Abstract
Major depressive disorder, especially in later life, has heterogeneous clinical characteristics and treatment responses. Symptomatically, psychomotor retardation, lack of energy, and apathy tends to be more common in people with late-onset depression (LOD). Despite recent advances in psychopharmacologic treatments, 20% to 30% of patients with mood disorders experience inadequate responses to medication, often resulting in a trial of electroconvulsive therapy (ECT). However, the therapeutic mechanism of ECT is still unclear. By using 18F-fluorodeoxy-D-glucose positron emission tomography-computed tomography (18F-FDG PET/CT), we can obtain the status of brain metabolism in patients with neuropsychiatric disorders and changes during psychiatric treatment course. The object of this case report is evaluating the effect of ECT on brain metabolism in treatment-refractory LOD by PET/CT and understanding the mode of action of ECT. In this case report, we presented a 55-year-old female patient who suffered psychotic depression that was resistant to pharmacological treatment. Several antidepressants and atypical anti-psychotics were applied but there was no improvement in her symptoms. The patient presented not only depressed mood and behaviors but also deficit in cognitive functions. We found decreased diffuse cerebral metabolism in her brain 18F-FDG PET/CT image. ECT resulted in amelioration of the patients’ symptoms and another brain PET imaging 7 weeks after the last ECT course showed that her brain metabolism was normalized.
Keywords: Electroconvulsive therapy, Depression, Fluorodeoxyglucose F18, Positron emission tomography
INTRODUCTION
Major depressive disorder, especially in later life, has heterogeneous clinical characteristics and treatment responses. Classification of depression according to age of onset may be a useful approach to understanding its intricate and complex properties. Several studies of late-life depression have focused on age of onset between 40 and 60 years.1,2) However, although many studies have been conducted regarding differences between early-onset depression (EOD) and late-onset depression (LOD), only a few consistent differences between these disorders have been identified. Research has shown that EOD is associated with more chronic features3) and a higher tendency toward suicidal behavior.4) In patients with EOD, there are more commonly feeling of worthlessness, suicidal ideation and anxiety, whereas psychomotor retardation, lack of energy, and apathy tend to be more common in patients with LOD.1,5) The person with LOD have more problems in cognitive function, neurological abnormalities and increased medical comorbidity.
Despite recent advances in psychopharmacologic treatments, 20% to 30% of patients with mood disorders experience inadequate responses to medication, often resulting in a trial of electroconvulsive therapy (ECT).6) A large body of evidence suggests ECT as an effective treatment especially for severe and treatment-refractory depression,7) and studies indicate that up to 90% of people with severe treatment-refractory depression improve dramatically with ECT.8)
With 18F-fluorodeoxy-D-glucose positron emission tomography-computed tomography (18F-FDG PET/CT) we can obtain the status of brain metabolism of patients with neuropsychiatric disorders and the metabolic changes due to ECT. Several studies have described the relationship between depressive disorder and brain metabolism. In this report, we described a case of 55-year-old female patient who suffered treatment refractory depression and cognitive deficit. She presented recovered brain metabolic function by undergoing ECT. The changes of brain metabolism before and after ECT were presented by 18F-FDG PET/CT.
