Abstract
Mesenchymal stem cells (MSCs) are undergoing intensive testing in clinical trials as a promising new therapy for many inflammatory diseases and for regenerative medicine, but further optimization of current MSC-based therapies is required. In this study, we found that in addition to direct complement–mediated attack through the assembly of membrane attack complexes (MACs) that we and others have recently reported, of the released complement activation products, C5a, but not C3a, activates neutrophils in the blood to further damage MSCs through oxidative burst. In addition, we have developed a simple method for painting factor H, a native complement inhibitor, onto MSCs to locally inhibit complement activation on MSCs. MSCs painted with factor H are protected from both MAC- and neutrophil-mediated attack and are significantly more effective in inhibiting antigen-specific T cell responses than the mock-painted MSCs both in vitro and in vivo.
Introduction
Mesenchymal stem cells (MSCs), adult stem/progenitor cells found in many tissues, such as bone marrow, adipose tissue, and umbilical cord, can differentiate into different types of cells, including adipocytes, osteoblasts, and chondrocytes[1]. In addition to this property, which makes them potentially useful in regenerative medicine, MSCs are also strongly immunosuppressive and can inhibit cells both in the adaptive immune system, including T and B cells[2], and in the innate immune system, including NK cells and γδ T cells[3]. Because of their potent immunosuppressive activity, MSCs are being extensively tested in clinical studies as a potential therapy for many inflammatory diseases, such as graft versus host disease (GVHD)[4], multiple sclerosis[5], diabetes[6], colitis[7], and transplant rejection[8]. Despite all the efforts over the years and more than 400 registered MSC-based clinical trials (www.clinicaltrials.gov), there has been no approved MSC product in the US for treating inflammatory diseases. However, even though the results from a recently completed Phase III clinical trial using MSCs to treat GVHD failed to meet the primary endpoints when all patients were considered[9], subsequent analyses demonstrated treatment efficacy in the pediatric subgroup[10]. Although the results of these clinical studies and promising results from previous in vitro studies and in vivo studies in animals strongly argue that MSCs have potential for development as a new therapy for inflammatory diseases, current MSC-based therapies need to be improved in order to be successful.
Complement is an important component of the innate immune system and its primary role is to defend the host from infection[11]. Complement factors are abundant in the blood and, after activation, can assemble membrane attack complexes (MACs) on target cells to lyse or damage them. At the same time, the small molecular weight components, C3a and C5a, known as anaphylatoxins, are generated and released from the site of complement activation and bind to their respective receptors, C3aR and C5aR, both of which are G protein-coupled receptors present on different types of cells, especially myeloid cells. This results in chemoattraction and activation of nearby cells, such as neutrophils, triggering inflammation to damage the invading pathogens (foreign cells)[12].
Previous studies have suggested that MSCs are immunoprivileged and can escape from host immune system surveillance partially due to their potent immunosuppressive activities[13]. Because of this belief and other advantages, such as cost and convenience, many MSCs in clinical development are allogeneic MSCs. We and others previously reported that, although MSCs seem to be able to escape from adaptive immune surveillance, infused MSCs can be recognized by complement from the innate immune system[14–16]. Using serum as a source of complement, we further demonstrated that MACs are assembled on MSCs and directly damage the cells, leading to cellular injury and impaired function14.
In practice, when MSCs are administrated, they encounter not only complement, but also other cells in the blood. In this study, we investigated the impact of neutrophils, the most abundant circulating leukocytes in the blood in mammals, on the fate of MSCs after administration and the underlying mechanisms. In addition, we also studied the effects of locally “painting” MSCs with complement factor H (CFH)[17, 18], a native complement inhibitor, on their viability and function after administration. We found that MAC generation and activation of neutrophils by C5a synergistically damage MSCs, leading to reduced MSC viability and impaired function. We also demonstrated that the simple CFH painting approach protects MSCs from both MAC-mediated and neutrophil-mediated attack, leading to significantly improved MSC survival and function both in vitro and in vivo.
Reagents and Methods
MSCs, mice and normal human serum
Primary human MSCs were isolated from bone marrow from healthy donors at the MSC Core Facility at Case Western Reserve University as described previously[14] and were used at passage numbers 4–7 were used. MSCs were cryopreserved in a liquid nitrogen and before each experiment, MSCs were thawed and cultured for ~ 1 week in complete media under standard culturing conditions. Pooled normal human serum (NHS) were ordered from Innovative Research Inc (Novi, MI), and aliquoted NHS were stored in a −80°C freezer until needed. OT-II mice, C3 knockout mice (both are on C57BL/6 background) and C57BL/6 mice, purchased from the Jackson Laboratory (Bar Harbor, Maine), were maintained in the animal facility at the Cleveland Clinic. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees of the Cleveland Clinic.
In vivo neutrophil activation assay
1×106 MSCs in 300 μl of sterile PBS or PBS alone was injected into WT or C3 KO mice by tail vein injection, then, after 40 min, activation of neutrophils in the blood was assessed by staining peripheral blood cells for reactive oxygen species (oxidative burst) with 10 μM dihydrorhodamine 123[19] (DHR123; Sigma, St. Louis, MO) for 20 min at room temperature. After washing and lysis of red blood cells, neutrophil activation (DHR123+) was measured using a flow cytometer (FACSCalibur BD Bioscience).
In vivo neutrophil depletion
Neutrophils were depleted by intraperitoneal injection of mice with an anti-neutrophil monoclonal antibody (NIMP-R14; kindly provided by Dr. Eric Pearlman, Case Western Reserve University) (0.5 mg/mouse) 24 h before the experiment following a previously published protocol[20–22] and neutrophil depletion was confirmed by staining the peripheral white blood cells with phycoerythrin (PE)-labeled anti-mouse Gr-1 monoclonal antibody (mAb) (2μg/ml Biolegend, San Diego, CA) for 15 min at 4°C, followed by flow cytometry analysis.
Complement-mediated MSC damage assay
MSC damage after incubation with serum was assessed using a 2′, 7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) leakage assay[14]. In brief, 2×104 MSCs were labeled by incubation for 30 minutes at 37°C w ith 5 μM BCECF-AM (ThermoFisher, Waltham, MA). After washing, the labeled MSCs were incubated 30 minutes at 37°C with different concentrations of pooled normal human serum (NHS) in 100 μL of GVB++ buffer (0.1 % gelatin, 5 mM Veronal, 145 mM NaCl, 0.15 mM CaCl2 and 0.5mM MgCl2, pH 7.3, Complement technology Inc, Tyler, TX), then released BCECF in the supernatants was measured by a fluorescence GEMINI XS Microplate Reader (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths of 485 nm and 538 nm, respectively. To calculate the percentage of BCECF release (complement-mediated injury), the following equation was used as reported previously[23, 24]: percentage of BCECF release (cell damage) = [(A−B)/(C−B)]×100%, where A represents the mean experimental BCECF release, B represents the mean spontaneous BCECF release, and C represents the mean maximum BCECF release induced by incubating cells with 0.1% Triton X-100.
