Abstract
Objective
Electroconvulsive therapy (ECT) is used in the treatment of many psychiatric diseases and this therapy may be effective on antioxidant defence system. In this study, we aimed to evaluate the effects of ECT on oxidative stress.
Methods
Fourteen major depression, 11 schizophrenia and 8 bipolar affective disorder patients diagnosed and received ECT treatment, and 37 healthy volunteers enrolled in the study. ECT was applied to all patients. Before ECT, after the first and last ECTs, serum samples were obtained. Serum total antioxidant status (TAS), total oxidant status (TOS), and calculated oxidative stress index (OSI) were measured in patients before and after ECTs.
Results
TOS values before ECT were higher in major depression (p=0.005) and schizophrenia (p=0.001) groups compared to the control group. TAS values were lower in major depression (p=0.0001), schizophrenia (p=0.004), bipolar affective disorder (p=0.004) groups compared to the controls. Also OSI values were higher in major depression (p=0.0001), schizophrenia (p=0.001), bipolar affective disorder (p=0.009) groups compared to healthy group. After the last ECT, TOS values were significantly lower compared to TOS values before ECT in major depression (p=0.004) and schizophrenia patients (p=0.004). TAS values after the first ECT were higher compared to values before ECT in major depression patients (p=0.004). After last ECT, OSI values were significantly lower compared to before ECT in schizophrenia patients (p=0.006).
Conclusion
As a result, it can be said that ECT did not increase oxidative stress. However, further studies with more patients are needed.
Keywords: Electroconvulsive therapy, Total oxidant status, Total antioxidant status, Oxidative stress index
INTRODUCTION
Atoms or molecules containing one or more unpaired electronsare called oxidant or free radicals, and in biological reactions involving oxygen, some free radicals may be produced. These toxic products mayhave a role in the etiology of the disease by damaging carbohydrates, proteins, lipids and DNA structures incells. The substances eliminating the harmful effects of oxidative products are called antioxidants. Antioxidant defence system comprises enzymatic components (e.g., superoxide dismutase [SOD], catalase [CAT], glutathione peroxidase [GPx] and glutathione reductase) and non-enzymatic components (e.g., albumin, uric acid, bilirubin, vitamin E, vitamin C, and β-carotene).1) Oxidative stres occurs as a result of increase in levels of oxidants and/or reduction in the level of antioxidants.
The increase in oxidative stress was determined more than 100 different diseases.2) There are several studies on the effects of free radicals in psychiatric disorders. For a long time, scientific evidence is available about the deterioration in the balance of oxidative metabolism in psychiatric disorders such as schizophrenia, bipolar disorder and anxiety disorders. Cause-effect relationship between psychiatric disorders and increase in oxidants is still not fully elucidated. In other words, it is not clear that whether increased oxidant parameters cause psychiatric disorders or psychiatric diseases cause increases in oxidant parameters. The available data suggest that oxidants may lead to psychiatric disorders.3–5)
Electroconvulsive therapy (ECT) is considered to be one of the effective treatment options for many psychiatric disorders such as major depression, bipolar disorder, schizophrenia and schizoaffective disorder.6) Which mechanisms that ECT carried out its clinical benefitsis currently under discussion. The effects of ECT on the oxidant-antioxidant status were studied and different results were found. In one study, while oxidative lipid damage was observed only in the frontal cortex in rats after ECT, such effect could not be found in hippocampus, cerebellum and pons.7) In another study, it has been determined that, malondialdehyde (MDA), an indicator of oxidative lipid damage, decreased, GPx and SOD activities increased in the brain samples after single and repeated ECT compared with before values.8) In patients with schizophrenia, while MDA levels decreased after 9th ECT, this finding was not observed after the first ECT. CAT activity, glutathione, and nitric oxide levels did not change after ECT.9) Since the measurement of different oxidant and antioxidant molecules is not practical and cheap and their effects are additive, we measured total oxidant status (TOS) and total antioxidant status (TAS).2) TOS, TAS, and OSI which allow us to make an exact comment on the oxidant and antioxidant balance are studied as oxidative stress markers for human.10)
The effect of ECT on oxidative metabolism is waiting for being elucidated with new studies which will be performed.
