Abstract
Objective
The present study aims to analyze the levels of resistin, tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-18, and C-reactive protein (CRP) in patients with Alzheimer’s disease (AD) and also investigate a potential relationship between resistin levels and TNF-α, IL-1β, IL-6, IL-18, and CRP levels in patients with AD.
Methods
The study included fifty patients with AD and 30 healthy controls with normal cognitive functions. The serum resistin, TNF-α, IL-1β, IL-6, IL-18, and CRP levels were assessed. We performed a Mini-Mental State Examination (MMSE) to evaluate the general cognitive performance.
Results
The mean serum resistin, IL-1β, IL-18, and TNF-α levels were significantly higher in patients with AD compared with the controls (p=0.026, p=0.002, p=0.003, and p=0.038, respectively). The IL-6 and CRP levels did not differ between the groups (p=0.874 and p=0.941). The resistin levels were positively correlated with the levels of CRP and IL-18 (r=0.526, p<0.001; r=0.402, p=0.004, respectively). MMSE scores and inflammatory markers were not correlated (p>0.05 for all).
Conclusion
Serum resistin levels were significantly increased and correlated with some inflammatory markers in AD patients, suggesting that resistin might play a role in the inflammatory process of AD.
Keywords: Resistin, Inflammatory cytokines, Alzheimer disease, Inflammation
INTRODUCTION
Alzheimer’s disease (AD) is the most widespread neurodegenerative disorder and the most common cause of dementia in elderly people. AD is characterized by progressive learning and memory loss and an impairment of other cognitive functions, ultimately resulting in dementia and death. It has been suggested that almost 10% of the population aged ≥65 years have been affected by AD.1) It is estimated that more than 44 million people suffered from AD in the world in 2014, which is expected to be doubled by 2030 as the world population ages.1,2)
Although the cause of AD remains elusive, amyloid beta (Aβ)-induced neurotoxicity has been largely accepted to be the hallmark in pathogenesis.1) It has been also suggested that inflammation contributes to the pathogenesis of AD.3)
Cytokines have been detected to play a crucial role in the neuroinflammation of AD.4) Several studies have reported that the AD brain exhibits an over-expression of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-6, and IL-18.3,5,6) The generation of these proinflammatory cytokines causes astrogliosis and microglial activation and lead to the release of other pro-inflammatory molecules, increasing the effects of cytokine. Although the actual impact of changed cytokines on brain functions and AD-associated degeneration remains unclear, many investigations suggest that these inflammatory molecules may potently affect amyloidosis, neurodegeneration, learning, and memory in AD.7)
Resistin is a kind of adipokine secreted from adipocytes and is linked to the insulin resistance. Recently, resistin has been found to be involved in inflammation and regulation of other cytokines as well.8) On the other hand, some proinflammatory cytokines can induce the expression of resistin.9) Several studies reported an increased level of resistin in various inflammation-related conditions such as ankylosing spondylitis, rheumatoid arthritis, and inflammatory bowel disease.8,10–12) Moreover, it was shown that resistin levels were positively correlated with the C-reactive protein (CRP), a marker of inflammation, in several diseases.13,14) To the best of our knowledge, there have been only three studies investigating the resistin levels in patients with AD.3,15,16) These studies report significantly elevated resistin levels compared with the controls. However, the association between resistin levels and IL-1β, IL-6, and IL-18 has not yet been investigated in patients with AD. An elucidation of the inflammatory mediators involved in the pathogenesis of AD is strongly needed to recognize new therapeutic approaches in order to prevent and treat the disease.3) In this context, the present study aimed to investigate the levels of resistin, TNF-α, IL-1β, IL-6, IL-18, and CRP in non-obese AD patients and compare the results with those of non-obese healthy subjects. We also aimed to assess the possible association between the resistin level and the TNF-α, IL-1β, IL-6, IL-18, and CRP levels and evaluate the relationship between the Mini-Mental State Examination (MMSE) and the laboratory parameters.
