Abstract
Objective
Antiepileptic drugs (AED) have chronic teratogenic effects, the most common of which are congenital heart disease, cleft lip/palate, urogenital and neural tube defects. The aim of our study is to examine teratogenic effects of AED and the correlation between these malformations and AED in single or multiple pregnancies.
Methods
This is a retrospective study of malformations in children born to mothers currently followed up by our outpatient clinics who used or discontinued AED during their pregnancy. Their children were then investigated using echocardiography, urinary ultrasound, cranial magnetic resonance image, and examined by geneticists and pediatric dentists.
Results
One hundred and seventeen children were included in the study. Ninety one of these children were exposed to AED during pregnancy. The most commonly used AED were valproic acid and carbamazepine in monotherapy. The percentage of major anomaly was 6.8% in all children. Dysmorphic features and dental anomalies were observed more in children exposed especially to valproic acid. There were 26 mothers with two and four mothers with three pregnancies from the same fathers. No correlation was found between the distribution of malformations in recurring pregnancies and AED usage.
Conclusion
Our study has the highest number of dysmorphism examined in literature, found in all the children exposed to valproic acid, which may account for the higher rate of facial dysmorphism and dental anomalies. On lower doses of valproic acid, major malformations are not seen, although the risk increases with polytherapy. Our data also indicate possible effects of genetic and environmental factors on malformations.
Keywords: Dysmorphic features, Dental anomalies, Congenital malformations, Antiepileptic drugs, Teratogenesis
INTRODUCTION
Epilepsy ranks first among neurological disorders requiring continuous treatment during pregnancy, since antiepileptic drugs (AEDs) have frequent chronic teratogenic effects, the most common of which are congenital heart disease, cleft lip/palate, urogenital defects, and neural tube defects.1–4) A two to three fold increase in major malformations was shown in children exposed to AEDs during pregnancy.5–8) Minor malformations and cognitive delay have been examined in terms of their relation to long term adverse effects of AEDs.9–11) Certain patterns of minor malformations were related to causes including certain AEDs, developmental problems and epilepsy.12–16) Likewise, various factors have been indicated for early developmental delay in children born to women with epilepsy.17,18) Not only AEDs but also genetic dispositions have been suggested to increase the probability of malformation in children born to women with epilepsy following the birth of another child with malformation.19,20) In this long-term retrospective study, we report the developmental, neuroradiological, cardiological, urological, dental and dysmorphic features of children exposed to AEDs during pregnancy in comparison to children unexposed to AEDs. Our aim is to make an analysis of the data on children born from women who either used or discontinued AEDs during pregnancy to determine major or minor malformations, drug specific effects and the association among the results of various investigation methods including cranial magnetic resonance image (MRI), ultrasound and echocardiography. This is a more comprehensive study than found in literature in that a medical geneticist and a pediatric dentist were also included in the research group. We also examine teratogenic consequences of AEDs on different births of the same parents to verify the effects of AEDs.
METHODS
This is a retrospective study initiated in 2006 involving children born from mothers with epilepsy, who got pregnant between 1990–2006, while they were either followed up by the epilepsy outpatient clinics of Department of Neurology, Istanbul Faculty of Medicine, Istanbul University; or Department of Neurology, Bakirkoy Research and Training Hospital for Psychiatry, Neurology, and Neurosurgery; or applied to our clinic after birth. Data were collected until 2007 and mothers willing to contribute to the study were invited to the clinic with their children, and data from mothers who declined to participate were not included. Mothers were divided into two groups depending on their use or non-use of AEDs (AED group and non-AED group). If the mother stopped taking their AEDs after learning she was pregnant, she was included in the AED group considering that the fetus had been exposed to AED until the time of disuse. The demographic and clinical data of patients (age of onset, type of epilepsy, dosages of AEDs used during pregnancy), pregnancy details (age of pregnancy, frequency of seizures and alcohol use or smoking duration of pregnancy), information about the baby (age and sex, febrile convulsions or epilepsy diagnosis), and folic acid usage were recorded retrospectively from patient files. Epilepsy syndromes were grouped according to the International League Against Epilepsy (ILAE) 2010 classification. Routine biochemistry, complete blood count, thyroid function tests were done for each child. Urinary ultrasound was performed by a pediatric radiologist; echocardiography was checked by a pediatric cardiologist. Cranial MRIs with thin slices were taken from children with sufficient cooperation at 1.5 tesla, T1, T2, flair, coronal, sagittal, and axial. Parents and children were examined and photographed by a medical geneticist according to a list of 47 items comprised of dysmorphism and dental anomalies assembled from the results of previous studies, and their dysmorphic features of the face and the extremities (if any) were determined.
