Abstract
Objective
Prenatal infection is implicated in the etiology of schizophrenia. The objective of this paper is to study the role of complement protein C1q in the psychosis of adult offspring after maternal immune activation (MIA). In addition, effect of 7,8-dihydroxyflavone (7,8-DHF: a tropomyosin receptor kinase B [TrkB] agonist) was also examined.
Methods
Western blot analysis of C1q in the brain regions from adult offspring after prenatal poly(I:C) (5.0 mg/kg/day from E12 to E17) exposure was performed. 7,8-DHF or vehicle was given from 4 to 8-weeks old.
Results
Expression of C1q in the prefrontal cortex (PFC) of adult offspring from poly(I:C)-treated pregnant mice was significantly higher than that of control group. Early treatment with 7,8-DHF during juvenile and adolescent stages could prevent an increase of C1q in the PFC of adult offspring after MIA.
Conclusion
Therefore, it is likely that increased C1q expression in the frontal cortex may play a role in the behavioral abnormalities of adult offspring after MIA. Furthermore, supplementation with a TrkB agonist such as 7,8-DHF during the prodromal stage may have prophylactic effects on the behavioral abnormalities after MIA.
Keywords: Brain-derived neurotrophic factor, Complement protein C1q, Maternal immune activation, Prefrontal cortex, TrkB
INTRODUCTION
Prenatal infection could be implicated in the etiology of schizophrenia.1,2) The offspring of prenatal rodents exposed to polyriboinosinic-polyribocytidylic acid (poly(I:C)) mimics schizophrenia-like behavioral abnormalities in adulthood. Therefore, maternal immune activation (MIA) in rodents has been widely used as an animal model of neuro-developmental disorders such as schizophrenia.3,4)
Complement protein C1q (complement component 1, q subcomponent), the recognition molecule of the classical pathway, performs a diverse range of complement and non-complement function. Recent evidence suggests that C1q plays an important role in pregnancy where its deficiency and dysregulation can have adverse effects, leading to preeclampsia, missed abortion, miscarriage or spontaneous loss, and various infections.5,6) Furthermore, the C1q seropositivity was significantly associated with schizophrenia group compared to control group.7) Interestingly, levels of C1q IgG were significantly higher in mothers whose offspring developed psychoses as adults compared to control mothers.8) In case mothers only, C1q was significantly correlated with antibodies to both food and infections antigens including gluten, herpes simplex virus type 2, and adenovirus.8) These all findings suggest that exposure to maternal C1q activity during pregnancy may be a risk factor for the development of schizophrenia and psychosis in offspring. Therefore, it is of interest to study whether C1q expression is altered in the brain regions of adult offspring after MIA.
Recently, we reported decreased brain-derived neurotrolphic factor (BDNF) and its receptor tropomyosin receptor kinase B (TrkB) signaling in the prefrontal cortex (PFC) of juvenile and adult offspring after MIA.9) Interestingly, we found that supplementation of TrkB agonist 7,8-dihydroxyflavone (7,8-DHF)10,11) during juvenile and adolescent stages prevented behavioral abnormalities and loss of parvalbumin (PV)-immunoreactivity in the PFC of adult offspring after MIA.9) In the present study, we investigated whether C1q expression were altered in the brain regions from the adult offspring after prenatal poly(I:C) exposure. In addition, we examined the effect of 7,8-DHF supplementation during juvenile and adolescent stages on the alterations in C1q expression of adult offspring after MIA.
METHODS
Animals and Chemicals
Pregnant ddY mice (SLC Japan, Hamamatsu, Japan) were injected intraperitoneally with poly(I:C) (5.0 mg/kg/day from E12 to E17) dissolved in 1% phosphate buffer saline (PBS) or vehicle (10 ml/kg) as previously reported.9,12) The offspring mice were treated with vehicle or 7,8-DHF (1 mg/ml; Tokyo Chemical Industry Co. Ltd., Tokyo, Japan) in drinking water for consecutive from 4 to 8-weeks old. 7,8-DHF was dissolved in PBS containing 17% dimethyl sulfoxide to generate a stock solution at 100 mg/ml concentration. The stock solution (1 ml) was added to 100 ml of Hydropac (Hydro-Pac, Inc., Fairview, PA, USA) water that contained 1% sucrose (pH=7.4). Subsequently, normal water was given into all mice from 8 to 10-weeks old. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee (No. 28-274).
