Abstract
Genomic sequencing of the β-proteobacterium Wautersia (previously Ralstonia) metallidurans CH34 revealed the presence of three genes encoding proteins of the cation diffusion facilitator (CDF) family. One, CzcD, was previously found to be part of the high-level metal resistance system Czc that mediates the efflux of Co(II), Zn(II), and Cd(II) ions catalyzed by the CzcCBA cation-proton antiporter. The second CDF protein, FieF, is probably mainly a ferrous iron detoxifying protein but also mediated some resistance against other divalent metal cations such as Zn(II), Co(II), Cd(II), and Ni(II) in W. metallidurans or Escherichia coli. The third CDF protein, DmeF, showed the same substrate spectrum as FieF, but with different preferences. DmeF plays the central role in cobalt homeostasis in W. metallidurans, and a disruption of dmeF rendered the high-level metal cation resistance systems Czc and Cnr ineffective against Co(II). This is evidence for the periplasmic detoxification of substrates by RND transporters of the heavy metal efflux family subgroup.
Cation diffusion facilitators (CDF proteins [TC 2.A.4.1.1]) constitute a family of membrane-bound proteins that occur in all domains of life (35, 45, 52). Members of the CDF family were initially identified in Saccharomyces cerevisiae and Alcaligenes eutrophus CH34 (later called Ralstonia metallidurans and now called Wautersia metallidurans CH34 [58]) (34, 41, 45). These transporters were initially identified as Co(II) or Zn(II) transporters (5, 36), but the family was subsequently shown to be able to transport Cd(II) (1, 18), Mn(II) (6), Ni(II) (46), or Fe(II) (12, 27) as well. For the zinc- and cadmium-transporting CDF protein CzcD from Bacillus subtilis, it was demonstrated that the mechanism of transport is electroneutral antiporting of the metal cation against H+ or K+ (18). Recently, kinetic parameters for transport for the Zn(II)-effluxing CDF ZitB from Escherichia coli were experimentally determined, suggesting that the ratio of Zn(II) to H+ is 1:1 and thus not electroneutral (3).
The first characterized bacterial member of the CDF family, CzcD (36) from W. metallidurans, was shown to mediate the efflux of Cd(II), Zn(II), and Co(II) (1). The Czc system also comprises another tripartite efflux machinery, CzcCBA, that spans the cytoplasmic and outer membranes and mediates high-level resistance against Cd(II), Zn(II), and Co(II) by active efflux driven by the proton motive force (9, 35).
Recently, the complete genome of W. metallidurans was made available. An analysis revealed the presence of two additional genes for CDF proteins in this organism. In contrast to czcD, these open reading frames are not located on one of the two indigenous megaplasmids (pMOL28 and pMOL30) but are carried by the bacterial chromosome. The first, named dmeF (divalent metal efflux), is located on contig 366 (gene number 7456), and the second, named fieF (ferrous iron efflux) after its orthologue from E. coli (12), is located on contig 369 (gene number 9480) (nomenclature is according to the web site http://www.genome.jgi-psf.org/draft_microbes/ralme/ralme.home.html).
Using several mutant strains of E. coli and W. metallidurans, we investigated the roles and metal specificities of the two new cation diffusion facilitators from W. metallidurans in E. coli and in their indigenous host. We show here that DmeF and FieF mediate resistance to a wide variety of divalent metal cations and that DmeF is essential for Co(II) detoxification by RND systems such as the Czc [for Co(II), Zn(II), and Cd(II)] or Cnr [for Co(II) and Ni(II)] system (28, 35). This suggests that RND systems utilize Co(II) only from the periplasm and thus that RND-driven transport occurs from the periplasm rather than from the cytoplasm to the outside.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli was grown in Luria-Bertani (LB) medium or Tris-buffered mineral salts medium (30) containing 2 ml of glycerol and 3 g of Casamino Acids per liter. Tris-buffered mineral salts medium containing 2 g of sodium gluconate per liter was used to cultivate Wautersia strains (30). Solid Tris-buffered medium contained 20 g of agar/liter. Antibiotics (chloramphenicol [15 to 20 μg/ml], kanamycin [25 μg/ml for E. coli and 1,500 μg/ml for W. metallidurans], or inducer anhydrotetracycline [200 μg/liter; IBA GmbH, Göttingen, Germany]) or metals were added when appropriate.
Genetic techniques.
Standard molecular genetic techniques were used (34, 53). For conjugative gene transfer, overnight cultures of the donor strain E. coli S17/1 (55) and of the Wautersia recipient strains grown at 30°C in complex medium were mixed (1:1) and plated onto nutrient broth agar. After overnight growth, the bacteria were suspended in saline (9 g of NaCl/liter), diluted, and plated onto selective media as previously described (34).
Construction of dmeF-lacZ and fieF-lacZ transcriptional fusions and of dmeF and fieF mutant strains.
For the construction of dmeF-lacZ and fieF-lacZ derivatives in W. metallidurans, an internal 300-bp fragment of dmeF or fieF, respectively, was PCR amplified as a PstI-XbaI fragment from the total DNA of W. metallidurans strain AE104, cloned into vector plasmid pGEM T-Easy (Promega, Madison, Wis.), and then subcloned as a PstI/XbaI fragment into plasmid pLO2-lacZ (26; also J. Scherer, unpublished data). Finally, the pLO2 hybrid plasmid carrying dmeF::lacZ or fieF::lacZ was used in a recombination event to insert the lacZ gene within dmeF or fieF as described previously (15). With the same 300-bp internal fragment of dmeF or fieF, respectively, cloned into the vector pLO2 (26), disruptions of dmeF or fieF in various W. metallidurans strains were constructed. The correct insertion and orientation of lacZ in these strains were verified by PCR, and the disruption of dmeF or fieF was additionally confirmed by Southern DNA-DNA hybridization.
Metal uptake.