Children older than six months were examined by a pediatric dentist for developmental dental anomalies such as delayed eruption of permanent teeth and malocclusion which may have been caused by the AEDs. All the examiners were blinded to AED and non-AED groups. Unusual morphological traits that are of no serious medical or cosmetic consequence to the patients were defined as ‘minor anomaly’.21) Any anatomical deformity that threatens life or necessitates medical or surgical intervention is termed as ‘major anomaly’.22)
This study is conducted under the permission of the Ethics committee of Istanbul Faculty of Medicine, Istanbul University. Before every examination, parents were asked to sign the approval form after reading and being explained verbally about every detail of the procedure.
The study was conducted according to the Declaration of Helsinki criteria and no pharmaceutical support was obtained.
Continuous variables are expressed as mean±standard deviation; categorical variables are presented as frequency and percentage. Differences between groups were assessed using unpaired t-test for normally distributed data. The chi-square and Fisher exact tests were used to compare the differences of categorical variables between the groups. SPSS statistical software ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A p value less than 0.05 was considered statistically significant.
RESULTS
In this study, a total of 117 children, born of 88 mothers with epilepsy, were included. Of 117, 91 were born to 71 of the mothers on AEDs and 26 born to 17 of the mothers were not exposed to AED during pregnancy. Four of the mothers had three, and 22 of the mothers had two children, which add up to 56 siblings. Data for 21 of the children were received from Bakirkoy Research and Training Hospital for Psychiatry, Neurology, and Neurosurgery.
According to the history of mothers, one mother had multiple sclerosis and another one antiphospholipid syndrome in addition to epilepsy. Age for epilepsy onset was close to each other (14.7±5.8 years in the AED group and 16.1±4.1 in the non-AED group). Average age of pregnancy was close in both groups, 29.9±4.5 years in the AED group and 31.3±6.1 years in the non-AED group, with no statistically significant difference between the groups (p>0.05). The frequency of seizure remained the same during pregnancy and there was no instance of status epilepticus in either group. There was no significant difference in seizure frequency in either group (p>0.05) (Table 1).
Table 1.
The features of mothers and children
| Variable | On AED | Non-AED | p value |
|---|---|---|---|
| Mother (n=88) | |||
| Epilepsy onset age (year) | 14.7±5.8 | 16.1±4.1 | 0.34 |
| Epilepsy type | |||
| Idiopathic epilepsy | 31 | 12 | |
| Unknown epilepsy | 30 | 5 | 0.08* |
| Symptomatic epilepsy | 10 | 0 | |
| Mean gestational age (year) | 29.9±4.5 | 31.3±6.1 | 0.69 |
| Seizure frequency during pregnancy | |||
| No change | 64 | 18 | |
| Increased | 13 | 6 | 0.39* |
| Decreased | 14 | 2 | |
| Children (n=117) | |||
| Age (yr) | 4.4±3.5 | 6.5±3.5 | 0.07 |
| Gender (female/male) | 46/45 | 16/10 | 0.32 |
Values are presented as mean±standard deviation, or number only.
AED, antiepileptic drug.
Fisher exact test.
In the AED group, 76 (83.5%) of the pregnancies were completed under monotherapy, and the most frequently used drugs were valproic acid (VPA; 32) and carbamazepine (CBZ; 24) (Table 2).
Table 2.
Antiepileptic drugs used by mothers and major anomaly (n=117)
| Variable | Data | Major anomaly* |
|---|---|---|
| Monotherapy | 76 (83.5) | 4 (5.3) |
| VPA | 32 | |
| CBZ | 24 | |
| PB | 8 | |
| OXC | 8 | |
| PHT | 2 | |
| CLZ | 2 | |
| Polytherapy | 15 (16.5) | 3 (20.0) |
| VPA+CBZ | 2 | |
| VPA+PB | 2 | |
| VPA+LTG | 1 | |
| VPA+LTG+LEV | 1 | |
| VPA+CBZ+PHT | 1 | |
| PB+PHT | 1 | |
| PB+LTG | 1 | |
| PB+PHT+CBZ | 2 | |
| PB+PHT+CBZ+VGB | 1 | |
| PHT+PRM | 1 | |
| PHT+OXC | 1 | |
| PHT+VGB+CBZ | 1 | |
| Non-AED group | 26 | 1 (3.8) |
Values are presented as number (%) or number only.
AED, antiepileptic drug; VPA, valproic acid; CBZ, carbamazepine; PB, phenobarbital; OXC, oxcarbazepine; PHT, phenytoin; CLZ, clonazepam; LTG, lamotrigine; LEV, levetirecetam; PRM, primidone; VGB, vigabatrin.
p>0.05 in AED (monotherapy and polytherapy) and non-AED groups.
The blood tests were normal for each child. In the urinary ultrasound, echocardiography and cranial MRI examinations, eight major malformations (6.8%) and 23 minor malformations (19.7%) were detected among all the children. The percentage of major anomaly in the non-AED group was 3.8% and 7.7% in the AED group which indicates no significant difference from the AED group (p>0.05) (odds ratio [OR], 0.48; 95% confidence interval [CI], 0.56–4.08). The percentage of major anomaly was 5.3% in monoteraphy group and 20% in polytherapy which was not statistically significant (p>0.05) (Table 3).
