Abstract
Open reading frame sll1556 in the cyanobacterium Synechocystis sp. strain 6803 encodes a putative type II isopentenyl diphosphate (IPP) isomerase. The His6-tagged protein was produced in Escherichia coli and purified by Ni2+ chromatography. The homotetrameric enzyme required NADPH, flavin mononucleotide, and Mg2+ for activity; KmIPP was 52 μM, and kcatIPP was 0.23 s−1.
The isomerization of isopentenyl diphosphate (IPP) to dimethylallyl diphosphate (DMAPP) is an essential reaction in the mevalonate (MVA) pathway to isoprenoids (13) and, although not essential, probably serves to balance the IPP and DMAPP pools in the methylerythritol phosphate (MEP) route (9). Two types of IPP isomerase are known. The type I enzyme, found in Eucarya and some of the Bacteria, is well characterized (16). Type II IPP isomerase, found in Archaea and some of the Bacteria, was discovered much more recently (10), and only a few studies of the protein have been reported. Analysis of the genome of Synechocystis sp. strain PCC 6803 indicated that the cyanobacterium synthesizes isoprenoid compounds by the MEP route. Although it was reported elsewhere that the bacterium does not have detectable IPP isomerase activity under phototrophic growth conditions (6, 7), open reading frame (ORF) sll1556 encodes a protein that is substantially similar to other type II IPP isomerases. We now report that purified ORF sll1556 protein is an active type II IPP isomerase.
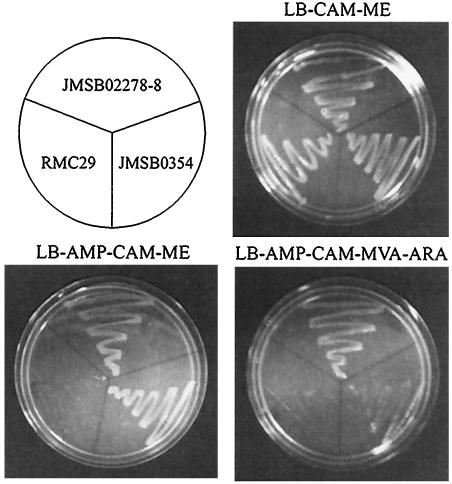
Evidence that Synechocystis sp. strain PCC 6803 ORF sll1556 encodes a functional type II IPP isomerase was obtained by complementation studies with Salmonella enterica serovar Typhimurium strain RMC29 (Table 1) (2). The chromosomal copy of idi (IPP isomerase) in RMC29 was disrupted with a chloramphenicol (CAM) marker, and dxs (deoxyxylulose synthase) was disrupted with a minioperon that includes the yeast genes required for biosynthesis of IPP from MVA and an ampicillin (AMP) marker. Thus, RMC29 is viable when supplemented with methylerythritol (ME) but does not grow on MVA unless idi activity is restored. RMC29 was transformed with plasmid pJMSB02278-8 bearing a copy of ORF sll1556 from Synechocystis sp. strain PCC 6803 and the expression plasmid without ORF sll1556. Both strains were resistant to AMP and expressed the plasmid-carried genes when induced with arabinose. As shown in Fig. 1, strains JMSB02278-8, JMSB0354, and RMC29 grew on LB broth-CAM-ME, demonstrating that they can utilize the MEP pathway to synthesize IPP and DMAPP without a functional IPP isomerase. Strains JMSB02278-8 and JMSB0354 contain plasmids conferring resistance to AMP and grew on LB-AMP-CAM-ME, while RMC29 did not. Only JMSB02278-8 grew on LB-AMP-CAM-MEV-arabinose by utilizing the IPP isomerase encoded by ORF sll1556 from Synechocystis sp. strain PCC 6803 to convert IPP synthesized from MVA to DMAPP.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli | ||
| M15 pREP4 | Host for pQE-30 overexpression vectors; Kan4 | Qiagen |
| JMSB0373a | M15 pREP4 containing pJMSB0373Sa | This work |
| S. enterica serovar Typhimurium | ||
| RMC29 | dxs::mevalonate operon (araC Pbad erg8 erg12 erg19 Kanr) and idi::Camr | 2 |
| JMSB02278-8 | RMC29 containing pJMSB02278-8 | This work |
| JMSB0354 | RMC29 containing pBAD Myc/HisC | This work |
| Plasmids | ||
| pBAD Myc/HisC | E. coli overexpression vector; Ampr | Invitrogen |
| pQE-30 | Expression vector with an N-terminal His6 tag; Ampr | Qiagen |
| pJMSB02278-8 | pBADA Myc/HisC with a 1.1-kb Xhol-KpnI insert corresponding to ORF sll1556 | This work |
| pJMSB0373Sa | pQE-30 with a 1.1-kb BamHI-Kpnl insert corresponding to ORF sll1556 | This work |
FIG. 1.
