Abstract
Context:
Rubella is a mild self-limiting disease all over the world; nevertheless, it is of significant public health importance due to its teratogenic effect of congenital rubella syndrome. Rubella vaccine is currently not included in the national immunization program in India. Rubella-specific IgG in the unvaccinated population is a marker of previous rubella infection. Rubella IgG estimation in children will provide data for initiation and necessary modification to the immunization strategy.
Aims:
In this background, this study was conducted with an aim to know the age-specific susceptibility of acquiring rubella infections and future risk of congenital rubella syndrome (CRS) among girls.
Settings and Design:
This was a community-based, observational study.
Participants and Methods:
The study was conducted at a randomly selected rural area Mavoor Panchayath of Kozhikode District, Kerala, among adolescent girls. The estimation of rubella-specific IgG antibody was done by quantitative enzyme-linked immunosorbent assay method. IgG titer value of >15 IU was taken positive, 8–15 IU as equivocal, and <8 IU as negative.
Statistical Analysis Used:
Statistical analysis was performed using Statistical program for Social science version 16 for Windows. Chi-square test was applied to find out significant difference and Fisher's exact test wherever applicable.
Results:
The data and blood sample collection was done from 250 girls. The mean IgG titer was 151.93 ± 128.78 IU, and as per the criteria, 68.3% were positive, 28.5% were negative, and 3.2% were equivocal. At this age, majority (68.3%) of the girls get protection by natural infection without any vaccine. Some girls (32%) may remain susceptible to infection during adulthood and pregnancy.
Conclusions:
Natural rubella infection was widely prevalent among child population and at this age. An immunization policy recommending rubella-containing vaccine is highly desirable to prevent rubella and CRS.
Keywords: Age shifting, congenital rubella syndrome, herd immunity, immunization policy, rubella-specific immunity
Introduction
Rubella is usually a mild self-limiting illness in children, presenting with fever and rash; however, in pregnant women, infection during the first 16 weeks of pregnancy can result in miscarriage, fetal death, or an infant born with congenital birth defects known as congenital rubella syndrome (CRS).[1,2,3,4] Rubella is of significant public health importance due to these teratogenic effects. Up to 60% of rubella cases may not present with a rash, many cases are not detected or reported. Seroprevalence surveys have documented widespread circulation of the rubella virus in all parts of the world.[4]
The presence of rubella-specific IgG in an unvaccinated population is a long-term marker of previous rubella infection and immunity status, and the antibodies persist life long, protecting the individual from further infections.[1,2] A study conducted in Tamil Nadu (South India) among unvaccinated girls aged 10–16 shows the presence of protective antibodies in 86.5%.[1] A similar study in North India (mean age of 10.7 years) reported that 90% have protective antibodies.[5] It was estimated that in India, about 50% of children acquire rubella antibodies by the age of 5 years and 80–90% become immune by 15 years by naturally acquired rubella.[1,5] All these studies were conducted before the widespread use of rubella vaccine in private sectors of India. Childhood exposure renders immunity to majority of women; however, periodic epidemics still occur among children and spread to involve a small portion of susceptible adult women, leading to epidemics of CRS.[6] Several sero-epidemiological surveys from other countries have reported that a substantial number of women reach childbearing age without acquiring natural immunity to rubella, making them more susceptible to infection.[4,7,8]
Due to the high incidence of subclinical infections during childhood, eliciting a reliable history is difficult making seroprevalence studies using representative sampling from different age groups superior in determining the age groups for vaccination.[4,9] Indian newborns consist of 20% of annual birth cohorts of the world. Regardless, no country-wide estimates of CRS burden and susceptibility to rubella infection are available due to lack of a national surveillance and registry for rubella.[2,3,10] There are no systematic reviews or nation-wide studies assessing the susceptibility of Indian population to rubella infection in general or specific to children or adolescent girls.[10]
The WHO evaluation studies validate the inclusion of childhood rubella vaccination as cost-effective in countries having measles vaccine coverage of more than 80%. Countries which can sustain the above coverage level can introduce rubella vaccination in their UIP program for the desired effects.[3,5] The latest data show that 140 of the 194 WHO member countries have incorporated rubella vaccine in combination with mumps and measles at the age of 12–18 months.[11] Rubella and CRS control was the goal established in the South-East Asian region as an initial step toward elimination and many developed countries have been successful in doing so.[11] Rubella vaccine is not yet included in the National childhood vaccination program (UIP) in India and it is given as an optional vaccine by private providers. This results in low coverage and thereby reduced herd immunity since only a small proportion of children from families who can afford the vaccine gets vaccinated. This unregulated use of vaccine may rise the “effective reproduction rate ‘R’ and ultimately lead to ‘age shifting’ and paradoxical increase of CRS in future.”[2,3,12] The state of Kerala, with best health indicators in the country, has been facing periodic epidemics of rubella among adult population in recent years. The Social Justice Department, with the help of Health and Education Department, in 2014, introduced the monovalent rubella vaccine among school girls but faced resistance from the community due to less perceived risks and resulted in low coverage (<25%).
