Abstract
Objective
CDKN2B-AS1 polymorphisms were shown to associate with the risk of stroke in European. The goal of this study was to evaluate the contribution of CDKN2B-AS1 rs1333049 to the risk of hemorrhagic stroke (HS) and brain tumor (BT) in Han Chinese.
Methods
A total of 142 HSs, 115 BTs, and 494 controls were included in the current association study. The genotyping test was performed using the melting temperature shift method.
Results
We failed to validate the association of CDKN2B-AS1 rs1333049 with the risk of brain disease. Significantly higher levels of low-density lipoprotein cholesterol (LDL-C) (p=0.027), high-density lipoprotein cholesterol (HDL-C) (p<0.001) and total cholesterol (TC) (p<0.001) were found in HSs in the genotype GG/GC carriers, but not the genotype CC carriers (p>0.05). The meta-analysis of 10 studies among 133,993 individuals concluded that rs1333049 of CDKN2B-AS1 gene was likely to increase a 16% incidence rate of cerebrovascular disease (CD) among various populations (odds ratio 1.16, 95% confidence interval 1.08–1.25; p<0.0001, random-effect method).
Conclusion
Our case-control study identified rs1333049 genotypes showed different association with the concentration of the LDL-C, HDL-C and TC in the HS patients. Meta-analysis supported the association between rs1333049 and CD risk in various populations, although we were unable to observe association between rs1333049 and the risk of HSs in Han Chinese.
Keywords: Hemorrhagic stroke, Cerebrovascular disease, CDKN2BAS1, rs1333049, Lipid concentration
INTRODUCTION
Hemorrhagic stroke (HS) accounts for approximately 30% of all strokes, and it tends to affect young adults and carries a high morbidity and mortality in the worldwide.1) Stroke, particularly HS, is becoming the most frequent and important vascular disorder in China.2) The stroke incidence is threefold higher in northern than that in southern Chinese cities, suggesting important environmental or genetic influences.3) Current studies have affirmed that inherited factor may play a key role in the pathogenesis of HS.4) Yet the mechanisms of brain diseases are not completely understood.
CDKN2B anti-sense RNA (CDKN2B-AS1) spans 126.3 kb, overlaps at its 5′ end with CDKN2B (p15), and includes 20 exons subjected to alternative splicing.5) CDKN2B-AS1, nearby the CDKN2A and CDKN2B genes, has been found to associate with the risk of multiple diseases including coronary heart disease (CHD),6) myocardial infarction (MI),7) hypertension,8) and stroke.9) CDKN2B-AS1 polymorphisms have been extensively identified as predictors of cardiovascular disease,10) cerebrovascular disease (CD),11) and also brain tumors (BTs).12) CDKN2B-AS1 is differentially expressed in a variety of tissues such as vascular endothelial cells and smooth coronary muscle cells.5) As a large antisense non-coding RNA, the CDKN2B-AS1 function is still unclear, but the CDKN2B-AS1 transcript level shows significant association with the severity of vascular diseases13) and cancers.14) CDKN2B-AS1 variants are showed to connect with CDKN2BAS expression in cardiovascular diseases.15) The single nucleotide polymorphism (SNP) rs1333049 is located in the 3′-untranslated region of the CDKN2B-AS1 gene. And this polymorphism may play a pivotal role in the development of cardio- or cerebra-vascular disease by altering the dynamics of vascular cell proliferation.16) CDKN2B-AS1 rs1333049 has been studied in different diseases such as CHD,17) atherosclerosis,14) MI,15) metabolic disease18) and Alzheimer’s disease.19) There are some published studies20–22) that focus on the association between rs1333049 and stroke, notwithstanding, no published study on Han Chinese population.
In this work, we recruited 257 patients with brain diseases (including 142 HSs and 115 BTs) and 494 controls, and performed a case-control test to validate the contribution of CDKN2B-AS1 rs1333049 to the risk of brain diseases in Han Chinese. In addition, we performed a meta-analysis of the available case-control studies between CDKN2B-AS1 rs1333049 and CD.
