Abstract
Single-strand gaps (SSGs) and double-strand breaks (DSBs) are the major initiation sites for recombination. In bacteria, the SSGs are repaired by RecFOR, while the DSBs are processed by RecBCD in gram-negative bacteria and AddAB in gram-positive bacteria. Unexpectedly, instead of recBCD genes, the addAB genes were found in members of the α-proteobacteria group (gram negative). Taking Rhizobium etli as a model, the role of recF and addAB genes in homologous recombination and repair of damaged DNA was evaluated. Inactivation of either recF or addA provoked strong sensitivity to UV radiation and mitomycin C, while an additive effect was observed in the recF-addA mutant. The DSBs generated by nalidixic acid caused low viability only in the addA mutant. The recombination frequency of large and small plasmids was reduced in the recF mutant (24- and 36-fold, respectively), whereas a slight decrease (threefold) in the addA mutant was observed. Moreover, an additive effect (47- and 90-fold, respectively) was observed in the double mutant, but it was not as dramatic as that in a recA mutant. Interestingly, the frequency of deletion and Campbell-type recombination was slightly affected in either single or double mutants. These results suggest that another pathway exists that allows plasmid and Campbell-type recombination in the absence of recF and addA genes.
Homologous recombination is a key process involved in the repair of damaged DNA, the appropriate segregation of chromosomes, and the generation of genetic diversity (11, 29). The genetic and biochemical studies in prokaryotes and eukaryotes have allowed the division of the process of DNA recombination into four successive steps, initiation, homologous pairing, branch migration, and resolution (29).
In the gram-negative bacterium Escherichia coli, about 20 gene products are implicated. In the initiation step, several protein complexes (RecBCD, RecFOR, RecJ, RecQ, RecN, etc.) act alternatively to generate the crucial 3′ single-stranded DNA (ssDNA) end. This end is required for the RecA-dependent pairing between homologous sequences, producing a four-strand intermediate known as a Holliday junction. The Holliday junction is processed by either the RuvAB complex (causing branch migration) or the RecG protein (favoring resection of the Holliday intermediate). Finally, RuvC resolves the RuvAB-cruciform complex specifically (29). Alternatively, replication enzymes (DNA polymerase III and primosome proteins) are recruited to resolve recombinants (30, 44). Alternative use of different protein complexes in the initiation step has revealed the existence of the RecBCD, RecFOR, and RecE recombination pathways (29). The first two pathways are widely distributed in E. coli, while the last pathway (encoded on a cryptic prophage) is restricted to some K-12 strains. Additionally, the RecBCD pathway is involved in the majority of conjugational recombination and in repairing double-stranded DNA breaks (DSBs) in wild-type bacteria. In contrast, the RecFOR pathway is implicated in plasmid recombination as well as in repairing single-stranded DNA gaps (SSGs) (30, 31).
A similar number of recombination genes, classified into five epistatic groups (α, β, γ, ɛ, and ζ), have been described in mutational studies of the gram-positive bacterium Bacillus subtilis. The α and β groups have functional counterparts in E. coli corresponding to the RecFOR and RecBCD pathways, respectively (1, 2, 17).
In bacteria, the 3′ ssDNA ends required for recombination are principally generated by the processing of SSGs and DSBs. These lesions are generated as a by-product of normal DNA metabolism (replication, transcription, etc.); their occurrence can be increased by DNA-damaging agents and other environmental conditions (11, 29, 31). Upon encountering an SSG, the RecFOR complex binds the 5′ terminus at the ssDNA-double-stranded DNA junction of the SSG, allowing the loading of the RecA protein onto ssDNA; alternatively, the helicase-exonuclease proteins (RecQ and RecJ) can process this structure to generate the 3′ ssDNA end required to recombinationally repair the SSGs (5, 43, 61, 63, 64). These genes are widely distributed in eubacteria (13).
In E. coli, the helicase-exonuclease activity of the RecBCD complex is needed to generate 3′ ssDNA ends at the DSBs in a process modulated by the RecD subunit in a sequence-dependent manner (45). The heterodimeric B. subtilis AddAB enzyme is the functional equivalent of RecBCD (26, 42). The distribution of recBCD/addAB genes is believed to be group specific; previous evaluations identified recBCD homologs in gram-negative bacteria, while the addAB genes are thought to be restricted to gram-positive bacteria (9).