Complementation of an idi::MVA knockout in S. enterica serovar Typhimurium with ORF sll1556 from Synechocystis sp. strain 6803. Strains are RMC29 (dxs::MVA operon; idi::CAM), JMSB0354 (RMC29; Ampr), and JMSB02278-8 (RMC29; Ampr; ORF sll1556). AR, arabinose.
Nickel-nitrilotriacetic acid affinity chromatography of the supernatant from a cell extract of Escherichia coli strain JMSB0373a yielded a protein that gave a single band upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis with an apparent molecular mass of ∼50 kDa. This band was isolated, and an in-gel tryptic digestion was performed. Analysis of the matrix-assisted laser desorption ionization mass spectrometry of the tryptic peptides revealed 69% coverage of the amino acids from the expressed sequence of ORF sll1556 from Synechocystis sp. strain PCC 6803 (data not shown). The state of aggregation of the protein was examined by sedimentation equilibrium. The data were best described by a tetramer-octamer equilibrium, with Kd being 0.2 μM (Fig. 2). The protein exists in a homotetrameric state at the concentrations used for assays (29 nM). Isomerase activity was measured by the acid lability technique (2). The optimal pH and concentrations for Mg2+, flavin mononucleotide, and NADPH were determined, and under these conditions (37°C, 50 mM HEPES buffer [pH 7.0], 20 μM flavin mononucleotide, 10 mM NADPH, 20 mM MgCl2, 50 μM dithiothreitol), KmIPP was 52 μM, kcatIPP was 0.23 s−1, and kcat/Km was 4.4 × 103 M−1 s−1.
FIG. 2.
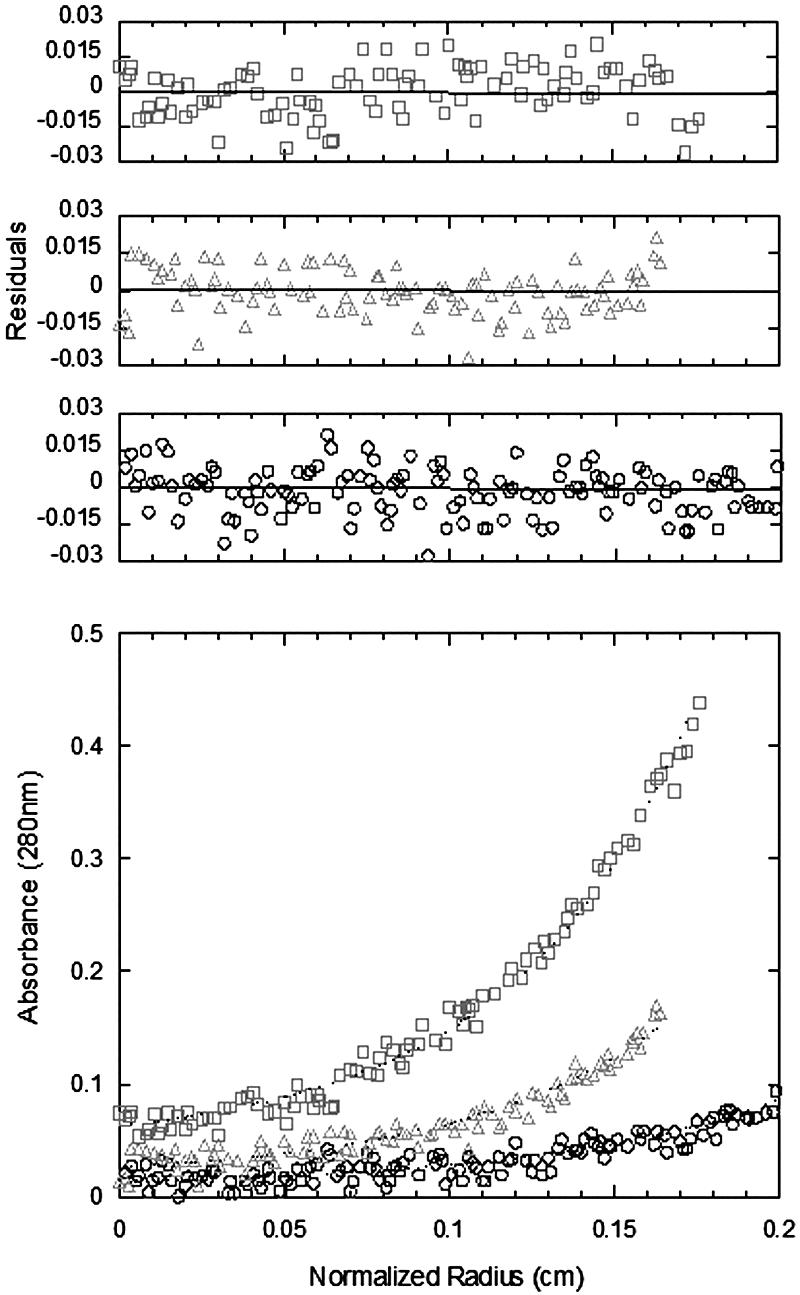
Sedimentation equilibrium data for Synechocystis sp. strain 6803 type II IPP isomerase. The lower panel shows experimental data points for three different loading concentrations (□, 2.8 μM; ▵, 1.4 μM; ○, 0.7 μM) of the protein with the corresponding calculated fit (dotted line). The upper panels show the residuals for these fits; all are small and random, indicating a good fit corresponding to a Kd of 0.2 μM.