Table 3.
Congenital malformations
| Malformations | Drug (mg/day) | Folic acid* |
|---|---|---|
| Major malformations (n=8) | ||
| Atrial septal defect+atrial septal aneurysm | VPA 300 | + |
| Atrial septal defect | CBZ 200 | + |
| Atrial septal defect | VPA 500+PB 150 | + |
| Patent foramen ovale+atrial septal defect+left persistent superior vena cava | PB 300+PHT 100+CBZ 300 | − |
| Hydronephrosis | PHT 150+OXC 1,200 | − |
| Syndactyly | VPA 500 | + |
| Congenital hip dislocation | VPA 1,000 | + |
| Ventricular septal defect | Non-AED | + |
| Minor malformations (n=23) | ||
| Left intraventricular band/t | VPA 2,000 | + |
| Patent foramen ovale+strabismus | PB 200 | + |
| Patent foramen ovale | CBZ 100 | − |
| Patent foramen ovale | CBZ 200 | − |
| Patent foramen ovale | VPA 500 | + |
| Patent foramen ovale | OXC 900 | − |
| Patent foramen ovale | Non-AED | + |
| Left intraventricular band/t | Non-AED | − |
| Bilateral medullar nephrocalcinosis | VPA 500 | + |
| Bilateral medullar nephrocalcinosis | CLZ 0.5 | − |
| Bifid renal pelvis+ptotic kidney | PB 100+PHT 100 | − |
| Ptotic kidney | PB 300+PRM 50 | − |
| Renal agenesis | VPA 1,000+LTG 100 | − |
| Dilatation in pericaliceal system | VPA 500 | + |
| Renal agenesis | Non-AED | − |
| Hyperintense noduler lesion | CLZ 50 | − |
| Choroid fissure cyst | PB 100 | − |
| Arachnoid cysts | VPA 500 | − |
| Ventricular asymmetry+deep white matter lesion | PB 100 | − |
| Periventricular leukomalacia+arachnoid cyst+cerebellar atrophy | VPA 200 | − |
| Inguinal hernia | Non-AED | + |
| Strabismus (n=2) | VPA 2000 | + |
AED, antiepileptic drug; VPA, valproic acid; CBZ, carbamazepine; PB, phenobarbital; PHT, phenytoin; OXC, oxcarbazepine; LTG, lamotrigine; CLZ, clonazepam; PRM, primidone.
p>0.05 major malformations and folic acid using.
Dysmorphism was detected in 79.7% of the children by the medical geneticist according to the list comprised for this study. The mean number of dysmorphic features was 3.2±7.7 in the AED group, and 0.9±2.5 in the non-AED group. Hence, the statistical difference with regards to dysmorphism frequency between the groups was significant (p<0.001). Dysmorphic features had no statistically significant correlation with either mono/polytherapy or type of epilepsy (p>0.05). The most common dysmorphic features were observed in children whose mothers used VPA, regardless of the daily dosage, with statistically significant difference (p<0.05) (Table 4, Fig. 1). Hence, all the children of mothers who were on VPA during pregnancy had five dysmorphic features on average. Our study has the highest number of dysmorphism examined in literature which may be attributed to the higher rate of dysmorphism in our patients.
Table 4.
Dysmorphic features and developmental dental anomalies
| Dysmorphic features* | Monotherapy (n=76) | Polytherapy (n=15) | Non-AED (n=26) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| VPA | CBZ | PB | PHT | OXC | CLZ | |||
| Eyea | 48 | 25 | 9 | 1 | 8 | 2 | 14 | 8 |
| Noseb | 35 | 8 | 4 | 0 | 1 | 0 | 14 | 5 |
| Earc | 3 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Mouthd | 23 | 20 | 7 | 1 | 2 | 2 | 13 | 10 |
| Jointe | 8 | 3 | 4 | 0 | 1 | 0 | 6 | 0 |
| Othersf | 14 | 8 | 2 | 0 | 2 | 0 | 6 | 1 |
| Dental anomaliesg | 11 | 9 | 6 | 1 | 2 | 2 | 9 | 4 |
| Total mean number of dysmorphic features† | 3.1±8.1 | 3.6±5.6 | 0.9±2.5 | |||||
| Total mean number of dysmorphic features for AED group | 3.2±7.7 | |||||||
| Ratio of of dental anomalies (%)‡ | 53.8 | 23.5 | ||||||
Values are presented as number only, mean±standard deviation, or percent only.
AED, antiepileptic drug; VPA, valproic acid; CBZ, carbamazepine; PB, phenobarbital; PHT, phenytoin; OXC, oxcarbazepine; CLZ, clonazepam.