Our interest in the study of recombination in the gram-negative α-proteobacterium Rhizobium etli is motivated by the fluidity displayed by the rhizobial genomes, where many rearrangements can occur by homologous recombination between repeated sequences present in the entire genome; these rearrangements may affect the symbiotic interaction of Rhizobium with leguminous plants (39-41, 51-54, 62). Therefore, the identification of genes that participate in the initial steps of recombination is particularly important. Although clear recF homologs were identified during this study, no convincing recBCD homologs were found. Surprisingly, BLAST searches in which the addAB sequences from B. subtilis or the rexAB sequences of Lactococcus lactis were used as queries revealed the presence of addAB homologs in several species of the α-proteobacteria group, including R. etli. In this work, we characterize the role of both recF and addA in the repair of damaged DNA and in the recombination of small and large plasmids in R. etli. Surprisingly, the inactivation of these two R. etli recombination pathways did not lead to a complete loss of recombination capacity, although repair of damaged DNA was seriously affected. This finding may indicate that R. etli possess additional recombination activities such as helicases or prophage recombinases.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The strains and plasmids used in this work are listed in Table 1. R. etli strains were grown in PY medium supplemented with CaCl2 (6) at 30°C. E. coli strains were grown in Luria-Bertani medium (55) at 37°C. Antibiotics were used at the following concentrations: nalidixic acid, 20 to 150 μg ml−1; spectinomycin, 100 μg ml−1; kanamycin, 30 μg ml−1; gentamicin, 5 μg ml−1; and tetracycline, 5 μg ml−1. Mitomycin C (Sigma-Aldrich) was used at concentrations ranging from 0.5 to 1 μg ml−1. UV treatment was carried out by using the UVC500 cross-linker (Hoefer Scientific Instruments). After DNA damage treatment (mitomycin C, UV radiation, or nalidixic acid), the cells were incubated at 30°C in the dark. β-Galactosidase-positive strains were identified by growth on plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). For positive selections involving the sacB gene, sucrose-resistant strains were selected in presence of 12.5% (vol/vol) sucrose.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strain | ||
| R. etli | ||
| CFN42 | Wild type, Nalr | 50 |
| CE3 | CFN42 derivative, Strr | 50 |
| CFNX101 | CE3 derivative, recA::Sp | 39 |
| CFNX693 | CE3 derivative, recF::loxPSp | This work |
| CFNX694 | CE3 derivative, addA::loxPSp | This work |
| CFNX695 | CE3 derivative with a deletion-substitution of addAaddB by ΔaddAB::loxPSp | This work |
| CFNX696 | CFNX693 derivative, but recF::loxP | This work |
| CFNX697 | CFNX696 derivative, but addA::loxPSp | This work |
| E. coli | ||
| DH5α | recA1 | Stratagene |
| S17.1 | recA13, mobilization host | 56 |
| Plasmid | ||
| pBluescript II SK(+) | Cbr, cloning vector | Stratagene |
| pSUP202 | Cmr Tcr Cbr, suicide vector | 56 |
| pKOK4 | Cmr Tcr Cbr, suicide vector | 23 |
| pJQ200SK+ | GmrsacB, suicide vector | 47 |
| pBX404-7 | Tcr 5′ del neo/3′ del neo Kms plasmid recombination system | 65 |
| pBBRMCS5 | Gmr, broad-host-range vector | 28 |
| pJMS2 | Spr, source of loxPSp cassette | 40 |
| pJMS08 | vector containing the loxP site-specific recombinase Cre | Martínez-Salazar et al., unpublished |
| pLGsym56 | pSUP202 derivative harboring a 6.0-kb BamHI fragment from R. etli pSym | 19 |
| pLG506 | pSUP202 derivative containing 2.0-kb HindIII ΩKm and MCS from pBluescript SK(+) into BgIII | Girard et al., unpublished |
| pRK2013 | Kmr, helper vector | 56 |
| c1343 | pLAFR1 cosmid harboring R. etli recF region | This work |
| c2486 | pLAFR1 cosmid harboring R. etli addAB region | This work |
| pJZC1 | pKOK4 derivative harboring the 3.0-kb EcoRI fragment of R. etli recF gene | This work |
| pJZC2 | pJZC1 derivative containing the R. etli recF::loxPSp allele | This work |
| pJZC3 | pSK derivative harboring a 6-kb EcoRI fragment of R. etli addA gene | This work |
| pJZC4 | pSK derivative harboring a 1.5-kb EcoRI PCR product with 225 bp upstream and 1,188 bp of R. etli addA gene | This work |
| pJZC5 | pJZC4 derivative harboring the R. etli addA::loxPSp allele | This work |
| pJZC6 | pJQ200SK derivative harboring the 3.5-kb EcoRI addA::loxPSp from pJZC5 into MCS | This work |
| pJZC7 | pJQ200SK derivative containing the 3.3-kb EcoRI-SalI 5′ del neo/3′ del neo Kms from pBX404-7 into MCS | This work |
| pJZC8 | pJZC7 derivative containing the 6.0-kb BamHI fragment from pLGsym56 into MCS | This work |
| pJZC9 | pBBMCS5 derivative harboring the addA gene in the same orientation to lacZ promoter | This work |
| pJZC10 | pBBMCS5 derivative harboring the addA gene in the opposite orientation to lacZ promoter | This work |
| pJZC11 | pBBMCS5 derivative harboring the addAB genes in the same orientation to lacZ promoter | This work |
| pJZC12 | pSK derivative containing the 1.2-kb SalI from pJZC3, corresponding to 585 bp of 3′ end of addA and 672 bp downstream (trxA and folC) | This work |
| pJZC13 | pJZC12 derivative harboring the 2.0-kb SalI loxPSp into the SalI site located at aa 988 of addA | This work |
| pJZC14 | pSK derivative harboring the 1.2-kb EcoRI-XhoI, corresponding to aa 63 to 469 of addB | This work |
| pJZC15 | pJZC13 derivative harboring 1.2-kb EcoRI-XhoI from pJZC14 into EcoRI-SalI, ΔaddAB::loxPSp | This work |
| pJZC16 | pLG506 derivative harboring the 4.4-kb EcoRI-XhoI from pJZC15 (ΔaddAB::loxPSp) | This work |
Recombinant DNA procedures.
Restriction enzymes, alkaline phosphatase, and T4 DNA ligase were purchased from Amersham Biosciences or Invitrogen Life Technologies and were used as recommended by the manufacturer. Plasmid DNA was isolated by an alkaline-sodium dodecyl sulfate lysis method and transformed into CaCl2-treated E. coli cells; other general DNA methods were done according to standard protocols (55). The transfer of plasmids from E. coli to R. etli was done by biparental or triparental matings with S17.1 or pRK2013 plasmid as a helper. Chromosomal DNA from R. etli and Southern blot hybridization were carried out as previously described (40). Probes were labeled with [α32-P]dCTP by using a Rediprime II kit (Amersham Biosciences).
The PCRs were carried out with Taq DNA polymerase (Invitrogen Life Technologies) as follows: 95°C for 1 min, 55°C for 1 min, and 72°C for 2 min for 30 cycles, with an extension step at 72°C for 10 min. For long PCR products (3 to 7 kb), the reactions were carried out with a PCR-XL kit (rTth polymerase; Perkin-Elmer) with the following cycling regime: 15 cycles of 92°C for 30 s, 60°C for 1 min, and 68°C for 10 min and 16 cycles of 92°C for 30 s, 60°C for 1 min, and 72°C for 10 min.
