Abstract
Background:
The knowledge of the current prevalence of lymphatic filariasis and its transmission will be helpful in its elimination. Thus, the present study is aimed to determine its prevalence among hydrocele patients which is a common presentation in chronically infected cases.
Materials and Methods:
One hundred patients suffering from hydrocele admitted to the surgical ward were included in the study. Blood samples were collected from the patients during the day hours for the detection of anti-filarial antibody and during night hours to detect the presence of microfilaria by smear examination. Blood samples were also collected from the family member attending the ward along with the patients to determine the presence of anti-filarial antibodies. Serum IgE level and eosinophil count were also determined in the patients showing a positive result for the anti-filarial antibody test.
Results:
Out of 100 hydrocele patients, 21% patients showed anti-filarial antibody card test positive with maximum patients belonging to age group of 20–40 years. Microfilaria was detected in 5% of the hydrocele patients, whereas none of the family members showed positive anti-filarial antibody test. Serum IgE level and eosinophil count were more than 1000 ng/ml and 500/mm3, respectively.
Conclusions:
The study has found a high prevalence of filariasis among hydrocele patients. It is suggested that more studies are needed to know the real time prevalence of the cases showing manifestations of the filariasis in the acute stage which will help the eradication program to formulate new strategies.
Keywords: Anti-filarial antibody, endemic filariasis, hydrocele, lymphatic filariasis
Introduction
Lymphatic filariasis most commonly caused by Wuchereria bancrofti is affecting almost 73 tropical and subtropical countries worldwide. Globally, around 1.4 billion people are estimated to be at risk, with 120 million already infected and 40 million seriously affected or disfigured by the disease. Among these affected populations, 25 million men are suffering from filariasis of genitals most commonly hydrocele. The World Health Organization (WHO) has launched a Global Programme to Eliminate Lymphatic Filariasis (GPLEF), in 2000, with the aim of elimination as a Public Health Problem by 2020.[1]
About one-third population of India lives at risk of developing lymphatic filariasis. Out of 289 (62%) district surveyed up to 1995, 257 districts were found to be endemic.[2] About 489.1 million people were exposed to the risk of infection and required massive drug administration.[3] Bihar has the highest endemicity followed by Kerala, Uttar Pradesh, Andhra Pradesh, and Tamil Nadu with endemicity over 17%, 15.7%, 14.6%, 10%, and 10%, respectively. Goa has the least endemicity of approximately 1% of all the states followed by Lakshadweep and Madhya Pradesh with more than 1.5% and 3% endemicity, respectively.[4] About 190 districts were not surveyed at any point of time to observe the prevalence of microfilaria.[5] The national average prevalence of microfilaria showed a declining trend from 1.24% in 2004 to 0.63% in 2008.[6]
Although most of the infected individuals appear clinically asymptomatic with subclinical disease, approximately one-third of patients present with lymphedema, lymphadenitis, lymphangitis, elephantiasis, hydrocele, lymphorrhagia, or recurrent infections due to damaged lymphatics.[7] Hydrocele, a very common manifestation of filariasis, occurs due to obstruction of lymph vessels of spermatic cord and exudation of lymphatic fluid into the scrotum. About 40–50% of men living in endemic areas develop hydrocele as a chronic consequence of disease.[7,8] In the endemic area, the early diagnosis of the disease during the asymptomatic stage by the primary care physicians may decrease the risk of development of symptoms and complications. Furthermore, the prevalence of infection is 10% more in males as compared to females. Studies have shown that the disease rate steadily increases from the age of 10 onward. Lymphangitis is a common manifestation in children below 15 years of age, whereas hydrocele, lymphedema, and elephantiasis are more common in adult above 20 years of age.[4]
The diagnosis of bancroftian filariasis till recently relied on the demonstration of microfilariae in blood specimens collected during night.[9] In cases of low microfilariae density, concentration techniques, such as diethylcarbamazine provocation test, which induce the release of microfilaria in peripheral blood even during day time showed a comparable specificity and positive predictive value to that of night blood samples.[10] With the development of recombinant DNA technology, a recombinant antigen WbSXP-1 has been evaluated and is highly sensitive for detection of specific circulating filarial antibody against W. bancrofti and Brugia malayi.[11,12,13] Use of specific circulating filarial antigens (CFAs) such as Og4C3 allows detection of W. bancrofti antigens in serum, plasma, and hydrocele fluid and has no cross reactivity with any other helminthic infections. The advantage of detection of CFA is that its level remains constant during the whole day, and thus, there is no need to take the blood sample during night.[14]
Lymphatic filariasis is an endemic disease in a major portion of the country and needed to be diagnosed during its early phase. Furthermore, the advent of rapid, highly sensitive, and specific diagnostic methods has improved the diagnosis of the disease even in the laboratory with limited resources available in most of the primary care centers. Hydrocele being a common chronic presentation of the disease and most of the patients seek the primary care center for its treatment; this study will be helpful for the primary care physicians to perceive the significance of lymphatic filariasis as a cause of hydrocele.