Uptake experiments were performed by filtration. Stationary-phase cultures were diluted to 30 Klett units in minimal medium. The cells were grown to an optical density of 60 Klett units, and gene expression from derivatives of plasmid pASK-IBA in E. coli was initiated with 200 μg of anhydrotetracycline per liter. After growth for 30 min, ascorbate (1 mM final concentration) and FeSO4 labeled with 55FeCl3 (5 μM final iron concentration) or NiCl2 labeled with 63NiCl2 (5 μM final nickel concentration) were added. The cells were incubated with shaking, and 0.5-ml aliquots were filtered through nitrocellulose membranes (0.45-μm pore size) at various times and immediately washed with 5 ml of 0.1 mM LiCl (iron uptake [22]) or 10 ml of buffer (0.02 M MOPS [pH 7], 0.1 M sucrose, 0.1 M K3PO4) (nickel uptake [11]). The membranes were dried, and radioactivities were measured in a liquid scintillation counter (LS6500; Beckman, Munich, Germany). The dry weight was determined from the optical density by use of a calibration curve. 55FeCl3 and 63NiCl2 were purchased from Perkin-Elmer (Boston, Mass.).
Membrane preparations.
Overnight cultures expressing different CDF genes from plasmid pASK-IBA3 were diluted 1:100 in fresh LB medium and cultivated with shaking at 30°C up to an optical density at 600 nm of 0.8. The expression of CDF genes was induced with 200 μg of anhydrotetracycline/liter, and incubation was continued for 3 h. The cells were harvested by centrifugation (10 min, 7,650 × g, 4°C), suspended in buffer W (100 mM Tris-HCl [pH 8.0], 1 mM EDTA), and lysed in a French press (1,250 lb/in2) (SLM Aminco; Sopra GmbH, Büttelborn, Germany) in the presence of a protease inhibitor cocktail (Sigma-Aldrich, Deisenhofen, Germany) and DNase I (10 mg/ml). Debris was removed by centrifugation (23,400 × g, 15 min, 4°C), and the membrane fraction was isolated by ultracentrifugation (100,000 × g, 2 h, 4°C). The membrane pellet was suspended in buffer W to a final protein concentration of 10 mg/ml. Protein concentrations were determined by a bicinchoninic acid assay (Sigma-Aldrich).
Immunoblotting.
Protein samples were separated in sodium dodecyl sulfate-polyacrylamide gels, blotted (SemiDry-Blot; Biometra, Göttingen, Germany) onto a polyvinylidene difluoride membrane, and developed as previously described (23). For the detection of CzcA, W. metallidurans was grown in Tris minimal broth, and cells were harvested by centrifugation. CzcA-specific polyclonal antibodies were employed (2, 9).
Miscellaneous.
The chromosomal DNA of W. metallidurans was isolated as described previously (21). PCRs were performed with Taq, Pwo, or Tgo DNA polymerase (Roche, Mannheim, Germany). β-Galactosidase activities in permeabilized cells were determined as published previously (36), with 1 U defined as the activity forming 1 nmol of o-nitrophenol per min (44, 57) at 30°C.
RESULTS
DmeF and FieF mediate resistance against zinc and increase tolerance towards iron in E. coli.
Studies to better understand the factors determining the function and metal specificity of a given transporter are often hampered by redundant transport systems. Therefore, E. coli strains that are deficient in relevant metal transport systems are useful tools for elucidating the functions of individual transporters. Previously, we created two E. coli strains with deletions in Zn(II) efflux or iron homeostasis factors. One strain (GG48) is devoid of ZntA, the Zn(II)/Cd(II)-translocating P-type ATPase (50), and ZitB, the CDF protein mediating Zn(II) tolerance in E. coli (10). Another strain (GG200) has deletions of the genes for the global iron repressor Fur (19) and the ferrous iron-detoxifying CDF protein FieF (previously YiiP) (3, 4, 12).
Both putative genes for new cation diffusion facilitators from W. metallidurans, dmeF and fieF, were heterologously expressed under an inducible promoter in these metal-sensitive E. coli strains to elucidate their substrate specificities. Cultures of zinc-sensitive E. coli strain GG48 (zntA::kan ΔzitB::cat) (10) carrying the plasmid pECD873(pASK-IBA3::dmeF) or pECD875(pASK-IBA3::fieF) were challenged with increasing concentrations of Zn(II) in liquid medium. The empty vector and the gene for the E. coli CDF protein involved in Zn(II) efflux, ZitB, cloned into the same vector (10), were employed as controls. Figure 1 demonstrates that cells expressing zitB in trans were much more resistant to Zn(II) than the negative control. Heterologous expression of the W. metallidurans gene dmeF or fieF in E. coli strain GG48 resulted in an intermediate phenotype. All CDF genes were successfully expressed in E. coli and located in the cytoplasmic membrane, as demonstrated by Western blotting, but the expression levels were different (Fig. 2, inset). The indigenous E. coli CDF transporter ZitB was more effective at protecting the cells against zinc, even though the biosynthesis level of either FieF protein was higher (Fig. 2, inset). Nevertheless, both DmeF and FieF from W. metallidurans are capable of mediating Zn(II) resistance in E. coli.
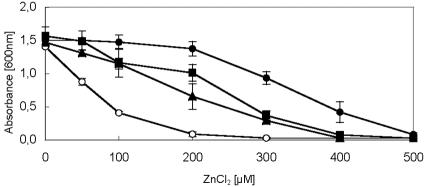
FIG. 1.
Zinc resistance of E. coli strains that express dmeF or fieF from W. metallidurans. Cultures grown overnight in LB medium were diluted 1:400 in fresh medium with the inducer anhydrotetracycline and increasing ZnCl2 concentrations. The cultures were cultivated for 6 h at 37°C, and the optical densities at 600 nm were determined. The strains tested included GG48 (zntA::kan ΔzitB::cat) (pASK-IBA3) (○) as a negative control, GG48(pZITB)(pASK-IBA3::zitB) (•) as a positive control, ECA166(pASK-IBA3::dmeF) (▴), and ECA168(pASK-IBA3::fieF) (▪). The data shown are the averages and standard deviations for three independent experiments.
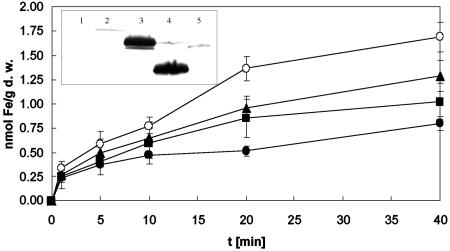
FIG. 2.