The oligonucleotides used to obtain the PCR products were the sense FrF1 (5′-GGC GAG AAC GGT GCG GGC AA-3′) and antisense RrF3 (5′-GTC GAG AAA GCG CCT GCG GT-3′) (corresponding to amino acids 33 to 40 and 137 to 144, respectively) of Sinorhizobium meliloti recF and the sense ReBUSLA (5′-GCG AGG ACG ACG ATG AGG-3′) and antisense ReBLSLA (5′-TCG AGC GGG AAC TGG TGC-3′) (corresponding to 12 bp upstream to aa 2 and 143 to 149, respectively) of the S. meliloti addA (SMc02760) gene. The primers used to obtain PCR products of the R. etli addAB region were the sense JadKp1 (5′-GCG GGT ACC GAA GCG GAG GAA ACA ATC-3′) (corresponding to 30 to 56 bp upstream of R. etli addB), the antisense JaddAE (5′-TCA GCG AAT TCG CGG TGC GGG TGA TCA GG-3′) (corresponding to aa 389 to 396 of the addA gene), and the antisense JadSp1a (5′-TGA GAC TAG TTT TCA CGG TAG CCA TAG G-3′) (corresponding to 76 to 96 bp downstream of the R. etli addA gene). The KpnI, EcoRI, and SpeI sites introduced are underlined. The sequencing primers were synthesized by Unidad de Síntesis, Instituto de Biotecnología, Universidad Nacional Autónoma de México.
Nucleotide sequence.
The R. etli cosmids c1343 and c2486, containing the recF and addAaddB genes respectively, were subcloned into pBluescript II SK(+) and sequenced by the Sanger dideoxy chain termination method using a Thermosequenase cycle sequencing kit (Amersham Biosciences). Primers for sequencing were 5′ end labeled with [γ-33P]dATP and T4 DNA kinase. For automated sequencing, a DYEnamic ET dye terminator cycle sequencing kit (Amersham Bioscienes) and the ABI-prism 310 (Applied Biosystems) were used.
Sequence analysis.
BLAST searches were performed with BLASTX, BLASTP, and PSI-BLAST programs at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov). Multiple protein alignment analyses were carried out with the ClustalW program (60), and the phylogenetic relationships were obtained by using the PHYLIP program (16) and visualized with TreeView software (46).
Plasmid construction.
pJZC1 is a pKOK4 derivative (23) containing the 3.0-kb EcoRI fragment from c1343. This vector is mobilizable by conjugation but unable to replicate in R. etli. pJZC2 is a pJZC1 derivative where the recF gene was interrupted by insertion of the 2.0-kb SalI loxPSp interposon from pJMS2 into the SalI site located at codon 240 (40). pJZC3 is a pBluescript II SK(+) derivative harboring the 6.0-kb R. etli addA gene-containing EcoRI fragment from c2486. pJZC4 is a pBluescript II SK(+) derivative containing a 1.5-kb EcoRI PCR product using the JaddAE and −20 primers from pJZC3 (having the 225 bp upstream and 1,188 bp of the coding addA gene). pJZC5 is a pJZC4 derivative with the 2.0-kb BamHI loxPSp interposon inserted into the BglII site located at codon 95 of addA. pJZC6 is a pJQ200SK+ (47) derivative carrying the 3.5-kb EcoRI fragment from pJZC5 (addA::loxPSp) in the multiple cloning site (MCS). pJZC7 is a pJQ200SK+ derivative containing the 3.3-kb EcoRI-SalI fragment from pBX404-7 (65) (contains the truncated but overlapping version of the kan/neo gene) in the MCS. pJZC8 is a pJZC7 derivative containing the 6.0-kb BamHI fragment from pLGsym56 (fragment of R. etli pSym) (19) in the MCS. This suicide and mobilizable plasmid allows measurement of Campbell-type recombination with pSym. After its integration, large-plasmid recombination with pSym (385 kb) was evaluated by restoration of the kanamycin gene or deletions that confer sucrose resistance and gentamicin sensitivity.
The 6-kb EcoRI fragment from pJZC3 was cloned into pBBRMCS5 in both orientations to place the addA gene in the same direction (pJZC9) or in the opposite direction (pJZC10) with respect to the lacZ promoter.
Primers JadKp1 and JadSp1a were used to obtain a 6-kb PCR product from the R. etli genome (containing the 76 bp upstream of addB and the entire addB and addA genes) that was cloned into pCR2.1-TOPO (Invitrogen Life Technologies) to create pTOPO::addAB. Plasmid pJZC11 is a pBBRMCS5 derivative containing the 6-kb SpeI fragment from pTOPO::addAB with both genes in the same direction as the lacZ promoter. Plasmid pJZC12 is a pBluescript II SK(+) derivative containing a 1.2-kb SalI fragment from pJZC3 comprising 585 bp of the 3′ end of the addA gene (aa 988 to 1183) and 672 bp downstream (corresponding trxA and folC genes). pJZC13 is a pJZ12 derivative with the 2.0-kb SalI loxPSp interposon from pJMS2 cloned into the SalI site located at codon 988 of the addA gene. Plasmid pJZC14 is a pBluescript II SK(+) derivative containing the addB 5′ end (corresponding to aa 63 to 469) in a 1.2-kb EcoRI-XhoI fragment. The pJZC14 1.2-kb EcoRI-XhoI fragment was inserted between the EcoRI and SalI sites of pJZC13 to generate pJZC15. The resulting plasmid has the deletion-substitution of the addAB genes by the loxPSp interposon (ΔaddAB::loxPSp) with a 2.5-kb deleted region comprising codon 406 of AddB (C terminus) to codon 195 of AddA (N terminus). Plasmid pJZC16 is a suicide plasmid derivative of pLG506 (M. L. Girard et al., unpublished data) harboring the 4.4-kb EcoRI-XhoI fragment from pJZC15 (ΔaddAB::loxPSp).
Construction of recF, addA, and recF-addA mutants.