In this study we aimed to investigate the total oxidant status and total antioxidant status in psychiatric patients undergone ECT; to evaluate oxidative metabolism by calculating the oxidative stress index (OSI) according to this conditions. Thus, we aimed to investigate the relationship between some psychiatric disorders and the oxidative metabolism and the changes occured by ECT in this metabolism.
METHODS
A total of 33 patients (11 with schizophrenia, 8 with bipolar disorder and 14 with major depression) were included this study; thirty seven healthy subjects with similar age and sex and matching with the same exclusion criteria with the group of patients were used as the control group.
The approval of Pamukkale University Medical Ethics Committee with date of November 8, 2010 and number of 2010/119 was included in the study. The study participants were asked to complete a written informed consent form. The medical records and follow-up notes of the patients were examined; socio-demographic variables such as age, sex, diseases, drugs used, regular exercise, smoking status were noted.
The individuals using alcohol, vitamin C, A, E, folic acid, and xanthine oxidase inhibitors, who have chronic diseases (cancer, diabetes, chronic kidney or liver disease) and severe neurological diseases such as Parkinson’s, Alzheimer’s, were excluded from this study.
Samples Collection
The blood samples were taken from all patients following the 8-hour of fasting before ECT, four hours after the first ECT and 4 hours after the termination of cure of ECT. Also, blood samples were taken from the control group following the 8-hour of fasting. Serum samples obtained were stored at −80°C to freezer until analysis date. TAS was determined using a novel-automated measurement method developed by Erel.2) Serum TOS levels were measured using an automated colorimetric method of Erel.11)
The TOS/TAS ratio was used as OSI. OSI was calculated as follows: OSI=[(TOS, μmol H2O2 equivalent/L)/(TAS, μmol Trolox equivalent/L) ×100].
Three patients were excluded from the study due to the fact that two patients (one major depression, one bipolar disorder) had grossly hemolyzed serum, one patient in bipolar disorder group could not complete the ECT treatment, and the data of these patients were not used in any analysis of study.
Statistical Analysis
The data were statistically analyzed using the SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) program package. Results are expressed as median (1st and 3rd quarter percentages). Mann-Whitney test following Kruskall Wallis were used for comparison of the groups as appropriate. The comparisons of serum TAS, TOS, and OSI values in patients before and after ECT were assessed by Wilcoxon signed rank test following Friedman test. Bonferroni correction was used to resolve a problem of multiple testing. Chi-square was used to test the association between categorical outcome variables. Spearman rank correlation test was used for analysis of correlations among results. The p value was set at 0.05. After Bonferroni correction of the 3 and 4 difference groups the significance levels were 0.016 and 0.0125.
RESULTS
The anthropometric characteristics of the patient and the control groups were given in Table 1. There was no difference among the groups in terms of age and gender (p> 0.05). Body mass index (BMI) values of the control group showed a statistically significant decrease in comparison with BMI values of patients with major depression (p<0.001). The number of smokers was significantly less than the number of non-smokers in the control group (p<0.001).
Table 1.
The anthropometric characteristics of the patients and the control group
| Control group (n=37) | Schizophrenia (n=11) | Bipolar disorder (n=8) | Major depression (n=14) | |
|---|---|---|---|---|
| Age (yr) | 29 (23.5–37) | 32 (26–41) | 23.5 (19.5–35.2) | 40 (23.5–53) |
| Sex | ||||
| Male | 17 (45.9) | 7 (63.6) | 4 (50.0) | 7 (50.0) |
| Female | 20 (54.1) | 4 (36.4) | 4 (50.0) | 7 (50.0) |
| Body mass index (kg/m2) | 24.0 (21.5–25.8) | 26.2 (20.8–28.2) | 29.8 (25.5–34.1) | 28.6 (26.9–31.6) |
| Smoking | 7 (18.9) | 4 (36.4) | 4 (50.0) | 7 (50.0) |
| Antidepressant | 4 (36.4) | 1 (12.5) | 14 (100) | |
| Antipsychotic | 11 (100) | 8 (100) | 14 (100) | |
| Anxiolytic | 1 (9.1) | 3 (37.5) | 4 (28.6) | |
| Mood regulator | 2 (18.2) | 8 (100) | 5 (35.7) | |
Values are presented as median (interquartile range) or number (%).