Medial deficiency of eyebrow, epicanthus, infraorbital grooves, hypertelorism, upward slanting palpebral fissures, downward slanting palpebral fissures, prominent eyelashes, telecanthus, blue sclera, supraorbital fullness;
Broad nasal root, short nose, anteverted nares, broad nasal tip, hypoplastic nasal alae, tubular nose;
Retroverted ears, low set ear;
Smooth philtrum, thin upper lip, thick lower lip, down turned corners of the mouth, large mouth, small mouth, high arched palate, prominent columella;
Hyperextensible joints, cubitus valgus, fetal finger pad, tapering fingers;
Broad forehead, high forehead, facial hirsuitism, nail hypoplasia, frontal bossing, micrognathia, mongolian spot, pectus excavatum;
Hypoplasia, delayed eruption, malocclusion, disturbances of shape, supernumerary teeth, hypodontia.
Every patient has more than one diysmorphic feature.
p<0.001 in AED and non-AED groups, but p>0.05 in monoteraphy and polyteraphy;
p<0.05 in AED and non-AED groups.
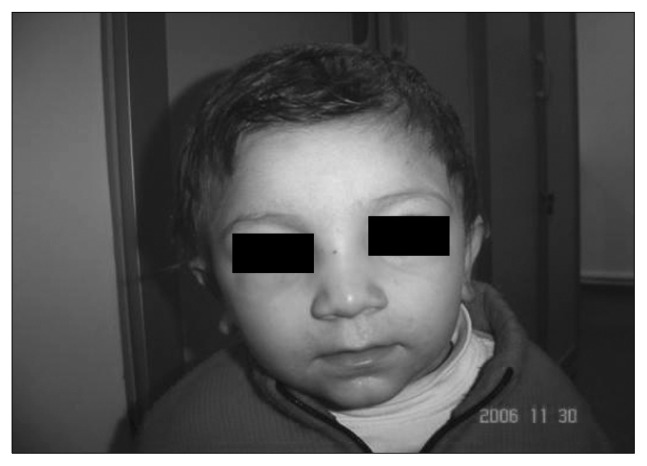
Fig. 1.
Epicanthic folds, hypertelorism, broad nasal root, short nose, thin upper lip (valproic acid, 500 mg/day).
Dental anomalies were observed in 48.4% of all the children. The most frequent dental anomalies were hypoplasia, delayed eruption and malocclusion. Moreover, 54% of the AED and 23.5% of the non-AED children had dental anomalies, which was again statistically noteworthy (p<0.05) (Table 4, Fig. 2). Our study is also among the few studies with a high number of dental features examined in children.
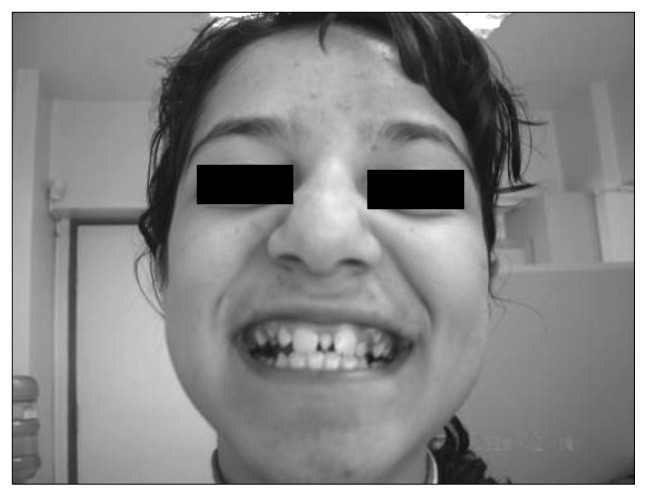
Fig. 2.
Supernumerary teeth (valproic acid, 600 mg/day).
In the study, 56 children were siblings from 30 mothers. The number of mothers with a second pregnancy was 26, and third pregnancy was four, all from the same fathers. The distribution of malformations with respect to the recurrence of pregnancies was found to be independent of AED use (Table 5). For instance, both siblings had renal agenesis although their mother was on AED (1,000 mg/day VPA and 100 mg/day lamotrigine [LTG]) during her first pregnancy but discontinued AEDs during her second. In another example of three siblings, their mother never used AED during pregnancies, the first and last children were healthy, whereas the second child had a major malformation. In the case of two other siblings, the first child was healthy despite being exposed to phenobarbital (25 mg/day) during pregnancy; the second child had a minor malformation, although not exposed to an AED during pregnancy.
Table 5.