CASE
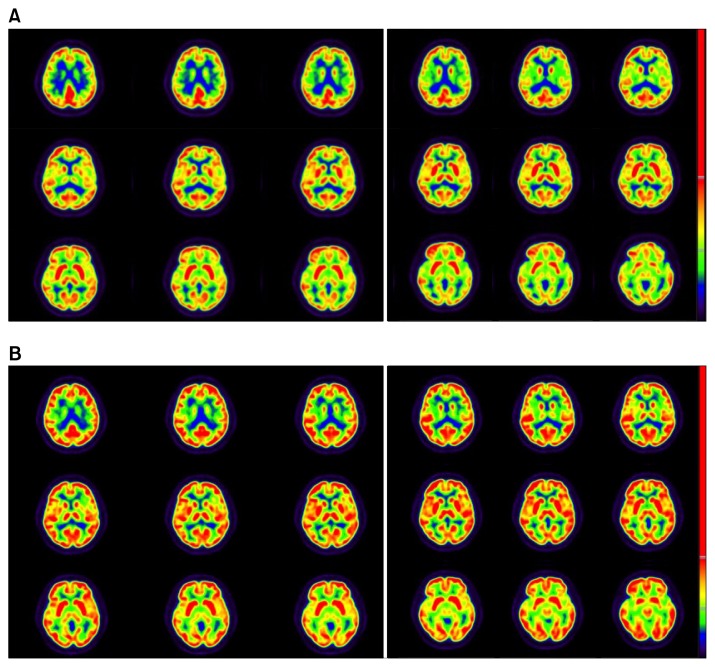
A 55-year-old female patient who had suicidal attempt with organophosphate was treated in the department of nephrology for a while and transferred to the department of psychiatry. She had experienced no earlier episode of mood change, but two months prior to admission she started feeling depressed mood and feelings of guilt towards her family members. She had paranoid delusion that her husband had another partner and wanted to murder her for her insurance. The patient had feelings of hopelessness, helplessness, worthlessness, and poor self-esteem. Her depressive symptoms were worsening and she presented with impaired concentration and decreased memory. A complete biochemical workup was negative, including CBC, thyroid hormones, HIV, and syphilis blood tests. She underwent full psychometric evaluation including Wechsler test, cognition and memory function test, REY-KIM memory test, Minnesota multiphasic personality inventory-II (MMPI-II), sentence completion test, symptom check list-90-revised (SCL-90-R), draw-a-person test, mini-mental state examination (MMSE), clinical dementia rating (CDR), Rorschach test, positive and negative syndrome scale (PANSS), and Hamilton rating scale for depression (HAM-D). Her HAM-D score was 43, PANSS score was 113 and MMSE was 20. The differential diagnosis comprised initially psychotic mood disorder, neurodegenerative disease and iatrogenic delirium secondary to medication. The patient presented as medication-resistant during the course of duloxetine, mirtazapine, trazodone, aripiprazole, and quetiapine. Medications for 4 weeks showed no significant improvement of symptoms. To rule out organic brain damages, the patient underwent brain magnetic resonance imaging but there were no significant findings. For evaluating her cerebral function, the patient underwent a 18F-FDG cerebral PET (Fig. 1A). 18F-FDG PET/CT scans were obtained with a BiographmCT 128 scanner (Siemens Healthcare, Knoxville, TN, USA). The patient fasted for 6 hours before the scans. She was intravenously injected with 185 MBq of 18F-FDG approximately 60 minutes before the imaging. The blood glucose level was <150.0 mg/dl before 18F-FDG injection. The patient was stable for 30 minutes prior to 18F-FDG injection and in the subsequent uptake phase. Each PET/CT scan was acquired from the vertex of skull to the skull base during 10 minutes. After CT scanning, a PET scan was performed in the three-dimensional mode. PET images were reconstructed with an iterative reconstruction algorithm with attenuation correction. There was diffuse hypometabolism in the whole brain.
Fig. 1.
(A) 18F-fluorodeoxy-D-glucose (18F-FDG) PET images of the patient before taking ECT. (B) 18F-FDG PET images secondarily taken after the electroconvulsive therapy (ECT) 7 weeks later to compare the images taken before the ECT procedure.