Age-specific seroprevalence among girls, which the WHO considers to be a sensitive tool for risk assessment of CRS, has never been done here.[3,12] This will also help in understanding the degree of impact of private sector/optional vaccine.
Rubella IgG estimation in children will provide data for the necessary modification in the immunization strategy. Although rubella and CRS are a public health problem, no studies were done from the state on this subject. With the aim to understand the age-specific susceptibility of acquiring rubella infections and future risk of CRS, estimation of rubella IgG antibodies among girls in the age group of 13–15 was conducted.
Participants and Methods
The study was conducted at a randomly selected rural area named Mavoor Panchayath of Kozhikode District, Kerala, among adolescent girls. Being the only high school in the area, with 100% school enrollment and zero dropouts, all the adolescent girls from the area were admitted in the selected school, hence representing the population of girls in the community. With an expected rubella-specific IgG prevalence of 80% and for precision of ± 5, the minimum sample size required was estimated using Epi Info software 7 World health organization (WHO) as 250.[1]
The protocol of the study was approved by the Institutional Ethics Committee.
The study was conducted in Mavoor Government high school, among students of 8th and 9th standard (ages 13–15) with the permission of the school authorities and the parent–teacher association (PTA). All the girls studying in 8–9th standards in the selected school (n = 280), whose parents have given written informed consent and accent by the girls, were enrolled as participants and they were selected consecutively according to the roll number in the school registers till we got the required sample size of 250. Data and blood sample collection was done by conducting screening camps in the school on fixed days from 10 am to 2 pm. Those who were absent on the fixed day, their data were collected during the subsequent visits.
A standardized questionnaire was used by the interviewer to collect the demographic, morbidity, and immunization data (specifically measles-mumps-rubella [MMR]). Some of the variables used include history of exanthematous fever and past and recent hospitalizations. The variables include history of any exanthematous fever, recently or past and hospitalization. Medical examination was conducted to detect any disease such as fever/exanthema/lymph node enlargement and anomaly-cataract, deafness, and congenital heart disease to rule out rubella or features of CRS.
From every participant, 5 ml venous blood was collected from the antecubital vein under aseptic precautions using vacutainers with gel separators. Serum was separated and brought to the microbiology department at the medical college in vaccine carriers maintained at a temperature of 4°C on the same day and stored at −20°C till further analysis was made.
Rubella-specific IgG antibody was measured by quantitative enzyme-linked immunosorbent assay using BEIA Rubella IgG Quant Technogenetics SRL Italy kit, with a relative sensitivity and specificity of 100%. The tests were done using standard procedures by a laboratory technician with adequate experience under the supervision of a microbiologist (author). The results are expressed in IU/ml. As per the product manual (BEIA Rubella IgG Quant Ed. 01-09-2006), IgG titer of >15 IU is considered positive, 8–15 as equivocal, and <8 IU as negative. About 10% of the samples were retested at Kasturba Medical College, Manipal Laboratory, a central laboratory accredited by the National Accreditation Board for Testing Laboratories of the Department of Science and Technology, Government of India, and the validity and consistency were assured.