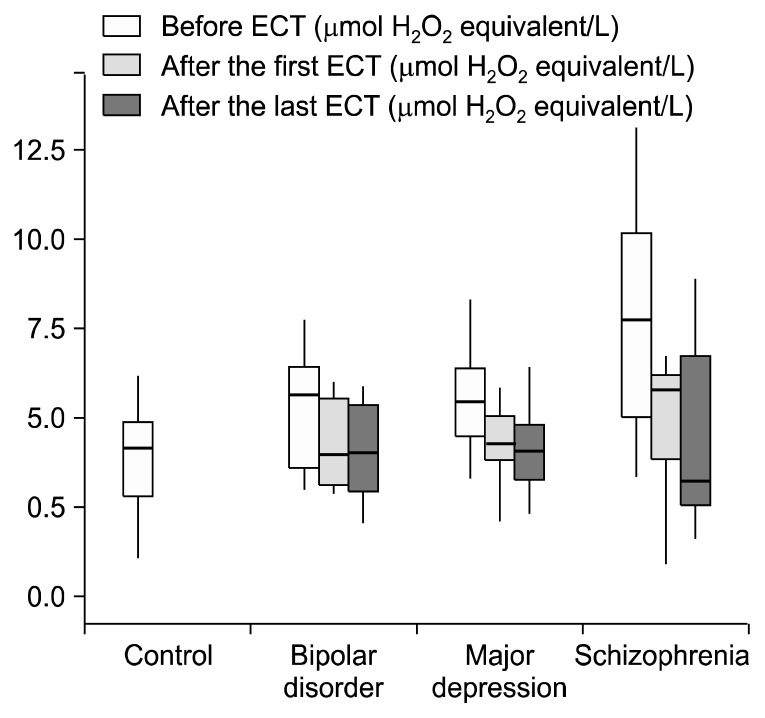
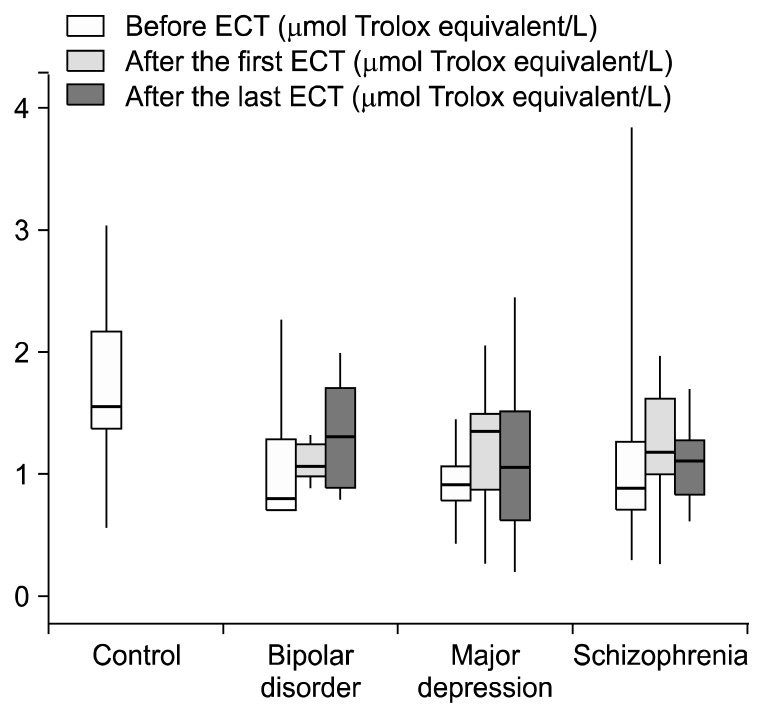
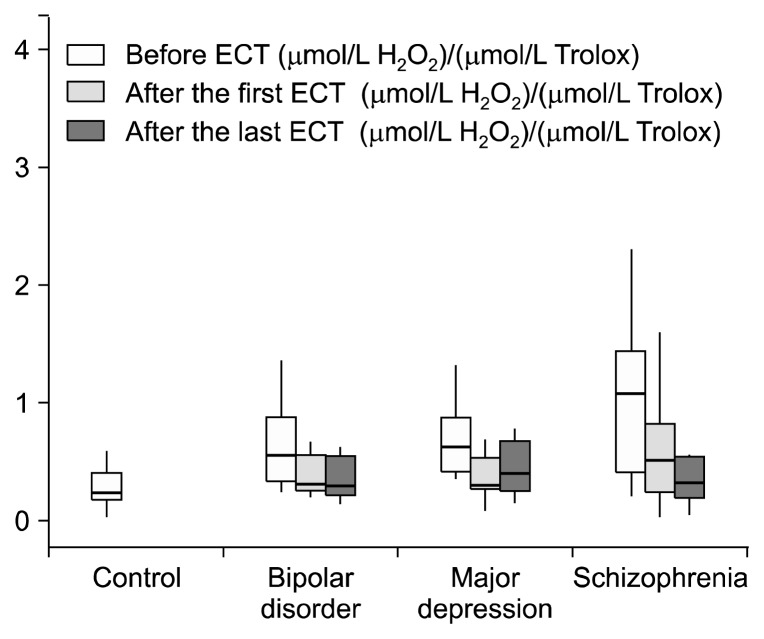
Before ECT, TOS values of the patients with schizophrenia (p=0.001), bipolar disorder and major depression (p=0.005) were higher than those of the control group, but this difference was not statistically significant only in the bipolar disorder group (p>0.05) (Fig. 1). TAS prior to ECT in patients with schizophrenia (p=0.004), bipolar disorder (p=0.004) and major depression (p<0.001) was significantly lower than in control group (Fig. 2). OSI values before ECT in the control group were significantly lower than those of the patients with schizophrenia (p=0.001), bipolar disorder (p=0.009) major depression (p<0.001) (Table 2, Fig. 3). When we controlled BMI in the statistical analyses we found that before ECT TOS and OSI levels were higher in the three patients groups than the control group (p<0.001). Also TAS levels were lower in the three patients groups than the control group (p<0.001). When we examine TOS, TAS, OSI parameters according to the smoking factor in patients, there were significant increseses in TOS and OSI levels between schizophrenia (p<0.001; p<0.001), bipolar disorder (p=0.005; p=0.001), major depression groups (p<0.001; p<0.001) and control group. Also TAS levels were lower in the schizophrenia (p<0.001), bipolar disorder (p=0.001), major depression (p<0.001) groups than the control group.