METHODS
Participants
The study included fifty patients with AD who referred to the outpatient clinic of neurology and 30 healthy controls with normal cognitive functioning. The diagnosis of AD was determined based on the Diagnostic and Statistical Manual of Mental Disorders 4th edition, text revision (DSM-IV-TR) criteria. All participants underwent an elaborate neurological and psychiatric examination by a neurologist and psychiatrist and also the MMSE, which sought to assess the general cognitive performance. Informed consents were obtained from all participants or their legal representatives. The study was approved by the ethics committee of Süleyman Demirel University, School of Medicine (17/12/2014 - 213).
Subjects with a history of malignant disease, diabetes mellitus, hypertension, liver failure, renal failure, ischemic heart disease, congestive heart failure, obesity, thyroid disorders, metabolic disease, body mass index (BMI) over 25, cerebrovascular accident, with active infection or other inflammatory diseases, and receiving anti-inflammatory drugs were excluded from the study. Participants’ age, gender, BMI, and MMSE scores were recorded. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Serum Samples
Venous blood samples were taken from all subjects and immediately centrifuged at a speed of 4,000 rpm for 10 minutes to obtain serum. All sera samples of the participants were stored at −80°C instantly after separation from peripheral blood prior to the analysis. Serum IL-1β, IL-6, IL-18, TNF-α, and resistin concentrations were determined by Specific ELISA kits (IL-1β, IL-6, TNF-α: AviBion, Orgenium Laboratories, Finland; IL-18: Invitrogen, Carlsbad, CA, USA; resistin: R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s recommendation. The absorbance of samples was measured at 450 nm using an Organon Teknika 530 Microplate Reader (Anthos Labtec Instruments GmbH, Salzburg, Austria). The IL-1β, IL-6, IL-18, TNF-α, and resistin concentrations were determined by interpolation on the standard curve created by the absorbance of the standards. Serum IL-1β, IL-6, IL-18, and TNF-α concentrations were expressed in pg/ml. Serum resistin concentrations were expressed in ng/mL. Serum CRP levels were measured by means of a particle-enhanced immunonephelometry using Siemens BN-ProSpec System (Siemens Health-care Diagnostics Products GmbH, Marburg, Germany). Serum CRP concentrations were expressed in mg/L.
Statistical Analysis
We performed a statistical analysis on the SPSS software (version 15; SPSS Inc., Chicago, IL, USA). The distribution of normality for all continuous variables was determined by a Kolmogorov-Smirnov test. We employed descriptive statistics to report the data analysis expressed as mean±standard deviation. A chi-square test was used to compare categorical variables presented as the number of cases and percentages. Differences between the groups were analyzed with the Student’s t-test and Mann-Whitney U-test. Correlations between the variables were identified using the Pearson’s and Spearman’s rank correlation tests.
RESULTS
The present study included fifty patients with AD (31 females and 19 males) and 30 healthy subjects (18 females and 12 males). There was no age and gender difference between the patients and controls (p=0.10, p=0.85, respectively). The mean MMSE scores were 17.7±5.62 in the AD group. The mean serum resistin, IL-1β, IL-18, and TNF-α levels were significantly higher in patients with AD compared with the controls (p=0.026, p=0.002, p=0.003, and p=0.038, respectively). The IL-6 and CRP levels were not significantly different in patients with AD than the control group (p=0.874 and p=0.941). The demographic and clinical features and serum resistin, IL-1β, IL-6, IL-18, TNF-α, and CRP levels are presented in Table 1.
Table 1.