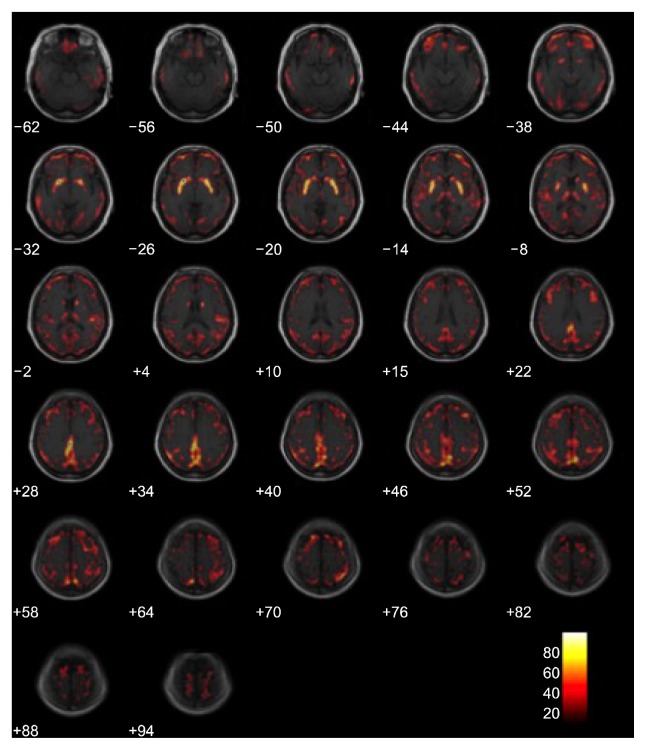
A depressive episode was still suspected clinically and, because medication had little effect, it was progressively reduced. Modified bilateral ECT was applied and the patient underwent 3 weeks of an index course ECT (9 times). Thereafter, there was impressive improvement in the patient’s global functioning with complete resolution of symptoms. Her HAM-D score was 4, PANSS score was 34, and MMSE was 29. The final diagnosis was major depressive disorder of high severity with mood congruent psychotic features. After the course of ECT, the patient was discharged on minimal dosage of mirtazapine, aripiprazole, and quetiapine. After discharge, she showed no signs of residual psychosis or mood disorder and greater emotional expressiveness and had no psychomotor retardation or cognitive deficit. Seven weeks after the last ECT, the patient underwent 18F-FDG cerebral PET (Fig. 1B) for evaluation of her brain metabolism. Image preprocessing was performed using SPM8 (University College of London, UK). Acquired pre/post PET images were co-registered and the relative standard uptake value changes were estimated by image subtraction algorithm. The image was compared with the 18F-FDG cerebral PET that was obtained before ECT procedure and showed recovery of cerebral metabolism without any evidence of a neurodegenerative process (Fig. 2).
Fig. 2.
Comparing positron emission therapy images between before and after electroconvulsive therapy procedure by color. The red area representing increased metabolic activity.
DISCUSSION
This case report presented the change of brain metabolism before and after ECT procedure in a patient with treatment-refractory LOD by PET/CT imaging. There was increased metabolic activity in cerebral cortex and basal ganglia during the course. In other studies, the patients with LOD showed decreased cerebral glucose metabolism than the healthy group in multiple areas of brain, which might be the cause of cognitive impairment that commonly accompanies LOD.9)
The use of PET as a probe of the cerebral metabolic rate of glucose (CMR) in depressive patients appears especially promising, because depression is reportedly associated with changes in CMR in different cerebral regions and can be used to display the “resting state” metabolism of the brain.10) Repeated PET provides an opportunity to explore changes in CMR associated with the use of ECT in vivo. Glucose represents the main source of energy for brain cells. Thus, persistent changes in glucose metabolism patterns are expected to provide a reliable estimate of neuroanatomic metabolism.11)
Drevets et al.12) reported reduced cerebral blood flow (CBF) and metabolism in the anterior cingulate cortex (ACC) ventral to the genu of the corpus callosum in patients with depression. And Bell et al.13) reported decreased CBF in dorsomedial/dorsoanterolateral prefrontal cortex in patients with depression. Reduced glucose metabolism in bilateral anterior and posterior frontal areas represented the most consistent finding of previous studies, despite considerable methodological heterogeneities. The study with 6 depressive patients treated ECT found that significant increases of CMR which were presented by PET in basal ganglia, brainstem and occipital lobe.14) Some researchers found that significant decreased metabolism in left subgenual ACC and hippocampal area in patients with depression; and ECT led to increased metabolism in these areas.15) However, there are different results that the ECT-induced seizures change from the site of initiation to other specific brain regions and decrease CBF in cingulate and left dorsolateral frontal cortex 30 minutes after induction of seizures.16) And Nobler et al.17) have reported reduced regional cerebral metabolic rate for glucose in 10 patients with depression in post-ECT state. Suwa et al.18) presented the synthetic view of these difference in metabolic change after ECT. In those studies, they have shown that ECT changed CBF and cerebral glucose metabolism which were decreased in depression patients. In our case, increases in glucose metabolism in the frontal, parietal, and occipital regions may have represented the antidepressant effect of ECT.