Fig. 1.
Total oxidant status levels of control and patients groups. ECT, electroconvulsive therapy.
Fig. 2.
Total antioxidant status levels of control and patients groups. ECT, electroconvulsive therapy.
Table 2.
Oxidative values before and after electroconvulsive therapy (ECT)
| Control group (n=37) | Schizophrenia (n=11) | Bipolar disorder (n=8) | Major depression (n=14) | |
|---|---|---|---|---|
| TOS before ECT | 4.42 (3.12–5.16) | 7.72 (4.80–10.3)* | 5.64 (3.40–6.54) | 5.47 (4.39–6.34)† |
| TOS after the first ECT | 5.76 (3.87–6.25) | 3.99 (3.07–5.72) | 4.27 (3.71–5.15) | |
| TOS after the last ECT | 3.22 (2.11–7.35)** | 4.01 (2.90–5.60) | 4.06 (3.26–4.75)** | |
| TAS before ECT | 1.55 (1.30–2.19) | 0.87 (0.66–1.56)‡ | 0.77 (0.66–1.26)‡ | 0.89 (0.77–1.05)§ |
| TAS after the first ECT | 1.16 (0.95–1.77) | 1.06 (0.96–1.27) | 1.34 (0.83–1.55)** | |
| TAS after the last ECT | 1.11 (0.81–1.28) | 1.30 (0.85–1.74) | 1.03 (0.61–1.56) | |
| OSI before ECT | 0.26 (0.17–0.39) | 1.08 (0.39–1.76)* | 0.56 (0.32–0.89)¶ | 0.63 (0.42–0.93) |
| OSI after the first ECT | 0.52 (0.23–1.02) | 0.32 (0.27–0.61) | 0.30 (0.27–0.55) | |
| OSI after the last ECT | 0.34 (0.19–0.57)†† | 0.30 (0.19–0.59) | 0.41 (0.24–0.69) |
Values are presented as median (interquartile range).