Neutrophil-mediated MSC damage assay
Human neutrophils, either purified from healthy donor peripheral blood mononuclear cells (PBMCs) by Ficoll centrifugation (provided by the Hematopoietic Stem Cells Core Facility at Case Western Reserve University) or differentiated from the neutrophil progenitor cell line HL-60 (ATCC)[25], were used. In some experiments, neutrophil-mediated MSC damage was assessed using a similar BCECF-based assay to that described above[14]. In brief, 2×104 MSCs were labeled with BCECF-AM as described above, then, after washing, the labeled MSC were incubated for 30 min at 37°C with different numbers of neutrophils in the presence or absence of 30% NHS in 100 μl of GVB++ buffer with various concentrations of the neutrophil function inhibitor wortmannin that inhibits oxidative burst[26] (Cayman Chemical, Ann Arbor, MI) and released BCECF in the supernatants was measured as described above. The percentage BCECF release (cell injury) was calculated as above. In some experiments, a C5aR antagonist (JPE1375, Calbiochem, Billerica, MA)[27] or a C3aR antagonist (SB 290157, Calbiochem, Billerica, MA)[28] were added, both at 10 μM, to assess the role of C3aR and C5aR on neutrophils, while, in other experiments, NHS was omitted and 50 ng/ml of purified C5a (Complement technology Inc, Tyler, TX) added instead.
Painting purified CFH onto MSCs
Primary MSCs were painted with purified human CFH (Complement technology Inc, Tyler, TX) after 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)-mediated activation of carboxyl groups. In brief, 2 mM EDC and 5 mM N-hydroxysulfosuccinimide (ThermoFisher, Waltham, MA) were added to CFH solution at the indicated concentration and allowed to react for 15 min at room temperature to create activated carboxyl groups, then 20 mM β-mercaptoethanol was added to quench the excess EDC and the activated CFH was separated using a desalting column (ThermoFisher, Waltham, MA) equilibrated with PBS. MSCs (2×104) were then incubated with different concentrations of activated CFH for 30 min at 37°C in 100 μl PBS, then the cells were washed with PBS and used for experiments.
Detection of painted CFH on MSCs
Painted CFH on MSCs were detected by flow cytometric analysis. In brief, 0.5 ×105 CFH- painted or mock-painted (buffers only) MSCs were stained for 30 min at 4°C with 2 μg/ml of goat anti-human CFH mAb (Complement technology Inc, Tyler, TX) or the same concentration of control goat IgG. After washing, the cells were incubated for 30 min at 4°C with 1 μg/ml of Alexa 488-labeled donkey anti-goat IgG antibodies (Jackson ImmunoResearch, West Grove, PA), followed by flow cytometric analysis on a flow cytometer (BD FACSCalibur).
Painted CFH stability assay
MSCs were painted with CFH (10 μg/ml) following the protocols described above. Levels of painted CFH on the cell surface (mean fluorescence intensity) were quantitated by flow cytometric analysis every 12 hr for the first 36 hr, then daily for up to 5 days.
MSC-activated complement assays
Complement activation by CFH-painted or mock-painted MSCs was evaluated by culturing the MSCs with NHS, then measuring levels of C3b/iC3b on the MSCs and C3a and C5a in the supernatants. In brief, MSCs (2×104) painted with different concentrations of activated CFH or mock-painted were incubated at 37°C for 30 min with 30% NHS in GVB++ buffer, then the MSCs were collected and C3b/iC3b deposited on the cell surface quantified by staining the cells with fluorescein isothiocyanate-conjugated anti-human C3 IgG (Cedarlane, Burlington, NC), followed by flow cytometric analysis, while the supernatants from some of these experiments were collected and C3a and C5a levels measured using an anaphylatoxin cytometric bead array kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions.
MSC in vitro function assay
MSC function was assessed by measuring their ability to inhibit the proliferation of, and cytokine production by, activated T cells. In brief, MSCs (2×104) were irradiated at 30-Gy to inhibit proliferation, then were painted for 30 min at 37°C in the presence or absence of activated CFH (10 μg/ml). After washing, these cells were incubated with 30% NHS in GVB++ buffer for 30 min at 37°C, washed, and incubated with different numbers of PBMCs (MSC: PBMC ratio 1:10, 1:20 and 1:40) in the presence of anti-CD3/28 Dynabeads (2 μl, ThermoFisher, Waltham, MA) and human IL-2 (30 U/ml; Biolegend, CA). After 48 h, BrdU (10 μM) was added to the co-cultures and culture supernatants were collected 72 h later and IFN-r levels measured by conventional ELISA (Biolegend, CA), while the cells were fixed and proliferation of the activated T cells assessed by measuring BrdU incorporation using a BrdU ELISA kit (Roche, Chicago, IN).
In vivo MSC damage and survival assays
To evaluate in vivo damage of MSCs after administration, MSCs (1×106) labeled with BCECF-AM were infused via the tail vein into WT mice in which neutrophils had been depleted using anti-NIMP antibody, WT mice which had been injected with the same amount of control IgG, and untreated C3 KO mice. For MSC injury assessment, at 0, 20, 40 and 60 min, the mice were bled from the tails and BCECF levels released from damaged MSCs quantified as described above. Mice were then euthanized at 6 h after cell transfer, and the lungs, liver, and spleen collected and single cell suspensions prepared either by collagenase digestion (lungs and livers) or by mashing (spleens), then the cells were stained using 2 μg/ml of Cy5.5-labeled anti-human CD105 mAb (Biolegend, San Diego, CA), and the number of surviving infused MSCs inside each organ analyzed by flow cytometry (BD FACSCalibur).
In vivo MSC function assay
The in vivo immunosuppressive activity of MSCs was first assessed by measuring their ability to inhibit systemic antigen-specific T cell responses in mice adaptively transferred with uveitigenic Th17 cells. In brief, following previously published protocols[29, 30], WT B6 mice were immunized subcutaneously (s.c.) with interphotoreceptor retinoid-binding protein peptide 1–20 (IRBP1–20) in complete Freund’s adjuvant (200 μg/mouse), then 2 weeks later, spleen cells were collected and re-stimulated with 10 μg/ml of IRBP1–20 peptide in vitro, together with 10 ng/ml of IL-23 (Biolegend, CA). After 72 h of culture, blasting T cells were enriched by Ficoll centrifugation and adoptively transferred into naïve B6 mice by tail vein injection (5 × 106/mouse), which were then randomly divided into 2 groups. One group received 0.5×105 CFH- painted MSCs and the other received the same number of mock-painted MSCs by tail vein injection, then, after 7 days, the mice were euthanized and splenocytes collected for IL-17a recall assays to compare the Th17 responses in the two groups.
The in vivo immunosuppressive activity of MSCs was also assessed by measuring their ability to inhibit local T cell responses using a dendritic cell (DC)-mediated T cell activation model[31]. In brief, T cells were enriched from OT-II mouse splenocytes using nylon wool and injected via the tail vein into naïve WT C57BL/6 mice (4 × 106 cells/mouse). After 24 h, syngeneic bone marrow-derived DCs loaded with ovalbumin (OVA) were injected s.c. into the hind footpads (0.4 × 106/mouse) and CFH- or mock-painted MSCs (1×106/mouse) were injected via the tail vein. After a further 24 h, 1 mg of BrdU was injected intraperitoneally into each mouse, then, another 24 h later, the draining lymph nodes (popliteal lymph nodes) were harvested and T cell proliferation measured by BrdU incorporation using a BrdU ELISA kit (Roche, Chicago, IN).
Data Analysis
All data were analyzed using the software Statistical Package for Social Sciences (SPSS, version 23.0; SPSS Inc., Chicago, IL) and GraphPad Prism (version 6.0; GraphPad software Inc., La Jolla, CA). To determine whether groups were statistically different, results were compared using Student’s t-tests, ANOVA, and Tukey post-hoc tests. A p value of <0.05 was considered significant.