In this case, we described a patient who did not have any other history of mental disorder before and presented severe cognitive deficit and treatment-resistance. The patient presented a severe and diffuse cerebral glucose hypo-metabolism on 18F-FDG. The ECT on this patient showed excellent clinical and radiological result. Awata et al.19) have reported similar recovered regional cerebral blood flow patterns were shown at 2 weeks and 12 weeks after ECT in depressive patients, because of that the post ECT image was taken 7 weeks later after the last ECT to minimize the effect of psychotropic agent and to evaluate the long-term treatment result. This case illustrates the potential of 18F-FDG brain PET to demonstrate the normalization of brain metabolism in major depressive episode in patients benefiting from ECT. It also illustrates the efficacy of ECT in the treatment of major depression and suggests that its clinical mechanism is related with changes in metabolic function. Alterations in brain metabolism from depression have been described in multiple regions. The multifactorial effect of numerous psychoactive drugs might have increased the severity of the deficits.20) We presented the cerebral metabolic changes in the patient with severe LOD and cognitive disturbance. There were several studies reported cerebral metabolic changes during the ECT in depressive patients, but the little of those researches concern with both LOD and cognition. This case report is not only the evidence of classical view of ECT in treatment resistant depression but also the treatment choice of LOD with cognitive deficit.
This case study has some limitations. First, we described one female patient and were unable to compare with other patient(s) with the same conditions. Second, our patient was unipolar depression and it is not certain that the ECT-induced brain metabolic change is replicable in bipolar depressions. Third, the patient had LOD, therefore findings might differ in EOD.
Despite these limitations, FDG-PET represents a promising marker of neuronal cell functions and reflects an epiphenomenon of a complex and dynamic interaction of different neurobiochemical processes. Regardless of these methodological difficulties, research into this area highlights the potential of functional neuroimaging. Changes in metabolic patterns have been correlated with changes in behavioral variables reflecting the severity of the underlying disease; in addition, a link between changes in frontal activity and improvements in clinical symptoms was identified.17)
Further study should include more patients who have depressive episode, and focus on objectifying clinical improvement of depression after ECT to allow a correlation with possible changes in CMR. Follow up PET scans after six or twelve months should be performed to assess possible long-term effects.21) Clearly, a separation of patients with unipolar depression from patients with bipolar depression should be considered and larger samples of patients are needed to increase statistical power and account for heterogeneity in study cohorts.
Acknowledgments
This work was supported by Soonchunhyang University. The authors have no conflict of interest to declare.