The values of TOS, TAS, OSI were given as μmol H2O2 equivalent/L, mmol Trolox equivalent/L, (μmol/L H2O2)/(μmol/L Trolox), respectively.
p=0.001,
p=0.005,
p=0.004,
p=0.0001, and
p=0.009 compared with controls;
p=0.004 and
p=0.006 compared with before ECT values.
TOS, total oxidant status; TAS, total antioxidant status; OSI, oxidative stress index.
Fig. 3.
Oxidative stress index levels of control and patients groups. ECT, electroconvulsive therapy.
After the last ECT, TOS values in patients with major depression and schizophrenia were significantly lower than the before ECT values (p=0.004 for both) (Table 2).
It has been demostrated that after the first ECT, TAS values were higher in patients with major depression compared to before ECT values (p=0.004). There was no statistically significant difference between after the last and the first ECT in terms of TAS values in three patient groups (Table 2).
OSI values after the first ECT were lower than in patients with bipolar disorder, major depression and schizophrenia to the before ECT values, but this reduction was not significant (p>0.05, p=0.019, p=0.033, respectively). Statistically significant difference between last ECT and before ECT OSI values was found only in the patients with schizophrenia (p=0.006) (Table 2).
When we analyzed the correlation between the TOS, TAS, and OSI levels, TOS and OSI a significant positive correlation was found between them in major depression and schizophrenia (r=0.667, p=0.009; r=0.918, p=0.0001 respectively); a negative correlation was found between the serum TOS and TAS levels in schizophrenia (r= −0.736, p=0.01). Also a negative correlation was found between the TAS and OSI levels in major depression, schizophrenia and bipolar disorder (r= −0.761, p=0.002; r= −0.891, p=0.0001; r= −0.833, p=0.01, respectively) (Table 3).
Table 3.
Correlation table
| TOS-TAS | TOS-OSI | TAS-OSI | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| r value | p value | r value | p value | r value | p value | |
| Major depression | −0.232 | >0.05 | 0.667 | 0.009** | −0.761 | 0.002** |
| Schizophrenia | −0.736 | 0.010** | 0.918 | 0.0001** | −0.891 | 0.0001** |
| Bipolar disorder | 0.048 | >0.05 | 0.452 | >0.05 | −0.833 | 0.010** |
p<0.05,
p<0.01, r correlation coefficient.
TOS, total oxidant status; TAS, total antioxidant status; OSI, oxidative stress index.