The R. etli recF (CFNX693), addA (CFNX694), and deletion-substitution ΔaddAaddB (CFNX695) mutants were obtained by homologous gene replacement of the wild-type allele with the recF::loxPSp (pJZC2), addA::loxPSp (pJZC6), and ΔaddAB::loxPSp (pJZC16) alleles, respectively. To that end, the corresponding plasmid was transferred by triparental mating from E. coli to R. etli CE3, and the double recombinants were selected on the basis of their respective phenotypes. The double mutant recF::loxP-addA::loxPSp (CFNX697) was generate in two steps. First, the loxPSp interposon in the CFNX693 strain was deleted by using the loxP-specific Cre recombinase expressed from pJMS08 (J. M. Martínez-Salazar et al., unpublished data). The loxPSp deletion and pJMS8 curing plasmid strain was selected by screening for the Sps Tcs phenotype. In a second step, the addA::loxPSp allele was introduced by marker exchange in the CFNX696 (recF::loxP) strain, and the double mutant was selected by the Spr Gms Sacr phenotype. All mutants were verified by Southern blot hybridization using the corresponding intermediate plasmids as probes.
Integrative recombination assay.
Campbell-type recombination was evaluated in proficient and mutant strains of R. etli by using the conjugative, nonreplicative pJZC8 plasmid (14.3 kb). This plasmid has a 6-kb region homologous to the symbiotic plasmid (371 kb), which generates pSym::pJZC8 after integration.
Small-plasmid recombination determination.
The small, broad-host-range vector pBX404-7 (13 kb) (65) was introduced into wild-type and mutant strains by triparental matings using pRK2013 as a helper. pBX404-7 contains two truncated but overlapping copies of the kan/neo resistance gene. Plasmid recombination (intermolecular and unequal sister exchange) frequency was measured by the restoration of the kanamycin resistance gene as described previously (40, 65).
Large-plasmid recombination assays.
To evaluate recombination on a large plasmid, pSym::pJZC8 was obtained by integration of pJZC8 into pSym in wild-type and mutant strains (see above). The strains were verified for sensitivity to DNA-damaging agents and normal plasmid profile by the Eckhardt method (6).
Nucleotide sequence accession numbers.
GenBank accession numbers for the nucleotide sequences of recF and addAB regions are AY552333 and AY552334, respectively.
RESULTS
Isolation of R. etli recF and addAB genes.
A bioinformatic approach was used to identify crucial genes involved in the initiation of recombination in the gram-negative α-proteobacteria group (Agrobacterium tumefaciens, Bradyrhizobium japonicum, Brucella melitensis, Brucella suis, Mezorhizobium loti, Rickettsia prowazekii, and S. meliloti). To that end, BLAST searches were performed by using the E. coli recF, recO, recR, recB, and recC genes; the B. subtilis addA and addB genes; and the L. lactis rexA and rexB genes as queries.
The analysis revealed that the recF, recO, and recR genes were present in this group of bacteria as well as in other reported bacterial genomes (13). Unexpectedly, recB and recC were absent in members of the α-proteobacterial group sequenced to date; instead, homologs of the addA and addB genes were found (see below). In addition, the addA and addB genes were found in another gram-negative bacterium (Ralstonia solanacearum, a β-proteobacterium). The AddAB and RecBCD enzymes (composed of two and three subunits, respectively) are both members of the helicase-exonuclease type V protein family and fulfill similar roles in the initiation of homologous recombination. Previous studies suggested that the recBCD genes were present only in gram-negative species, while addAB genes appear to be restricted to gram-positive bacteria (9).
To study the initiation of recombination in our R. etli CE3 strain model system, the recF and addA genes were isolated. An R. etli cosmid library (constructed with EcoRI fragments in pLAFR1) (40) was screened by Southern blot hybridization using PCR products corresponding to the recF and addA (SMc02760) genes from the related bacterium S. meliloti as probes. The cosmids c1343 (recF) and c2486 (addAB) were subcloned, and regions containing the genes were sequenced.
Conserved functional motifs in α-proteobacterial AddA proteins.
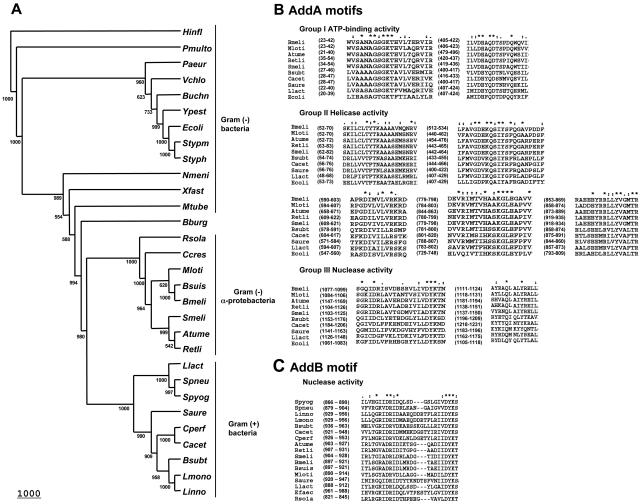
To gain insight into the identity of R. etli AddA and other homologs in α-proteobacteria, the corresponding amino acid sequences were compared with the reported AddA and RecB proteins. Figure 1A shows the phylogenetic relationships for RecB and AddA proteins in bacteria. Interestingly, the AddA homologs in α-proteobacterial species are more similar to those found in gram-positive species than to RecB genes of gram-negative bacteria. Moreover, the seven conserved helicase domains (25, 27) and the nuclease motif (48) of the protein family described previously are present (Fig. 1B). Convincing homologs of the addB genes are present upstream of all the α-proteobacterial addA genes. This organization mirrors the one found for addAB genes in the gram-positive group. The proteins encoded by these putative addB genes also contain a nuclease motif (Fig. 1C) characteristic of members of this family. Furthermore, the α-proteobacterial addB genes display a phylogenetic distribution fully congruent with that of the addA genes (data no shown). Despite the existence of a similar organization and high similarity of the α-proteobacterial addAB genes with members of the gram-positive clade, sequence features of the α-proteobacterial genes, such as codon usage and GC content, are consistent with the consensus found for each species (data not shown). These data do not support a recent horizontal transfer event from gram-positive bacteria as the origin of the α-proteobacterial homologs.
