects model. We evaluated heterogeneity of effects using the Higgins’ I2 and Q-statistic test. Heterogeneity was defined as I2 >25%, statistical significance was set at a P < 0.05 (two-tailed). Results are reported as summary point estimate (95% CI). We performed random effects meta-regression using method of moments to identify significant moderator variables in linear meta-regression analyses. To address publication bias, we used three methods: Visual inspection of funnel plots, Begg–Mazumdar test, and Egger test. Statistical analyses were performed using MedCalc for Windows, version 15.0 (MedCalc Software, Ostend, Belgium) and Comprehensive Meta-analysis, version 3.3 (Biostat, Englewood, NJ, USA).
Results
Study selection
Of 698 abstracts screened and 142 full-text articles reviewed, we included 13 studies[8,9,10,11,12,13,14,15,16,17,18,19,20] with a total of 494 patients receiving ECMO, in our final meta-analysis [Figure 1] after thorough appraisal. All studies were observational, either single center or multicenter cohorts or case series. There were no randomized controlled trials. Most studies were based on the 2009 H1N1 influenza pandemic in various parts of the world, which include Europe, North America, Australia/New Zealand, and Asia (Japan). There were several studies where data were derived from a common patient population reported to national registries such as the Australia and New Zealand Intensive Care Registry, the German ARDS Network, and the French REVA Registry. To avoid duplication, patient characteristics and outcomes were extracted from multiple sources, merged, and presented in the data tables. Overall, the study validity was adequate with a median score of 7 on the Newcastle–Ottawa Scale [Figure 2].
Figure 1.
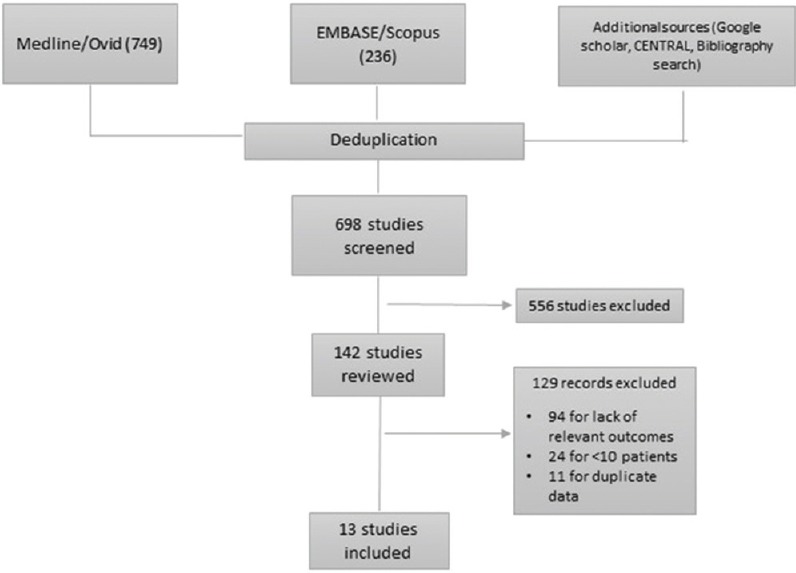
Preferred Reporting Items for Systematic Reviews and Meta-analyses diagram for study selection
Figure 2.