DISCUSSION
The results of investigations regarding to the effects of ECT on oxidative metabolism are controversial and inadequate.7,8,12,13) In an animal experiment, Feier et al.13) showed that, after single or eight electroconvulsive shocks, while lipid peroxidation product, MDA and protein carbonyl levels, showing protein damage, increased, the activity of SOD and CAT decreased in various regions of the brain of rats. In other words, there was an increase in oxidative stress and decrease in antioxidant enzymes activities after the electroconvulsive shock.12) In another study, Eraković et al.14) reported decreases in SOD and CAT activities after single and repeated electroconvulsive shocks, in some parts of the rat brain, and determined that these enzymes did not return to normal levels even after 24 and 48 hours. Zupan et al.7) found that the oxidative response in brain after single electroconvulsive shock showed regional differences, only frontal cortex was subjected to oxidative damage. Lipid peroxidation was not observed in some areas such as hippocampus, cerebellum, even increases in SOD and GPX activities were detected in these regions. Jornada et al.12) did not observe evidence of lipid peroxidation and protein damagein the hippocampus, cerebral cortex, cerebellum and striatumin 48th hour and on 7th day after electroconvulsive shock. Barichello et al.8) examined the oxidative changes in brain of rats occuring on the 0, 48th hours, 7th and 30th days after single and eight electroconvulsive shocks. Unlike the above-mentioned findings, a decrease in MDA levels in the brain after a single electroconvulsive shock on the 0 hour and 30th day and a decrease in protein carbonyl groups on the 7th and 30th days were determined. In the 48th hour after single electroconvulsive shock, only SOD activity was found to be increased; on the 7th day after repeated electroconvulsive shocks, SOD and CAT activities were found to be increased.8)
It has been reported that, convulsion increases the release of excitatory neurotransmitters, and causes oxidative damage in the central nervous system secondary to this increase.15) On the basis of this information, it is expected that, oxidative stress increases after electroconvulsive shock. However, excitation after electroconvulsive shock may not play a major role in oxidative stress unlike previous animal models, thus oxidative damage may not be observed. Also, oxidative stress may depend on variability in the dose or duration of electroconvulsive shock.
Although it is not in complete agreement with our study, the study of Barichello et al.8) supported our study demostrating that TAS levels increased and TOS levels decreased after ECT. However, it should not be forgotten that, the oxidant and antioxidant status examined in peripheral blood, which is one of the limitations of our study-may not reflect the oxidative stress in the brain exactly. Also, the presence of any psychiatric illness was not reported in animal studies and it is possible that, as opposed to ECT treatment in human studies, sufficient oxygenation is likely not to be achieved in experimental studies.
In our study, significant differences in TOS and OSI values were not observed when before and after the first ECT values were compared in all groups, but, only in major depression patients, TAS values after first ECT were higher than in before ECT values. But, there was no significant difference between before ECT and last ECT in terms of TAS levels in major depression patients. After the last ECT, TOS values significantly decreased in major depression and schizophrenia group, OSI values significantly decreased in schizophrenia group compared to before ECT values. From these data, it can be said that ECT may not lead to oxidative stress, contrary ECT may reduce oxidative stress. Decreasing TOS levels, in contrary to expectations after ECT treatment, may bring to mind that ECT reduces oxidative stress via a different mechanism. It may have protective effect against oxidative damage by increasing total antioxidant status (we found that TAS values after first ECT increased in major depression). However, repeated ECT application may not effective in increasing TAS levels. It may be speculated that recurrent ECT application may prevent the formation of reactive oxygen species without affecting the TAS values. Additionally, it may decrease oxidants via treatment of psychiatric illness. Prevention of muscle contraction with anesthesia and giving oxygen during the ECT application may also prevent the increase of oxidants.
Virit et al.16) investigated the effects of ECT on serum MDA, NO levels and, xanthine oxidase and SOD activities in patients with major depression and bipolar disorders and they found that SOD activity decreased after ECT. In the study conducted by Kartalci et al.9) on the patients with schizophrenia, it was observed that plasma MDA levels and CAT activity were higher in patients than in healthy controls. They could not find any significant difference between two groups before ECT treatment in terms of plasma levels of NO and activity of GSH. Although, before ECT, plasma MDA levels similar to after first ECT values, it has been determined that, after the 9th ECT, MDA levels significantly decreased compared to the values before ECT.9) This finding supports our finding (the decrease in TOS levels after the last ECT in patients with schizophrenia and major depression).
The relation of oxidant-antioxidant and drug treatment must be taken into account in these three disease groups when the changes in oxidative metabolism after ECT are being evaluated.
Furthermore, Herken et al.17) observed increase in SOD activities and decrease in XO activities and NO levels after 8 weeks of antidepressant treatment. Khanzode et al.18) found that, the antidepressant treatment reduced the levels of MDA and activities of SOD, increased the amount of ascorbic acid. Sarandol et al.19) did not observe any change of red blood cell (RBC) SOD activity, whole blood GPx, plasma vitamin E, vitamin C, carotenoid with anti-depressant treatment. Cumurcu et al.20) suggested that the levels of TOS and OSI decreased and the level of TAS increased in patients with major depression after treatment.