FIG. 1.
Phylogenetic relationships of the AddA and RecB subunits of the helicase-exonuclease type V protein family. A. The neighbor-joining cladogram was constructed with PHYLYP by using a CLUSTALW multiple-protein alignment of gram-negative bacteria [Gram (−)] RecB and gram-positive bacteria [Gram (+)] AddA sequences. The corresponding bootstrap values (1,000 replications) are shown at the edge of each clade. The GenBank accession numbers corresponding to each species are included in parentheses. Abbreviations for gram-negative bacteria: Mtube, Mycobacterium tuberculosis (accession number CAB07119); Xfast, Xylella fastidiosa (accession number AAF83233); Nmeni, Neisseria meningitidis (accession number AAF41198); Paeru, Pseudomonas aeruginosa (accession number AAG076672); Vchol, Vibrio cholerae (accession number AAF95464); Ypest, Yersinia pestis (accession number CAC89863); Ecoli, E. coli (accession number AAC75859); Styph, Salmonella enterica serovar Typhi (accession number CAD02818); Stypm, Salmonella enterica serovar Typhimurium (accession number AAL21870); Hinfl, H. influenzae (accession number ACC22966); Pmult, Pasteurella multocida (accession number AAK02600); Buchn, Buchnera sp. (accession number AP001119); Bburg, Borrelia burgdorferi (accession number AAC66981); Ccres, Caulobacter crescentus (accession number AAK25500); Mloti, Mezorhizobium loti (accession number BAB51593); Bmeli, Brucella melitensis (accession number AAL53204); Bsuis, Brucella suis (accession number ANN30993); Smeli, Sinorhizobium meliloti (accession number CAC41421); Atume, Agrobacterium tumefaciens (accession number AAK85848); Retli, R. etli (accession number AY552334); Rsola, Ralstonia solanacearum (accession number CAD14892). Abbreviations for gram-positive bacteria: Saure, Staphylococcus aureus (accession number BAB42067); Bsubt, B. subtilis (accession number AAA22200); Linno, Listeria innocua (accession number CAC97575); Lmono, Listeria monocytogenes (accession number EAL05437); Cacet, Clostridium acetobutylicum (accession number AAK80219); Cperf, Clostridium perfringens (accession number BAB79727); Llact, L. lactis (accession number AAK04102); Spneu, Streptococcus pneumoniae (accession number AAK99844); Spyog, Streptococcus pyogenes (accession number AAK33717). B. Conserved domains among AddA homologs of α-proteobacteria, AddA of gram-positive bacteria and RecB of E. coli (25, 27, 48). The asterisks indicate conserved amino acids, and colons indicate conservative replacements. C. Alignment of the nuclease motif present at the C termini of AddB homologs of α-proteobacteria and gram-positive bacteria proteins (48). The asterisks indicate conserved amino acids, and colons indicate conservative replacements.
Conversely and in concordance with the distribution previously reported (13), the phylogenetic distribution for the α-proteobacterial recF homologs is similar to that reported for recF genes of gram-negative bacteria (data no shown).
Inactivation of addA and recF genes.
To determine the biological role of AddA and RecF proteins in α-proteobacteria, the corresponding R. etli CE3 genes were inactivated. Mutants harboring the recF::loxPSp (CFNX693), the addA::loxPSp (CFNX694), or a deletion-substitution ΔaddAB::loxPSp (CFNX695) allele were generated by homologous gene replacement (see Materials and Methods). Additionally, inactivation of both recombination pathways was achieved by sequential introduction of mutations into both genes. To that end, the loxPSp interposon was excised from the strain CFNX693 (recF::loxPSp) by using the specific Cre recombinase. These excisants are still inactivated in the recF gene due to the retention of a 181-bp insertion. The introduction of the addA::loxPSp allele in these excisants by marker exchange gave rise to the recF::loxP-addA::loxPSp (CFNX697) mutant (see Materials and Methods). The inactivation of the addA gene showed a dramatic reduction in cell viability (20 to 30% of the wild type), while the effect was slight (80 to 90% of the wild type) in recF mutants.
The AddA and RecF proteins are required for repair of damaged DNA.
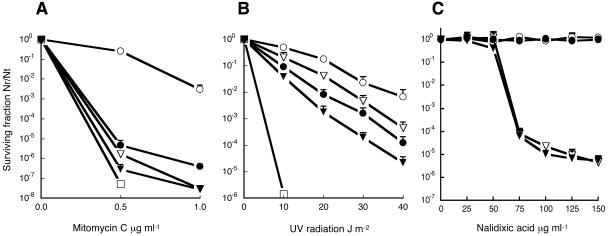
It is well known that recombination proteins play an active role in the recombinational repair of damaged DNA (11, 31). To study the roles of addA and recF in DNA repair, the corresponding R. etli mutants were evaluated for sensitivity towards several mutagenic agents. Mutants in either the recF or addA gene displayed a marked hypersensitivity to both mitomycin C and UV radiation (Fig. 2A and B), agents that may generate SSGs and DSBs (31). Interestingly, the sensitivity of the double mutant recF::loxP-addA::loxPSp was increased in an additive way (Fig. 2A and B), suggesting a joint participation of both systems in DNA repair.
FIG. 2.
Sensitivity of R. etli mutants to mitomycin C (A), UV radiation (B), and nalidixic acid (C). Data are the averages of three independent experiments. The surviving fraction is the number of viable cells after treatment with DNA-damaging agents divided by the number of viable cells without treatment. Symbols: CE3 (wild type), ○; CFNX693 (recF::loxPSp), •; CFNX694 (addA::loxPSp), ▿; CFNX697 (recF::loxP-addA::loxPSp), ▾; CFNX101 (recA::Sp), □.