Iron uptake into E. coli by FieF or DmeF from W. metallidurans. Cultures that were grown overnight in minimal medium were inoculated at 30 Klett units into fresh medium at 37°C and grown up to an optical density of 60 Klett units. Uptake was started by the addition of a reaction mix of 55Fe (1 μCi), FeSO4 (5 μM final concentration), and 1 mM ascorbate. At defined time points, cellular iron accumulation was determined by the filtration method. The data shown are for E. coli strains ECA187 (ΔfieF) (pASK-IBA3) (○), ECA187(pECD884)(pASK-IBA3::fieF [from E. coli]) (•), ECA187(pECD873)(pASK-IBA3::dmeF) (▴), and ECA187(pECD875)(pASK-IBA3::fieF [from W. metallidurans]) (▪). The data shown are the averages and standard deviations for three independent experiments. (Inset) Western blot of StreptagII-labeled CDF proteins from Fig. 1 and 2 in isolated E. coli membranes (100 μg of total protein for each lane): 1, vector control; 2, DmeF from W. metallidurans; 3, FieF from W. metallidurans; 4, FieF from E. coli; 5, ZitB from E. coli.
The growth of E. coli strain GG200 (ΔfieF::cat Δfur) was shown to be diminished in LB broth (12). This growth retardation could be relieved by adding 100 μM EDTA, which decreased the concentration of free divalent metal cations. However, the growth retardation was even more pronounced in the presence of 100 μM EDTA plus 100 μM FeSO4. Thus, when the concentration of iron was selectively increased over that of other divalent metal cations, the growth of the cells was inhibited (12) (Table 1). This indicated that the iron content of the LB medium was responsible for the inhibition of growth of E. coli strain GG200 (12).
TABLE 1.
Growth yields of derivatives of iron-sensitive E. coli strain GG200 (Δfur::cat ΔfieF)
| Additive(s)a | Dry wt (g/liter)
|
|||
|---|---|---|---|---|
| pASK-IBA3 | pASK-IBA3::fieF (E. coli) | pASK-IBA3::dmeF (W. metallidurans) | pASK-IBA3::fieF (W. metallidurans) | |
| None | 0.08 ± 0.02 | 0.31 ± 0.05 | 0.2 ± 0.02 | 0.31 ± 0.04 |
| 100 μM EDTA | 0.28 ± 0.04 | 0.27 ± 0.03 | 0.32 ± 0.06 | 0.30 ± 0.04 |
| 100 μM FeSO4 | 0.07 ± 0.01 | 0.31 ± 0.03 | 0.18 ± 0.03 | 0.32 ± 0.06 |
| 100 μM EDTA + 100 μM FeSO4 | 0.01 ± 0.01 | 0.34 ± 0.05 | 0.18 ± 0.02 | 0.37 ± 0.05 |
Cultures grown overnight in LB broth were diluted 1:400 in fresh medium and grown to early log phase (2 h). They were then diluted 1:400 in fresh LB medium buffered with 50 mM Tris-HCl (pH 7.0) with 1 mM ascorbate and the indicated additives. Cell growth was monitored as the optical density at 600 nm after 6 h of incubation at 37°C with shaking, and the dry weight was determined. Experiments were performed in triplicate, and averages and standard deviations were calculated.
The expression of fieF from E. coli in trans restored ferrous iron tolerance to this E. coli strain. Similarly, the expression of dmeF or fieF from W. metallidurans in strain GG200 also resulted in an iron-tolerant phenotype (Table 1). FieF from W. metallidurans was as effective as FieF from E. coli, whereas DmeF from W. metallidurans allowed only about half of the growth yield observed for the FieF protein from either bacterium (Table 1).
FieF from E. coli diminished iron accumulation in E. coli (12). Therefore, E. coli strain ECA187 (ΔfieF) was used to express the W. metallidurans genes dmeF and fieF in order to examine the capabilities of the W. metallidurans homologues in iron detoxification. The presence of either FieF protein (from E. coli or W. metallidurans) led to about the same level of diminished iron accumulation (Fig. 2). While DmeF was not as effective as FieF from either organism, it still led to a significantly lower level of iron accumulation than that in negative control cells without any CDF protein.
Taken together, these data demonstrated that genes for W. metallidurans CDF proteins are able to take part in zinc and iron homeostasis in E. coli, but with different substrate preferences.
Disruption of fieF or dmeF in W. metallidurans influences resistance against several metal cations.
W. metallidurans strain AE104 is a megaplasmid-free derivative of the high-level metal-resistant strain CH34(pMOL38, pMOL30) (30). Strain AE104 was used for studies of the functions of DmeF and FieF because metal resistance determinants located on the megaplasmids would not interfere. Mutants of dmeF or fieF were constructed by gene disruptions. The metal resistance of the resulting strains, AE104(dmeF::pLO2) and AE104(dmeF::pLO2), was tested. MICs were determined for Co(II), Ni(II), Zn(II), and Cd(II) on a solid low-complexing medium (Table 2), and dose-response curves for liquid medium were recorded (Fig. 3).
TABLE 2.
MICs of metal ions for W. metallidurans strains with mutations in genes for cation diffusion facilitatorsa
| Strain | Relevant genotype | Plasmid(s) | MIC of metal salt (μM)b
|
|||
|---|---|---|---|---|---|---|
| CoCl2 | NiCl2 | ZnCl2 | CdCl2 | |||
| AE104 | 400 | 400 | 200 | 250 | ||
| AE104 | dmeF::pLO2 | 4 | 300 | 200 | 100 | |
| AE104 | fieF::pLO2 | 300 | 400 | 200 | 200 | |
| CH34 | pMOL30, pMOL28 | 6,000 | 2,500 | 4,500 | 1,500 | |
| CH34 | dmeF::pLO2 | pMOL30, pMOL28 | 50 | 2,500 | 4,500 | 1,500 |
| CH34 | fieF::pLO2 | pMOL30, pMOL28 | 50 | 2,500 | 4,500 | 1,500 |
| AE128 | pMOL30 | 4,500 | ND | ND | ND | |
| DN182 | ΔczcD | pMOL30-4 | 2,500 | ND | ND | ND |
| AE128 | dmeF::pLO2 | pMOL30 | 15 | ND | ND | ND |
| AE128 | dmeF::pLO2 ΔczcD | pMOL30-4 | 4 | ND | ND | ND |
Strains were grown for 48 h in minimal medium, diluted 1:100, and streaked on minimal agar plates with increasing metal concentrations. Growth was monitored for 5 days at 30°C. The results were confirmed by at least three independent experiments.