METHODS
Sample Collection
With the informed consent of all patients and approval of the Ethical Committee of Ningbo First Hospital (2013–002), a total of 257 unrelated patients and 494 controls (274 males and 220 females) were collected from the Department of Neurosurgery of the Ningbo First Hospital between March 2013 and March 2014. Of these, 142 HS patients (89 males and 53 females) diagnosed by digital subtraction angiography and 115 BT patients (60 males and 55 females) diagnosed by way of brain magnetic resonance imaging or computed tomography. Control participants were without symptoms or history of diseases, including stroke, autoimmune diseases, and severe liver or kidney disease. Five milliliters of venous blood sample was collected from each subject, saved into 3.2% citrate sodium-treated tubes and stored at −20ºC till use. The levels of triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) were determined by standard enzymatic methods.23)
Single-nucleotide Polymorphism Genotyping
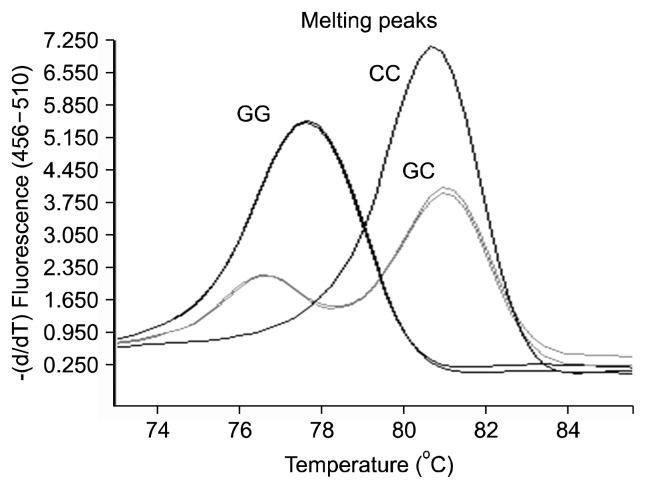
DNA extraction was performed though the magnetic bead isolation method.24) Temperature shift genotyping methods were used for the CDKN2B-AS1 rs1333049 genotyping test.25) The sequences of the primers of CDKN2B-AS1 rs1333049 are as follows: short primer 5′-gattaccg CCTCATACTAACCATATGATCAACAGTTG-3′; long primer 5′-gcgggcagggcggcCCTCATACTAACCATATG ATCAACAGTTC-3′; and one common primer 5′-GCTT ACCTCTGCGAGTGGCTGCT-3′. The polymerase chain reaction (PCR) amplification for genotyping was performed on the Roche Light Cycler® 480 (Roche, Mannheim, Germany) according to the manufacturer’s instructions. The PCR conditions included an initial denaturation at 95°C for 30 seconds, followed by 40 cycles of 95°C for 30 seconds, 59°C for 30 seconds, 72°C for 30 seconds, and a final extension for 30 seconds at 72°C. The SNP types were determined by the melting curve analysis using the analysis software from the quantitative PCR instrument (Fig. 1).
Fig. 1.
Genotyping results for the rs1333049 using the melting temperature shift polymerase chain reaction.
Statistical Analysis
Statistical analysis for the case-control study was performed using SPSS software ver. 16.0 (SPSS Inc., Chicago, IL, USA). The continuous variables were presented as means±standard deviation (SD). Student’s t test was performed to test the degree of difference between data from cases and controls. Genotype frequencies were tested for departure from Hardy-Weinberg equilibrium using the Arlequin program version 3.5 (University of Bern, Bern, Switzerland).26) The genotype distribution between case and control groups were analyzed by CLUMP22.27) The odds ratio (OR) and 95% confidence interval (CI) were calculated for the risk analysis. Power analysis was made by Power and Sample Size Calculation software version 3.0.43 (Nashville, TN, USA). The meta-analysis was performed using the Stata statistical software version 11.0 (Stata Corporation, College Station, TX, USA). The publication bias was evaluated by the egger test. Z test was used to conclude the pooled OR. A p value <0.05 was considered to be significant.