On the other hand, while inactivation of the addA gene led to increased sensitivity to nalidixic acid, the recF mutant remained as resistant as the wild-type strain towards this antibiotic (Fig. 2C). Strains harboring the simultaneous inactivation of both recF and addA (recF::loxP-addA::loxPSp) or with a loss of both addA and addB (ΔaddAB::loxPSp) showed sensitivity to nalidixic acid similar to that of the addA::loxPSp mutant (Fig. 2C and data not shown, respectively).
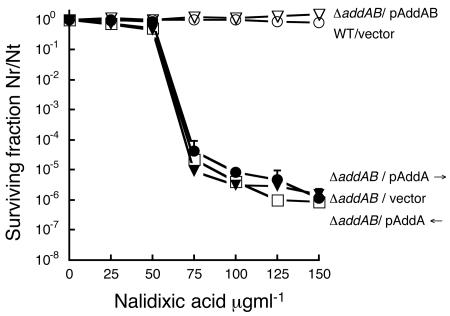
Complementation tests of the ΔaddAB::loxPSp mutant strain were performed by using vectors containing either the addA or addAB genes (see Materials and Methods). Ectopic expression of addA was sufficient to complement the addA mutant to wild-type levels with respect to nalidixic acid sensitivity and viability (Fig. 3 and data not shown). Full complementation of the ΔaddAB strain requires simultaneous expression of both addA and addB (Fig. 3). It is known that nalidixic acid causes an increase in DSB formation (58). The strong sensitivity towards nalidixic acid observed by inactivation of addAB genes, coupled with the results of complementation experiments, suggests that this enzymatic complex has a prime role in DSB repair.
FIG. 3.
R. etli DSB repair is dependent on both addA and addB genes. The CFNX695 (ΔaddAB::loxPSp) mutant was complemented by the addAB genes located in plasmid. The surviving fraction is the number of viable cells divided by the number of total cells. Each point is the average of three independent experiments. The arrows indicate that the addA gene is in the same (→) or in the opposite (←) orientation to the lacZ promoter of the vector.
Recombination of both small and large plasmids is dependent on RecF and AddA.
To gain further insight into the physiological relevance of RecF and AddA, we examined the effect of R. etli mutants on homologous recombination. To this end, reporter systems on plasmids were used to evaluate different recombination events. The recombination of small plasmids was evaluated with pBX404-7 (65), which harbors two unequally truncated and overlapping fragments of the kan/neo resistance gene, arranged in a direct orientation. A restoration of kanamycin resistance could be achieved by either intermolecular recombination of two plasmids or intramolecular unequal sister exchange recombination.
To evaluate if large plasmids have differential gene dependence for recombination, pBX404-7 (see above) was modified (pJZC8) to allow integration into one of the large plasmids (pSym, 371 kb) of R. etli, as described in Materials and Methods.
A mutation in recF led to a marked reduction in recombination frequency for both small and large plasmids (36- and 24-fold, respectively [Table 2 ]); in contrast, inactivation of addA showed only a slight decrease (threefold) in recombination in both systems. Moreover, the recF-addA strain showed an additive effect on recombination frequency, with 90- and 47-fold decreases on small and large plasmids, respectively. Both events were more negatively affected by a recA mutation (100-fold for small plasmids and 117-fold for large plasmids [Table 2]).
TABLE 2.
Recombination frequencies of R. etli strainsa
| Strain | Recombination frequency
|
|||
|---|---|---|---|---|
| Campbell-type recombinationb (10−4) | Small plasmidic recombinationc (10−4) | Large plasmidic recombinationd (10−5) | Deletion recombinatione (10−4) | |
| CE3 (wild type) | 6.17 ± 0.91 (1.0) | 4.71 ± 0.77 (1.0) | 5.76 ± 1.05 (1.0) | 5.57 ± 0.99 (1.0) |
| CFNX693 (recF) | 2.05 ± 0.87 (0.33) | 0.13 ± 0.007 (0.027) | 0.24 ± 0.02 (0.042) | 2.92 ± 1.06 (0.52) |
| CFNX694 (addA) | 5.73 ± 1.00 (0.92) | 1.64 ± 0.65 (0.34) | 1.60 ± 0.82 (0.27) | 1.86 ± 0.89 (0.33) |
| CFNX697 (recFaddA) | 4.13 ± 0.48 (0.66) | 0.05 ± 0.005 (0.011) | 0.12 ± 0.00 (0.021) | 0.69 ± 0.55 (0.12) |
| CFNX101 (recA) | 0.0009 ± 0.0007 (0.0015) | 0.02 ± 0.005f (0.01) | 0.049 ± 0.033 (0.0085) | 0.30 ± 0.07 (0.053) |
Data reported are the averages of four independent experiments plus the standard deviations. The numbers in parentheses correspond to frequencies relative to the wild type.
The Campbell-type recombination frequency is the number of gentamicin-resistant colonies divided by the total number of viable cells.
The small plasmidic recombination frequency is the number of kanamycin-resistant colonies divided by the number of tetracycline-resistant cells.
The large plasmidic recombination frequency is defined as the number of kanamycin-resistant colonies divided by the number of viable cells.
The deletion recombination frequency is the number of sucrose-resistant colonies divided by the total number of viable cells. The deletion was verified by gentamicin sensitivity.
Data previously reported (39).
These results led us to conclude the following: (i) RecF constitutes the major pathway for recombination of small plasmids in R. etli, as shown in other systems (10, 22, 32, 36, 37); (ii) although the AddA route plays a minor role in small-plasmid recombination, the near-additive effect observed in the double mutant indicates that the two pathways are required in this system; (iii) large- and small-plasmid recombinations are achieved in similar ways, processed principally by a RecF pathway with a minor contribution of the AddA pathway; and (iv) other activities may participate in large-plasmid recombination (as seen for a recA mutant) since it is not abolished in the double mutant.
Participation of RecF and AddA in deletions.