Isoprenoid metabolites play major roles during photosynthesis (8). Carotenoids are essential for the normal development of photosynthetic membranes, for dissipation of light energy, and for protecting plants from excess light (3-5, 15). Carotenoids quench singlet oxygen, and their absence is lethal in plants and oxygenic photosynthetic bacteria such as Synechocystis sp. strain PCC 6803. Chlorophyll is anchored to membranes by a hydrophobic phytyl chain. DMAPP is the essential allylic diphosphate primer for the biosynthesis of geranylgeranyl diphosphate required for carotenoids and phytol. In the MEP pathway DMAPP and IPP are synthesized simultaneously from hydroxydimethylallyl diphosphate to give a nonequilibrium (∼6:1) mixture favoring IPP (1). The isomerization of IPP and DMAPP results in a ∼1:2.5 ratio of IPP to DMAPP (14). In those organisms containing the MEP pathway and a type II IPP isomerase, such as cyanobacteria, the ratio of DMAPP relative to IPP can be increased in order to balance the pools of IPP and DMAPP during periods of high carotenoid biosynthesis where only three molecules of IPP are required for each DMAPP in the chain elongation reaction to produce geranylgeranyl diphosphate.
Prior to the discovery of type II IPP isomerase, Gannt and Ershov reported that the chromosome of Synechocystis sp. strain PCC 6803 did not appear to encode an IPP isomerase and that a cell extract from the bacterium did not have IPP isomerase activity (6). While this paper was under review, Poliquin and coworkers reported that the Synechocystis ORF sll1556 protein was inactive in a type II IPP isomerase assay, although the Streptomyces type II enzyme was active under the same conditions (12). This is in marked contrast to our findings for the ORF sll1556 protein, which has IPP isomerase activity comparable to that of other type II enzymes (Table 2). Given the similarities between the constructs and protocols used to obtain purified protein and the procedures used to assay for activity, we cannot explain the difference between our results and theirs.
TABLE 2.
Kinetic constants for type II IPP isomerasesa
| Organism | KMIPP (μM) | kcat (s−1) | kcat/KM (M−1 s−1) |
|---|---|---|---|
| Synechocystis sp. strain 6803 | 52 | 0.23 | 4.4 × 103 |
| Bacillus subtilis | 0.39 | ||
| Methanobacter thermoauto trophicus | 64 | 1.6 | 2.5 × 104 |
| Staphylococcus aureus | 19 | 1.3 | 6.8 × 104 |
| Streptomyces sp. strain C1190 | 450 | 0.70 | 1.6 × 103 |
Kinetic constants for Bacillus subtilis (11), Methanobacter thermoautotrophicus (2), Staphylococcus aureus (10), and Streptomyces sp. strain CL190 (10) are compared to those for Synechocystis sp. strain 6803.
Acknowledgments
We thank Cindy Chepanoske for providing matrix-assisted laser desorption ionization-time of flight mass spectra and Lisa Jones for assistance with the sedimentation equilibrium experiments.
This work was supported by Public Health Service grant GM 25521 from the Institute of General Medical Sciences.