The conditions of children from the same parents with two or more pregnancies
| Patient | 1. Pregnancy AED (mg/day) | Malformation | 2. Pregnancy AED (mg/day) | Malformation | 3. Pregnancy AED (mg/day) | Malformation |
|---|---|---|---|---|---|---|
| 1 | CBZ 100 | - | CBZ 100 | PFO | ||
| 2 | CBZ 800 | Teeth | CBZ 800 | Teeth | ||
| 3 | CBZ 200+VPA 500 | - | CBZ 200 | PFO | ||
| 4 | CBZ 300 | - | CBZ 600 | - | ||
| 5 | VPA 500 | Dilatation in pericalyxial system | VPA 500 | - | ||
| 6 | VPA 2,000 | Strabismus | VPA 2,000 | Strabismus | ||
| 7 | VPA 1,000 | - | VPA 1,000 | - | ||
| 8 | VPA 600 | - | VPA 200 | - | ||
| 9 | VPA 1,500 | Teeth | VPA 1,000 | - | ||
| 10 | VPA 500 | Teeth | VPA 500 | - | VPA 500 | - |
| 11 | PB 200 | Teeth | VPA 500 | - | ||
| 12 | PB 100 | Teeth | PB 300 | Teeth | ||
| 13 | PHT 300 | Teeth | PHT 150+PB 150 | Hydronephrosis | ||
| 14 | OXC 1,000 | PFO | OXC 1,000 | - | ||
| 15 | CLZ 0.5 | Teeth | CLZ 0.5 | Teeth | ||
| 16 | PB 25 | - | Non-AED | PFO | ||
| 17 | CBZ 300+PB 300+PHT 300 | Teeth | CBZ 300+PB 300 +PHT 100 | Teeth+PFO+ASD+ left persistant superior vena cava* | CBZ 200+PB 300 +PHT 100 | Teeth |
| 18 | Non-AED | Renal agenesis | VPA 1,000 | Renal agenesis | ||
| 19 | Non-AED | - | Non-AED | VSD | Non-AED | - |
| 20 | Non-AED | - | Non-AED | Left intraventricular band/t | ||
| 21 | Non-AED | - | VPA 500 | Syndactyly | ||
| 22–25 | Non-AED | - | Non-AED | - | ||
| 26 | Non-AED | - | Non-AED | - | Non-AED | - |
AED, antiepileptic drug; CBZ, carbamazepine; VPA, valproic acid; PB, phenobarbital; PHT, phenytoin; OXC, oxcarbazepine; CLZ, clonazepam; PFO, patent foramen ovale; ASD, atrial septal defect; VSD, ventricular septal defect.
The number of mothers on VPA, with 1,000 mg/day and above, as mono- or part of a poly-therapy, was 15. There was no significant statistical difference between VPA doses of mothers and the malformations in their babies (p>0.05). Furthermore, distribution of malformations according to not only VPA monotherapy, but also polytherapy including VPA and AEDs other than VPA was not found to be statistically significant (p>0.05) (OR, 0.53; 95% CI, 0.11–2.5). This may be due to the limitation of our sample size.
While folic acid usage was prevalent among mothers on AED (59.3%), there was no statistically significant difference between its use and the occurrence of a major malformation (p>0.05) (OR, 3.48; 95% CI 0.67–18.0).
DISCUSSION
Using safe AEDs in pregnancy is important not only to render the mother seizure-free during pregnancy but also to prevent teratogenic side effects in the baby. There have been a significant number of researches on this subject in recent years.
From the mothers’ point of view, it is known that seizure frequency does not change during pregnancy in 47% to 83% of the mothers.23–26) Similarly, in our study, seizure frequency remained the same in 70.1% of the cases.
In this study, all investigations including urinary ultrasound, echocardiography and cranial MRI were used, which is particularly important for the detection of asymptomatic malformations. Two siblings who had renal agenesia unknown to their parents but detected in our investigations are a case in point. There is no study on asymptomatic children in literature.
While malformation rates in the general population range from 2% to 3%, it was reported to be 3.65% in the Turkish population.27–29) The ratio of major malformation due to AED use during pregnancy has been documented to be 2.8–10%, and higher in mothers on AED polytherapy, although there are arguments to the contrary.24,26,30) We found major malformation in 7.7% of the cases in the AED group, which was statistically not significant with 5.3% in the monotherapy, and 20% in the polytherapy group. In line with the figures for the general Turkish population, the percentage of major malformation in the non-AED group was 3.8%, not statistically significant with respect to AED group. However, the OR was 0.48, which indicates that the percentage of major malformation in the AED group was 2.08 times higher.
Long-term teratogenic effects of older AEDs are well-known whereas the same cannot be argued for new AEDs. Our information on the teratogenic effects of new AEDs is limited to case reports and pregnancy registries. Oxcarbazepine (OXC) is reported to cause spina bifida, cardiac, urinary and skeletal system malformations, LTG to cause orofacial cleft, urinary system malformations.31,32) We had 15 cases on new generation AEDs, three of whom had malformations (one major and two minor). We observed patent foramen ovale with 900 mg/day OXC, renal agenesis with 100 mg/day LTG and 1,000 mg/day VPA and hydronephrosis with 1,200 mg/day OXC and 150 mg/day phenytoin. In our study we report no results on levetirecetam, pregabalin, vigabatrin, topiramat although they can be found in the market in Turkey.