Materials and Methods
Study design
The present study was a prospective, cross-sectional, observational study to determine the presence of anti-filarial antibody among the hydrocele patients living in an endemic area for filariasis. The present study was conducted in the Department of Microbiology of the institution.
Study population
The patients suffering from hydrocele admitted to the surgical ward were included in the study.
Sample size
One hundred nonduplicate samples collected from the patients suffering from hydrocele.
Selection criteria
Following inclusion and exclusion criteria was used.
Inclusion criteria
1. Patients suffering from hydrocele neither being diagnosed nor treated for filariasis earlier.
Exclusion criteria
1. Clinical symptoms of filariasis other than hydrocele
2. Presence of chronic illness such as diabetes, hypertension, leprosy, and tuberculosis.
Data collection
After enrollment of the patients on the basis of inclusion and exclusion criteria, detailed information was recorded on preformed questionnaire, which includes personal detail, demographic variables, medical history, and physical examination.
Sample collection
After obtaining written informed consent from each patient, 2 ml each of venous blood was collected in a plain vial and ethylenediaminetetraacetic acid (EDTA) vial following universal precautions. Another 1 ml of venous blood in EDTA vial was collected between 22:00 and 01:00 h in patients showing a positive result for the anti-filarial antibody test. One milliliter of venous blood in the plain vial was collected from the family members of filariasis patients living in the same house after obtaining written consent from the each member. All blood samples were labeled properly and transferred immediately to the laboratory for further processing. In the case of delay, it was refrigerated at a temperature between 2°C and 8°C.
Sample processing
The collected blood sample was processed as follows.
Anti-filarial antibody test
The blood sample collected in the plain vial was used for detection of anti-filarial antibodies. Serum was separated from the blood. The test was performed using an immunochromatographic test kit (OnSite Filariasis IgG/IgM combo rapid test) available commercially. The test is based on flow through immunochromatographic method and employs purified recombinant antigen (WbSXP-1) to detect specific anti-filarial antibodies against both W. bancrofti and B. malayi. The test performed and interpreted as per the manufacturer instructions.
Demonstration of microfilaria in peripheral blood smear
The blood sample collected in EDTA vial during day hours and night hours was examined for the presence of microfilariae. The blood sample was concentrated by Knott's concentration technique, in which 1 ml blood was placed in 9 ml of 2% formalin and centrifuge 500 × g for 1 min. A thick and thin blood smear was prepared from the sediment on a clean glass slide. The slide was stained by Giemsa stain and examined microscopically for the detection of microfilariae.
Detection of eosinophil count
The blood sample collected in EDTA vial from filariasis patients was used for eosinophil count. A thin smear was prepared on a clean glass slide and stained by Leishman stain. The smear was examined microscopically for determination of the percentage of eosinophils in differential leukocyte count. The eosinophil count of the blood was determined using hemocytometer chamber.