Results
Neutrophils in the blood are activated by complement after MSC administration
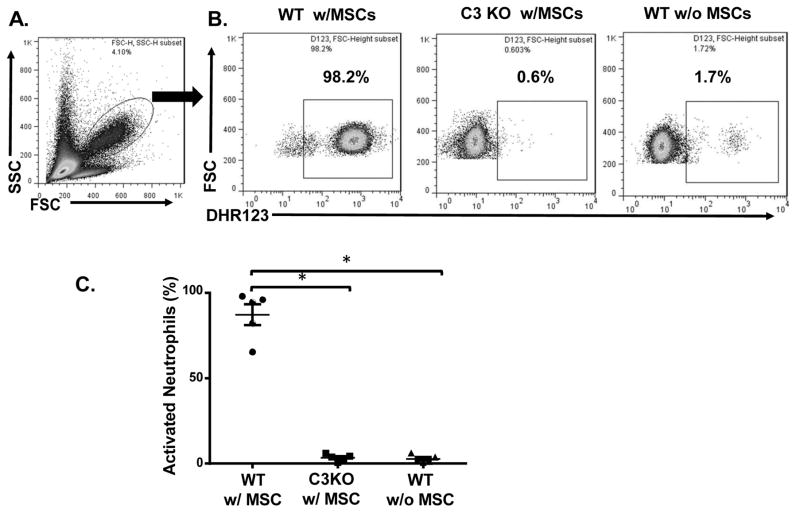
To test whether neutrophils were activated after contact of human MSCs with blood following administration and whether complement was involved in this process, because C3 KO mice are most commonly used to demonstrate a role of complement in vivo, we first infused MSCs into WT and C3 KO mice by tail vein injection, then, after 40 min, assessed neutrophil activation in the peripheral blood by dihydrorhodamine 123 (DHR123) which stains for reactive oxygen species[32], followed by flow cytometry analysis. As shown in Fig. 1, the results showed that, while the majority of neutrophils were quiescent in naïve WT mice, after MSC infusion, most neutrophils in the WT mice were activated, as indicated by positive DHR123 staining for reactive oxygen species. In contrast, C3 KO mice showed markedly reduced neutrophil activation after receiving the same numbers of MSCs. These results showed that neutrophils are activated shortly after MSC infusion in vivo and that complement is the primary mechanism by which MSCs activate neutrophils.
Figure 1. Neutrophils are activated by complement after MSC infusion.
MSCs were infused into WT or C3 KO mice (1×106/mouse) by tail vein injection, then, after 40 min, peripheral blood was collected and activation of neutrophils in the blood assessed by staining the cells with DHR123, followed by flow cytometric analysis. WT mice without MSC infusion were included as controls. (A) Neutrophils in the peripheral blood. The oval gate indicates the cells analyzed in B and C. (B) Representative results showing activated neutrophils (DHR123+) in WT mice after MSC infusion (WT w/MSCs), C3 KO mice after MSC infusion (C3 KO w/MSCs), and control WT mice without MSC infusion (WT w/o MSCs). (C) Combined results for neutrophil activation assessment after MSC infusion. n=5 in each group and show the mean ± SD, One-way ANOVA and Tukey post-hoc test were used for data analysis, *p<0.05
MSCs survive better in C3 KO or neutrophil-depleted WT mice than in control WT mice
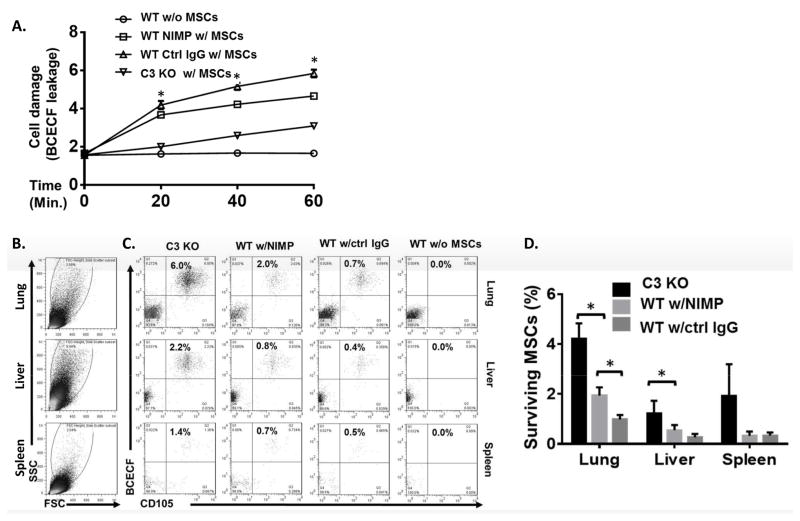
We then evaluated the impact of the activated neutrophils on the fate of MSCs after administration by measuring MSC injury and surviving MSCs in different organs. MSCs labeled with the fluorescent dye BCECF were infused into C3 KO mice, neutrophil-depleted WT mice (injected with NIMP-R14 anti-neutrophil antibody 24 h before MSC injection), or control IgG- injected WT mice, then, at 0, 20, 40, and 60 min, levels of released BCECF in the blood were measured to monitor in vivo MSCs damage. The mice were then euthanized six hours after MSC infusion and single cell suspensions prepared from the lungs, liver, and spleen and surviving MSCs in these organs measured by flow cytometry by analyzing the percentage of BCECF+ CD105+cells. Fig. 2A shows that the greatest BCECF leakage (more severe damage) from MSCs occurred in the control IgG-injected WT mice, less severe damage was seen with the neutrophil-depleted mice, and the least damage was seen in the C3 KO mice. In accordance with these results, flow cytometric analyses in different tissues showed that more surviving MSCs were found in the C3 KO mice, fewer in the neutrophil-depleted WT mice, and least in the control IgG-treated WT mice (Fig. 2B–D).
Figure 2. MSCs are damaged by complement-activated neutrophils after administration.
MSCs (1×106) were labeled with BCECF-AM and infused into WT mice depleted of neutrophils using anti-NIMP Ab (WT NIMP w/MSCs), WT controls not depleted of neutrophils (WT Ctrl IgG w/MSCs), and C3 KO mice (C3 KO w/MSCs). WT mice without MSC infusion (WT w/o MSCs) were included as controls. (A) At 0, 20, 40 and 60 min, blood was collected and levels of BCECF released from damaged MSCs were measured. Two-way ANOVA tests showed a significant difference between each group, * p<0.05. (B–D) Six hours after MSC infusion, the lung, liver, and spleen were collected and single cell suspensions prepared and incubated with Cy5.5-labeled anti-human CD105 mAb, a marker of human MSCs, followed by flow cytometric analysis. (B) shows large and living cells analyzed in C and D. (C and D) The large and living cells were then analyzed for the presence of surviving MSCs (BCECF+ CD105+); (C) shows representative results for surviving MSCs and (D) shows the combined results for all mice studied. n=4 in each group. One-way ANOVA and Tukey post-hoc test were used for data analysis, * p<0.05
Complement-activated neutrophils and MACs have synergistic effects in damaging MSCs
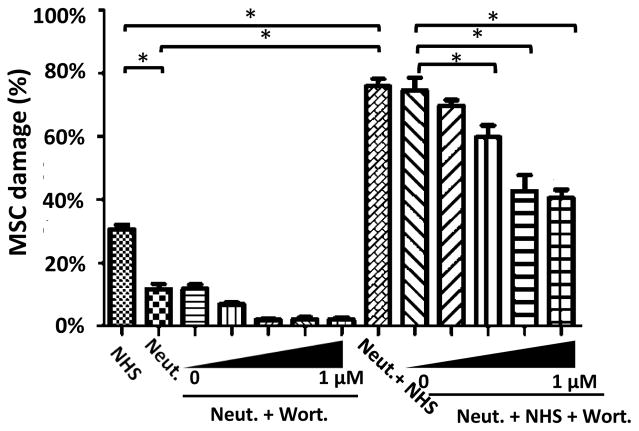
To elucidate the mechanism underlying the above in vivo results, we carried out in vitro MSC damage assays using neutrophils derived from the progenitor cell line HL-60 in the presence or absence of NHS and in the presence or absence of a known neutrophil function inhibitor, wortmannin, which inhibits oxidative burst[26]. Fig. 3 shows that “quiescent” neutrophils caused some damage to MSCs in the absence of complement (no NHS; left panel) and that this damage was reduced in a dose-dependent manner by wortmannin. When MSCs were incubated with NHS alone, MSCs were damaged by complement membrane attack complexes (left column in left panel), as shown in our previous report[14]. However, when MSCs were incubated with both neutrophils and NHS (right panel), the damage was markedly increased and damage was gradually reduced by increasing concentrations of wortmannin, finally plateauing at a level similar to that seen with NHS alone. These results demonstrate that neutrophils in the blood can act synergistically with complement to damage MSCs after administration.