We detected 4 major and 9 minor malformations in the group on VPA and no significance was observed in major malformations between patients who were on VPA and those who were not. However, the odd ratio was 0.53, which indicates that the percentage of major malformation in the VPA group was 1.9 times higher. There is evidence in literature to suggest that the ratio of major malformation increases due to VPA monotherapy or polytherapy including VPA, especially with higher doses, and has a negative impact on the long-term cognitive development of the child.18,33–38) There was one child with autism while 28 children were below the age of two, hence impossible to diagnose at the time of the study. Therefore, the ratio of autism in our sample needs to be revised at a later date. In our previous study, higher cognitive impairment was detected in children exposed to polytherapy and VPA, whereas the electroencephalography abnormality was statistically significant among children in the AED group, more common under VPA, than the non-AED group.39) We demonstrated the importance of maximum reduction possible in AED dosage for the healthy cognitive and behavioral development of a child.40)
Mothers on VPA who had a child with malformation are reported to have higher risk for malformation in their children in following pregnancies.19) Likewise, the ratio of malformation is known to be higher in recurrent pregnancies with VPA and topiramat.20) In comparison to literature, our study is one of the few studies with a high number of mothers with multiple pregnancies. We observed noteworthy findings with respect to recurrent pregnancies although the results are not statistically significant due to the small size of the group. For instance, we had one mother who had two children with renal agenesis although she was on AED (VPA 1,000 mg/day and LTG 100 mg/day) in one of her pregnancies and the other one was non-AED. It must be pointed out that the children’s uncle also had renal agenesis with no other systemic disease or AED use during his mother’s pregnancy, which suggests that there might be a genetic disposition rather than AED effect. Another mother with three children, who never used AED during her pregnancies, had her first and last child healthy, whereas her second child had a major malformation. Yet another mother, who used phenobarbital (25 mg/day) during her first pregnancy, had a healthy child, but her second child had a minor malformation, although she did not use an AED during her second pregnancy. These examples illustrate the difficulty of associating malformations with the teratogenic effects of AEDs only.
As for mothers with multiple pregnancies on VPA, there were major malformations in two siblings born to a mother on 2,000 mg/day VPA. On the other hand, another patient who had a minor malformation in her first-born child was on 1,500 mg/day VPA, but reduced her dose to 1,000 mg/day during her second pregnancy which may account for the fact that her second child was healthy. Our usual protocol is to administer 500 mg/day VPA and we have few mothers on doses higher than the standard. The presence of malformations of various degrees in children exposed to high or different doses of VPA during pregnancy might be considered an important finding—although not statistically significant—as it suggests a correlation between higher doses of VPA and congenital malformations, consistent with literature.14,35)
Minor dysmorphic features such as epicanthic folds, small mouth, abnormal philtrum, dysplastic ears, hypoplastic digits and nails were described in AED exposed infants. After assessing the dysmorphisms of the subjects according to the checklist put together in accordance to the previous studies, it was observed that children exposed to VPA, CBZ and polytherapy had more minor dysmorphic features compared to other groups. Furthermore, patients with multiple minor dysmorphic features may be at increased risk of cognitive impairment.9) As for the non-AED group, approximately 25% of the non-exposed subjects were scored with respect to their one or more dysmorphic features. These included features such as epicanthic folds, hypertelorism, high arched palate which is among normal variations in the population. Epicanthic folds, broad nasal root and tip, anteverted nares, short nose, depressed nasal tip, smooth philtrum, thin upper lip and hypertelorism were commonly seen in our patients with VPA exposure. These findings were in correlation with the typical VPA gestalt mentioned in previous reports.9,41) Patient in Fig. 1 is a typical example for fetal valproate syndrome faces.
Overall, our study resonates with previous studies in that subjects receiving VPA, CBZ or polytherapy during pregnancy have more minor dysmorphic findings than non-AED exposed subjects and subjects exposed to different AEDs. Dysmorphic features most commonly observed in our study were in the children of mothers who used VPA during pregnancy. Similarly, Kini et al.,9) had described more facial dismorphic features in the VPA exposed children than non-exposed AEDs.
AED use during pregnancy has been associated with mineralization defects of teeth. Dental anomalies and delayed eruption were found in 10% of children born to mothers on AED.41) In our study, the percentage of dental anomaly is significantly higher in all children (48.4%) with 39.3% in children exposed to VPA. In our study, enamel hypoplasia and delay of eruption were the most common dental anomalies among children of mothers on AED during pregnancy. This is the first study to examine such a high number of both dental and dysmorphic abnormalities together. This is particularly important since both dental and dysmorphic anomalies are not considered malformations and frequently disregarded.
While it has been reported in 2009 that daily use of 2.5–5 mg folic acid before pregnancy reduced malformation, there are also studies that suggest the contrary.42,43) In our study, daily folic acid dose was 5 mg (59.3%) with no statistically significant difference in the two groups of mothers in terms of major malformations. However, the odd ratio was 3.48, which indicates that the percentage of major malformation was three times higher in patients who did not use folic acid.