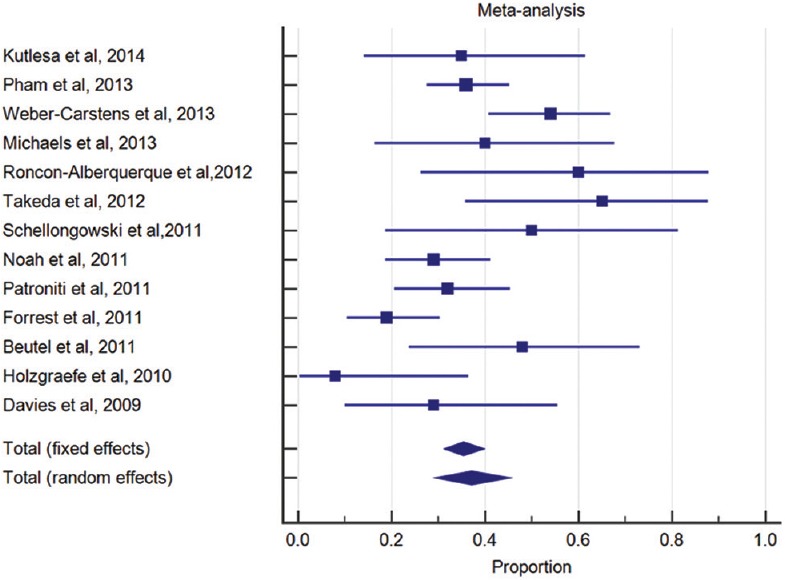
Forest plot for mortality diamond indicates overall summary estimate for the analysis (width of the diamond represents 95% confidence interval and size of the shaded square indicates population size)
Study characteristics
A total of 1175 patients with H1N1 influenza infection with respiratory failure needing admission to the ICU have been included. Of these, 494 (42%) patients received ECMO. Median age of those receiving ECMO was 40 years, and 55% were men, 40% were obese, 13% had diabetes, 14% had preexisting lung disease, and 8% were peripartum. The median sequential organ failure assessment (SOFA) score was 9.5 and lung injury score was 3.8. Veno-venous (VV)-ECMO was used in 94% cases, with a median duration of pre-ECMO mechanical ventilation of 2 days and pre-ECMO PaO2/FiO2 ratio of 58 [Table 1]. Details of cannulation for ECMO are shown in Table 2.
Table 1.
Clinical characteristics of included patients
| Study | Design | Setting | Total patients in ICU (n) | ECMO (n) | Age (median in years) | Male sex (%) | Obese (%) | DM (%) | PELD (%) | Peripartum (%) | SOFA (median) | Lung injury score (median) | Newcastle–Ottawa score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kutleša, 2014 (Croatia) | Case series | Single center | 17 | 17 | 43 | 71 | NA | 17 | 5 | NA | NA | 7 | |
| Pham, 2013 (France) | Observational cohort | Multicenter | 589 | 123 | 42 | 50 | 40 | NA | NA | 15 | 9.5 | 3.4 | 9 |
| Weber-Carstens, 2013 (Germany) | Observational cohort | Multicenter | 116 | 61 | 42 | 55 | NA | 13 | 16 | 6 | 12.8 | NA | 9 |
| Michaels, 2013* (USA) | Case series | Single center | 15 | 15 | 34 | 47 | 26 | 13 | NA | 10 | NA | NA | 6 |
| Roncon-Albuquerque, 2012 (Portugal) | Observational cohort | Single center | 51 | 10 | 40 | 60 | 40 | 10 | NA | NA | NA | 3.5 | 6 |
| Takeda, 2012 (Japan) | Case series | Multicenter | 14 | 14 | 54 | 85.7 | 57 | NA | NA | 7 | 15.5 | NA | 6 |
| Schellongowski, 2011 (Austria) | Case series | Single center | 17 | 10 | 39 | 52 | 29 | NA | NA | NA | NA | 4 | 6 |
| Noah, 2011 (UK) | Observational cohort | Multicenter | 80 | 69 | 36.5 | 38 | 11 | 5 | 10 | 26 | 9.1 | 3.5 | 7 |
| Patroniti, 2011 (Italy) | Observational cohort | Multicenter | 153 | 60 (11#) | 39 | 57 | 39 | 6 | 12 | 8 | 7 | NA | 8 |
| Forrest, 2011 (Australia) | Case series | Multicenter | 17 | 17 | 34 | 50 | 48 | 14 | 42 | 5 | 8 | 3.75 | 8 |
| Beutel, 2011 (Germany) | Observational cohort | Single center | 25 | 17 | 45 | 64 | 40 | 12 | 16 | NA | 11 | NA | 6 |
| Holzgraefe, 2010 (Sweden) | Case series | Single center | 13 | 13 | 31 | 61 | 38 | 23 | 8 | 23 | NA | 3.6 | 6 |
| Davies, 2009 (Australia/New Zealand) | Observational cohort | Multicenter | 68 | 68 (7#) | 36 | 48 | NA | 15 | 30 | NA | NA | 3.8 | 6 |
#Figures in brackets show suspected but not confirmed cases of H1N1, *Data are presented as mean excluded from analysis. Lung injury score (Murray score):[21] Used to assess the extent of pulmonary damage. Comprises of a point score system which includes four criteria: Extent of consolidation on chest X-ray, PaO2/FiO2 ratio, level of applied PEEP and respiratory system compliance. Scores >2.5 represent severe lung injury. Newcastle-Ottawa Score:[7] The Newcastle-Ottawa Scale is a quality assessment tool for nonrandomized studies in systematic review, using three broad perspectives: selection of study groups, comparability of the groups and ascertainment of exposure or outcome of interest. NA: Data not available, PEEP: Positive end-expiratory pressure, DM: Diabetes mellitus, PELD: Preexisting lung disease, ICU: Intensive Care Unit, ECMO: Extracorporeal membrane oxygenation, SOFA: Sequential organ failure assessment
Table 2.