Brain Collection and Western Blot Analysis
The mice (n=5 or 6) were used for C1q Western blot analysis. The brain samples of PFC, CA1, CA3 and dentate gyrus (DG) of the hippocampus, and nucleus accumbens (NAc) were collected as described previously.9) Basically, tissue samples were homogenized in Laemmli lysis buffer. Aliquots (10 μg) of protein were measured using the DC protein assay kit (Bio-Rad, Hercules, CA, USA), and incubated for 5 minutes at 95°C, with an equal volume of 125 mM Tris/HCl, pH 6.8, 20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol, 4% sodium dodecyl sulfate, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, using 10% mini-gels (Mini-PROTEAN® TGX™ Precast Gel; Bio-Rad). Proteins were transferred onto polyvinylidenedifluoride membranes using a Trans Blot Mini Cell (Bio-Rad). For immunodetection, the blots were blocked with 2% bovine serum albumin in TBST (Tris buffer saline+0.1% Tween-20) for 1 hour at room temperature, and kept with primary antibodies overnight at 4°C. The following primary antibodies were used: anti-C1q antibody (1:500, sc-25856; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and β-actin (1:10,000, Sigma-Aldrich, St. Louis, MO, USA). The next day, blots were washed three times in TBST and incubated with horseradish peroxidase conjugated anti-rabbit antibody or anti-mouse antibody for 1 hour at room temperature. After final three washes with TBST, bands were detected using enhanced chemiluminescence plus the Western Blotting Detection system (GE Healthcare Bioscience, Tokyo, Japan). Images were captured with a Fuji LAS3000-mini imaging system (Fujifilm, Tokyo, Japan) and immunoreactive bands were quantified.
Statistical Analysis
The data are expressed as the mean±standard error of the mean (SEM). The data were analysed by two-way ANOVA, followed by post hoc Fisher’s least significant difference test. Statistical significance was set at p<0.05.
RESULTS
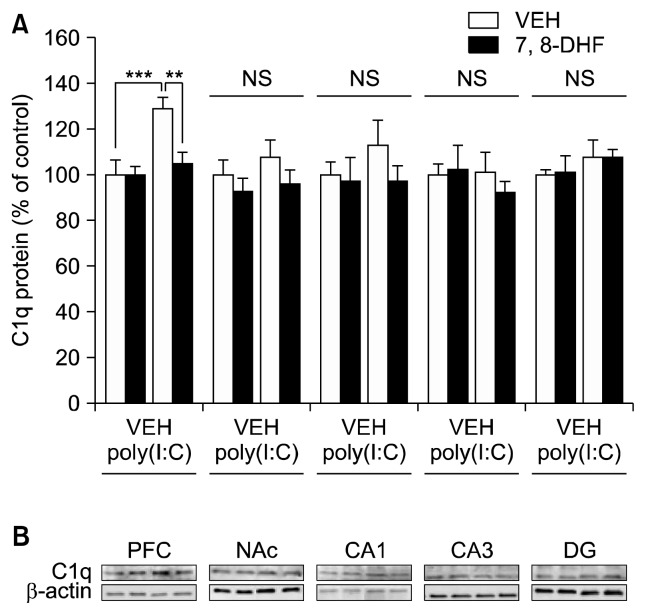
We performed Western blot analysis of C1q in the PFC, NAc, and CA1, CA3, DG after prenatal poly(I:C) exposure. Two-way ANOVA analysis of data in the PFC revealed statistical significant differences (poly(I:C): F1,20=11.16, p=0.003; 7,8-DHF: F1,20=5.411, p=0.031; interaction: F1,20=5.803, p=0.026) (Fig. 1). Interestingly, supplementation with 7,8-DHF during juvenile and adolescent periods stages could prevent an increase in the C1q expression in the PFC of adult offspring after prenatal poly(I:C) exposure (Fig. 1). Two-way ANOVA analysis of data in the other regions revealed no differences (Fig. 1). These results suggest that adult offspring from pregnant mice exposed to poly(I:C) showed increased C1q expression in the PFC, and that supplementation with 7,8-DHF from 4- to 8-weeks old could prevent an increase in the C1q in the PFC at adulthood after MIA.
Fig. 1.