The small sample size and the restrospective study design are limitations on the generalizability of our results as well as the limited follow-up period and exclusion of data on abortions. The study would have benefitted from a control group comparison, a limitation unfortunately found in many studies.
Even with these limitations, our study conveys significant results. On lower doses of VPA, major malformations are not seen, although the risk increases with polytherapy. As a final note, we argue that development of malformations may be associated with not only exposure to AEDs during pregnancy but also with genetic and environmental factors, and hence is multifactorial.
Acknowledgments
This study was supported by the Research Fund of Istanbul University (571/140820006). We would like to thank all the patients who participated in this study.
REFERENCES
- 1.Fairgrieve SD, Jackson M, Jonas P, Walshaw D, White K, Montgomery TL, et al. Population based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674–675. doi: 10.1136/bmj.321.7262.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes LB. The teratogenicity of anticonvulsant drugs: a progress report. J Med Genet. 2002;39:245–247. doi: 10.1136/jmg.39.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennell PB. Pregnancy in women who have epilepsy. Neurol Clin. 2004;22:799–820. doi: 10.1016/j.ncl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Meador KJ. Effects of in utero antiepileptic drug exposure. Epilepsy Curr. 2008;8:143–147. doi: 10.1111/j.1535-7511.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battino D, Binelli S, Caccamo ML, Canevini MP, Canger R, Como ML, et al. Malformations in offspring of 305 epileptic women: a prospective study. Acta Neurol Scand. 1992;85:204–207. doi: 10.1111/j.1600-0404.1992.tb04029.x. [DOI] [PubMed] [Google Scholar]
- 6.Samrén EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko S, Battino D, Andermann E, Wada K, Kan R, Takeda A, et al. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999;33:145–158. doi: 10.1016/S0920-1211(98)00084-9. [DOI] [PubMed] [Google Scholar]
- 8.Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 9.Kini U, Adab N, Vinten J, Fryer A, Clayton-Smith J Liverpool and Manchester Neurodevelopmental Study Group. Dysmorphic features: an important clue to the diagnosis and severity of fetal anticonvulsant syndromes. Arch Dis Child Fetal Neonatal Ed. 2006;91:90–95. doi: 10.1136/adc.2004.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meador KJ, Baker GA, Browning N, Cohen MJ, Clayton-Smith J, Kalayjian LA, et al. NEAD Study Group. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134(Pt 2):396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, et al. NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones KL, Lacro RV, Johnson KA, Adams J. Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661–1666. doi: 10.1056/NEJM198906223202505. [DOI] [PubMed] [Google Scholar]
- 13.Ornoy A, Cohen E. Outcome of children born to epileptic mothers treated with carbamazepine during pregnancy. Arch Dis Child. 1996;75:517–520. doi: 10.1136/adc.75.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mawer G, Clayton-Smith J, Coyle H, Kini U. Outcome of pregnancy in women attending outpatient epilepsy clinic: adverse features associated with higher doses of sodium valproate. Seizure. 2002;11:512–518. doi: 10.1016/S1059-1311(02)00135-8. [DOI] [PubMed] [Google Scholar]
- 15.Gaily E, Granström ML. Minor anomalies in children of mothers with epilepsy. Neurology. 1992;42(4 Suppl 5):128–131. [PubMed] [Google Scholar]
- 16.Nulman I, Scolnik D, Chitayat D, Farkas LD, Koren G. Findings in children exposed in utero to phenytoin and carbamazepine monotherapy: independent effects of epilepsy and medications. Am J Med Genet. 1997;68:18–24. doi: 10.1002/(SICI)1096-8628(19970110)68:1<18::AID-AJMG4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Gaily E, Kantola-Sorsa E, Granström ML. Intelligence of children of epileptic mothers. J Pediatr. 1988;113:677–684. doi: 10.1016/S0022-3476(88)80377-9. [DOI] [PubMed] [Google Scholar]
- 18.Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vajda FJ, O’Brien TJ, Lander CM, Graham J, Roten A, Eadie MJ. Teratogenesis in repeated pregnancies in antiepileptic drug-treated women. Epilepsia. 2013;54:181–186. doi: 10.1111/j.1528-1167.2012.03625.x. [DOI] [PubMed] [Google Scholar]
- 20.Campbell E, Devenney E, Morrow J, Russell A, Smithson WH, Parsons L, et al. Recurrence risk of congenital malformations in infants exposed to antiepileptic drugs in utero. Epilepsia. 2013;54:165–171. doi: 10.1111/epi.12001. [DOI] [PubMed] [Google Scholar]
- 21.Smith DW, Jones KL. Recognizable patterns of human malformations: genetic, embryologic, and clinical aspects. 3rd ed. Philadelphia: W.B. Saunders; 1982. p. 573. [Google Scholar]