Detection of serum IgE level
The blood sample collected in the plain vial from filariasis patients was used for detection of serum IgE level. The serum IgE level was detected by chemiluminescence method.
Results
In the present study, 100 patients suffering from hydrocele admitted to the surgical ward were included. Out of these 100 patients, 21 (21%) showed positive anti-filarial antibody test. Of these 100 patients, highest number of patients (72%) were in age group of 20–40 years. The anti-filarial IgG and IgM positivity were maximum (15% and 4%, respectively) in the age group of 20–40 years. IgG antibody were also found in all the patients tested positive for IgM antibody test [Table 1].
Table 1.
Distribution of anti-filarial antibody test positive patients on the basis of age (n=100)
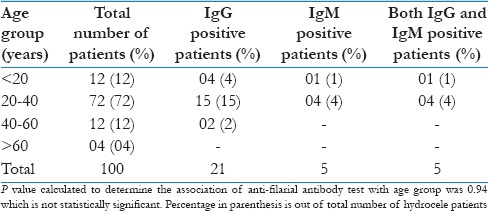
The family members of the hydrocele patients attending the hospital with the patients were also examined for the anti-filarial antibody test. None of the attending family members showed positive anti-filarial antibody test. Furthermore, none of the family members have given the history and clinical presentation of lymphatic filariasis or hydrocele during the examination.
Microfilaremia detected by peripheral blood smear examination showed that out of 21 anti-filarial antibody positive patients, 5 (23.8%) patients showed microfilaria in their blood. All the five patients showing microfilaria in their blood were positive for IgM antibody. Only the samples collected during night hours showed microfilaria and none of the day samples showed microfilaria on examination.
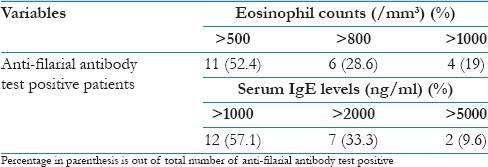
Table 2 demonstrated the eosinophil counts and serum IgE levels of the patients showing anti-filarial antibody. Eosinophil count was >500/mm3 in all the patients showing a positive result for the anti-filarial antibody. Nineteen percent patients had eosinophil count of more than 1000/mm3. Serum IgE levels were >1000 ng/ml in all the patients showing a positive result for anti-filarial antibody, whereas 9.6% patients showing the level of more than 5000 ng/ml.
Table 2.
Eosinophil count and serum IgE level of anti-filarial antibody test positive patients
Discussion
Lymphatic filariasis commonly caused by W. bancrofti is an endemic disease in India. Although most of the individuals suffering from the disease remain asymptomatic, it causes morbidity among the patients presenting with clinical manifestations such as elephantiasis, hydrocele, and recurrent infections due to damage of the lymphatic vessels. Hydrocele is a common manifestation of the disease affecting approximately 40–50% of males residing in endemic areas. The WHO has started a programme GPLEF with a target to eliminate the disease from India by 2020.[1,7,8] The diagnosis of the disease among individuals carrying the parasite will intercept its transmission and thus help in the elimination of the disease. This can be achieved by diagnosis of the disease among the patients presenting with either no symptoms or with chronic symptoms in the primary care center by the physicians. Thus, primary care physicians may play an important role in the elimination of the disease from the country by diagnosing the disease among the suspected cases of lymphatic filariasis.
In the present study, 21% hydrocele patients showed anti-filarial antibody. Goel et al.[15] has found anti-filarial antibody among 14% of hydrocele patients, whereas Shah and Mulla[16] has detected infection in 11.40% of hydrocele patients. A higher (34.6%) prevalence of filariasis among hydrocele patients was found by Rocha et al.[17] and very high prevalence was found by Dandapat et al.[18] with 43% patients having hydrocele definitely due to filariasis. This difference in prevalence may be attributed to variation in the geographical distribution of the disease as well as methods of detection.