The demographic, clinical and laboratory parameters in patients with Alzheimer’s disease (AD) and controls
| AD (n=50) | Controls (n=30) | p value | |
|---|---|---|---|
| Age (yr) | 75.8±8.01 | 73.1±5.73 | 0.101 |
| Sex (F/M) | 31 (62.0)/19 (38.0) | 18 (60.0)/12 (40.0) | 0.859 |
| MMSE | 17.7±5.62 | 27.4±1.19 | < 0.001 |
| BMI (kg/m2) | 22.9±1.82 | 22.8±2.03 | 0.824 |
| CRP (mg/L) | 6.97±6.28 | 6.62±5.68 | 0.941 |
| IL-1β (pg/ml) | 11.8±51.8 | 1.69±4.95 | 0.002 |
| IL-6 (pg/ml) | 19.4±88.5 | 14.0±50.7 | 0.874 |
| IL-18 (pg/ml) | 117.97±133.59 | 61.5±40.2 | 0.003 |
| TNF-α (pg/ml) | 148.94±202.21 | 64.0±84.8 | 0.038 |
| Resistin (ng/mL) | 2.42±0.74 | 2.11±0.56 | 0.026 |
Values are presented as mean±standard deviation or number (%).
F, female; M, male; BMI, body mass index; MMSE, Mini-Mental State Examination; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor alpha.
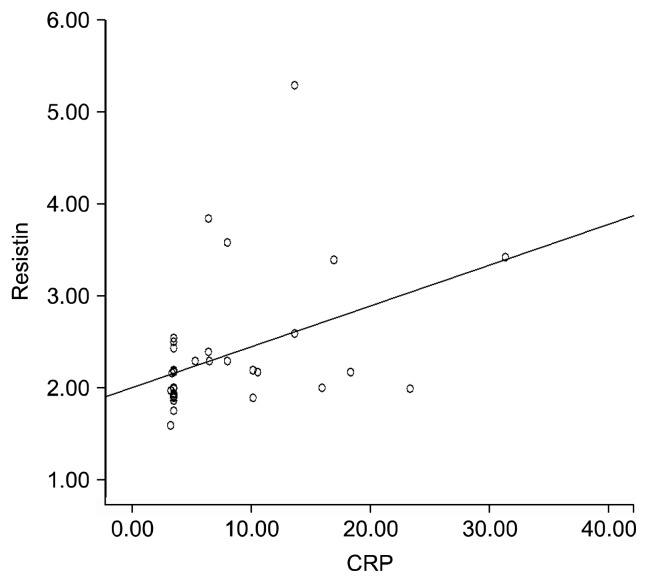
The resistin levels were positively correlated with the levels of CRP and IL-18 (r=0.526, p<0.001 and r=0.402, p=0.004). Correlation between the levels of resistin and CRP are demonstrated in Figure 1. The MMSE scores were not statistically correlated with the levels of resistin, IL-1β, IL-6, IL-18, TNF-α, and CRP (p>0.05 for all). The correlations between resistin and other laboratory parameters as well as those between MMSE and laboratory parameters are shown in Table 2.
Fig. 1.
Correlation between the levels of resistin and C-reactive protein (CRP).
Table 2.
The correlation between MMSE and laboratory parameters and between resistin and other laboratory parameters.
| MMSE | Resistin (ng/mL) | |||
|---|---|---|---|---|
|
|
|
|||
| r | p value | r | p value | |
| CRP (mg/L) | −0.125 | 0.432 | 0.526 | <0.001 |
| IL-1β (pg/ml) | −0.117 | 0.419 | 0.279 | 0.050 |
| IL-6 (pg/ml) | −0.114 | 0.431 | 0.118 | 0.414 |
| IL-18 (pg/ml) | −0.075 | 0.603 | 0.402 | 0.004 |
| TNF-α (pg/ml) | −0.206 | 0.151 | 0.128 | 0.375 |
| Resistin (ng/mL) | −0.198 | 0.169 | - | - |
MMSE, Mini-Mental State Examination; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor alpha.
DISCUSSION
The present study found that the levels of resistin, IL-1β, IL-18, and TNF-α were significantly higher in patients with AD than healthy controls. The resistin levels were positively correlated with the levels of CRP and IL-18.