RESULTS
Distribution of rs1333049 in Neurosurgical Patients and Controls
The genotype and allele frequencies of rs1333049 in cases and controls were shown in Table 1. The rs1333049-C allele frequencies were 0.494 in all patients, 0.487 in BT cases, 0.500 in HS participants and 0.466 in healthy controls. There were no significant differences in the genotype and allele distribution between controls and each of the three case groups (allele model, dominant model and recessive model, p>0.05; Table 1). A breakdown analysis by gender demonstrated a negative association of rs1333049 with brain diseases under any genetic models (p>0.05; Table 1). In addition, our age-stratified association test was unable to detect any relationship of rs1333049 with neurosurgical patients (p>0.05; Table 1).
Table 1.
The distribution of genotypic and allelic frequencies in rs1333049 between cases and controls
| Variable | Group (n) | Genotype (n), GG/GC/CC | Allele (n), G/C | Allele model | Dominant model | Recessive model |
|---|---|---|---|---|---|---|
| All | Controls (494) | 134/260/100 | 528/460 | |||
| All patients (257) | 60/140/57 | 260/254 | 1.12 (0.91–1.39) | 1.12 (0.78–1.62) | 1.22 (0.86–1.73) | |
| BT (115) | 25/68/22 | 118/112 | 1.09 (0.82–1.45) | 0.93 (0.56–1.56) | 1.34 (0.84–2.19) | |
| HS (142) | 35/72/35 | 142/142 | 1.15 (0.88–1.50) | 1.29 (0.83–2.00) | 1.14 (0.74–1.75) | |
| Gender | ||||||
| Male | Controls (274) | 76/134/64 | 286/262 | |||
| All patients (149) | 34/80/148 | 148/150 | 1.11 (0.83–1.47) | 1.01 (0.63–1.61) | 1.30 (0.82–2.07) | |
| BT (60) | 13/36/11 | 62/58 | 1.02 (0.69–1.52) | 0.74 (0.36–1.50) | 1.39 (0.71–2.71) | |
| HS (89) | 21/44/24 | 86/92 | 1.17 (0.83–1.64) | 1.21 (0.70–2.09) | 1.24 (0.71–2.17) | |
| Female | Controls (220) | 58/126/36 | 242/198 | |||
| All patients (108) | 26/60/22 | 112/104 | 1.13 (0.92–1.57) | 1.31 (0.73–2.36) | 1.13 (0.66–1.93) | |
| BT (55) | 12/32/11 | 56/54 | 1.18 (0.78–1.79) | 1.28 (0.60–2.71) | 1.28 (0.63–2.60) | |
| HS (53) | 14/28/11 | 56/50 | 1.09 (0.71–1.67) | 1.34 (0.63–2.84) | 1.00 (0.51–1.97) | |
| Age (yr) | ||||||
| <65 | Controls (373) | 101/202/70 | 404/342 | |||
| All patients (196) | 44/106/46 | 194/198 | 1.21 (0.94–1.54) | 1.33 (0.87–2.02) | 1.28 (0.85–1.92) | |
| >65 | Controls (121) | 33/58/30 | 124/118 | |||
| All patients (61) | 16/34/11 | 66/56 | 0.89 (0.58–1.38) | 0.67 (0.31–1.44) | 1.05 (0.53–2.12) | |
Values are presented as number only or odds ratio (95% confidence interval).
BT, brain tumor; HS, hemorrhagic stroke.