- 22.EUROCAT Central Registry. EUROCAT Guide 1.3 and reference documents. Instructions for the registration and surveillance of congenital anomalies [Internet] Newtownabbey: EUROCAT Central Registry; 2005. Sep, [cited at 2015 Nov 1]. Available from: http://www.eurocat-network.eu/content/EUROCAT-guide-1.3.pdf. [Google Scholar]
- 23.Harden CL, Hopp J, Ting TY, Pennell PB, French JA, Hauser WA, et al. American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy-focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:126–132. doi: 10.1212/WNL.0b013e3181a6b2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viinikainen K, Heinonen S, Eriksson K, Kälviäinen R. Community-based, prospective, controlled study of obstetric and neonatal outcome of 179 pregnancies in women with epilepsy. Epilepsia. 2006;47:186–192. doi: 10.1111/j.1528-1167.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 25.Vajda FJ, Hitchcock A, Graham J, O’Brien T, Lander C, Eadie M. Seizure control in antiepileptic drug-treated pregnancy. Epilepsia. 2008;49:172–176. doi: 10.1111/j.1528-1167.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 26.Endo S, Hagimoto H, Yamazawa H, Kajihara S, Kubota S, Kamijo A, et al. Statistics on deliveries of mothers with epilepsy at Yokohama City University Hospital. Epilepsia. 2004;45( Suppl 8):42–47. doi: 10.1111/j.0013-9580.2004.458009.x. [DOI] [PubMed] [Google Scholar]
- 27.Yerby MS. The use of anticonvulsants during pregnancy. Semin Perinatol. 2001;25:153–158. doi: 10.1053/sper.2001.24900. [DOI] [PubMed] [Google Scholar]
- 28.Yerby MS. Clinical care of pregnant women with epilepsy: neural tube defects and folic acid supplementation. Epilepsia. 2003;44( Suppl 3):33–40. doi: 10.1046/j.1528-1157.2003.t01-1-44703.x-i1. [DOI] [PubMed] [Google Scholar]
- 29.Tunçbilek E, Boduroğlu K, Alikaşifoğlu M. Results of the Turkish congenital malformation survey. Turk J Pediatr. 1999;41:287–297. [PubMed] [Google Scholar]
- 30.Harden CL, Meador KJ, Pennell PB, Hauser WA, Gronseth GS, French JA, et al. American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:133–141. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunnington M, Ferber S, Quartey G International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Effect of dose on the frequency of major birth defects following fetal exposure to lamotrigine monotherapy in an international observational study. Epilepsia. 2007;48:1207–1210. doi: 10.1111/j.1528-1167.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- 32.Eisenschenk S. Treatment with oxcarbazepine during pregnancy. Neurologist. 2006;12:249–254. doi: 10.1097/01.nrl.0000215743.02301.17. [DOI] [PubMed] [Google Scholar]
- 33.Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK epilepsy and pregnancy register. J Neurol Neurosurg Psychiatry. 2006;77:193–198. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas SV, Ajaykumar B, Sindhu K, Francis E, Namboodiri N, Sivasankaran S, et al. Cardiac malformations are increased in infants of mothers with epilepsy. Pediatr Cardiol. 2008;29:604–608. doi: 10.1007/s00246-007-9161-4. [DOI] [PubMed] [Google Scholar]
- 35.Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurol Scand. 2005;112:137–143. doi: 10.1111/j.1600-0404.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 36.Wide K, Winbladh B, Källén B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr. 2004;93:174–176. doi: 10.1111/j.1651-2227.2004.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 37.Vinten J, Adab N, Kini U, Gorry J, Gregg J, Baker GA, et al. Liverpool and Manchester Neurodevelopment Study Group. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954. doi: 10.1212/01.WNL.0000154514.82948.69. [DOI] [PubMed] [Google Scholar]
- 38.Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–1878. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 39.Guveli B, Gürses C, Atakli D, Bebek N, Dörtcan N, Baykan B, et al. Are there any EEG changes in children whose mothers with epilepsy had used antiepileptic drug during pregnancy? J Neurol Sci (Turk) 2012;29:192–200. [Google Scholar]
- 40.Güveli BT, Gürses C, Ataklı D, Akça Kalem Ş, Dirican A, Bebek N, et al. Behavioral characteristics and cognitive development among school age children born to women with epilepsy. Neurol Res. 2015;37:295–300. doi: 10.1179/1743132814Y.0000000449. [DOI] [PubMed] [Google Scholar]
- 41.Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrell MJ. Folic acid and epilepsy. Epilepsy Curr. 2002;2:31–34. doi: 10.1046/j.1535-7597.2002.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harden CL, Pennell PB, Koppel BS, Hovinga CA, Gidal B, Meador KJ, et al. American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142–149. doi: 10.1212/WNL.0b013e3181a6b325. [DOI] [PMC free article] [PubMed] [Google Scholar]