Technical details of extracorporeal membrane oxygenation cannulation wherever available
| Study | Site of insertion | Cannula size |
|---|---|---|
| Kutleša, 2014 (Croatia) | Outflow: Femoral vein (17) Return: Jugular vein (14), femoral vein (3) |
21-23 Fr outflow, 19-21 Fr return |
| Roncon-Albuquerque, 2012 (Portugal) | Outflow: Right femoral vein or right femoral artery Return: Right internal jugular vein |
21-23 Fr outflow, 17-19 Fr return |
| Takeda, 2012 (Japan) | Outflow: Femoral vein (14), IVC (10), RA (4) Inflow: Jugular vein (12), SVC (8), RA (4), IVC (2) |
18-21.5 Fr outflow, 12-21 Fr return |
| Patroniti, 2011 (Italy) | Femorojugular (33), femoro-femoral (26); veno-arterial (1) | NA |
| Forrest, 2011 (Australia) | Outflow: Femoral or right internal jugular Return: Femoral artery or vein |
NA |
| Holzgraefe, 2010 (Sweden) | Outflow: Right internal jugular Inflow: Right or left femoral vein; femoral artery if veno-arterial |
23-29 Fr outflow, 19-23 Fr return |
| Davies, 2009 (Australia/New Zealand) | All patients had peripheral approach (jugular or femoral), one patient had central cannula | NA |
SVC: Superior vena cava, NA: Data not available, RA: Right atrium, IVC: Inferior vena cava
Outcomes
Outcomes were highly variable with an in-hospital or short-term mortality ranging from 8% to 65%, likely reflecting heterogeneity in the patient population, disease severity, and treatment received. The overall mortality was 37.1% (95% CI: 30–45%) limited by underlying heterogeneity (I2 = 65%, P value of Q statistic = 0.006). The median duration for ECMO was 10 days, mechanical ventilation was 19 days, and ICU length of stay was 33 days [Table 3]. The causes of death in patients receiving ECMO (wherever available) are shown in Table 4.
Table 3.
Key procedural details and outcomes
| Study | VV ECMO (%) | Pre-ECMO MV Median (days) | Pre-ECMO PaO2/FiO2 ratio | Median (days) | Mortality (%) | ||
|---|---|---|---|---|---|---|---|
| ECMO duration | MV | ICU LOS | |||||
| Kutleša, 2014 | 100 | 2 | 58 | 8 | NA | NA | 35 |
| Michaels, 2013 | 46 | 3.5 | 62 | 9.8 | NA | 21 | 40 |
| Pham, 2013 | 87 | 2 | 63 | 11 | 28 | 33 | 36 |
| Weber-Carstens, 2013 | NA | 2.6 | 87 | NA | 32 | 33 | 54 |
| Roncon-Albuquerque, 2012 | 90 | 9.3 | 69 | 22 | 32 | 36 | 60 |
| Takeda, 2012 | 100 | 5 | 50 | 8.5 | NA | NA | 65 |
| Beutel, 2011 | 100 | NA | 85 | 10 | 19 | NA | 48 |
| Forrest, 2011 | 94 | 2 | 57 | 10 | NA | 36 | 19 |
| Noah, 2011 | 84 | 4 | 55 | 9 | NA | NA | 29 |
| Patroniti, 2011 | 98 | 2 | 63 | 10 | 18 | 22 | 32 |
| Schellongowski, 2011 | 80 | NA | 56 | 13 | 17 | 21 | 50 |
| Holzgraefe, 2010 | 92 | 1 | 53 | 16 | NA | NA | 8 |
| Davies, 2009 | 93 | 2 | 56 | 10 | 18 | 27 | 29 |
NA: Data not available, ICU: Intensive Care Unit, ECMO: Extracorporeal membrane oxygenation, VV: Veno-venous, LOS: Length of stay, MV: Mechanical ventilation
Table 4.