Figure 3. Activated neutrophils act synergistically with complement to damage MSCs.
MSCs (2×104) were labeled with BCECF and incubated for 30 min with 30% NHS alone (NHS) or 2×104 HL60 cell-derived neutrophils alone (Neut) or in the presence of various concentrations of wortmannin (0, 0.001, 0.01, 0.1, and 1 μM) (Neut + Wort), or 30% NHS plus 2×104 neutrophils alone (Neut + NHS) or in the presence of various concentrations of wortmannin (0, 0.001, 0.01, 0.1 and 1 μM) (Neut + NHS + Wort), then MSC damage was quantified using the BCECF release assay. The data are representative results in triplicate for 3 independent experiments and show the mean ± SD, One-way ANOVA and Tukey post-hoc tests were used for data analysis, *p<0.05
The C5aR, but not the C3aR, on neutrophils is involved in the neutrophil-mediated MSC damage
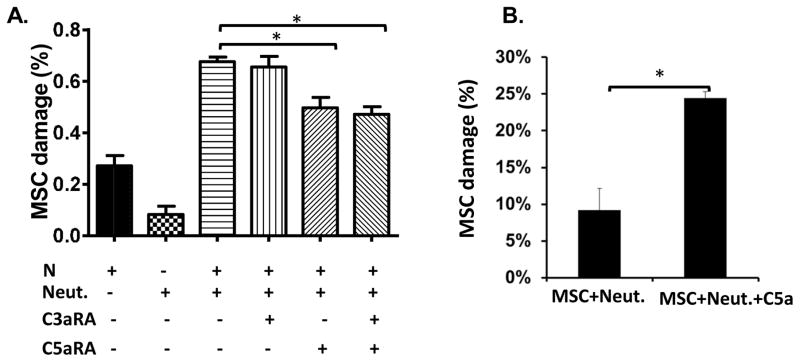
Neutrophils express both the C3aR and the C5aR[33, 34]. When complement is activated, C3a and C5a, the ligands of these complement receptors, are produced and released into the fluid phase, and can bind to their respective receptor on neutrophils and activate neutrophils to kill. To distinguish the respective roles of C3a and C5a in the newly discovered neutrophil-mediated MSC injury, we repeated the HL60-derived neutrophil-mediated and NHS- mediated MSC damage assay in the presence and absence of a C3aR antagonist and/or a C5aR antagonist. Fig. 4A shows that, although the C3aR antagonist alone had no appreciable effect, the C5aR antagonist caused a significant reduction in neutrophil-mediated MSC damage which was not increased in the presence of the C3aR antagonist, showing that the C5aR, but not the C3aR, on neutrophils contributes to the observed neutrophil-mediated MSC damage.
Figure 4. C5aR, but not C3aR, signaling activates neutrophils to injure the administrated MSCs.
(A) MSCs (2×104) were labeled with BCECF, then incubated for 30 min with different combinations of 30% NHS (NHS), HL60 cell-derived neutrophils (2×104) (Neut.), a C3aR antagonist (C3aRA, 10 μM), and/or a C5aR antagonist (C5aRA, 10 μM), then MSC damage was quantified using the BCECF release assay. (B) MSCs (2×104) were labeled with BCECF, then were incubated for 30 min with primary neutrophils (2×104) purified from peripheral blood in the presence or absence of purified C5a (50 ng/ml), then MSC damage was assessed as above assay. The data are representative results in triplicate for 3 independent experiments mean ± SD, One-way ANOVA and Tukey post-hoc tests were used for data analysis, *p<0.05
C5a-activated primary neutrophils damage MSCs
In the above experiments, we used HL-60-derived neutrophils. To test whether primary neutrophils could also induce MSC damage after C5a activation, we incubated MSCs for 4 h with freshly purified primary neutrophils in the absence or presence of purified human C5a, then assessed MSC damage. Fig. 4B shows that consistent with results obtained using HL-60- derived neutrophils and NHS, primary neutrophils also induced significant MSC damage after stimulation with purified C5a.
Purified CFH can be painted onto the MSC surface
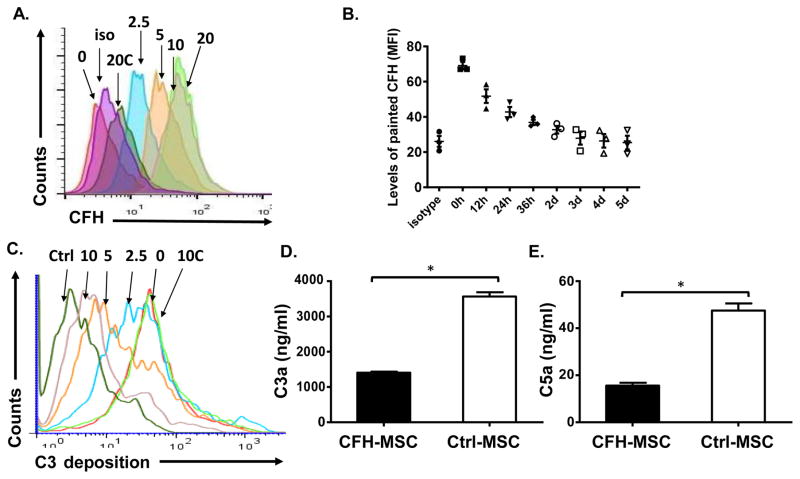
The above results and our previous report[14] demonstrated that complement in the blood is activated by the infused MSCs. The activated complement assembles MACs to directly injure MSCs, and, at the same time, the released C5a activates neutrophils in the blood to further damage MSCs. These results suggest that local inhibition of complement activation on MSCs should greatly preserve MSC viability and function by inhibiting not only MAC-mediated, but also neutrophil-mediated, attack. To try to achieve this goal, we developed a protocol for painting CFH onto MSCs in which carboxylic acid groups in the purified CFH were activated with EDC, and were then reacted with amine groups on the MSC surface to covalently cross-link (paint) CFH onto MSCs. Fig. 5A shows flow cytometric results when MSCs were incubated with 0, 2.5, 5, 10, or 20 μg of EDC-activated CFH or 20 μg of non-activated CFH, then with goat anti- CFH antibodies and Alexa 488-labeled donkey anti-goat IgG, demonstrating that activated CFH was bound in a dose-dependent manner. In experiments studying the stability of the painted CFH on MSCs, we found that the painted CFH can stay on MSCs for 2 days (Fig. 5B).