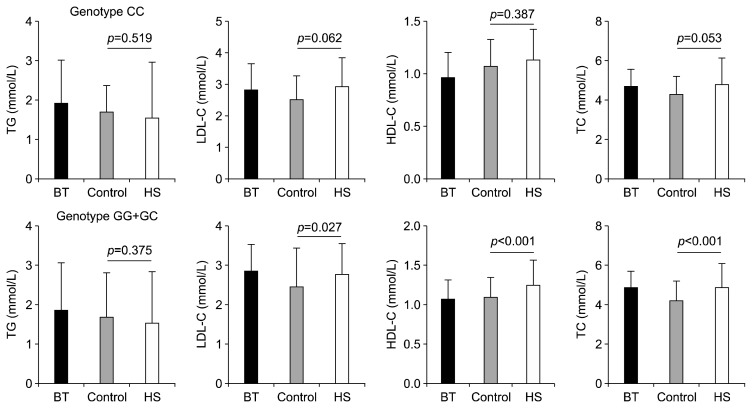
Previous study showed that rs1333049 genotypes might play a key pharmacogenomics role in hypercholesterolemia. Therefore, we further stratified the data analysis in the different lipids by rs1333049 genotypes. As shown in Figure 2, there were significantly higher levels of LDL-C (p=0.027), HDL-C (p<0.001) and TC (p<0.001) in HSs than in controls in the genotype GG/GC carriers, but not the genotype CC carriers (p>0.05).
Fig. 2.
The comparison of characteristics between cases and controls in different genotypes.
BT, brain tumor; HS, hemorrhagic stroke; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol.
Meta-analysis of the Association between rs1333049 and the Risk of CD
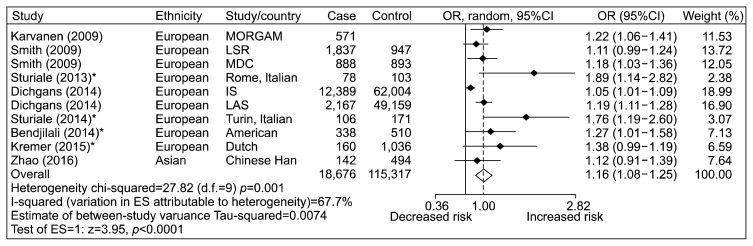
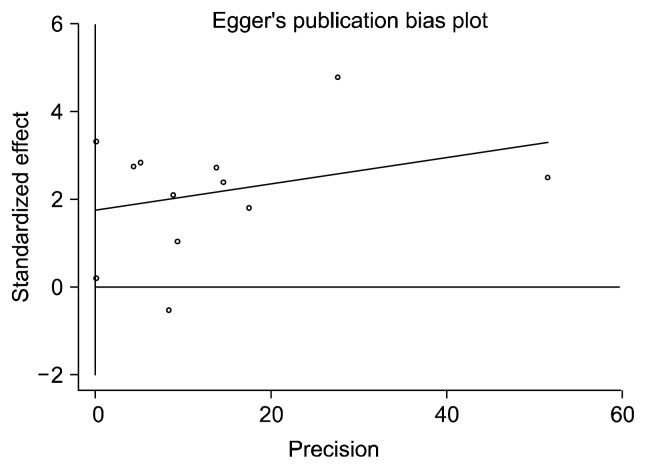
A total of 10 study stages11,20–22,28–30) (including 18,676 cases and 115,317 controls) were selected into the meta-analysis for the association of rs1333049 of CDKN2B-AS1 gene with CD. Since substantial heterogeneity were observed among the overall studies (p=0.001, I2=67.7%), random-effect method was applied for the meta-analysis. As shown in Figure 3, the data showed that CDKN2B-AS1 rs1333049 was a risk factor of CD (overall OR=1.16, 95% CI=1.08–1.25, p<0.0001, random-effect method). The publication bias of studies was analyzed by the Egger test. As shown in Figure 4, the results did not show any evidence of publication bias for the analysis (p(Egger)=0.056).
Fig. 3.
Meta-analysis of the studies between rs1333049 and cerebrovascular disease risk.