Major causes of death in individual studies wherever ascertainable, during or after extracorporeal membrane oxygenation therapy
| Study | Causes of death | |||||
|---|---|---|---|---|---|---|
| Multiple organ failure | Significant hemorrhage | Noscomial infection/sepsis | Refractory circulatory failure | Refractory respiratory failure | Others | |
| Kutleša, 2014 (Croatia) | 2 | 1 | 1 | 3 | 1 | |
| Pham, 2013 (France) | 22 | 5 | 1 | 6 | 8 | 3 |
| Weber-Carstens, 2013 (Germany) | NA | NA | NA | NA | NA | NA |
| Roncon-Albuquerque, 2012 (Portugal) | 1 | 2 | 1 | 1 | - | - |
| Takeda, 2012 (Japan) | 4 | 3 | - | - | 3 | 2 |
| Schellongowski, 2011 (Austria) | 3 | 4 | - | - | - | - |
| Noah, 2011 (UK) | 5 | 10 | 1 | 1 | 2 | 3 |
| Patroniti, 2011 (Italy) | 10 | 4 | 5 | 1 | - | 8 |
| Forrest, 2011 (Australia) | 2 | 1 | - | - | 3 | - |
| Beutel, 2011 (Germany) | 12 | - | - | - | - | - |
| Holzgraefe, 2010 (Sweden) | - | 1 | - | - | - | - |
| Davies, 2009 (Australia/NZ) | - | 10 | 1 | - | 4 | - |
Significant hemorrhage includes intracranial hemorrhage. All numbers indicated in the columns reflect number of patients who were thought to have died from the cause mentioned. NA: Data not available
Exploratory meta-regression did not identify any statistically significant moderator of mortality (P < 0.05), except for the duration of pre-ECMO mechanical ventilation in days [coefficient 0.19, standard error: 0.09, Z = 2.01, P < 0.04, R2 = 0.16, Figure 3].
Figure 3.
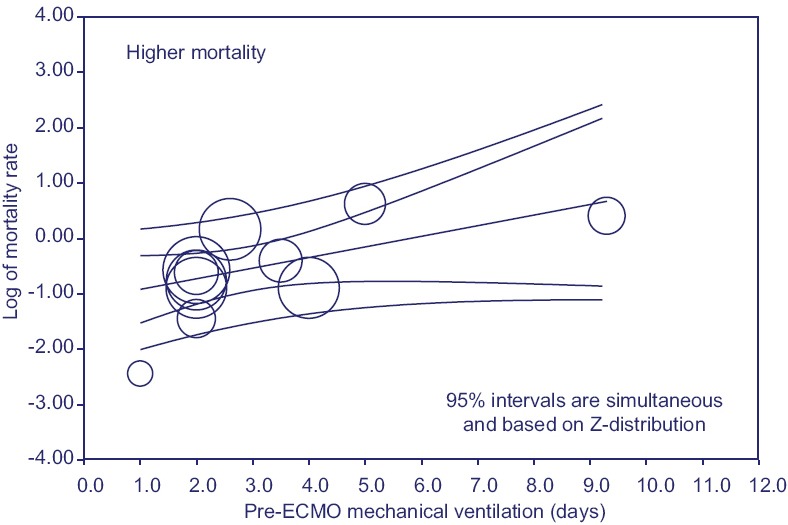
Regression of mortality on duration of pre-ECMO mechanical ventilation in days
The funnel plot did not show asymmetry suggesting bias for all outcomes [Figure 4]. This was confirmed also after quantifying the observed bias with others method (Begg–Mazumdar test and Egger test P > 0.05). The individual study quality appraisals of the included studies are summarized in Table 1. Overall, the study validity was adequate with a median score of 7 on the Newcastle–Ottawa Scale.