The collected data were processed in an excel data sheet with codes, with personal anonymity, and statistical analysis was performed using SSPS program. Chi-square test was applied to find out significant difference and Fisher's exact test wherever applicable.
Results
Data and blood sample collection was done from 250 girls. The age ranged from 13 to 15 with a mean age of 14.2 ± 0.7 years.
IgG estimation was done on 224 samples (90%), and due to sample lysis, the rest was discarded. Out of which 3 girls had received MMR vaccine who were also excluded from the analysis, so 221 samples were finally included for the analysis.
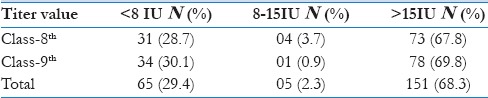
Age wise distribution of girls were 24.5%, 51%, 24.5% respectively in 13, 14 and 15 years. The mean IgG titer was 151.93 ± 128.78 IU, and as per the criteria, 68.3% were positive, 28.5% negative, and 3.2% equivocal [Table 1]. The class-wise details of IgG values are given in Table 2. There is no association between the titer values and the age of the participants (Pearson's correlation: 0.043, P = 0.53).
Table 1.
Prevalence of IgG antibody titer among girls
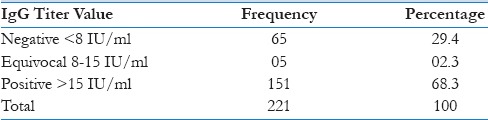
Table 2.
Class wise distribution of IgG antibody titer among girls
The childhood immunization details were collected from parents by crosschecking immunization cards and it was found that 61% had given Bacillus Calmette–Guérin, 57% had given three doses of OPV and DPT, and 54% had given measles vaccine.
Twenty-four girls had a history of mumps. None of the participants showed features of rubella (fever, rashes, and cervical nodes), congenital anomalies, or features of CRS on clinical examination.
The susceptibility to rubella may considerably vary with socioeconomic strata. As a proxy of this, the educational status of both parents was collected, this revealed a 100% literacy rate. Nearly 87% of fathers and 85% of mothers have completed >10 years of schooling. There was no variation of IgG status between the strata (P = 0.2, 0.4) in both parents meaning that rubella is uniformly transmitting among all income groups [Figures 1 and 2].
Figure 1.
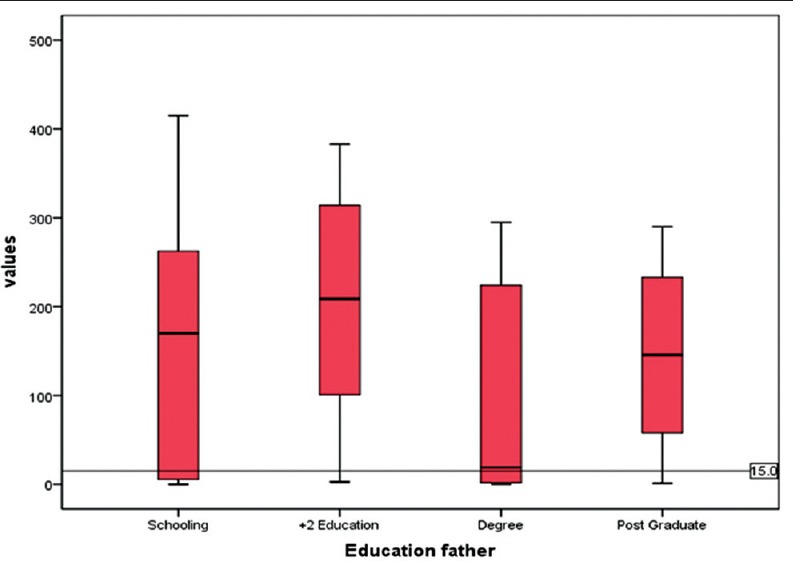
Relation of IgG tire values with father's education status*. *10th standard, Plus two, Degree, Postgraduate
Figure 2.