Effect of 7,8-dihydroxyflavone (7,8-DHF) supplementation on C1q protein in the brain regions of adult offspring of prenatal mice exposed to poly(I:C). (A) C1q protein. **p<0.01, ***p<0.001 compared with poly(I:C)+vehicle (VEH) group. The value is expressed as the mean±standard error of the mean (n=5 or 6). (B) Representative bands for Western blot analysis of C1q andβ-actin in the brain regions of adult offspring after maternal immune activation.
NS, not significant; PFC, prefrontal cortex; NAc, nucleus accumbens; DG, dentate gyrus.
DISCUSSION
Recently, we reported that supplementation with 7,8-DHF during the juvenile and adolescent stages of offspring of prenatal mice exposed to poly(I:C) led to the prevention of behavioral changes (e.g., cognitive deficits and PPI deficits), decreased BDNF-TrkB signaling in PFC and CA1 of hippocampus, and loss of PV in the prelimbic (PL) of medial PFC and CA1 of hippocampus at adulthood after MIA.9) Here, we found an increased C1q expression in the PFC of adult offspring from poly(I:C)-treated pregnant mice. Interestingly, there was an increased C1q-circulating immune complexes level and complement receptor type 1 (CR1) expression on blood cells, elevated number of CR1 positive erythrocytes and reduced number of CR1 positive lymphocytes and monocycytes in patients with schizophrenia compared to controls.13) Considering immune system abnormalities in schizophrenia,14) it is likely that increased C1q expression in the PFC may be involved in the behavioral abnormalities of adult offspring after MIA.
In this study, we also found that early treatment with 7,8-DHF (a TrkB agonist) during juvenile and adolescent stages could prevent an increase of C1q in the PFC of adult offspring after prenatal poly(I:C) exposure, suggesting a role of BDNF-TrkB signaling. Therefore, it is likely that supplementation with a TrkB agonist such as 7,8-DHF during the prodromal stage may have prophylactic effects on offspring after prenatal immune activation. This is a first paper showing the role of BDNF-TrkB signaling in the expression of C1q in the PFC. Precise mechanisms underlying the role of BDNF-TrkB signaling in the C1q expression in the PFC are currently unknown. Further study on the role of BDNF-TrkB signaling in C1q expression will be needed.
In conclusion, this study suggests that increased C1q expression in the PFC may play a role in the behavioral abnormalities of adult offspring after MIA. Furthermore, supplementation of a TrkB agonist in young subjects at ultra-high risk for psychosis may prevent the onset of psychosis.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.H.), the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and development, AMED (to K.H.). Dr. Mei Han was supported by Postdoctoral Fellowship for Overseas Researchers of the Japan Society for the Promotion of Science (JSPS) (Tokyo, Japan).
REFERENCES
- 1.Sullivan PF. The genetics of schizophrenia. PLoS Med. 2005;2:e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204:322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal V, Blom AM. Roles of complement C1q in pneumococcus-host interactions. Crit Rev Immunol. 2015;35:173–184. doi: 10.1615/CritRevImmunol.2015012177. [DOI] [PubMed] [Google Scholar]
- 6.Kouser L, Madhukaran SP, Shastri A, Saraon A, Ferluga J, Al-Mozaini M, et al. Emerging and novel functions of complement protein C1q. Front Immunol. 2015;6:317. doi: 10.3389/fimmu.2015.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severance EG, Gressitt KL, Halling M, Stallings CR, Origoni AE, Vaughan C, et al. Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia. Neurobiol Dis. 2012;48:447–453. doi: 10.1016/j.nbd.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Severance EG, Gressitt KL, Buka SL, Cannon TD, Yolken RH. Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr Res. 2014;159:14–19. doi: 10.1016/j.schres.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han M, Zhang JC, Yao W, Yang C, Ishima T, Ren Q, et al. Intake of 7,8-dihydroxyflavone during juvenile and adolescent stages prevents onset of psychosis in adult offspring after maternal immune activation. Sci Rep. 2016;6:36087. doi: 10.1038/srep36087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014;18:pyu077. doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Arakelyan A, Zakharyan R, Khoyetsyan A, Poghosyan D, Aroutiounian R, Mrazek F, et al. Functional characterization of the complement receptor type 1 and its circulating ligands in patients with schizophrenia. BMC Clin Pathol. 2011;11:10. doi: 10.1186/1472-6890-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller BJ, Goldsmith DR. Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology. 2016;42:299–317. doi: 10.1038/npp.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]