To evaluate the deletion frequency in the recF (CFNX693), addA (CFNX694), and recF-addA (CFNX697) mutants, a cointegrate pSym::pJZC8 was selected for each mutant (data not shown). In these large plasmids (385 kb), the whole pJZC8 is flanked by two 6-kb direct repeats generated during cointegration. Unequal sister exchange recombination or intramolecular recombination between the 6-kb direct repeats led to the deletion of pJZC8, which was evaluated by scoring for sucrose-resistant and gentamicin-sensitive derivatives.
Inactivation of recF, addA, or both had a weaker effect on deletion recombination in large plasmids (two-, three-, and eightfold, respectively [Table 2]) compared to plasmidic recombination. Interestingly, the reduction in recombination frequency observed in a recA mutant was also more modest in this system (18-fold) than in the large plasmidic assay (117-fold). These results indicate that for the substrate analyzed, recF and addA play a minor role in the generation of deletions. This finding raises the possibility of alternative recombination pathways for the processing of this substrate (see Discussion).
Effect of recF and addAB genes on Campbell-type recombination.
The recF and addA weak effect observed on deletion recombination prompted us to evaluate other types of recombination. One of these is Campbell-type recombination leading to cointegration. The frequency of this event was scored by cointegration of pJZC8 into pSym using a 6-kb homologous region. The recombination frequency of this event was slightly decreased in the recF and recF-addA mutants (30 and 60%, respectively, with respect to wild type) but was not significantly affected in the addA mutant (Table 2). However, the Campbell-type integration was decreased by 666-fold by inactivation of the recA gene. These results suggest the existence of additional recombination pathways responsible for Campbell-type integration (see Discussion).
DISCUSSION
We employed a bioinformatic approach to identify crucial recombination genes in members of the gram-negative α-proteobacteria group. The study focused on genes implicated in the presynaptic formation of the 3′ ssDNA ends at SSGs and DSBs, the major sites of initiation for recombination in bacteria. We found recF, recO, and recR genes in this group, as expected due to their ubiquitous distribution in other bacterial genomes (13). In a previous report, the distribution of recBCD genes was clustered in gram-negative bacteria, while addAB was found exclusively in gram-positive bacteria (9). However, recB and recC were absent in members of the α-proteobacteria, and addA and addB homologs were found (see Results). The α-proteobacterial homologs are part of a single operon, with a genetic organization similar to that observed in the gram-positive bacteria (14, 24). Moreover, both homologs are expressed in R. etli from an upstream promoter of the galF-like gene, which encodes a nucleotidyl transferase not involved in the repair-recombination process (unpublished data). In addition, the GC content and codon usage analysis do not indicate a recent lateral transfer event from gram-positive bacteria to α-protobacteria (data not shown).
Although RecBCD and AddAB/RexAB perform a similar role, several differences are observed: (i) RecBCD is constituted by three subunits, while AddAB/RexAB have two; (ii) although the identity between AddA and RecB is low, both share conserved regions corresponding to helicase and nuclease motifs (25, 27, 48) (Fig. 1B); (iii) only one nuclease motif is present in the RecB subunit of RecBCD, while AddAB have two active nuclease motifs, one in each subunit (48) (Fig. 1C); and (iv) RecBCD has two helicase activities performed by the RecB and RecD subunits, while in AddAB, only AddA has helicase activity (12, 20, 27, 49, 59). Based on these features, the homologs found in the α-proteobacteria group (including R. etli) and in Ralstonia solanacearum (β-proteobacterium) correspond to AddAB. These data indicate that the AddAB pathway is distributed in both gram-positive and gram-negative bacteria.
To evaluate the role of the RecF and AddA proteins in α-proteobacteria, the corresponding genes were interrupted in R. etli CE3. The single recF and addA mutants showed a hypersensitivity to both mitomycin C and UV radiation, both inducers of SSGs and DSBs (31), while the recF-addA double mutant showed an additive effect to these agents (Fig. 2A and B, respectively). Furthermore, only addA and addAB mutants were markedly sensitive to nalidixic acid (DSB inducer) (Fig. 2C and 3). Moreover, ectopic expression of both addA and addB in the ΔaddAB::loxPSp mutant background was needed to repair DSBs. The results observed for R. etli are in agreement with those reported for other bacteria; in both E. coli (gram negative) and B. subtilis (gram positive), the inactivation of recB or addA genes produces a marked decrease in the viability and hypersensitivity to DSB inducers (i.e., nalidixic acid and γ radiation), while the viability of the recF mutant is slightly affected but a strong sensitivity to SSGs inducers is observed (UV radiation and mitomycin C). Finally, a double knockout in either recF-recB (E. coli) or recF-addA (B. subtilis) shows a synergistic effect to DNA-damaging agents (1, 17, 29, 31, 42, 43).
To elucidate the role of RecF and AddA, several homologous recombination events were evaluated in recF, addA, and recF-addA genetic backgrounds. The 36-fold reduction of small-plasmid recombination observed in R. etli clearly established its dependence on recF, while the inactivation of addA caused only a slight effect (threefold). In the double mutant, an additive effect was observed (90-fold), but it was not as dramatic as that in the recA mutant. The results are in agreement with the RecBCD independence of small plasmids lacking chi sequences in E. coli (10, 22, 32, 37). However, plasmid recombination shows RecBCD dependence in an sbcB background if a chi sequence is present (66). Furthermore, by using plasmids lacking a chi sequence, the recombination rate in the recB-recF mutant is similar to that observed in the recF mutant (10, 22, 32, 37), or an enhanced activity is observed in the wild-type or recF backgrounds after recB inactivation (36).
Recombination pathways for large plasmids (>100 kb) have been infrequently explored in bacteria, even though a high frequency of rearrangements of R-type plasmids, mainly through an active RecF pathway, was observed in E. coli (38). In R. etli, large-plasmid recombination was mainly recF dependent (24-fold), with a minor participation of addA (threefold). Moreover, an additive effect was observed in the double mutant recF-addA (47-fold). These results indicate that small- and large-plasmid recombination in R. etli uses primarily the RecF pathway, with a minor role for the AddAB pathway, and suggest that pBX404-7 and pSym (371 kb) may have a low density of the R. etli chi sequence. However, the remaining recombination levels still observed in the recF-addA mutant (compare with the recA mutant [Table 2]) suggest the participation of an additional large-plasmid recombination route.