Figure 5. Factor H can be painted onto MSCs to locally inhibit complement activation.
(A) MSCs were incubated for 30 min with 0, 2.5, 5, 10, or 20 μg of EDC-activated CFH or 20 μg of non-activated CFH (20C) in 100 μl of PBS for painting, then, after washes, CFH on MSCs was measured by incubation for 30 min at 4°C with goat anti-CFH antibodies and for 30 min at 4°C with Alexa 488-labeled donkey anti-goat IgG antibodies, followed by flow cytometric analysis. iso, isotype control for the anti-CFH IgGs on cells painted with 20 μg of EDC-activated CFH. The result shown is representative of those obtained in 5 independent experiments. (B) To evaluate how long the painted CFH can stay on MSCs, MSCs were painted with CFH (10 μg/ml), and cultured in wells of 6-well plates. Levels of painted CFH on the cell surface (mean fluorescence intensity) were quantitated by flow cytometric analysis every 12 hr for the first 36 hr, then daily for up to 5 days. (C) To assess local complement activation, MSCs were first incubated for 30 min with 0, 2.5, 5, or 10μg of EDC-activated CFH or 10 μg of non-activated CFH (10C) for painting, then, after washing, the cells were incubated with or without (Ctrl) 30% NHS in GVB++ for 30 min, then C3b/iC3b deposited on the cell surface were quantified by flow cytometry after staining the cells with a fluorescein isothiocyanate-conjugated anti human C3 IgG. The result show is representative of those obtained in 3 independent experiments. (D and E) MSCs painted with 10 μg of EDC-activated CFH (CFH-MSC) or mock-painted (Crtl-MSC) were incubated for 30 min with 30% NHS, then the supernatants were collected and C3a (D) and C5a (E) measured using an anaphylatoxin cytometric bead array kit. The data are representative results in triplicate for 3 independent experiments and are the mean ± SD, multiple t-test was used for data analysis, *p<0.05
CFH painted onto MSCs inhibits C3b/iC3b deposition on MSCs and C3a/C5a release after serum contact
To test whether the painted CFH inhibited complement activation on MSCs, we incubated MSCs painted with different concentrations of CFH with or without NHS in GVB++ buffer, then assessed local complement activation by measuring levels of C3b/iC3b on MSCs using flow cytometry. As shown in Fig. 5C, painting CFH onto MSCs greatly reduced C3b/iC3b deposition on MSCs in a dose-dependent manner. We also measured released C3a/C5a in the supernatants after incubating MSCs painted using 10 μg/ml of activated CFH or mock-painted MSCs with NHS in GVB++ and found that levels of released C3a (Fig. 5D) and C5a (Fig. 5E) were significantly reduced in the culture supernatants from CFH-painted MSCs, showing that the painted CFH locally inhibited complement activation on MSCs. In subsequent studies, 10 μg/ml of CFH was used.
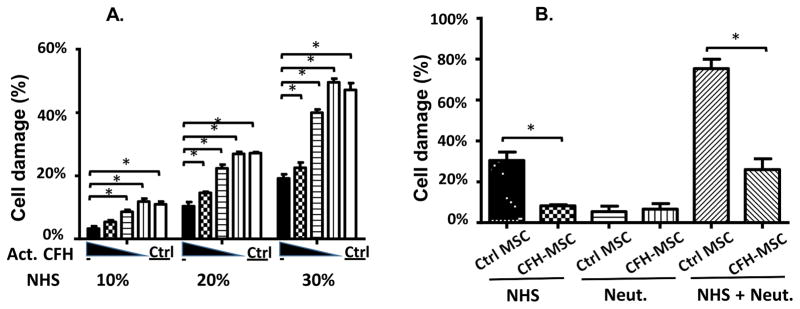
CFH-painted MSCs are better protected from MAC-mediated direct attack than mock-painted MSCs
We then tested whether CFH-painted MSCs were better protected from complement- mediated direct attack than mock-painted MSCs using the BCECF leakage-based cytotoxicity assay. We first incubated MSCs with 0, 2.5, 5, or 10 μg of activated CFH for painting, using MSCs incubated with10 μg of non-activated CFH as controls, then, after washes, labeled the cells with BCECF, incubated them with 10%, 20%, or 30% NHS in GVB++ for 30 min, and measured MSC damage by measuring levels of released BCECF. As shown in Fig. 6A, painting CFH onto MSCs significantly reduced complement-mediated MSC damage in a dose- dependent manner at all concentrations of NHS (complement) used.
Figure 6. Painting factor H onto MSCs protects MSCs from the synergistic effect of complement and neutrophils.
(A) MSCs (2×104) were incubated with 0, 2.5, 5, or 10 μg of EDC-activated CFH (Act. CFH) or 10 μg of non-activated CFH (Ctrl) for painting, then were labeled with BCECF, washed, then incubated with 30% NHS in GVB++ for 30 min and MSC damage was quantified using the BCECF leakage assay. Two-way ANOVA tests were used for data analysis, * p<0.05. (B) MSCs (2×104) were painted with (CFH-MSC) or without (Ctrl-MSC) 10 μg of EDC-activated CFH, labeled with BCECF, and then were incubated for 30 min with 30% NHS alone (NHS), 2×104 neutrophils alone (Neut.) or 30% NHS plus with 2×104 neutrophils (NHS+Neut), when MSC damage was measured using the BCECF leakage assay. The data are representative of results in triplicate for 4 independent experiments and show the mean ± SD, One-way ANOVA and Tukey post-hoc tests were used for data analysis, *p<0.05
CFH-painted MSCs are also better protected from neutrophil-mediated attack than mock- painted MSCs
Since painting CFH onto MSCs inhibited the production of C5a (Fig. 5D), and since C5a activated neutrophils to further damage the MSCs (Fig. 4), CFH-painted MSCs should also be better protected from neutrophil-mediated damage than mock-painted MSCs. To test this, we incubated BCECF-labeled and CFH-painted MSCs or mock-painted MSCs with NHS alone, neutrophils alone, or both NHS and neutrophils for 4 h, then quantified the damage to MSCs. As shown in Fig. 6B, the results demonstrated that, consistent with previous experiments described above, CFH-painted MSCs showed reduced cellular injury compared to mock-painted MSCs when incubated with NHS alone (left panel). When incubated with neutrophils in the absence of complement (“quiescent” neutrophils), there was no significant difference in terms of MSC injury between the painted and mock-painted MSCs, with little BCECF release in both cases (center panel), but, when incubated with both NHS and neutrophils, the CFH-painted MSCs showed significantly reduced cell injury compared to mock-painted MSCs (right panel), showing that the CFH-painted MSCs are also better protected from attack by complement-activated neutrophils.