ND, not done.
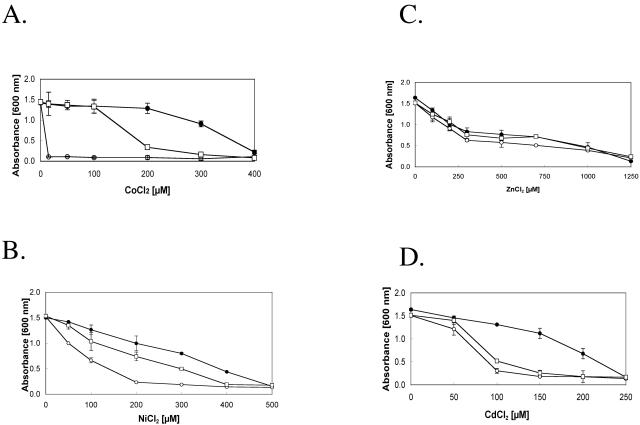
FIG. 3.
Influence of disruptions of dmeF or fieF on metal resistance of W. metallidurans strain AE104. Cultures grown in minimal medium for 48 h were diluted 1:100 in fresh medium and cultivated with shaking for 24 h at 30°C. The cultures were inoculated 1:50 into fresh minimal medium with several metal salt concentrations and cultivated for 18 h, after which the optical densities at 600 nm were determined. The metals tested included Co(II) (A), Ni(II) (B), Zn(II) (C), and Cd(II) (D), and the strains were AE104 (plasmid free) (•), AE104 dmeF::pLO2 (○), and AE104 fieF::pLO2 (□). The experiments were performed in triplicate, and averages and standard deviations were calculated.
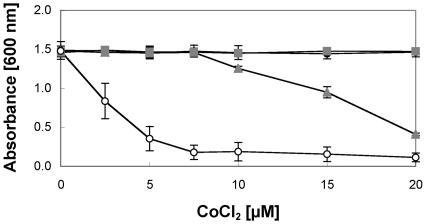
The deletion of dmeF resulted in a strong decrease in resistance against Co(II), Cd(II), and Ni(II). The level of Co(II) resistance (as demonstrated by the MIC) decreased to 1% of the MIC for control strain AE104; Ni(II) and Cd(II) resistance decreased to 35 and 60%, respectively. The deletion of fieF caused a less pronounced decrease in Co(II) and Cd(II) resistance than that observed for dmeF (Table 2). However, the resistance levels were below those for strain AE104, as demonstrated by dose-response curves (Fig. 3). The disruption of fieF or dmeF had no influence on the tolerance to Zn(II).
Because dmeF and fieF were disrupted by insertion mutagenesis, possible polar effects of these mutations on downstream genes had to be investigated. Therefore, both dmeF and fieF were expressed in trans from a broad-host-range vector in mutant derivatives of W. metallidurans AE104. The restoration of a metal-resistant phenotype in liquid medium was observed only when dmeF or fieF was expressed (data not shown). However, only partial complementation could be achieved because these strains were not able to mediate the full resistance displayed by the parental strain AE104. Still, both genes were able to complement the mutated strains in trans, and thus the observed phenotypes of the CDF gene disruptions were the result of the loss of the CDF proteins rather than of polar effects on downstream genes.
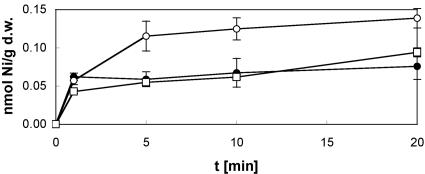
Figure 4 shows that the deletion of dmeF from W. metallidurans strain AE104 led to increased nickel accumulation, indicating the occurrence of Ni(II) detoxification by DmeF. On the other hand, the deletion of fieF had no apparent effect on Ni(II) accumulation. These different effects of DmeF and FieF on nickel accumulation correlated with the different results these proteins had on nickel resistance on solid (Table 2) and in liquid medium (Fig. 3B).
FIG. 4.
Nickel uptake into a dmeF or fieF mutant of W. metallidurans strain AE104. Cultures grown in minimal medium for 48 h were inoculated at 30 Klett units into fresh medium at 30°C and grown up to an optical density of 60 Klett units. Uptake was started by the addition of a reaction mix of 63Ni (1 μCi) and NiCl2 (5 μM final concentration), and incubation was continued with shaking at 30°C. At defined time points, nickel samples were withdrawn and the cellular accumulation of nickel was determined for W. metallidurans strains AE104 (•), AE104 dmeF::pLO2 (○), and AE104 fieF::pLO2 (□). The data shown are the averages and standard deviations for three independent experiments.
Expression of dmeF and fieF is constitutive and is not induced by metals.
To investigate the metal-cation-dependent regulation of the new CDF-encoding genes, we constructed strains harboring β-galactosidase reporter genes by the insertion of a promoterless lacZ gene into either dmeF or fieF. W. metallidurans strains AE104 dmeF::pLO2-lacZ and AE104 fieF::pLO2-lacZ were challenged with different metal cations (2.5 μM CoCl2, 100 μM NiCl2, 150 μM ZnCl2, 50 μM CdCl2, or 100 μM FeSO4) that may be substrates of DmeF or FieF. However, none of the metals tested led to a significant increase in lacZ expression, and the basal expression levels of both genes were rather low. Both dmeF- and fieF-lacZ mutant β-galactosidase activities without added metal cations were determined to be 9 ± 2 U/mg of dry weight, and the activities with added metal cations ranged from 8 ± 1 to 9 ± 3 U/mg of dry weight.
This clearly indicated that the expression of the chromosomally encoded CDF proteins was constitutive and low under the conditions tested. Additionally, CDF-LacZ reporters were also not inducible in strains AE126(pMOL28), AE128(pMOL30), and CH34(pMOL28, pMOL30) by any metal cation tested (data not shown): the expression levels remained at a low constitutive level throughout, similar to the values observed for strain AE104. This indicated that no megaplasmid-carried genes were needed for the metal-dependent induction of dmeF or fieF.