Resistin, a member of a secretory protein family, has proinflammatory properties.9) The primary sources of resistin in humans include macrophages, polymorphonuclear cells, and bone marrow cells. Similar to several other inflammatory cytokines, circulating resistin down-regulates the expression of endothelial nitric oxide, upregulates endothelial permeability, elevates adhesion molecule expression and oxidative stress, activates the proliferation and migration of smooth muscle cells, and causes endothelial dysfunction, which results in vascular dysfunction.8,9,17,18) Several studies demonstrated that resistin played an importance role in a variety of conditions such as ankylosing spondylitis, rheumatoid arthritis, atherosclerosis, and inflammatory bowel disease.8–12) Recently, Dong et al.18) reported that those patients with acute cerebral infarction had increased levels of resistin compared to healthy subjects, suggesting an association between resistin levels and elevated risk of acute cerebral infarction. Zhang et al.19) found increased serum resistin levels in patients with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis and anti-acetylcholine receptor antibody-positive myasthenia gravis with thymoma and reported a correlation between serum resistin levels and the severity of disease. Moreover, a study investigating resistin levels in episodic migraine demonstrated that pretreatment ictal resistin levels increased with elevating pain severity and reduced after successful treatment.20)
Currently, very little is known about the impact of resistin on AD. Hu et al.16) found that the resistin level of cerebrospinal fluid was different in AD patients compared to the healthy subjects with normal cognitive functions. The researchers suggested that resistin was particularly related to AD alongside Aβ42 and thrombospondin-1. A recent study by Kizilarslanoğlu et al.3) analyzed the resistin levels in patients with AD. The researchers demonstrated that resistin levels of patients with AD were significantly higher than healthy controls while h-CRP and TNF-α were not different between the groups. Also, they reported that overall accuracy of resistin in determining AD was 70.66% and resistin was negatively correlated with MMSE. Leung et al.15) reported that resistin was significantly associated with abnormal cerebrospinal fluid Aβ1–42 levels. In line with the results of the previous studies,3,16) we found that resistin levels were significantly higher in AD patients compared with the controls.
Although there have been several hypotheses available for the etiopathology of AD, it is well-known that there is a chronic inflammatory process in the brain of AD.21,22) Activated microglia and astrocytes excessively secrete inflammatory components including pro-inflammatory cytokines, reactive oxygen species, chemokines, macrophage inflammatory proteins, and complements.7) Those inflammatory molecules secreted, certain cytokines in particular, disturb the balance of normal neurophysiologic condition, which is related to cognition, learning, and memory.7,23) Several studies have analyzed various cytokines and other inflammatory marker levels in AD.3,21) Tan et al.24) reported that individuals whose peripheral blood mononuclear cells spontaneously produced TNF-α or IL-1 were exposed to a higher risk of developing AD. Malaguarnera et al.25) reported higher concentrations of IL-18 in AD patients compared to the patients with vascular dementia and non-demented subjects. Moreover, several studies reported elevated peripheral IL-6 levels in patients with AD.21,26) However, Lanzrein et al.27) did not find significant differences between patients with AD and healthy subjects in terms of average serum IL-6, IL-1β, soluble TNF receptors types I and II, IL-1 receptor antagonist, TNF-α, and alpha 1-antichymotrypsin levels. Further, although several studies have reported a relationship between the hs-CRP and AD,28,29) some studies do not support this relationship.3,24,30) A meta-analysis investigating the cytokines in AD demonstrated that peripheral blood levels of IL-1β, IL-6, IL-12, IL-18, TNF-α, and transforming growth factor-β were significantly elevated in AD patients compared with the healthy subjects, while the levels of interferon-γ, IL-4, IL-8, IL-10 and CRP did not differ between the groups.30) In the present study, we found that levels of IL-1β, IL-18, and TNF-α were significantly higher in AD patients compared with the healthy controls but the levels of IL-6 and CRP did not differ between patients with AD and healthy controls. Such contradictory results may be explained by the biological instability and short half-lives of the cytokines, and different sample sizes.21,31)
A positive association has been demonstrated between resistin and many inflammatory markers such as TNF-α, CRP, and lipoprotein-associated phospholipase A2 under various pathophysiological conditions.13,14) In the present study, in addition to CRP and TNF-α, we also evaluated the association between levels of resistin and IL-1β, IL-6, and IL-18 in patients with AD for the first time. Our study showed that resistin levels were positively correlated with CRP and IL-18 levels. Therefore, our results suggest that resistin may play a role in the inflammatory process of AD. However, regarding the MMSE, we were unable to find a statistically significant correlation of the levels of resistin and other inflammatory markers with MMSE scores. Kizilarslanoğlu et al.3) reported a negative correlation between resistin levels and MMSE scores. Yasutake et al.32) found no correlation between the IL-1β, TNF-α levels and the MMSE scores in patients with AD. A study by Wu et al.33) showed no correlation between neurocognitive scores and levels of IL-6, IL-18, fractalkine, and tumor necrosis factor-related apoptosis-inducing ligand. This indifference of our results may be related to the small sample size.