MORGAM, an international pooling of cardiovascular cohorts; LSR: the Lund Stroke Register; MDC, the Malmo Diet and Cancer Study; IS, ischemic stroke; LAS, large artery stroke; OR, odds ratio; CI, confidence interval; d.f., degree of freedom; ES, effect size.
*rs1333049 showed high linkage disequilibrium with rs1333040 (0.81≤r2≤0.97) in the previous studies.36,37)
Fig. 4.
Egger test for the publication bias of the enrolled studies.
DISCUSSION
In the present study, we aimed to explore the significant association between the CDKN2B-AS1 rs1333049 with the risk of brain disease through the case-control study and meta-analysis. Our case-control study couldn’t find obvious relationship with the risk of BTs or HSs. But, the results showed that rs1333049 genotypes were strongly connected with the lipid concentration in HS patients. Our meta-analysis enrolled 133,993 subjects concluded that CDKN2B-AS1 rs1333049 contributed to the risk of CD, although substantial heterogeneity was shown in the involved studies.
Epidemiologic studies had demonstrated that blood lipid lipoprotein abnormalities were connected with the clinical manifestations in different diseases.31,32) And genetic variants could influence the circulating lipid levels in patients.33) Ahmed et al.34) found that the CDKN2B-AS1 rs1333049 genotype could influence the statin therapy in the hyperlipidemia families. The patients on statin therapy with the rs1333049-CC genotype had significantly lower LDL-C and TC as compared to the rs1333049-CG/GG carriers.34) In current study, our results showed that the genotype GG/GC carriers had significantly higher levels of LDL-C (p=0.027), HDL-C (p<0.001) and TC (p< 0.001) in HSs than in controls, but not the genotype CC carriers (p>0.05). We suspected that this phenomenon was related to increased plasma levels of pharmacologically active metabolites.
SNP rs1333049 in CDKN2B-AS1 was located on chromosome 9p21.3, which was considered as the most widely and consistently replicated risk locus for cardiovascular and CDs.29) Previous study showed that CDKN2B-AS1 polymorphism significantly associated with the risk of glioma by affecting the gliomagenesis.35) The CDKN2B-AS1 polymorphism rs1333040 was located on the risk locus chromosome 9p21.3, and showed high linkage disequilibrium with rs1333049 (0.81≤r2≤0.97) in previous studies.36,37) Sturiale et al.28) suggested that the distribution of the rs1333040 genotypes was statistically different between sporadic brain arteriovenous malformations and controls. And the rs1333040 was significantly associated with BAVMs in all the three genetic models.29) CDKN2B-AS1 rs1333049-C allele was certified to increase the risk of stroke in the MORGAM Project.20) In the large-scale genetic association study, Smith et al.21) detected that rs1333049 was a common genetic determinant for ischemic stroke on chromosome 9p21. In the following study, Dichgans et al.22) demonstrated that rs1333049 was associated with a significant excess risk for ischemic stroke and particularly for the large artery stroke subtype with CHD. The meta-analysis of 10 studies (including our study) among 133,993 individuals concluded that rs1333049 of CDKN2B-AS1 gene was likely to increase a 16% incidence rate of CD among various populations. Power analyses showed that our meta-analysis of rs1333049 had a perfect power (power=98%) to detect a susceptibility locus at the nominal type1 error rate of 0.05. In the case-control study, the results had only less than 30% power in the association tests. Thus, our results could not find any significant association between rs1333049 and BTs or HSs in Han Chinese. The lack of association may be due to the genetic heterogeneity of this locus or a lack of power in our sample for this minor-effect genetic marker in Chinese.
In present study, we failed to validate the association of CDKN2B-AS1 rs1333049 with the risk of brain disease in Han Chinese. The sample size of the case-control study was comparatively small, and the age and gender were not well matched. Therefore, we could not exclude a chance of random positive finding of the genotype association. Further studies utilizing a well-matched group with a larger sample size are warranted to increase the confidence of our findings.