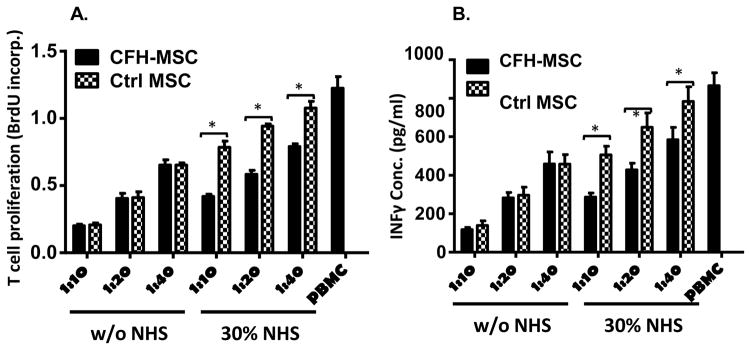
Painting MSCs with CFH does not interfere with their T cell inhibitory activity before contact with serum and preserves MSC function after contact with serum
We then examined whether CFH painting of MSCs affected cell function before and after their contact with complement. To address this question, we incubated CFH-painted MSCs or mock-painted MSCs for 30 min with 30% NHS in GVB++ or in GVB++ alone, irradiated them, and mixed them with different numbers of human T cells activated by anti-CD3/CD28 Dyna Beads, then compared their ability to inhibit the proliferation of, and inflammatory cytokine production by, the activated T cells using BrdU incorporation and IFNγ ELISA. Figure 7A shows that, at all MSC : T cell ratios tested, CFH-painted MSCs and mock-painted MSCs showed comparable efficacy in inhibiting the proliferation of activated T cells when pre-incubated with buffer alone, and that, after pre-incubation with 30% serum, CFH-painted MSCs were significantly more effective than mock-painted MSCs in inhibiting the proliferation of activated T cells. Figure 7B shows similar results for IFNγ production by the activated T cells. These data suggest that CFH painting would not interfere with MSC immunosuppressive function before administration and that CFH-painted MSCs would show better retention of function than mock- painted MSCs after administration.
Figure 7. Painting CFH onto MSCs preserves their viability and function after contact with complement in vitro.
MSCs were painted with (CFH-MSCs) or without (Ctrl-MSCs) 10 μg of EDC-activated CFH, then were incubated for 30 min with 30% NHS in GVB++ (NHS) or GVB++ alone (w/o NHS). After washing and irradiation, 2×104 of the MSCs were cultured in each well of a 96 well plate with different numbers of PBMCs in the presence of anti-CD3/CD28 beads and 30 U/ml of IL-2. After 48 h, 10 μM BrdU was added in each well, then, after 24 h, (A) the cells were harvested and proliferation of activated T cells by measuring levels of incorporated BrdU using a BrdU ELISA kit, and (B) supernatants were collected to measure IFNγ produced by the activated T cells. The data are results in triplicate representative of those in 4 independent experiments and are the mean ± SD, Two-way ANOVA tests were used in data analysis, *p<0.05
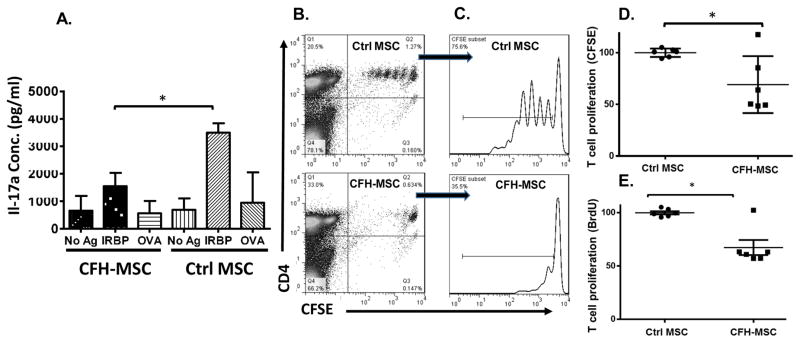
CFH-painted MSCs are more effective at inhibiting in vivo antigen-specific T cell responses than mock-painted MSCs
The above studies strongly suggested that CFH painting of MSCs might be a valid approach for improving the ability of MSCs to inhibit T cell responses in vivo by protecting the cells from complement- and neutrophil-mediated attack. To test this, we first compared the ability of CFH-painted MSCs and mock-painted MSCs to inhibit pre-activated antigen-specific T cells systemically transferred into naïve mice. In brief, we first immunized WT mice with IRBP1–20 peptide in CFA to induce antigen-specific T cells, then adoptively transferred 5×106 in vitro propagated IRBP1–20-specific T cells and 0.5×106 CFH- or mock-painted MSCs into naïve WT mice by i.v. injection, then, one week later, prepared splenocytes from the mice and re- stimulated the T cells in the presence of IRBP1–20, a non-relevant peptide, or no peptide for 72 h, then measured IL-17 levels in the culture supernatants. As shown in Fig. 8A, CFH-painted MSCs were significantly more effective in inhibiting the IRBP1–20-specific Th17 response in vivo than mock-painted MSCs.
Figure 8. Painting CFH onto MSCs improves their viability and function in vivo.
(A) Naïve WT mice were injected with 5×106 in vitro propagated IRBP1–20-specific Th17 cells via the tail vein injection, then half received 0.5 ×106 MSCs painted with 10 μg/ml of CFH (CFH- MSC) and the other half the same number of mock-painted MSCs (Ctrl MSCs). After 7 days, the spleens were collected and 1×106 splenocytes from each mouse incubated for 72 h in the presence of 20 μg/ml of IRBP1–20 (IRBP), 20 μg/ml of irrelevant OVA323–339 peptide (OVA), or no peptide (no Ag), then IRBP1–20-specific Th17 responses were assessed by measuring IL-17 in the culture supernatants using conventional ELISA. The results are the mean± SD, n=4 in each group, One-way ANOVA and Tukey post-hoc tests were used for data analysis, *p<0.05 comparing the indicated groups. (B–D) Naïve WT mice were injected with 4×106 CFSE-labeled OT II T cells via the tail vein, then, after 24 h, syngeneic bone marrow-derived DCs loaded with 1 μg/ml of OVA (0.4 × 106/mouse) were injected s.c. into the hind footpads and CFH-painted MSCs (CFH-MSC) or mock-painted MSCs (Ctrl MSC) (1×106/mouse) were injected via the tail vein. After another 24 h, 1 mg of BrdU was injected i.p. into each mouse, then, 24 h later, the draining lymph nodes (popliteal lymph nodes) were harvested and T cells proliferation quantified by measuring CFSE dilution and flow cytometry and by measuring incorporated BrdU using a BrdU ELISA. Representative T cell proliferation results based on CFSE-dilution measurements from Ctrl-MSC or CFH-MSC treated mice are presented in dot plot (B) and histogram (C). T cell proliferation assessments based on CFSE-dilution measurements (D) and BrdU incorporation (E) results from three different experiments were normalized against the average value from the Ctrl MSC-treated mice (as 100%) in each experiment and combined. n=6 in each group, mean ± SEM, multiple t-test was used for data analysis, *p<0.05
We also repeated the in vivo MSC function study by comparing the ability of CFH- painted MSCs and mock-painted MSCs to inhibit antigen-specific T cell responses that were locally activated by dendritic cells (DCs) in the draining lymph nodes. In brief, we adoptively transferred 4×106 OT-II T cells into naïve WT C57BL/6 mice, then, one day later, injected 0.4×106 CFSE-labeled OVA323–339 peptide-loaded DCs into the hind footpads and 1×106 of CFH- painted MSCs or mock-painted MSCs via the tail vein, then, after 48 h, assessed the proliferation of the activated OVA-specific T cells in the popliteal lymph nodes by flow cytometry (CFSE dilution) and by ELISA (BrdU incorporation). As shown in Fig. 8B–D, at the time point of examination, OT-II T cells in the mice injected with mock-painted MSCs showed extensive proliferation, but this was markedly reduced in mice injected with CFH-painted MSCs. Fig. 8E shows that the above results were supported by the BrdU incorporation results, as significantly lower BrdU incorporation was seen in the draining lymph nodes of the mice injected with CFH- painted MSCs than in those from mice injected with the same number of mock-painted MSCs.