In this study, the highest number (72%) of hydrocele patients belongs to the age group of 20–40 years. This finding is consistent with the study conducted by Shah and Mulla[16] which has found 56% hydrocele patients in this group. Similarly, Rocha et al.[17] has also documented 73% hydrocele patients in the age group of 20–40 years. Maximum number of anti-filarial antibody was detected in the patients of 20–40 years of age in our study. Earlier studies have reported a higher prevalence of filariasis in the younger age of <20 years contrary to our finding.[19,20,21] This may be due to the reason that most of the patients belong to rural areas and chronically ill patients suffering from the disease not consulted the clinicians.
Microfilaremia in our study was detected in 5% of hydrocele patients which is consistent with the finding of Shah and Mulla[16] which has detected microfilaria in 4.4% of patients. However, Goel et al.[15] have found microfilaria in only one out of 100 hydrocele patients. Since hydrocele is a manifestation of obstructive lymphangiopathy, the chances of detection of microfilaria in blood are quite less.
It is known that in allergic conditions total IgE level and eosinophil count are increased in the blood. Since filariasis has also allergic-like manifestations in the form of blood eosinophilia, this study has observed an eosinophil count more than 500/mm3 among all hydrocele patients. Similarly, in such patients, a high level of serum IgE was also observed.
Conclusions
The present study has found a high prevalence of filariasis among hydrocele patients with maximum patients belonging to the age group of 20–40 years. Since this area is covered under filariasis eradication program, the prevalence of the disease in younger age group (<20 years) is very low. At the same time, finding of filarial hydrocele in the age group of 20–40 years shows that disease is still endemic and effective measures are needed to eradicate it. It is suggested that more studies are needed to know the real time prevalence of the cases showing manifestations of the filariasis in the acute stage which will help the eradication program to formulate new strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The present study was conducted as an ICMR STS project for the year 2015 (Reference ID: 2015-07157).
References
- 1.World Health Organisation. Lymphatic Filariasis Fact Sheet No. 102; March. 2014. [Last accessed on 2015 Sep 29]. Available from: http://www.who.int/mediacentre/factsheets/fs102/en/
- 2.Sabesan S, Palaniyandi M, Das PK, Michael E. Mapping of lymphatic filariasis in India. Ann Trop Med Parasitol. 2000;94:591–606. doi: 10.1080/00034983.2000.11813582. [DOI] [PubMed] [Google Scholar]
- 3.WHO (World Health Organization). Global programme to eliminate lymphatic filariasis: Progress report for 2004. Wkly Epidemiol Rec. 2013;89:409–20. [Google Scholar]
- 4.Sabesan S, Vanamail P, Raju KH, Jambulingam P. Lymphatic filariasis in India: Epidemiology and control measures. J Postgrad Med. 2010;56:232–8. doi: 10.4103/0022-3859.68650. [DOI] [PubMed] [Google Scholar]
- 5.Sabesan S, Raju KH, Subramanian S, Srivastava PK, Jambulingam P. Lymphatic filariasis transmission risk map of India, based on a geo-environmental risk model. Vector Borne Zoonotic Dis. 2013;13:657–65. doi: 10.1089/vbz.2012.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. [Last cited on 2010 Jan 13]. Available from: http://www.nvbdcp.gov.in/filariasis-new.html .