In conclusion, our case-control study identified rs1333049 genotypes showed different association with the concentration of the LDL-C, HDL-C and TC in the HS patients. Meta-analysis supported the association between rs1333049 and CD risk in various populations. However, this study was designed as a pilot study and further investigations are needed to confirm our results and to elucidate unresolved questions.
Acknowledgments
This study was supported by the grants from the Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ17H090002, Ningbo People of Science and Technology Projects No. 2015C50005, Ningbo Natural Science Foundation No. 2014A610260, 2015A610232, and 2016A610154, Zhejiang Traditional Chinese Medicine Science and Technology Projects No. 2015ZB100, Ningbo Youth and Doctor Foundation, Ningbo High Level Innovative Talents Program.
REFERENCES
- 1.Labeyrie PE, Courthéoux P, Babin E, Bergot E, Touzé E, Pelage JP. Neurological involvement in hereditary hemorrhagic telangiectasia. J Neuroradiol. 2016;43:236–245. doi: 10.1016/j.neurad.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Wei JW, Huang Y, Wang JG, Liu M, Wong LK, Huang Q, et al. Current management of intracerebral haemorrhage in China: a national, multi-centre, hospital register study. BMC Neurol. 2011;11:16. doi: 10.1186/1471-2377-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Z, Yue Y, Leung CC, Chan MT, Gelb AW. Clinical diagnostic tools for screening of perioperative stroke in general surgery: a systematic review. Br J Anaesth. 2016;116:328–338. doi: 10.1093/bja/aev452. [DOI] [PubMed] [Google Scholar]
- 4.Ren C, Kobeissy F, Alawieh A, Li N, Li N, Zibara K, et al. Assessment of serum UCH-L1 and GFAP in acute stroke patients. Sci Rep. 2016;6:24588. doi: 10.1038/srep24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarinova O, Stewart AF, Roberts R, Wells G, Lau P, Naing T, et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler Thromb Vasc Biol. 2009;29:1671–1677. doi: 10.1161/ATVBAHA.109.189522. [DOI] [PubMed] [Google Scholar]
- 6.Dehghan A, Bis JC, White CC, Smith AV, Morrison AC, Cupples LA, et al. Genome-wide association study for incident myocardial infarction and coronary heart disease in prospective cohort studies: the CHARGE consortium. PLoS One. 2016;11:e0144997. doi: 10.1371/journal.pone.0144997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuoka R, Abe S, Tokoro F, Arai M, Noda T, Watanabe S, et al. Association of six genetic variants with myocardial infarction. Int J Mol Med. 2015;35:1451–1459. doi: 10.3892/ijmm.2015.2115. [DOI] [PubMed] [Google Scholar]
- 8.Bayoglu B, Yuksel H, Cakmak HA, Dirican A, Cengiz M. Polymorphisms in the long non-coding RNA CDKN2B-AS1 may contribute to higher systolic blood pressure levels in hypertensive patients. Clin Biochem. 2016;49:821–827. doi: 10.1016/j.clinbiochem.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Bai Y, Nie S, Jiang G, Zhou Y, Zhou M, Zhao Y, et al. Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p.C10X) with ischemic stroke. Stroke. 2014;45:383–388. doi: 10.1161/STROKEAHA.113.003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Ye H, Hong Q, Xu X, Jiang D, Xu L, et al. Association of CDKN2BAS polymorphism rs4977574 with coronary heart disease: a case-control study and a meta-analysis. Int J Mol Sci. 2014;15:17478–17492. doi: 10.3390/ijms151017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer PH, Koeleman BP, Pawlikowska L, Weinsheimer S, Bendjilali N, Sidney S, et al. Evaluation of genetic risk loci for intracranial aneurysms in sporadic arteriovenous malformations of the brain. J Neurol Neurosurg Psychiatry. 2015;86:524–529. doi: 10.1136/jnnp-2013-307276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adel Fahmideh M, Lavebratt C, Schüz J, Röösli M, Tynes T, Grotzer MA, et al. CCDC26, CDKN2BAS, RTEL1 and TERT Polymorphisms in pediatric brain tumor susceptibility. Carcinogenesis. 2015;36:876–882. doi: 10.1093/carcin/bgv074. [DOI] [PubMed] [Google Scholar]