Discussion
In our previous report, we showed that, after MSCs are infused into the blood, complement is activated, resulting in assembly of MACs to directly damage MSCs[14]. In the present study, we found that, in addition to the direct MAC-mediated attack on MSCs, C5a generated by MSC-mediated activation of complement activated neutrophils in the blood, and that these activated neutrophils have a synergistic effect with MACs in damaging MSCs and impairing their function. We then developed a method for painting CFH onto the surface of MSCs without negatively interfering with MSC function and demonstrated that the painted CFH locally inhibited complement activation on MSCs and reduced C5a release. Consequently, the CFH-painted MSCs showed significantly reduced cell damage, enhanced survival, and improved function both in vitro and in vivo, suggesting that this simple approach could be effective in improving the outcome of current MSC-based therapies for inflammatory diseases.
Largely because of their potent T cell inhibitory activity, MSCs are under intensive clinical development as a new therapy for treating inflammatory diseases. However, despite hundreds of registered clinical trials using MSCs in treating various disease conditions, no product has yet been approved in the US for clinical use. It is also well known that, in both animal and human studies, MSCs rarely engraft and only survive for a very short period of time after administration. As a matter of fact, even the observed short-term survival of MSCs after administration might be an overestimate, because, in most of these studies, MSCs were either labeled with luciferase or fluorescence or isotopes[35, 36] and residual signals were used to indirectly measure the survival of the cells in vivo; however, damaged MSCs with impaired function or even MSC debris can still retain the label, resulting in false positive signals. In our experiment, we used flow cytometry analysis to gate on “live cells” based on the forward scatter and side scatter patterns, then identified the surviving MSCs by their specific marker (human CD105). This approach should, at least in theory, provide more accurate data reflecting the real status of the injected MSCs than conventional indirect methods measuring residual MSC fluorescence/isotope labels.
The well-documented short life of MSCs after administration and the promising results shown in both human and animal studies led to the “hit-and-run” theory[37], speculating that, even if MSCs only survive for a short period of time after administration, this limited window is enough to allow them to be beneficial in treated patients/animals through yet-to-be-determined mechanisms. This theory, which is gaining more and more ground in the field, suggests that even a small improvement in MSC survival and/or function after administration could have a significant impact on augmenting the efficacy of treatment with MSCs. Indeed, even the painted CFH only stayed on MSC cell surface for up to two days, we found that the improved MSC viability and function after local complement inhibition by painting CFH onto MSCs resulted in significantly increased efficacy of MSCs in inhibiting antigen-specific T cell responses in vivo, suggesting that painting CFH onto the MSCs before administration might be a valid approach for improving MSC viability and function, therefore markedly improving the outcome of current MSC-based therapies.
Although MSCs have been considered to be hypoimmunogenic or immunoprivileged and can escape from host immune surveillance[38], we[14] and others[15, 16] have reported that MSCs activate complement in the blood, leading to cell damage and impairment of function. In our previous study[14], we focused on the direct effect of complement activation on MSC viability and function, i.e., direct MAC-mediated MSC injury, using serum as the source of complement in the absence of blood cells. However, under physiological conditions, immediately after infusion, MSCs encounter both complement and cellular components in the blood. Neutrophils, the most abundant leukocytes in the blood and an important component of the innate immune cells, can quickly respond to danger signals and attack and damage target cells by multiple mechanisms, including sudden release of reactive oxygen species (oxidative burst). C3a and C5a, anaphylatoxins released from activated C3 and C5 during complement activation, can recruit and activate neutrophils, with C5a being about 50–100 times more potent than C3a in activating neutrophils[39]. Consistent with these previous reports, we found that neutrophils were activated after MSC infusion in vivo, and, by using a C3aR antagonist, a C5aR antagonist, and purified C5a, demonstrated that C5a generated from MSC-initiated complement activation stimulated an oxygen burst by neutrophils that acted synergistically with MACs to damage MSCs.
CFH is the most potent complement regulator in serum, and impaired CFH function as a result of either certain gene polymorphisms or the development of anti-CFH autoantibodies leads to many diseases, including age-related macular degeneration and atypical hemolytic uremic syndrome due to excessive complement activation[40]. Despite the facts that MSCs express all known intrinsic cell surface complement inhibitors[14] and secrete factor H themselves[41], and that factor H exists at relatively high concentrations in the blood, immediately after MSC contact with the blood, MSC-activated complement overwhelms all these protective mechanisms to form MACs on MSCs and release C5a to mobilize neutrophils to damage the injected MSCs. These data further demonstrate the importance of local complement inhibition on MSCs in improving the treatment efficacy of MSCs. In addition, because, after MSC administration, complement activation primarily occurs on the MSCs, CFH in the blood cannot efficiently control activated complement on MSCs, making the painting of CFH onto MSCs even more critical for protecting the cells from complement-mediated direct and indirect attack. Although CFH is recognized as a complement alternative pathway inhibitor in the fluid phase, and we previously showed that all three complement activation pathways are involved in damaging MSCs[14], because the alternative pathway serves as an amplification mechanism for all complement activation pathways[42] and CFH also has co-factor activity for factor I to convert C3b into iC3b, which inhibits further complement activation from all three complement activation pathways[43], thus, painted CFH on MSCs should inhibit complement activation initiated from all three activation pathways, leading to robust protection of the MSCs from complement-mediated direct and indirect attack, as demonstrated in our in vitro and in vivo studies. At a concentration of 0.2–0.5 mg/ml in normal human plasma, purified CFH can be prepared on an industrial scale at a reasonable cost. Moreover, contrasting with other complement components in the blood, which are readily deactivated, CFH is stable even after incubation at 95°C, exposure to extreme pH, and sto rage at room temperature for several months[44], making the purification process relatively easy. These unique characteristics make the CFH-painting approach a practical method for improving current MSC-based therapies. We envision that current MSCs under development could be further amended by the simple CFH “painting” method before infusion, and this approach should improve MSC survival and function in patients, thereby could help to successfully demonstrate the potential of MSCs in treating different diseases.
In summary, we found that after administration, MSCs activate complement of the innate immune system. Activated complement leads to MAC assembly on MSCs to directly damage the cells, and it also produces C5a to stimulate neutrophils to further attack the administrated MSCs, resulting in impaired MSC survival and function. CFH, a potent native complement inhibitor can be activated and covalently painted onto MSCs to locally inhibit complement activation on MSCs, leading to significantly improved MSC viability and function after contact with complement and neutrophils both in vitro and in vivo. Our results suggest that painting CFH onto MSCs before the administration could be a simple and effective approach to improve the outcome of current MSC-based therapies.
Supplementary Material
Acknowledgments
We thank Dr. David Wald, Director of the MSC Core Facility at National Center of Regenerative Medicine, Case Western Reserve University for providing MSCs and primary neutrophils, and Dr. Eric Pearlman, Case Western Reserve University for providing the neutrophil-depleting mAb anti-NIMP-R14. This work was supported, in part, by NIH grants DK103581 (FL) and AR061564 (FL).