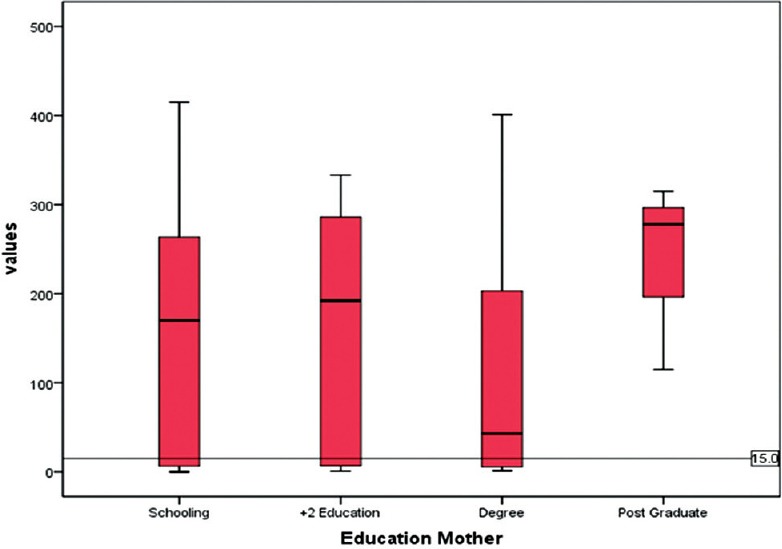
Relation of IgG tire values with mother's education status*. *10th standard, Plus two, Degree, Postgraduate
Discussion
Documentation of rubella infection has been difficult due to the challenges in diagnosing the disease due to its subclinical and atypical presentations. Hence, serological surveys play a precise role in defining infectious disease epidemiology, especially in the case of rubella, in determining its potential health impact, and also to help policymakers to decide the vaccination policies to contain the consequences of the disease.
In our study, even though girls were not immunized against rubella, 68.3% of the girls demonstrated a protective level of IgG by naturally acquired childhood infection. Since most of them were subclinical or with mild symptoms, their parents could not recall the past infections.
The IgG prevalence reported here was less than reported earlier from Tamil Nadu (86.5%) Maharashtra (76%), and New Delhi (90%).[1,5,6] This may be due to the improved socioeconomic status and higher standard of living in the state. A similar rate of prevalence (67.3%) was reported among girls aged 11–18 from Jammu and Kashmir.[13] These figures throw light on the widespread reach of rubella among the children of the state, thereby rendering them lifelong immunity.[4,12]
The IgG prevalence among girls did not differ significantly by religion, educational status, or family income, which is consistent with the reports from a hospital-based study previously conducted in the state.[14]
It is seen that more than 30% of the cohort was susceptible to rubella. It is noteworthy that the majority of girls get protection until they attain adolescence; however, not all were protected and therefore some girls may remain susceptible to infection during adulthood and pregnancy. The WHO reports that even when the susceptibility levels in women are below 10%, there is a chance of CRS in the future.[15] With the buildup of such susceptible cohorts, pregnant women can acquire rubella infection from younger children, leading to epidemics among adult population. Studies from different states of the country on acquired rubella infection among pregnant women during pregnancy period reported 3%, 6.7%, and 8.3% prevalence from Kerala, Andhra Pradesh, and New Delhi, respectively, as evidenced by IgM estimation.[14,16] With an average annual incidence of 5 lakh deliveries, we could expect about 15,000 infections from Kerala among pregnant women and proportional CRS.