- 13.Holdt LM, Hoffmann S, Sass K, Langenberger D, Scholz M, Krohn K, et al. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet. 2013;9:e1003588. doi: 10.1371/journal.pgen.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bochenek G, Häsler R, El Mokhtari NE, König IR, Loos BG, Jepsen S, et al. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 2013;22:4516–4527. doi: 10.1093/hmg/ddt299. [DOI] [PubMed] [Google Scholar]
- 15.Haslacher H, Perkmann T, Ratzinger F, Grimm G, Exner M, Keller A, et al. 9p21.3 risk locus is associated with first-ever myocardial infarction in an Austrian cohort. J Cardiovasc Med (Hagerstown) 2016;17:595–600. doi: 10.2459/JCM.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 16.Cunnington MS, Santibanez Koref M, Mayosi BM, Burn J, Keavney B. Chromosome 9p21 SNPs associated with multiple disease phenotypes correlate with ANRIL Expression. PLoS Genet. 2010;6:e1000899. doi: 10.1371/journal.pgen.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dechamethakun S, Ikeda S, Arai T, Sato N, Sawabe M, Muramatsu M. Associations between the CDKN2A/B, ADTRP and PDGFD polymorphisms and the development of coronary atherosclerosis in Japanese patients. J Atheroscler Thromb. 2014;21:680–690. doi: 10.5551/jat.22640. [DOI] [PubMed] [Google Scholar]
- 18.Hannou SA, Wouters K, Paumelle R, Staels B. Functional genomics of the CDKN2A/B locus in cardiovascular and metabolic disease: what have we learned from GWASs? Trends Endocrinol Metab. 2015;26:176–184. doi: 10.1016/j.tem.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Popov N, Gil J. Epigenetic regulation of the INK4b-ARF-INK4a locus: in sickness and in health. Epigenetics. 2010;5:685–690. doi: 10.4161/epi.5.8.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karvanen J, Silander K, Kee F, Tiret L, Salomaa V, Kuulasmaa K, et al. The impact of newly identified loci on coronary heart disease, stroke and total mortality in the MORGAM prospective cohorts. Genet Epidemiol. 2009;33:237–246. doi: 10.1002/gepi.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JG, Melander O, Lövkvist H, Hedblad B, Engström G, Nilsson P, et al. Common genetic variants on chromosome 9p21 confers risk of ischemic stroke: a large-scale genetic association study. Circ Cardiovasc Genet. 2009;2:159–164. doi: 10.1161/CIRCGENETICS.108.835173. [DOI] [PubMed] [Google Scholar]
- 22.Dichgans M, Malik R, König IR, Rosand J, Clarke R, Gretarsdottir S, et al. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke. 2014;45:24–36. doi: 10.1161/STROKEAHA.113.002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Nie S, Zhou S, Li K, Sun J, Zhao J, et al. PPARD rs2016520 polymorphism and circulating lipid levels connect with brain diseases in Han Chinese and suggest sex-dependent effects. Biomed Pharmacother. 2015;70:7–11. doi: 10.1016/j.biopha.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Zhao J, Sun J, Nie S, Li K, Gao F, et al. Sex-dichotomous effects of NOS1AP promoter DNA methylation on intracranial aneurysm and brain arteriovenous malformation. Neurosci Lett. 2016;621:47–53. doi: 10.1016/j.neulet.