- 7.Babu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Semin Immunopathol. 2012;34:847–61. doi: 10.1007/s00281-012-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumaraswami V. The clinical manifestations of lymphatic filariasis. In: Nutman TB, editor. Lymphatic Filariasis. London: Imperial College Press; 2000. pp. 103–25. [Google Scholar]
- 9.Nicolas L. New tools for diagnosis and monitoring of bancroftian filariasis parasitism: The Polynesian experience. Parasitol Today. 1997;13:370–5. doi: 10.1016/s0169-4758(97)01125-3. [DOI] [PubMed] [Google Scholar]
- 10.Bhumiratana A, Siriaut C, Koyadun S, Satitvipawee P. Evaluation of a single oral dose of diethylcarbamazine 300 mg as provocative test and simultaneous treatment in Myanmar migrant workers with Wuchereria bancrofti infection in Thailand. Southeast Asian J Trop Med Public Health. 2004;35:591–8. [PubMed] [Google Scholar]
- 11.Rao KV, Eswaran M, Ravi V, Gnanasekhar B, Narayanan RB, Kaliraj P, et al. The Wuchereria bancrofti orthologue of Brugia malayi SXP1 and the diagnosis of bancroftian filariasis. Mol Biochem Parasitol. 2000;107:71–80. doi: 10.1016/s0166-6851(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 12.Baskar LK, Srikanth TR, Suba S, Mody HC, Desai PK, Kaliraj P. Development and evaluation of a rapid flow-through immuno filtration test using recombinant filarial antigen for diagnosis of brugian and bancroftian filariasis. Microbiol Immunol. 2004;48:519–25. doi: 10.1111/j.1348-0421.2004.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 13.Ambily VR, Pillai UN, Kanaran PP. Immunological diagnosis of lymphatic filariasis in dogs of Kerala, India using filarial antibody detection immunospot test. J Immunol Tech Infect Dis. 2014;3:2. [Google Scholar]
- 14.Rocha A, Braga C, Belém M, Carrera A, Aguiar-Santos A, Oliveira P, et al. Comparison of tests for the detection of circulating filarial antigen (Og4C3-ELISA and AD12-ICT) and ultrasound in diagnosis of lymphatic filariasis in individuals with microfilariae. Mem Inst Oswaldo Cruz. 2009;104:621–5. doi: 10.1590/s0074-02762009000400015. [DOI] [PubMed] [Google Scholar]
- 15.Goel RS, Verma NS, Mullan SA, Ashdown AC. Detection of filarial antigen and antibody in serum and hydrocele fluid of 100 patients of hydrocele. Int J Urol. 2006;13:565–8. doi: 10.1111/j.1442-2042.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 16.Shah AP, Mulla SA. Circulating filarial antigen in serum and hydrocele fluid from individuals living in an endemic area for bancroftian filariasis. Indian J Med Microbiol. 2007;25:253–5. doi: 10.4103/0255-0857.34769. [DOI] [PubMed] [Google Scholar]
- 17.Rocha A, Lima G, Medeiros Z, Aguiar-Santos A, Alves S, Montarroyos U, et al. Circulating filarial antigen in the hydrocele fluid from individuals living in a bancroftian filariasis area – Recife, Brazil: Detected by the monoclonal antibody Og4C3-assay. Mem Inst Oswaldo Cruz. 2004;99:101–5. doi: 10.1590/s0074-02762004000100018. [DOI] [PubMed] [Google Scholar]
- 18.Dandapat MC, Mohapatro SK, Mohanty SS. The incidence of Filaria as an aetiological factor for testicular hydrocele. Br J Surg. 1986;73:77–8. doi: 10.1002/bjs.1800730131. [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan PK, Das PK, Subramanian S, Vanamail P, Ramaiah KD. Bancroftian filariasis in Pondicherry, South India: 1. Pre-control epidemiological observations. Epidemiol Infect. 1989;103:685–92. doi: 10.1017/s0950268800031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das PK, Manoharan A, Subramanian S, Ramaiah KD, Pani SP, Rajavel AR, et al. Bancroftian filariasis in Pondicherry, South India – Epidemiological impact of recovery of the vector population. Epidemiol Infect. 1992;108:483–93. doi: 10.1017/s0950268800049992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramaiah KD, Pani SP, Balakrishnan N, Sadanandane C, Das LK, Mariappan T, et al. Prevalence of bancroftian filariasis & its control by single course of diethyl carbamazine in a rural area in Tamil Nadu. Indian J Med Res. 1989;89:184–91. [PubMed] [Google Scholar]