Interplay of DmeF and FieF with CzcCBA and CnrCBA efflux pumps.
The W. metallidurans wild-type strain CH34 harbors a plethora of additional high-level resistance determinants on its indigenous megaplasmids in addition to those that are carried on the chromosome. Since DmeF and FieF were shown to be involved in divalent metal resistance, the interplay of the chromosomally encoded CDF transporters with plasmid-borne metal resistance systems, especially interactions with the well-studied RND systems CzcCBA and CnrCBA from megaplasmids pMOL30 and pMOL28, respectively, was investigated.
Therefore, the dmeF or fieF gene was disrupted in W. metallidurans strains AE126(pMOL28), with plasmid pMOL28 harboring cnr; AE128(pMOL30), with pMOL30 containing czc; and wild-type CH34(pMOL28, pMOL30), with plasmids carrying both resistance determinants. The mutation of fieF in these strains had only a minor effect on Co(II) resistance and no apparent influence on the resistance against Cd(II), Zn(II), or Ni(II) (Table 2 and data not shown).
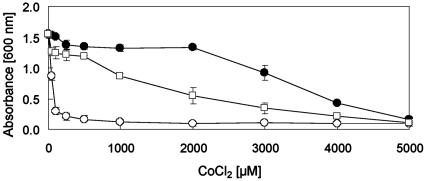
On the other hand, the disruption of dmeF resulted in decreased cobalt resistance in AE126 (data not shown), AE128, and wild-type CH34 (Table 2 and Fig. 5). The Co(II) MIC was reduced to 0.8% of the MIC for the wild-type strain CH34 when dmeF was disrupted. This decrease in resistance on solid medium was comparable to that for a dmeF disruption in the plasmid-free derivative AE104 (Table 2). However, in strain CH34 no influence of a dmeF disruption on resistance against Ni(II), Zn(II), or Cd(II) was detected (Table 2), indicating that other resistance systems may obscure the influence of DmeF on resistance against these metal cations.
FIG. 5.
Influence of disruption of dmeF or fieF on Co(II) resistance of W. metallidurans wild-type strain CH34(pMOL30, pMOL28). Cultures were grown as described in the legend for Fig. 3. The W. metallidurans strains were CH34(pMOL30, pMOL28) (•), CH34 dmeF::pLO2(pMOL30, pMOL28) (○), and CH34 fieF::pLO2(pMOL30, pMOL28) (□).
Such a remarkable role of DmeF in Co(II) homeostasis was also evident in growth experiments with liquid medium under Co(II) stress (Fig. 5). This clearly identified DmeF as the single most important factor for Co(II) homeostasis in W. metallidurans CH34. Without DmeF, the high-level efflux systems CzcCBA and CnrCBA from strain CH34 were rendered ineffective, suggesting that DmeF may be needed for the proper functioning of these heavy metal efflux (HME)-RND systems.
CzcD mediates low-level Co(II) resistance in the absence of DmeF in W. metallidurans strain AE128(pMOL30).
The gene of the third CDF protein from W. metallidurans, CzcD, is carried as part of the czc determinant (40). Why is one CDF protein part of a Czc resistance system, when apparently another CDF protein is essential for a functional Czc-mediated resistance? To address this question, we constructed a dmeF czcD double mutant strain in W. metallidurans AE128(pMOL30) and determined its resistance level against Co(II).
The deletion of czcD alone decreased Co(II) resistance on solid medium by one-half (Table 2), whereas the deletion of dmeF alone decreased Co(II) resistance to 3% of the MIC for strain AE128(pMOL30) (Table 2). The deletion of both genes, however, diminished the MIC to <0.1% [equaling 4 μM Co(II)] that for the parental strain [4.5 mM Co(II)]. This value of 4 μM Co(II) was also the MIC for the dmeF mutant in the megaplasmid-free, czc-negative strain AE104 (Table 2). Dose-response curves for the AE128(pMOL30) mutant strains in liquid medium matched these results (Fig. 6). Immunoblotting using a CzcA-specific antibody confirmed that equal amounts of the CzcA protein were present in these strains (data not shown). This confirmed that the observed phenotype was not due to a secondary down-regulation of the czcA gene, which encodes the major RND-type pump protein of the CzcCBA efflux complex. Thus, the absence of both Co(II)-detoxifying CDF proteins CzcD and DmeF rendered the high-level resistance-mediating efflux system CzcCBA completely ineffective. CzcCBA was obviously not able to function without the contribution of at least one CDF protein with the same substrate specificity.
FIG. 6.
CzcD mediates low-level Co(II) resistance in the absence of DmeF in W. metallidurans strain AE128(pMOL30). Cultures were grown as described in the legend for Fig. 3. The W. metallidurans strains were AE128(pMOL30) (•), DN182(pMOL30-14 ΔczcD) ( ), AE128 dmeF::pLO2(pMOL30) (
), AE128 dmeF::pLO2(pMOL30) ( ), and AE128 dmeF::pLO2(pMOL30-14 ΔczcD) (○).
), and AE128 dmeF::pLO2(pMOL30-14 ΔczcD) (○).
DISCUSSION
The β-proteobacterium W. metallidurans can be considered a model organism for bacterial metal homeostasis. Members of all major resistance mechanisms are abundantly found in this bacterium, as recently reviewed (29, 37). Cation diffusion facilitators and P-type ATPases are most important for these mechanisms and are responsible for the translocation of metal cations from the cytoplasm through the cytoplasmic membrane into the periplasmic space. Whereas ATPases, such as ZntA or CadA (25), are needed for the highly efficient transport of Zn(II) or Cd(II) energized by ATP hydrolysis, secondary CDF transporters, such as CzcD or DmeF, probably exchange protons for metal cations (3, 18, 45).
The data presented in this publication may help to solve several questions. First, is there a specialization of CDF protein function in bacteria that contain more than one member of this group? Second, what is the function of CDF proteins in bacterial metal cation homeostasis? Third, and of paramount importance, from where do the RND-driven efflux systems take up their substrates, the cytoplasm or the periplasm?
Specialization of CDF proteins and their contribution to bacterial heavy metal homeostasis.