The present study is subject to a series of limitations which must be considered while interpreting the results. The cross-sectional structure is one of them. The number of patients included in this study was relatively small to project the findings to the general population. Moreover, our study was performed in a single institute, which also limited the generalizability of findings to the AD population. The missing data on lipid parameters were another limitation.
In conclusion, our study revealed a significant increase in resistin levels in patients with AD than the controls. Also, levels of resistin were found to be positively correlated with the levels of CRP and IL-18. These findings may be linked to the chronic inflammation in AD. The present study may contribute to further studies including a wider range of populations in order to investigate a novel therapy for reversing or arresting the disease in the case of AD.
REFERENCES
- 1.Choi DY, Lee YJ, Hong JT, Lee HJ. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res Bull. 2012;87:144–153. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease International. World alzheimer report 2014: Dementia and risk reduction-an analysis of protective and modifiable factors [Internet] London: Alzheimer’s Disease International; 2014. [cited at 2015 Feb]. Available from: http://www.alz.co.uk/research/WorldAlzheimerReport2014. [Google Scholar]
- 3.Kizilarslanoğlu MC, Kara Ö, Yeşil Y, Kuyumcu ME, Öztürk ZA, Cankurtaran M, et al. Alzheimer disease, inflammation, and novel inflammatory marker: resistin. Turk J Med Sci. 2015;45:1040–1046. doi: 10.3906/sag-1403-55. [DOI] [PubMed] [Google Scholar]
- 4.Alam Q, Alam MZ, Mushtaq G, Damanhouri GA, Rasool M, Kamal MA, et al. Inflammatory process in Alzheimer’s and Parkinson’s diseases: Central role of cytokines. Curr Pharm Des. 2016;22:541–548. doi: 10.2174/1381612822666151125000300. [DOI] [PubMed] [Google Scholar]
- 5.Cacabelos R, Alvarez XA, Fernández-Novoa L, Franco A, Mangues R, Pellicer A, et al. Brain interleukin-1 beta in Alzheimer’s disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- 6.Sutinen EM, Pirttilä T, Anderson G, Salminen A, Ojala JO. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J Neuroinflammation. 2012;9:199. doi: 10.1186/1742-2094-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang ZG, Li Y, Ng CT, Song YQ. Inflammation in Alzheimer’s disease and molecular genetics: Recent update. Arch Immunol Ther Exp (Warsz) 2015;63:333–344. doi: 10.1007/s00005-015-0351-0. [DOI] [PubMed] [Google Scholar]
- 8.Filková M, Haluzík M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cell Mol Immunol. 2006;3:29–34. [PubMed] [Google Scholar]
- 10.Konrad A, Lehrke M, Schachinger V, Seibold F, Stark R, Ochsenkühn T, et al. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007;19:1070–1074. doi: 10.1097/MEG.0b013e3282f16251. [DOI] [PubMed] [Google Scholar]
- 11.Senolt L, Housa D, Vernerová Z, Jirásek T, Svobodová R, Veigl D, et al. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis. 2007;66:458–463. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocabas H, Kocabas V, Buyukbas S, Melıkoglu MA, Sezer I, Butun B. The serum levels of resistin in ankylosing spondylitis patients: a pilot study. Rheumatol Int. 2012;32:699–702. doi: 10.1007/s00296-010-1651-7. [DOI] [PubMed] [Google Scholar]
- 13.Axelsson J, Bergsten A, Qureshi AR, Heimbürger O, Bárány P, Lönnqvist F, et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006;69:596–604. doi: 10.1038/sj.ki.5000089. [DOI] [PubMed] [Google Scholar]