Figure 4.
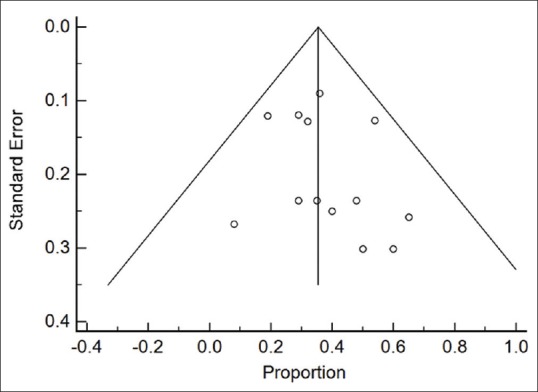
Funnel plot for mortality
Discussion
This systematic review pools data on patient characteristics and outcomes of 454 patients with suspected or confirmed H1N1 influenza with respiratory failure treated by ECMO, described in 13 studies. It provides an updated to an existing review by Zangrillo et al., 2013,[21] by including five additional studies which were published later. There were no randomized trials with a direct comparison of ECMO versus no ECMO for this patient population. All of these studies were either case series or observational cohort studies.
Many patients with respiratory failure and severe pandemic H1N1 pneumonia were young, with obesity, diabetes, and preexisting lung disease as significant comorbidities. A number of young peripartum women were also affected by this disease. The association of younger age, obesity, and pregnancy has been well described and studied for pandemic H1N1.[21,22,23,24] The protective effect of increased age has been attributed to preexisting cross-reactive antibodies in older persons from prior exposure to similar strains.[25] The loss of the leptin receptor leads to decreased viral clearance in obese mice and has been suggested to be one of the factors which may explain excess mortality in obese patients.[26] The subgroup of peripartum women with respiratory failure and severe H1N1 has been described by Saad et al. in a separate meta-analysis, which showed a pooled mortality of 25.4%.[27]
The majority of patients in all studies received VV-ECMO, except in Michaels et al.,[11] where 52% patients received veno-arterial (VA)-ECMO. There was no significant impact of the type of ECMO on mortality by meta-regression although VV-ECMO is associated with fewer vascular complications and easier access, at the cost of lesser hemodynamic support and oxygenation, when compared to VA-ECMO.[28]
Our point estimate of overall pooled mortality for respiratory failure with severe H1N1 is 37.1%, which should be viewed in the context of the overall mortality in ALI/ARDS, which ranges from 15% to 75%, with a pooled point estimate of 43% (95% CI: 40–46%).[29] The high initial median SOFA scores and lung injury scores, duration of mechanical ventilation, and ICU stay all probably contribute to the high mortality seen in these patients. Pappalardo et al. suggest that in patients undergoing VV-ECMO with severe H1N1 respiratory failure, initial extrapulmonary organ failure scores may best predict mortality, and have suggested an ECMONet scoring system, which includes duration of hospital stay before ECMO initiation, creatinine, bilirubin, hematocrit, and mean arterial pressure as significant determinants.[30]
Of note, on exploratory meta-regression, the only statistically significant moderator of mortality was the duration of pre-ECMO mechanical ventilation. Although these results should be interpreted cautiously and in light of the various limitations of meta-regression analysis,[31] this finding is consistent with the experience of the Italian ECMONet, where the duration of mechanical ventilation before ECMO was an independent predictor of mortality.[16] In addition, the results of the CESAR trial,[32] and propensity score matching analysis by Noah et al.,[15] further suggest that referral and transfer to an ECMO center are associated with a 50% reduction in mortality.
Although it is difficult to disentangle the effect of ECMO therapy itself from probably better overall intensive care provided at ECMO referral centers which are usually centers of excellence,[4] it is not unreasonable to surmise that an early referral and transfer to such an ECMO center, probably saves lives in patients with respiratory failure and severe H1N1 pneumonia.