REFERENCES
- 1.Adam, P., S. Hecht, W. Eisenreich, J. Kaiser, T. Grawert, D. Arigoni, A. Bacher, and F. Rohdich. 2002. Biosynthesis of terpenes: studies on 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase. Proc. Natl. Acad. Sci. USA 99:12101-12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkley, S. J., R. M. Cornish, and C. D. Poulter. 2003. Identification of an archaeal type II isopentenyl diphosphate isomerase in Methanothermobacter thermautotrophicus. J. Bacteriol. 186:1811-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bialek-Bylka, G. E., T. Tomo, K. Satoh, and Y. Koyama. 1995. 15-Cis-beta-carotene found in the reaction center of spinach photosystem II. FEBS Lett. 363:137-140. [DOI] [PubMed] [Google Scholar]
- 4.Bobik, T. A., M. Ailion, and J. R. Roth. 1992. A single regulatory gene integrates control of vitamin B12 synthesis and propanediol degradation. J. Bacteriol. 174:2253-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demmig-Adams, B., and W. W. Adams III. 1993. The xanthophyll cycle, p. 206-252. In A. J. Young and G. Britton (ed.), Carotenoids in photosynthesis. Chapman and Hall, London, United Kingdom.
- 6.Ershov, Y. V., R. R. Gantt, F. X. Cunningham, and E. Gantt. 2000. Isopentenyl diphosphate isomerase deficiency in Synechocystis sp. strain PCC6803. FEBS Lett. 473:337-340. [DOI] [PubMed] [Google Scholar]
- 7.Ershov, Y. V., R. R. Gantt, F. X. Cunningham, Jr., and E. Gantt. 2002. Isoprenoid biosynthesis in Synechocystis sp. strain PCC6803 is stimulated by compounds of the pentose phosphate cycle but not by pyruvate or deoxyxylulose-5-phosphate. J. Bacteriol. 184:5045-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin, T. W. 1980. The biochemistry of the carotenoids. Chapman and Hall, London, United Kingdom.
- 9.Hahn, F. M., A. P. Hurlburt, and C. D. Poulter. 1999. Escherichia coli open reading frame 696 is idi, a nonessential gene encoding isopentenyl diphosphate isomerase. J. Bacteriol. 181:4499-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneda, K., T. Kuzuyama, M. Takagi, and H. Seto. 2001. An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp. strain CL190. Proc. Natl. Acad. Sci. USA 98:932-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laupitz, R., S. Hecht, S. Amslinger, F. Zepeck, J. Kaiser, G. Richter, N. Schramek, S. Steinbacher, R. Huber, D. Arigoni, A. Bacher, W. Eisenreich, and F. Rohdich. 2004. Biochemical characterization of Bacillus subtilis type II isopentenyl diphosphate isomerase, and phylogenetic distribution of isoprenoid biosynthetic pathways. Eur. J. Biochem. 271:2658-2669. [DOI] [PubMed] [Google Scholar]
- 12.Poliquin, K., Y. V. Ershov, F. X. Cunningham, T. T. Woreta, R. R. Gantt, and E. Gantt. 2004. Inactivation of sll1556 in Synechocystis strain PCC 6803 impairs isoprenoid biosynthesis from pentose phosphate cycle substrates in vitro. J. Bacteriol. 186:4685-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulter, C. D., and H. C. Rilling. 1981. Prenyl transferases and isomerase, p. 162-224. In J. W. Porter (ed.), Biosynthesis of isoprenoid compounds, vol. 1. John Wiley & Sons, Inc., New York, N.Y. [Google Scholar]
- 14.Street, I. P., D. J. Christensen, and C. D. Poulter. 1990. Hydrogen exchange during the enzyme catalyzed isomerization of isopentenyl diphosphate and dimethylallyl diphosphate. J. Am. Chem. Soc. 112:8577-8578. [Google Scholar]
- 15.Tracewell, C. A., J. S. Vrettos, J. A. Bautista, H. A. Frank, and G. W. Brudvig. 2001. Carotenoid photooxidation in photosystem II. Arch. Biochem. Biophys. 385:61-69. [DOI] [PubMed] [Google Scholar]
- 16.Wouters, J., Y. Oudjama, S. J. Barkley, C. Tricot, V. Stalon, L. Droogmans, and C. D. Poulter. 2003. Catalytic mechanism of E. coli isopentenyl diphosphate isomerase involves Cys67, Glu116 and Tyr104 as suggested by crystal structures of complexes with transition state analogues and irreversible inhibitors. J. Biol. Chem. 278:11903-11908. [DOI] [PubMed] [Google Scholar]