Raffa et al.21) claimed that antioxidants such as glutathione, the activity of the RBC SOD did not alter by treatment of patients with schizophrenia. Dakhale et al.22) suggested that MDA and SOD values decreased and ascorbic acid increased with treatment. Padurariu et al.23) demonstrated the increase in the values of SOD in patients with schizophrenia treated with antipsychotics.
As a result of the chronic treatment of patients with bipolar disorder with valproate, which is a mood stabilizer, Wang et al.24) observed that glutathione-S-transferase iso-enzymes increased mRNA expression in cultured neurons. Again, Wang et al.25) found that mood regulators protected neuronal lipids and proteins against oxidative damage. Selek et al.26) claimed that NO levels decreased, SOD activity increased after treatment in patients with bipolar disorders. It has been demostrated that, there was a significant improvement in depressive symptoms, functioning and quality of life with the addition of glutathione precursors N-acetylcysteine which is an antioxidant, in the treatment of patients with bipolar disorder.27)
It has been demonstrated that, antidepressant, anti-psychotic and mood regulators had a protective effect against neuronal damage in patients with psychiatric disorders by eliminating or reducing oxidative stress often appears to be increased. These data are important for our study because in human studies, ECT and drug therapy were combined and the effect of this combined treatment on oxidative metabolism was examined. In our study, drug therapy was combined with ECT. Thus differences in the results may be attributed to ECT. In the studies, without making combination of ECT with drug therapy, examing the effect of ECT only can give more scientific results. However, to accomplish this seems not to be possible due to unwanted situations such as propensity to commit suicide can emerge in psychiatric patients during the time without drug therapy.
Smoking affects oxidant-antioxidant balance in favour of oxidation. In the study conducted by Ustundag et al.,28) while statistical difference was not demostrated in TAS values healthy smoker and nonsmoker participants, it has been detected that TAS levels in smoking patients with schizophrenia were lower than non-smokers ones. In the study conducted by Kosecik et al.,29) it has been found that, the values of TOS and OSI were higher and TAS lower in children, who were passive smokers, than the non passive smoker children. In our study, the control and patient groups were evaluated among themselves, it has been observed that, there was no difference between smokers and non-smokers in terms of TOS, TAS, and OSI.
There are some limitations in this study. The sample size was small and we did not evaluate the other oxidant and antioxidant parameters. And we do not make any measurements of TAS, TOS, and OSI between first and last ECT.
As a conclusion, ECT may not be cause of oxidative stress contrary to this decrease. Our study is the first study evaluating OSI by examining TOS and TAS after ECT in patients with schizophrenia. However, it is the first study evaluating OSI by examining TOS and TAS after first ECT in these psychiatric disorders.
However, further studies with more patients are needed.