Limitations
Our meta-analysis has several potential limitations. First, all included studies were observational, as no randomized trials exist for this topic. While pooling results from observational studies may meaningful and guide further research, the definitive determination of efficacy and safety of ECMO in severe H1N1 should come from randomized clinical trials designed to specifically address these issues. Potential biases are likely to be greater for observational studies compared with RCTs; therefore, results should always be interpreted with caution when they are included in reviews and meta-analyses. Second, this is a meta-analysis performed on study-level data rather than individual patient-level data. It is known that study-level analyses can lead to biased assessments and have some limitations in explaining the heterogeneity.[33] Third, data such as time from onset of symptoms to diagnosis of H1N1, timing, and duration of antiviral therapy, steroid therapy, or ventilator strategies involved were not available across trials. All these factors probably play a large role in determining outcomes and were not accounted for in this analysis. Fourth, the selection criteria for ECMO referral and initiation of therapy were diverse between studies. Fifth, the observational nature of meta-regressions carries major unavoidable limitations, including the risk of incorrect conclusions caused by ecological fallacy. Sixth, the wide variation in the number of subjects in different studies would result in a higher weightage assigned to the larger study, and the overall point estimate of the primary outcome of mortality would perhaps be more reflective of that institution or regions experience with the disease and care delivery. On the other hand, despite these limitations, the consistency of the magnitude and direction of the overall effect and the stability of the results after the sensitivity analyses support our conclusions.
Conclusions
ECMO therapy may be used as an adjunct or salvage therapy for severe H1N1 pneumonia with respiratory failure; however, no definite conclusions can be drawn due to the lack of randomized trials. It is associated with a prolonged duration of ventilator support, ICU length of stay, and high mortality. Initiating ECMO early once the patient has been instituted on mechanical ventilation may result in improved survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vasilyev S, Schaap RN, Mortensen JD. Hospital survival rates of patients with acute respiratory failure in modern respiratory intensive care units. An international, multicenter, prospective survey. Chest. 1995;107:1083–8. doi: 10.1378/chest.107.4.1083. [DOI] [PubMed] [Google Scholar]
- 2.DeLaney E, Smith MJ, Harvey BT, Pelletier KJ, Aquino MP, Stone JM, et al. Extracorporeal life support for pandemic influenza: The role of extracorporeal membrane oxygenation in pandemic management. J Extra Corpor Technol. 2010;42:268–80. [PMC free article] [PubMed] [Google Scholar]
- 3. [Last accessed on 2016 Apr 28]. Available from: http://www.cdc.gov/flu/pdf/weekly/overview.pdf .
- 4.Wallace DJ, Milbrandt EB, Boujoukos A. Ave, CESAR, morituri te salutant! (Hail, CESAR, those who are about to die salute you!) Crit Care. 2010;14:308. doi: 10.1186/cc8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane Handbook for Systematic Reviews of Interventions. [Last accessed on 2016 May 08]. Available from: http://www.handbookcochrane.org/
- 7.Wells GA, Shea B, O’connell D, Peterson JE, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Last accessed on 2016 May 08]. Available from: http://www.ohrica/programs/clinical_epidemiology/oxford.htm .