- 14.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 15.Leung YY, Toledo JB, Nefedov A, Polikar R, Raghavan N, Xie SX, et al. Identifying amyloid pathology-related cere-brospinal fluid biomarkers for Alzheimer’s disease in a multicohort study. Alzheimers Dement (Amst) 2015;1:339–348. doi: 10.1016/j.dadm.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, Shaw LM, et al. Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol. 2010;119:669–678. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BJ, Lee SH, Ryu WS, Kim CK, Yoon BW. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci. 2012;312:117–122. doi: 10.1016/j.jns.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Dong XL, Xu SJ, Zhang L, Zhang XQ, Liu T, Gao QY, et al. Serum resistin levels may contribute to an increased risk of acute cerebral infarction. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9751-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Zhang DQ, Wang R, Li T, Li X, Qi Y, Wang J, et al. Remarkably increased resistin levels in anti-AChR antibody-positive myasthenia gravis. J Neuroimmunol. 2015;283:7–10. doi: 10.1016/j.jneuroim.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Chai NC, Gelaye B, Tietjen GE, Dash PD, Gower BA, White LW, et al. Ictal adipokines are associated with pain severity and treatment response in episodic migraine. Neurology. 2015;84:1409–1418. doi: 10.1212/WNL.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savas S, Kabaroglu C, Alpman A, Sarac F, Yalcin MA, Parıldar Z, et al. No relationship between lipoprotein-associated phospholipase A2, proinflammatory cytokines, and neopterin in Alzheimer’s disease. Exp Gerontol. 2016;77:1–6. doi: 10.1016/j.exger.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Ma SL, Lam LC. Panel of genetic variations as a potential non-invasive biomarker for early diagnosis of Alzheimer’s disease. Clin Psychopharmacol Neurosci. 2011;9:54–66. doi: 10.9758/cpn.2011.9.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp (Warsz) 2012;60:251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 25.Malaguarnera L, Motta M, Di Rosa M, Anzaldi M, Malaguarnera M. Interleukin-18 and transforming growth factor-beta 1 plasma levels in Alzheimer’s disease and vascular dementia. Neuropathology. 2006;26:307–312. doi: 10.1111/j.1440-1789.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 26.Dursun E, Gezen-Ak D, Hanağası H, Bilgiç B, Lohmann E, Ertan S, et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J Neuroimmunol. 2015;283:50–57. doi: 10.1016/j.jneuroim.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Lanzrein AS, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1beta, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-alpha, the soluble tumor necrosis factor receptors I and II, and alpha1-antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12:215–227. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Song IU, Chung SW, Kim YD, Maeng LS. Relationship between the hs-CRP as non-specific biomarker and Alzheimer’s disease according to aging process. Int J Med Sci. 2015;12:613–617. doi: 10.7150/ijms.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol. 2010;67:87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Parker DC, Mielke MM, Yu Q, Rosenberg PB, Jain A, Lyketsos CG, et al. Plasma neopterin level as a marker of peripheral immune activation in amnestic mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2013;28:149–154. doi: 10.1002/gps.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasutake C, Kuroda K, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2006;256:402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- 33.Wu YY, Hsu JL, Wang HC, Wu SJ, Hong CJ, Cheng IH. Alterations of the neuroinflammatory markers IL-6 and TRAIL in Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2015;5:424–434. doi: 10.1159/000439214. [DOI] [PMC free article] [PubMed] [Google Scholar]