Since rubella vaccine was not included in the childhood vaccination program in India only three of our subjects have gave history of previous rubella vaccination. They received it from private providers as an optional vaccine with out-of-pocket expenditure. Currently, about 15–25% of the child population are getting rubella vaccine (RA 27/3) combined with mumps and measles (MMR). Reviews have shown that RA 27/3 vaccine has a duration of protection ranging from 10 to 21 years in children with a seropositivity rate of 95%.[9] As per the district level household surveys (DLHS I, II. III), the immunization coverage for the Kerala state is 84%, 78.6%, and 83%, with few districts below the state average, and at country average, it is only 65%.[17] Hence, with the current status of subpopulation immunization leading to “age shifting,” oscillating herd immunity, the probability of paradoxical rubella outbreaks among adult population and CRS, as occurred in Greece, cannot be ruled out in India.[3,11,12,18]
This study shows that rubella virus infection is prevalent in Kerala in all socioeconomic strata, and more than 30% girls reach childbearing age without acquiring natural immunity against the disease. Studies conducted across India suggest similar baseline information on the susceptibility profile of women of childbearing age.[19] Although rubella vaccine is safe and effective, clear policy regarding rubella immunization of children either at 15 months or young girls at 9–12 years has not been outlined in India. For controlling CRS, the “indirect strategy” is to immunize children and reduce transmission of rubella, and the “direct strategy” is to immunize adolescents to prevent rubella infection and CRS.[18,19,20] The first may pose a risk to adults and the second cannot prevent rubella transmission as described, so both are needed in a combined, coordinated form.
Consider a scenario, in which combination MMR vaccine has been introduced in the UIP of India. The state with expected average immunization coverage of 80% and vaccine efficacy of 95% will produce 24% birth cohort without immunity to rubella in each year. Considering the birth rate of 15 per thousand, within a 10–15 year period, it will produce a large number of rubella-susceptible youth population in the state, later leading to CRS. In other states where the immunization coverage is poor, the extrapolation of the data predicts a worse situation.
As supported by the WHO, Gavi, Kerala, India, may introduce MMR vaccine in UIP for children in the near future. Many authors have warned that Rubella vaccination: must not be business as usual. The childhood immunization programs against rubella without specifically addressing the countries epidemiology will not achieve the desired results.[19,20,21] To prevent these negative impacts, efforts should be taken to maintain the “herd immunity threshold” at a level above 83% among the adult population without regional variation within the country.[3,20,21] Therefore, the best results can be achieved only by a combined immunization policy as adopted by Denmark, Sweden, most of the European countries, and the USA, where the first dose is offered as MMR vaccine at 15–18 months of age and the second dose as MMR vaccine at 6–12 years, or only rubella vaccine exclusively to girls at 12–14 years of age is best for India too to eliminate CRS in the near future along with surveillance of rubella.[5,18]
Conclusion
Substantial numbers of women reach childbearing age without immunity against rubella and thus are at a risk of passing the infection to their fetuses, who can then develop subsequent congenital defects leading to CRS. An immunization policy recommending rubella-containing vaccine is highly desirable to prevent rubella and CRS.
Financial support and sponsorship
This study was financially supported by the State Board of Medical Research, Government of Kerala.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was funded by the State Board of Medical Research (SBMR) – Directorate of Medical Education, Government of Kerala. The authors express thanks to the staff and PTA students of Mavoor, Government high school. Thanks to Dr. Thomas Bina, Head of the Department, Community Medicine, for the help, and Dr. Rema Devi, Former Head of the Department of Microbiology, for providing laboratory support for the study.