REFERENCES
- 1.Gallagher D, Mhaolain AN, Greene E, Walsh C, Denihan A, Bruce I, et al. Late life depression: a comparison of risk factors and symptoms according to age of onset in community dwelling older adults. Int J Geriatr Psychiatry. 2010;25:981–987. doi: 10.1002/gps.2438. [DOI] [PubMed] [Google Scholar]
- 2.Janssen J, Beekman AT, Comijs HC, Deeg DJ, Heeren TJ. Late-life depression: the differences between early- and late-onset illness in a community-based sample. Int J Geriatr Psychiatry. 2006;21:86–93. doi: 10.1002/gps.1428. [DOI] [PubMed] [Google Scholar]
- 3.Korten NC, Comijs HC, Lamers F, Penninx BW. Early and late onset depression in young and middle aged adults: differential symptomatology, characteristics and risk factors? J Affect Disord. 2012;138:259–267. doi: 10.1016/j.jad.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Grayson L, Thomas A. A systematic review comparing clinical features in early age at onset and late age at onset late-life depression. J Affect Disord. 2013;150:161–170. doi: 10.1016/j.jad.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Corruble E, Gorwood P, Falissard B. Association between age of onset and symptom profiles of late-life depression. Acta Psychiatr Scand. 2008;118:389–394. doi: 10.1111/j.1600-0447.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 6.Thase ME, Rush AJ. Treatment-resistant depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Rave Press; 1995. [Google Scholar]
- 7.Janicak PG, Davis JM, Gibbons RD, Ericksen S, Chang S, Gallagher P. Efficacy of ECT: a meta-analysis. Am J Psychiatry. 1985;142:297–302. doi: 10.1176/ajp.142.3.297. [DOI] [PubMed] [Google Scholar]
- 8.Getz GE, Edner BJ, Nickell PV. The effect of electro-convulsive therapy on executive functioning in a treatment-resistant man with depression: a case report. J ECT. 2014;30:e11–e12. doi: 10.1097/YCT.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 9.Marano CM, Workman CI, Lyman CH, Kramer E, Hermann CR, Ma Y, et al. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res. 2014;222:84–90. doi: 10.1016/j.pscychresns.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oda K, Okubo Y, Ishida R, Murata Y, Ohta K, Matsuda T, et al. Regional cerebral blood flow in depressed patients with white matter magnetic resonance hyperintensity. Biol Psychiatry. 2003;53:150–156. doi: 10.1016/S0006-3223(02)01548-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt EZ, Reininghaus B, Enzinger C, Ebner C, Hofmann P, Kapfhammer HP. Changes in brain metabolism after ECT-positron emission tomography in the assessment of changes in glucose metabolism subsequent to electroconvulsive therapy--lessons, limitations and future applications. J Affect Disord. 2008;106:203–208. doi: 10.1016/j.jad.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 13.Bell KA, Kupfer DJ, Drevets WC. Decreased glucose metabolism in the dorsomedial prefrontal cortex in depression. Biol Psychiatry. 1999;45(8 Suppl 1):118S. [Google Scholar]
- 14.Henry ME, Schmidt ME, Matochik JA, Stoddard EP, Potter WZ. The effects of ECT on brain glucose: a pilot FDG PET study. J ECT. 2001;17:33–40. doi: 10.1097/00124509-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 15.McCormick LM, Boles Ponto LL, Pierson RK, Johnson HJ, Magnotta V, Brumm MC. Metabolic correlates of antidepressant and antipsychotic response in patients with psychotic depression undergoing electroconvulsive therapy. J ECT. 2007;23:265–273. doi: 10.1097/yct.0b013e318150d56d. [DOI] [PubMed] [Google Scholar]
- 16.Enev M, McNally KA, Varghese G, Zubal IG, Ostroff RB, Blumenfeld H. Imaging onset and propagation of ECT-induced seizures. Epilepsia. 2007;48:238–244. doi: 10.1111/j.1528-1167.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 17.Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, et al. Decreased regional brain metabolism after ECT. Am J Psychiatry. 2001;158:305–308. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- 18.Suwa T, Namiki C, Takaya S, Oshita A, Ishizu K, Fukuyama H, et al. Corticolimbic balance shift of regional glucose metabolism in depressed patients treated with ECT. J Affect Disord. 2012;136:1039–1046. doi: 10.1016/j.jad.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Awata S, Konno M, Kawashima R, Suzuki K, Sato T, Matsuoka H, et al. Changes in regional cerebral blood flow abnormalities in late-life depression following response to electroconvulsive therapy. Psychiatry Clin Neurosci. 2002;56:31–40. doi: 10.1046/j.1440-1819.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 20.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/S0924-977X(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 21.Guze BH, Baxter LR, Jr, Schwartz JM, Szuba MP, Liston EH. Electroconvulsive therapy and brain glucose metabolism. Convuls Ther. 1991;7:15–19. [PubMed] [Google Scholar]