To date, the mechanism behind the substrate specificities of CDF proteins remained obscure even though CDF proteins with different substrates could be classified into several subgroups (37). Bacteria and archaea usually contain between zero and four CDF proteins, with one subgroup 2 and one subgroup 3 protein as a rule, and subgroup 3 proteins are the most prominent CDF proteins in prokaryotes (37). W. metallidurans contains members of all three subgroups (37): DmeF is from subgroup 1, CzcD is from subgroup 2, and FieF is from subgroup 3.
Our results established that the subgroup 1 protein DmeF is a broad-range metal ion transporter that functions in E. coli and W. metallidurans. Subgroup 1 proteins were previously characterized only for eukaryotes (37), and DmeF is the first bacterial member of this group. The data presented here indicate that DmeF may be predominantly a Co(II) resistance system, but with a broad substrate specificity. To our knowledge, DmeF is the CDF protein with the broadest reported substrate spectrum. Relatively specific CDF transporters for several divalent metal cations were described previously (1, 6, 10, 12, 27, 46). The majority of known CDF substrates are divalent heavy metal cations with a diameter of 74 ± 2 pm (37), which rules out Mg(II) as a substrate. Since all CDF substrates may enter the cells by fast, nonspecific magnesium uptake systems (39), CDF proteins may be necessary to relieve the bacterial cells of surplus heavy metal cations that enter the cell by these uptake systems.
A unique feature of DmeF that separates it from all other bacterial CDF proteins mentioned in this study is its expanded histidine-rich region between transmembrane segments IV and V, located on the cytoplasmic side of the membrane. Typically, CDF proteins that transport Zn(II) have histidine-rich stretches located at either their N or C terminus or both (10, 23, 45).
Group 2 CDF proteins include CzcD from W. metallidurans and B. subtilis, ZitB from E. coli, and a variety of proteins from eukaryotes (37). These proteins seem to be predominantly transporters of Zn(II). However, as shown for CzcD from W. metallidurans (1), these transporters can also have quite broad substrate specificities.
FieF from W. metallidurans seems to be involved in the export of Fe(II), like its namesake from E. coli (12). Not many additional members of the abundant group 3 of CDF proteins have been investigated so far. One that has been studied is MamB from the magnetotactic bacterium Magnetospirillum gryphiswaldense (17, 54), which may be involved in the biosynthesis of magnetosomes (16) and is therefore probably also an iron transporter. FieF from W. metallidurans decreased Fe(II) accumulation in E. coli as effectively as the native FieF protein from E. coli, but it was also involved in the homeostasis of Ni(II), Co(II), and to a lesser extent, Cd(II) in W. metallidurans. There was no effect of FieF from W. metallidurans on zinc resistance in this bacterium, probably because the two P-type ATPases, CadA and ZntA (25), were present in the cells and protected them against this metal cation. The presence of CadA could also explain the small effect of FieF on cadmium resistance. While both DmeF and FieF from W. metallidurans mediated resistance in a Zn(II)- and Cd(II)-sensitive E. coli strain that lacked its native P-type ATPase ZntA and the CDF protein ZitB, no influence of DmeF or FieF on Zn(II) resistance in their host, W. metallidurans, was observed. Again, this fact was owed to the presence of the two P-type ATPases of W. metallidurans, ZntA [Zn(II)/Cd(II)] and CadA [Cd(II)/Zn(II)] (25), which probably masked the effects of the secondary transporters of the CDF family. In contrast to the case for E. coli, no Zn-sensitive mutant strain is yet available for W. metallidurans.
Since FieF from E. coli is also able to transport Zn(II) when it is overexpressed (12) and was recently shown to bind Zn(II), Cd(II), and Hg(II) as well (4), FieF-like proteins may be predominantly iron efflux systems that have rather broad substrate specificities. Hence, the widespread distribution of CDF group 3 members in bacteria (37) may mirror the high importance of iron over other divalent heavy metal cations (38).
Studies of iron homeostasis in W. metallidurans were not possible at this time because multiple deletions of chromosomally encoded genes that can be easily created in E. coli are very difficult to construct in W. metallidurans due to the lack of available markers and vectors. Furthermore, only a little is known about probable iron efflux transporters for targeted deletion mutagenesis.
Interplay of CDF and RND transport systems.
The answers to questions about CDF functions in homeostasis and interactions with other systems are closely connected. RND-driven efflux systems of the HME group (56) such as CzcCBA are large membrane-bound protein complexes that are composed of the central RND pump protein, a membrane fusion protein, and an outer membrane factor (40, 51). These protein complexes span the cell envelopes of gram-negative bacteria (20). CzcCBA mediates a strong increase in resistance to Co(II), Zn(II), and Cd(II) in W. metallidurans (24) by cation efflux (41). As discussed in detail elsewhere (37), substrates for CzcCBA may come from the periplasm, the cytoplasm, or both cellular compartments. Another subfamily of RND proteins is involved in resistance to organic substances in bacteria (56). The three-dimensional structure of one of these proteins, AcrB (32), and other experimental data concerning this and related proteins are consistent with RND-driven efflux complexes receiving their substrates from the periplasm or, in the case of lipophilic substrates, from the cytoplasmic membrane (31, 33, 37).
Another line of evidence favoring the periplasmic transport of metal cations by HME-RND systems can be derived from the study of copper detoxification in E. coli. E. coli harbors a copper efflux ATPase, CopA (47, 48); a multicopper oxidase, CueO (13, 14); and a RND efflux system, CusC(F)BA (7, 8, 43). Whereas CzcA and CnrA transport divalent metal cations, the substrate of CusA is probably Cu(I) since Cus also mediates resistance against Ag(I) (7). Strains with deletions in CopA are as copper sensitive as strains with deletions in both copA and cusA, and a triple deletion strain with deletions in copA, cueO, and cusA is no more sensitive than a strain with deletions in cusA and cueO (14). Thus, when copper is not transported from the cytoplasm to the periplasm by CopA, the CusC(F)BA complex is not able to confer metal resistance, suggesting that Cus receives its substrate from the periplasm and not from the cytoplasm (14, 49).