Footnotes
AUTHOR CONTRIBUTIONS
Y.L., W.Q., and L.Z. performed the experiments, analyzed the data, and helped in manuscript preparation. J. F. contributed to the data analysis, discussion, and editing of the manuscript. F.L. conceived the study, analyzed data, and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–64. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 2.Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013;13:856–67. doi: 10.2174/1566524011313050016. [DOI] [PubMed] [Google Scholar]
- 3.Spaggiari GM, Moretta L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol. 2013;91:27–31. doi: 10.1038/icb.2012.62. [DOI] [PubMed] [Google Scholar]
- 4.Munneke JM, Spruit MJ, Cornelissen AS, van Hoeven V, Voermans C, Hazenberg MD. The Potential of Mesenchymal Stromal Cells as Treatment for Severe Steroid-Refractory Acute Graft-Versus-Host Disease: A Critical Review of the Literature. Transplantation. 2015 doi: 10.1097/TP.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 5.Sadan O, Melamed E, Offen D. Bone-marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin Biol Ther. 2009;9:1487–97. doi: 10.1517/14712590903321439. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med. 2012;1:59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, et al. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180–92. doi: 10.1007/s12016-012-8347-6. [DOI] [PubMed] [Google Scholar]
- 8.Cortinovis M, Casiraghi F, Remuzzi G, Perico N. Mesenchymal stromal cells to control donor-specific memory T cells in solid organ transplantation. Curr Opin Organ Transplant. 2015;20:79–85. doi: 10.1097/MOT.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 9.Allison M. Genzyme backs Osiris, despite Prochymal flop. Nat Biotechnol. 2009;27:966–7. doi: 10.1038/nbt1109-966. [DOI] [PubMed] [Google Scholar]
- 10.Vaes B, Van’t Hof W, Deans R, Pinxteren J. Application of MultiStem((R)) Allogeneic Cells for Immunomodulatory Therapy: Clinical Progress and Pre-Clinical Challenges in Prophylaxis for Graft Versus Host Disease. Front Immunol. 2012;3:345. doi: 10.3389/fimmu.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadjeva M. The complement system. Overview. Methods Mol Biol. 2014;1100:1–9. doi: 10.1007/978-1-62703-724-2_1. [DOI] [PubMed] [Google Scholar]
- 12.Peng Q, Li K, Sacks SH, Zhou W. The role of anaphylatoxins C3a and C5a in regulating innate and adaptive immune responses. Inflamm Allergy Drug Targets. 2009;8:236–46. doi: 10.2174/187152809788681038. [DOI] [PubMed] [Google Scholar]
- 13.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120:3436–43. doi: 10.1182/blood-2012-03-420612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moll G, Rasmusson-Duprez I, von Bahr L, Connolly-Andersen AM, Elgue G, Funke L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem cells. 2012;30:1565–74. doi: 10.1002/stem.1111. [DOI] [PubMed] [Google Scholar]
- 16.Soland MA, Bego M, Colletti E, Zanjani ED, St Jeor S, Porada CD, et al. Mesenchymal stem cells engineered to inhibit complement-mediated damage. PLoS One. 2013;8:e60461. doi: 10.1371/journal.pone.0060461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whaley K, Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976;144:1147–63. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiler JM, Daha MR, Austen KF, Fearon DT. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976;73:3268–72. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson MP, Ayliffe MJ, Helbert M, Davies EG. A simple flow cytometry assay using dihydrorhodamine for the measurement of the neutrophil respiratory burst in whole blood: comparison with the quantitative nitrobluetetrazolium test. J Immunol Methods. 1998;219:187–93. doi: 10.1016/s0022-1759(98)00136-7. [DOI] [PubMed] [Google Scholar]
- 20.Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984;57:489–94. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 21.de Vries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, et al. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883–9. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- 22.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, et al. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–36. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 23.Quigg RJ, Holers VM, Morgan BP, Sneed AE., 3rd Crry and CD59 regulate complement in rat glomerular epithelial cells and are inhibited by the nephritogenic antibody of passive Heymann nephritis. J Immunol. 1995;154:3437–43. [PubMed] [Google Scholar]
- 24.Quigg RJ, Nicholson-Weller A, Cybulsky AV, Badalamenti J, Salant DJ. Decay accelerating factor regulates complement activation on glomerular epithelial cells. J Immunol. 1989;142:877–82. [PubMed] [Google Scholar]
- 25.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978;75:2458–62. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sue AQAK, Fialkow L, Vlahos CJ, Schelm JA, Grinstein S, Butler J, et al. Inhibition of neutrophil oxidative burst and granule secretion by wortmannin: potential role of MAP kinase and renaturable kinases. Journal of cellular physiology. 1997;172:94–108. doi: 10.1002/(SICI)1097-4652(199707)172:1<94::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 27.Schnatbaum K, Locardi E, Scharn D, Richter U, Hawlisch H, Knolle J, et al. Peptidomimetic C5a receptor antagonists with hydrophobic substitutions at the C-terminus: increased receptor specificity and in vivo activity. Bioorg Med Chem Lett. 2006;16:5088–92. doi: 10.1016/j.bmcl.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 28.Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, et al. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001;166:6341–8. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- 29.Tu Z, Li Y, Smith D, Doller C, Sugita S, Chan CC, et al. Myeloid suppressor cells induced by retinal pigment epithelial cells inhibit autoreactive T-cell responses that lead to experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:959–66. doi: 10.1167/iovs.11-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An F, Li Q, Tu Z, Bu H, Chan CC, Caspi RR, et al. Role of DAF in protecting against T-cell autoreactivity that leads to experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2009;50:3778–82. doi: 10.1167/iovs.08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt S, Qin J, Bennett C, Qian S, Fung JJ, Hamilton TA, et al. All-trans retinoic acid induces arginase-1 and inducible nitric oxide synthase-producing dendritic cells with T cell inhibitory function. J Immunol. 2014;192:5098–108. doi: 10.4049/jimmunol.1303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JA, Weidemann MJ. Further characterization of the neutrophil oxidative burst by flow cytometry. J Immunol Methods. 1993;162:261–8. doi: 10.1016/0022-1759(93)90391-j. [DOI] [PubMed] [Google Scholar]
- 33.Martin U, Bock D, Arseniev L, Tornetta MA, Ames RS, Bautsch W, et al. The human C3a receptor is expressed on neutrophils and monocytes, but not on B or T lymphocytes. J Exp Med. 1997;186:199–207. doi: 10.1084/jem.186.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–7. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 35.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 36.Weir C, Morel-Kopp MC, Gill A, Tinworth K, Ladd L, Hunyor SN, et al. Mesenchymal stem cells: isolation, characterisation and in vivo fluorescent dye tracking. Heart Lung Circ. 2008;17:395–403. doi: 10.1016/j.hlc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 37.von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem cells. 2012;30:1575–8. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 38.Trivedi P, Tray N, Nguyen T, Nigam N, Gallicano GI. Mesenchymal stem cell therapy for treatment of cardiovascular disease: helping people sooner or later. Stem Cells Dev. 2010;19:1109–20. doi: 10.1089/scd.2009.0465. [DOI] [PubMed] [Google Scholar]
- 39.Ehrengruber MU, Geiser T, Deranleau DA. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS letters. 1994;346:181–4. doi: 10.1016/0014-5793(94)00463-3. [DOI] [PubMed] [Google Scholar]
- 40.MKL, Atkinson JP. Complement regulators in human disease: lessons from modern genetics. J Intern Med. 2015;277:294–305. doi: 10.1111/joim.12338. [DOI] [PubMed] [Google Scholar]
- 41.Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem cells and development. 2010;19:1803–9. doi: 10.1089/scd.2009.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–10. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 43.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977;146:257–70. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kask L, Villoutreix BO, Steen M, Ramesh B, Dahlback B, Blom AM. Structural stability and heat-induced conformational change of two complement inhibitors: C4b-binding protein and factor H. Protein Sci. 2004;13:1356–64. doi: 10.1110/ps.03516504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.