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Zhou S, Zhao J, Wang Z, Li K, Nie S, Gao F, et al. Association study of BUD13-ZNF259 gene rs964184 polymorphism and hemorrhagic stroke risk. Int J Clin Exp Med. 2015;8:22503–22508. [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Zhou J, Ye H, Xu L, Le Y, Yang X, et al. Relationship between chemokine (C-X-C motif) ligand 12 gene variant (rs1746048) and coronary heart disease: case-control study and meta-analysis. Gene. 2013;521:38–44. doi: 10.1016/j.gene.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Huang Y, Huang RS, Wang F, Xu L, Le Y, et al. A case-control study provides evidence of association for a common SNP rs974819 in PDGFD to coronary heart disease and suggests a sex-dependent effect. Thromb Res. 2012;130:602–606. doi: 10.1016/j.thromres.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Sturiale CL, Gatto I, Puca A, D’Arrigo S, Giarretta I, Albanese A, et al. Association between the rs1333040 polymorphism on the chromosomal 9p21 locus and sporadic brain arteriovenous malformations. J Neurol Neurosurg Psychiatry. 2013;84:1059–1062. doi: 10.1136/jnnp-2012-304045. [DOI] [PubMed] [Google Scholar]
- 29.Sturiale CL, Fontanella MM, Gatto I, Puca A, Giarretta I, D’Arrigo S, et al. Association between polymorphisms rs1333040 and rs7865618 of chromosome 9p21 and sporadic brain arteriovenous malformations. Cerebrovasc Dis. 2014;37:290–295. doi: 10.1159/000360752. [DOI] [PubMed] [Google Scholar]
- 30.Bendjilali N, Nelson J, Weinsheimer S, Sidney S, Zaroff JG, Hetts SW, et al. Common variants on 9p21.3 are associated with brain arteriovenous malformations with accompanying arterial aneurysms. J Neurol Neurosurg Psychiatry. 2014;85:1280–1283. doi: 10.1136/jnnp-2013-306461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakaya N, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Oikawa S, et al. Large-scale cohort study on the relationship between serum lipid concentrations and risk of cerebrovascular disease under low-dose simvastatin in Japanese patients with hypercholesterolemia: sub-analysis of the Japan Lipid Intervention Trial (J-LIT) Circ J. 2005;69:1016–1021. doi: 10.1253/circj.69.1016. [DOI] [PubMed] [Google Scholar]
- 32.Bharosay A, Bharosay VV, Bandyopadhyay D, Sodani A, Varma M, Baruah H. Effect of lipid profile upon prognosis in ischemic and haemorrhagic cerebrovascular stroke. Indian J Clin Biochem. 2014;29:372–376. doi: 10.1007/s12291-013-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010;30:2264–2276. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed W, Ali IS, Riaz M, Younas A, Sadeque A, Niazi AK, et al. Association of ANRIL polymorphism (rs1333049: C>G) with myocardial infarction and its pharmacogenomic role in hypercholesterolemia. Gene. 2013;515:416–420. doi: 10.1016/j.gene.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 35.Wibom C, Späth F, Dahlin AM, Langseth H, Hovig E, Rajaraman P, et al. Investigation of established genetic risk variants for glioma in prediagnostic samples from a population-based nested case-control study. Cancer Epidemiol Biomarkers Prev. 2015;24:810–816. doi: 10.1158/1055-9965.EPI-14-1106. [DOI] [PubMed] [Google Scholar]
- 36.Chen SN, Ballantyne CM, Gotto AM, Jr, Marian AJ. The 9p21 susceptibility locus for coronary artery disease and the severity of coronary atherosclerosis. BMC Cardiovasc Disord. 2009;9:3. doi: 10.1186/1471-2261-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoppmann P, Erl A, Türk S, Tiroch K, Mehilli J, Schömig A, et al. No association of chromosome 9p21.3 variation with clinical and angiographic outcomes after placement of drugeluting stents. JACC Cardiovasc Interv. 2009;2:1149–1155. doi: 10.1016/j.jcin.2009.08.021. [DOI] [PubMed] [Google Scholar]