In contrast, purified CzcA reconstituted into proteoliposomes was able to transport Zn(II), Co(II), and Cd(II) with a low affinity across a single membrane (9). Additionally, CzcA without CzcC and CzcB is able to mediate low-level metal resistance (51). However, the observed residual resistance of the single CzcA protein may be accounted for by the ability of CzcA to recruit other membrane fusion proteins and outer membrane factors from related systems encoded by the chromosome to form a functional transport complex (37). Moreover, the Km of 10 mM Zn(II) observed for CzcA in vitro (9) is beyond the physiological concentration of this trace metal within the cell (42) and may be owed to the fact that the CzcC and CzcB parts of the transport complex were not present.
Unexpectedly, it was recently demonstrated that there is an interplay of the P-type ATPases CadA and ZntA of W. metallidurans with the CzcCBA system (25). Though neither single nor double gene deletions of zntA or cadA affected Zn(II) resistance in the presence of czcCBA, the Cd(II) resistance of the cadA zntA double mutant could only be partially increased by CzcCBA. Therefore, without any Cd(II)-transporting ATPase to translocate Cd(II) from the cytoplasm to the periplasm, the RND system CzcCBA was rendered inactive.
In this study, we showed that the same is true for the interplay of RND transporters and CDF proteins in Co(II) detoxification. CzcCBA increased the resistance of W. metallidurans to Co(II) by a factor of 11, DmeF alone in the absence of CzcCBA increased the resistance by a factor of 100, and both systems plus CzcD increased the resistance by a factor of 1,125 (Table 2). However, in the absence of DmeF and CzcD, CzcCBA did not increase the cobalt resistance at all. This indicates that the action of CzcCBA essentially requires the transport activity of DmeF or at least CzcD, which is consistent with CzcCBA and DmeF catalyzing subsequent steps of cobalt export as follows: DmeF exports Co(II) from the cytoplasm to the periplasm and this reaction is followed by transport by CzcCBA from the periplasm to the outside of the cell.
There are now several lines of evidence that RND transporters of the HME subfamily utilize periplasmic rather than cytoplasmic metal cations as substrates. Conversely, this also indicates that there is probably no transport from the cytoplasm to the periplasm by HME-RND systems such as CzcA or CnrA. Taken together, strong, albeit indirect, data are accumulating that show that RND-driven metal efflux complexes take their substrates from the periplasm.
Acknowledgments
This work was supported by grants Ni262/4-1 and GR2061/1-1 from the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie to D.H.N. and G.G.
We thank Grit Schleuder for skillful technical assistance and Judith Scherer for constructing pLO2-lacZ. Thanks are due to Christopher Rensing for critically reading the manuscript.
REFERENCES
- 1.Anton, A., C. Grosse, J. Reissmann, T. Pribyl, and D. H. Nies. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 181:6876-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloss, T., S. Clemens, and D. H. Nies. 2002. Characterization of the ZAT1p zinc transporter from Arabidopsis thaliana in microbial model organisms and reconstituted proteoliposomes. Planta 214:783-791. [DOI] [PubMed] [Google Scholar]
- 3.Chao, Y., and D. Fu. 2004. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J. Biol. Chem. 279:12043-12050. [DOI] [PubMed] [Google Scholar]
- 4.Chao, Y., and D. Fu. 2004. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J. Biol. Chem. 279:17173-17180. [DOI] [PubMed] [Google Scholar]
- 5.Conklin, D. S., J. A. McMaster, M. R. Culbertson, and C. Kung. 1992. COT1, a gene involved in cobalt accumulation in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:3678-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delhaize, E., T. Kataoka, D. M. Hebb, R. G. White, and P. R. Ryan. 2003. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 15:1131-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke, S., G. Grass, and D. H. Nies. 2001. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147:965-972. [DOI] [PubMed] [Google Scholar]
- 8.Franke, S., G. Grass, C. Rensing, and D. H. Nies. 2003. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 185:3804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg, M., T. Pribyl, S. Juhnke, and D. H. Nies. 1999. Energetics and topology of CzcA, a cation/proton antiporter of the resistance-nodulation-cell division protein family. J. Biol. Chem. 274:26065-26070. [DOI] [PubMed] [Google Scholar]
- 10.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grass, G., B. Fan, B. P. Rosen, K. Lemke, H. G. Schlegel, and C. Rensing. 2001. NreB from Achromobacter xylosoxidans 31A is a nickel-induced transporter conferring nickel resistance. J. Bacteriol. 183:2803-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grass, G., H. Haney, M. Otto, C. Rensing, D. H. H. Nies, and D. Munkelt. Fief (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol., in press. [DOI] [PubMed]
- 13.Grass, G., and C. Rensing. 2001. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 286:902-908. [DOI] [PubMed] [Google Scholar]
- 14.Grass, G., and C. Rensing. 2001. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 183:2145-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosse, C., G. Grass, A. Anton, S. Franke, A. N. Santos, B. Lawley, N. L. Brown, and D. H. Nies. 1999. Transcriptional organization of the czc heavy-metal homeostasis determinant from Alcaligenes eutrophus. J. Bacteriol. 181:2385-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grünberg, K., E.-C. Müller, A. Otto, R. Reszka, L. Linder, M. Kube, R. Reinhardt, and D. Schüler. 2004. Biochemical and proteomic analysis of the magnetosome membrane in Magnetospirillum gryphiswaldense. Appl. Environ. Microbiol. 70:1040-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grünberg, K., C. Wawer, B. M. Tebo, and D. Schüler. 2001. A large gene cluster encoding several magnetosome proteins is conserved in different species of magnetotactic bacteria. Appl. Environ. Microbiol. 67:4573-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guffanti, A. A., Y. Wei, S. V. Rood, and T. A. Krulwich. 2002. An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+. Mol. Microbiol. 45:145-153. [DOI] [PubMed] [Google Scholar]
- 19.Hantke, K. 1984. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol. Gen. Genet. 197:337-341. [DOI] [PubMed] [Google Scholar]
- 20.Higgins, M. K., E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis. 2004. Structure of the periplasmic component of a bacterial drug efflux pump. Proc. Natl. Acad. Sci. USA 101:9994-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. L. 1981. Genetic characterization, p. 450-572. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods of general bacteriology. American Society for Microbiology, Washington, D.C.