REFERENCES
- 1.Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 2.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savas HA, Gergerlioglu HS, Armutcu F, Herken H, Yilmaz HR, Kocoglu E, et al. Elevated serum nitric oxide and superoxide dismutase in euthymic bipolar patients: impact of past episodes. World J Biol Psychiatry. 2006;7:51–55. doi: 10.1080/15622970510029993. [DOI] [PubMed] [Google Scholar]
- 5.Yu YW, Chen TJ, Wang YC, Liou YJ, Hong CJ, Tsai SJ. Association analysis for neuronal nitric oxide synthase gene polymorphism with major depression and fluoxetine response. Neuropsychobiology. 2003;47:137–140. doi: 10.1159/000070582. [DOI] [PubMed] [Google Scholar]
- 6.Sengul MC, Kenar AN, Hanci E, Sendur İ, Sengul C, Herken H. Practice of acute and maintenance electroconvulsive therapy in the psychiatric clinic of a university hospital from Turkey: between 2007 and 2013. Clin Psychopharmacol Neurosci. 2016;14:57–63. doi: 10.9758/cpn.2016.14.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zupan G, Pilipović K, Hrelja A, Peternel S. Oxidative stress parameters in different rat brain structures after electroconvulsive shock-induced seizures. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:771–777. doi: 10.1016/j.pnpbp.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Barichello T, Bonatto F, Feier G, Martins MR, Moreira JC, Dal-Pizzol F, et al. No evidence for oxidative damage in the hippocampus after acute and chronic electroshock in rats. Brain Res. 2004;1014:177–183. doi: 10.1016/j.brainres.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Kartalci S, Karabulut AB, Ozcan AC, Porgali E, Unal S. Acute and chronic effects of electroconvulsive treatment on oxidative parameters in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1689–1694. doi: 10.1016/j.pnpbp.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Pazvantoglu O, Selek S, Okay IT, Sengul C, Karabekiroglu K, Dilbaz N, et al. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin Neurosci. 2009;63:693–700. doi: 10.1111/j.1440-1819.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 11.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Jornada LK, Feier G, Barichello T, Vitali AM, Reinke A, Gavioli EC, et al. Effects of maintenance electroshock on the oxidative damage parameters in the rat brain. Neurochem Res. 2007;32:389–394. doi: 10.1007/s11064-006-9214-8. [DOI] [PubMed] [Google Scholar]
- 13.Feier G, Jornada LK, Barichello T, Vitali AM, Bonatto F, Moreira JC, et al. Long lasting effects of electroconvulsive seizures on brain oxidative parameters. Neurochem Res. 2006;31:665–670. doi: 10.1007/s11064-006-9064-4. [DOI] [PubMed] [Google Scholar]
- 14.Eraković V, Zupan G, Varljen J, Radosević S, Simoniv A. Electroconvulsive shock in rats: changes in superoxide dismutase and glutathione peroxidase activity. Brain Res Mol Brain Res. 2000;76:266–274. doi: 10.1016/S0169-328X(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 15.Dal-Pizzol F, Klamt F, Vianna MM, Schröder N, Quevedo J, Benfato MS, et al. Lipid peroxidation in hippocampus early and late after status epilepticus induced by pilocarpine or kainic acid in Wistar rats. Neurosci Lett. 2000;291:179–182. doi: 10.1016/S0304-3940(00)01409-9. [DOI] [PubMed] [Google Scholar]
- 16.Virit O, Dakılıç A, Bulut M, Bülbül F, Altındağ A, Armutçu F, et al. Decreased superoxide dismutase activity after ECT and correlation between higher oxidant levels and poor response to ECT in depression. Arch Neuropsychiatr. 2010;47:247–251. [Google Scholar]
- 17.Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247–252. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 19.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 20.Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63:639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 21.Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–1183. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Dakhale G, Khanzode S, Khanzode S, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–209. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- 23.Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479:317–320. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 24.Wang JF, Shao L, Sun X, Young LT. Glutathione S-transferase is a novel target for mood stabilizing drugs in primary cultured neurons. J Neurochem. 2004;88:1477–1484. doi: 10.1046/j.1471-4159.2003.02276.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang JF, Azzam JE, Young LT. Valproate inhibits oxidative damage to lipid and protein in primary cultured rat cerebrocortical cells. Neuroscience. 2003;116:485–489. doi: 10.1016/S0306-4522(02)00655-3. [DOI] [PubMed] [Google Scholar]
- 26.Selek S, Savas HA, Gergerlioglu HS, Bulbul F, Uz E, Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord. 2008;107:89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Ustundag B, Atmaca M, Kirtas O, Selek S, Metin K, Tezcan E. Total antioxidant response in patients with schizophrenia. Psychiatry Clin Neurosci. 2006;60:458–464. doi: 10.1111/j.1440-1819.2006.01532.x. [DOI] [PubMed] [Google Scholar]
- 29.Kosecik M, Erel O, Sevinc E, Selek S. Increased oxidative stress in children exposed to passive smoking. Int J Cardiol. 2005;100:61–64. doi: 10.1016/j.ijcard.2004.05.069. [DOI] [PubMed] [Google Scholar]