- 8.Kutleša M, Novokmet A, Josipovic Mraovic R, Filar B, Mardešic P, Baršic B. Extracorporeal membrane oxygenation treatment for H1N1-induced acute respiratory distress syndrome (ARDS): Results of the Croatian Referral Center for Respiratory ECMO. Int J Artif Organs. 2014;37:748–52. doi: 10.5301/ijao.5000356. [DOI] [PubMed] [Google Scholar]
- 9.Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–85. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 10.Weber-Carstens S, Goldmann A, Quintel M, Kalenka A, Kluge S, Peters J, et al. Extracorporeal lung support in H1N1 provoked acute respiratory failure: The experience of the German ARDS Network. Dtsch Arztebl Int. 2013;110:543–9. doi: 10.3238/arztebl.2013.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaels AJ, Hill JG, Bliss D, Sperley BP, Young BP, Quint P, et al. Pandemic flu and the sudden demand for ECMO resources: A mature trauma program can provide surge capacity in acute critical care crises. J Trauma Acute Care Surg. 2013;74:1493–7. doi: 10.1097/TA.0b013e31828d636e. [DOI] [PubMed] [Google Scholar]
- 12.Roncon-Albuquerque R, Jr, Basílio C, Figueiredo P, Silva S, Mergulhão P, Alves C, et al. Portable miniaturized extracorporeal membrane oxygenation systems for H1N1-related severe acute respiratory distress syndrome: A case series. J Crit Care. 2012;27:454–63. doi: 10.1016/j.jcrc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Takeda S, Kotani T, Nakagawa S, Ichiba S, Aokage T, Ochiai R, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) severe respiratory failure in Japan. J Anesth. 2012;26:650–7. doi: 10.1007/s00540-012-1402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schellongowski P, Ullrich R, Hieber C, Hetz H, Losert H, Hermann M, et al. A surge of flu-associated adult respiratory distress syndrome in an Austrian tertiary care hospital during the 2009/2010 Influenza A H1N1v pandemic. Wien Klin Wochenschr. 2011;123:209–14. doi: 10.1007/s00508-011-1557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–68. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 16.Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: Preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–57. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrest P, Ratchford J, Burns B, Herkes R, Jackson A, Plunkett B, et al. Retrieval of critically ill adults using extracorporeal membrane oxygenation: An Australian experience. Intensive Care Med. 2011;37:824–30. doi: 10.1007/s00134-011-2158-8. [DOI] [PubMed] [Google Scholar]
- 18.Beutel G, Wiesner O, Eder M, Hafer C, Schneider AS, Kielstein JT, et al. Virus-associated hemophagocytic syndrome as a major contributor to death in patients with 2009 influenza A (H1N1) infection. Crit Care. 2011;15:R80. doi: 10.1186/cc10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzgraefe B, Broomé M, Kalzén H, Konrad D, Palmér K, Frenckner B. Extracorporeal membrane oxygenation for pandemic H1N1 2009 respiratory failure. Minerva Anestesiol. 2010;76:1043–51. [PubMed] [Google Scholar]
- 20.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 21.Zangrillo A, Biondi-Zoccai G, Landoni G, Frati G, Patroniti N, Pesenti A, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: A systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17:R30. doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. European communicable disease bulletin. 2008;14:127–36. doi: 10.2807/ese.14.33.19309-en. [DOI] [PubMed] [Google Scholar]
- 23.Díaz E, Rodríguez A, Martin-Loeches I, Lorente L, del Mar Martín M, Pozo JC, et al. Impact of obesity in patients infected with 2009 influenza A (H1N1) Chest. 2011;139:382–6. doi: 10.1378/chest.10-1160. [DOI] [PubMed] [Google Scholar]
- 24.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: A pattern of changing age distribution. J Infect Dis. 1998;178:53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 25.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 26.Radigan KA, Morales-Nebreda L, Soberanes S, Nicholson T, Nigdelioglu R, Cho T, et al. Impaired clearance of influenza A virus in obese, leptin receptor deficient mice is independent of leptin signaling in the lung epithelium and macrophages. PLoS One. 2014;9:e108138. doi: 10.1371/journal.pone.0108138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad AF, Rahman M, Maybauer DM, Fraser JF, Costantine MM, Pacheco LD, et al. Extracorporeal membrane oxygenation in pregnant and postpartum women with H1N1-related acute respiratory distress syndrome: A systematic review and meta-analysis. Obstet Gynecol. 2016;127:241–7. doi: 10.1097/AOG.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 28.Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care. 2011;15:243. doi: 10.1186/cc10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 30.Pappalardo F, Pieri M, Greco T, Patroniti N, Pesenti A, Arcadipane A, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: The ECMOnet score. Intensive Care Med. 2013;39:275–81. doi: 10.1007/s00134-012-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 32.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 33.Teramukai S, Matsuyama Y, Mizuno S, Sakamoto J. Individual patient-level and study-level meta-analysis for investigating modifiers of treatment effect. Jpn J Clin Oncol. 2004;34:717–21. doi: 10.1093/jjco/hyh138. [DOI] [PubMed] [Google Scholar]