- 22.Kammler, M., C. Schon, and K. Hantke. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 175:6212-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, S. M., G. Grass, C. J. Haney, B. Fan, B. P. Rosen, A. Anton, D. H. Nies, and C. Rensing. 2002. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol. Lett. 215:273-278. [DOI] [PubMed] [Google Scholar]
- 24.Legatzki, A., S. Franke, S. Lucke, T. Hoffmann, A. Anton, D. Neumann, and D. H. Nies. 2003. First step towards a quantitative model describing Czc-mediated heavy metal resistance in Ralstonia metallidurans. Biodegradation 14:153-168. [DOI] [PubMed] [Google Scholar]
- 25.Legatzki, A., G. Grass, A. Anton, C. Rensing, and D. H. Nies. 2003. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J. Bacteriol. 185:4354-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenz, O., E. Schwartz, J. Dernedde, M. Eitinger, and B. Friedrich. 1994. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J. Bacteriol. 176:4385-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, L., and J. Kaplan. 1997. Characterization of two homologous yeast genes that encode mitochondrial iron transporters. J. Biol. Chem. 272:28485-28493. [DOI] [PubMed] [Google Scholar]
- 28.Liesegang, H., K. Lemke, R. A. Siddiqui, and H. G. Schlegel. 1993. Characterization of the inducible nickel and cobalt resistance determinant cnr from pMOL28 of Alcaligenes eutrophus CH34. J. Bacteriol. 175:767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mergeay, M., S. Monchy, T. Vallaeys, V. Auquier, A. Benotmane, P. Bertin, S. Taghavi, J. Dunn, D. van der Lelie, and R. Wattiez. 2003. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 27:385-410. [DOI] [PubMed] [Google Scholar]
- 30.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. Van Gijsegem. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middlemiss, J. K., and K. Poole. 2004. Differential impact of MexB mutations on substrate selectivity of the MexAB-OprM multidrug efflux pump of Pseudomonas aeruginosa. J. Bacteriol. 186:1258-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami, S., R. Nakashima, E. Yamashita, and A. Yamaguchi. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587-593. [DOI] [PubMed] [Google Scholar]
- 33.Murakami, S., N. Tamura, A. Saito, T. Hirata, and A. Yamaguchi. 2004. Extramembrane central pore of multidrug exporter AcrB in Escherichia coli plays an important role in drug transport. J. Biol. Chem. 279:3743-3748. [DOI] [PubMed] [Google Scholar]
- 34.Nies, D., M. Mergeay, B. Friedrich, and H. G. Schlegel. 1987. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J. Bacteriol. 169:4865-4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nies, D. H. 1995. The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligenes eutrophus functions as a cation-proton antiporter in Escherichia coli. J. Bacteriol. 177:2707-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nies, D. H. 1992. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 174:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nies, D. H. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313-339. [DOI] [PubMed] [Google Scholar]
- 38.Nies, D. H. 2004. Essential and toxic effects of elements on microorganisms, p. 257-276. In K. Anke, M. Ihnat, and M. Stoeppler (ed.), Metals and their compounds in the environment. Wiley-VCH, Weinheim, Germany.
- 39.Nies, D. H. 1999. Microbial heavy metal resistance. Appl. Microbiol. Biotechnol. 51:730-750. [DOI] [PubMed] [Google Scholar]
- 40.Nies, D. H., A. Nies, L. Chu, and S. Silver. 1989. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc. Natl. Acad. Sci. USA 86:7351-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nies, D. H., and S. Silver. 1989. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J. Bacteriol. 171:896-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488-2492. [DOI] [PubMed] [Google Scholar]
- 43.Outten, F. W., D. L. Huffman, J. A. Hale, and T. V. O'Halloran. 2001. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 276:30670-30677. [DOI] [PubMed] [Google Scholar]
- 44.Pardee, A. B., and L. S. Prestidge. 1959. On the nature of the repressor of beta-galactosidase synthesis in Escherichia coli. Biochim. Biophys. Acta 36:545-547. [DOI] [PubMed] [Google Scholar]
- 45.Paulsen, I. T., and M. H. Saier, Jr. 1997. A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol. 156:99-103. [DOI] [PubMed] [Google Scholar]
- 46.Persans, M. W., K. Nieman, and D. E. Salt. 2001. Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc. Natl. Acad. Sci. USA 98:9995-10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen, C., and L. B. Moller. 2000. Control of copper homeostasis in Escherichia coli by a P-type ATPase, CopA, and a MerR-like transcriptional activator, CopR. Gene 261:289-298. [DOI] [PubMed] [Google Scholar]
- 48.Rensing, C., B. Fan, R. Sharma, B. Mitra, and B. P. Rosen. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 97:652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rensing, C., and G. Grass. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197-213. [DOI] [PubMed] [Google Scholar]
- 50.Rensing, C., B. Mitra, and B. P. Rosen. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 94:14326-14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rensing, C., T. Pribyl, and D. H. Nies. 1997. New functions for the three subunits of the CzcCBA cation-proton antiporter. J. Bacteriol. 179:6871-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saier, M. H., Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 54.Schübbe, S., M. Kube, A. Scheffel, C. Wawer, U. Heyen, A. Meyerdierks, M. H. Madkour, F. Mayer, R. Reinhardt, and D. Schüler. 2003. Characterization of a spontaneous nonmagnetic mutant of Magnetospirillum gryphiswaldense reveals a large deletion comprising a putative magnetosome island. J. Bacteriol. 185:5779-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 56.Tseng, T.-T., K. S. Gratwick, J. Kollman, D. Park, D. H. Nies, A. Goffeau, and M. H. J. Saier. 1999. The RND superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1:107-125. [PubMed] [Google Scholar]
- 57.Ullmann, A. 1984. One-step purification of hybrid proteins which have beta-galactosidase activity. Gene 29:27-31. [DOI] [PubMed] [Google Scholar]
- 58.Vaneechoutte, M., P. Kampfer, T. De Baere, E. Falsen, and G. Verschraegen. 2004. Wautersia gen. nov., a novel genus accommodating the phylogenetic lineage including Ralstonia eutropha and related species, and proposal of Ralstonia (Pseudomonas) syzygii (Roberts et al. 1990) comb. nov. Int. J. Syst. Evol. Microbiol. 54:317-327. [DOI] [PubMed] [Google Scholar]