References
- 1.Ramamurty N, Murugan S, Raja D, Elango V, Mohana, Dhanagaran D. Serosurvey of rubella in five blocks of Tamil Nadu. Indian J Med Res. 2006;123:51–4. [PubMed] [Google Scholar]
- 2.Indian Association of Paediatrics (IAP) Committee on Immunization 2005-2006. Indian Association of Paediatrics. 4th ed. 2007. Jan, pp. 23–4. [Google Scholar]
- 3. [Last accessed on 2009 Dec 10];Rubella vaccine WHO position paper. Wkly Epidemiol Rec. 2000 75:161–72. Available from: http://www.who.int/wer . [Google Scholar]
- 4.Controlling rubella and preventing congenital rubella syndrome – Global progress, 2009. Wkly Epidemiol Rec. 2010;85:413–8. [PubMed] [Google Scholar]
- 5.Yadav S, Wadhwa V, Chakarvarti A. Prevalence of rubella antibody in school going girls. Indian Pediatr. 2001;38:280–3. [PubMed] [Google Scholar]
- 6.Sharma HJ, Padbidri VS, Kapre SV, Jadhav SS, Dhere RM, Parekh SS, et al. Seroprevalence of rubella and immunogenicity following rubella vaccination in adolescent girls in India. J Infect Dev Ctries. 2011;5:874–81. doi: 10.3855/jidc.1847. [DOI] [PubMed] [Google Scholar]
- 7.Yadav S, Gupta S, Kumari S. Seroprevalence of rubella in women of reproductive age. Indian J Pathol Microbiol. 1995;38:139–42. [PubMed] [Google Scholar]
- 8.Gupta E, Dar L, Broor S. Seroprevalence of rubella in pregnant women in Delhi, India. Indian J Med Res. 2006;123:833–5. [PubMed] [Google Scholar]
- 9.Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2011;86:301–16. [PubMed] [Google Scholar]
- 10.Dewan P, Gupta P. Burden of congenital rubella syndrome (CRS) in India: A systematic review. Indian Pediatr. 2012;49:377–99. doi: 10.1007/s13312-012-0087-4. [DOI] [PubMed] [Google Scholar]
- 11.Grant GB, Reef SE, Dabbagh A, Gacic-Dobo M, Strebel PM. Rubella and congenital rubella syndrome control and elimination – Global progress, 2000-2014, WHO. Wkly Epidemiol Rec. 2015;90:510–16. [Google Scholar]
- 12.Papa A, Gioula G, Antoniadis A, Kyriazopoulou-Dalaina V. Rubella epidemic strain, Greece, 1999. Emerg Infect Dis. 2004;10:1696–7. doi: 10.3201/eid1009.040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma H, Chowdhari S, Raina TR, Bhardwaj S, Namjoshi G, Parekh S. Sero-surveillance to assess immunity to rubella and assessment of immunogenicity and safety of a single dose of rubella vaccine in school Girls. Indian J Community Med. 2010;35:134–7. doi: 10.4103/0970-0218.62575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padmaja M, Radhakrishna PM, Varghese SJ. Seroprevalence of immunity to rubella in pregnant women. Natl Med J India. 2010;23:248–9. [PubMed] [Google Scholar]
- 15.Guidelines for Surveillance of Congenital Rubella Syndrome and Rubella: Field Test Version, May 1999. Geneva: Department of Vaccines and Biologicals, World Health Organization; 1999. [Google Scholar]
- 16.Ramana BV, Reddy BK, Murty DS, Vasudevanaidu KH. Seroprevalence of rubella in women with bad obstetric history. J Family Med Prim Care. 2013;2:44–6. doi: 10.4103/2249-4863.109943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Institute for Population Sciences (IIPS), 2014. District Level Household and Facility Survey (DLHS-4), 2012-13: India. Kerala, Mumbai: IIPS; 2014. [Google Scholar]
- 18.Panagiotopoulos T, Antoniadou I, Valassi-Adam E. Increase in congenital rubella occurrence after immunisation in Greece: Retrospective survey and systematic review. BMJ. 1999;319:1462–7. doi: 10.1136/bmj.319.7223.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taneja DK, Sharma P. Targeting rubella for elimination. Indian J Public Health. 2012;56:269–72. doi: 10.4103/0019-557X.106413. [DOI] [PubMed] [Google Scholar]
- 20.Cutts FT, Metcalf CJ, Lessler J, Grenfell BT. Rubella vaccination: Must not be business as usual. Lancet. 2012;380:217–8. doi: 10.1016/S0140-6736(12)61215-X. [DOI] [PubMed] [Google Scholar]
- 21.Jayakrishnan T. Newer vaccines in the universal immunization program. Indian J Med Ethics. 2012;7:107–11. doi: 10.20529/IJME.2011.039. [DOI] [PubMed] [Google Scholar]