Our evaluation of deletion events in R. etli indicates that they are slightly affected in the recF, addA, and recF-addA mutants. These results indicate that recF and addA have a minor but additive effect in deletions. Interestingly, this event was affected only 18-fold in the recA mutant. It was previously reported that a 120-kb deletion generated by recombination between 5-kb direct repeats in the same plasmid was affected about 100-fold in a recA mutant (39, 53). Together, these results suggest that in R. etli, (i) closely spaced repeated sequences recombine in a recA-independent way and (ii) the degree of recA dependence for deletion is affected by the distance between the repeated sequences. The results are in agreement with those reported for E. coli, where the distance between and the length of homologous repeats determine the RecA dependence of recombination (3, 34-36).
It has been reported that inactivation of either addA (B. subtilis) or recB (E. coli) produces a strong decrease in postconjugational recombination events (Hfr and plasmid integration). Moreover, the effect is synergistic in the corresponding double knockout recF-addA (B. subtilis) or recF-recB (E. coli) (1, 29). In R. etli, the postconjugational integration of pJZC8 was unaffected by addA inactivation and only weakly dependent on the RecF pathway (Table 2) although it was recA dependent (666-fold [Table 2]). Similar recombinational frequencies were obtained by using other sites for integration in pSym or in the chromosome (data not shown). These results indicate the existence of another presynaptic route for Campbell-type recombination.
The unaltered rate of Campbell-type recombination in the recF-addA mutant raises the possibility that other as-yet-undescribed activities participate in the initiation of homologous recombination. For example, some helicase activities like RecQ, UvrD, HelD, etc., could be used with this type of substrate, or the absence of some exonucleases, like RecJ, ExoI, ExoX, etc., could allow the persistence of this type of substrate. In fact, the inactivation of the ssDNA exonucleases recJ, exoI, and exoX improves the recombination frequency of both homologous and homeologous substrates in E. coli (18, 21).
BLAST searches of the Rhizobiaceae genome database have identified recN, recQ, and recJ genes whose products suppress the absence of RecBCD and RecFOR systems at the initiation steps in E. coli (30). However, their role in repair and recombination is observed only when the two principal systems (RecBCD/AddAB and RecFOR) are mutated (17, 29).
The RecBCD and AddAB systems are modulated by a chi sequence. This species-specific sequence has been reported for E. coli, Haemophilus influenzae, B. subtilis, and L. lactis (4, 7, 8, 14, 15, 33, 49, 57). Furthermore, the basal level of plasmid recombination in E. coli and L. lactis is increased by incorporation of a chi sequence (14, 66). Therefore, it is possible that the integrative plasmids used in our work lack the R. etli chi sequence.
It is clear from the results presented that other systems for initiation of homologous recombination, beside those described in this paper, seem to be present in R. etli. Thus, our system provides the tool for the search of genes that, when mutated, provoke dramatic decrease in the recombinational capacity of R. etli.
Acknowledgments
Partial financial support was provided by grants 31753-N from CONACyT and IN203897 from PAPIIT-DGAPA and 202302 from PAEP-DGEP (both from de Universidad Nacional Autónoma de México). J.Z.-C. received scholarships from Consejo Nacional de Ciencia y Tecnología (México) and Dirección General de Estudios de Posgrado (Universidad Nacional Autónoma de México).
We are indebted to Sonia Davila for phylogenetic analysis, Rosa Elena Gómez for sequencing assistance, Paul Gaytán and Eugenio López for oligonucleotide synthesis, and Laura Cervantes and Javier Rivera for skillful technical assistance. We thank R. Myers and the two anonymous reviewers for helpful comments on the manuscript.
REFERENCES
- 1.Alonso, J. C., R. H. Tailor, and G. Lüder. 1988. Characterization of recombination-deficient mutants of Bacillus subtilis. J. Bacteriol. 170:3001-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, J. C., G. Lüder, and R. H. Tailor. 1991. Characterization of Bacillus subtilis recombinational pathways. J. Bacteriol. 173:3977-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi, X., and L. F. Liu. 1994. recA-independent and recA-dependent intramolecular plasmid recombination: differential homology requirement and distance effect. J. Mol. Biol. 235:414-423. [DOI] [PubMed] [Google Scholar]
- 4.Biswas, I., E. Magin, S. D. Ehrlich, and A. Gruss. 1995. A 7-base-pair sequence protects DNA from exonucleolytic degradation in Lactococcus lactis. Proc. Natl. Acad. Sci. USA 92:2244-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bork, J. M., M. M. Cox, and R. B. Inman. 2001. The RecOR proteins modulate RecA protein functions at the 5′-ends of single-strand DNA. EMBO J. 20:7313-7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brom, S., A. García de los Santos, T. Stepkowsky, M. Flores, G. Dávila, D. Romero, and R. Palacios. 1992. Different plasmids of Rhizobium leguminosarum bv. phaseoli are required for optimal symbiotic performance. J. Bacteriol. 174:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chédin, F., P. Noirot, V. Biaudet, and S. D. Ehrlich. 1998. A five-nucleotide sequence protects DNA from exonucleolytic degradation by AddAB, the RecBCD analogue of Bacillus subtilis. Mol. Microbiol. 29:1369-1377. [DOI] [PubMed] [Google Scholar]
- 8.Chédin, F., S. D. Ehrlich, and S. C. Kowalczykowski. 2000. The Bacillus subtilis AddAB helicase/nuclease is regulated by its cognate chi sequence in vitro. J. Mol. Biol. 298:7-20. [DOI] [PubMed] [Google Scholar]
- 9.Chédin, F., and S. C. Kowalczykowski. 2002. A novel family of regulated helicases/nucleases from Gram-positive bacteria: insights into the initiation of DNA recombination. Mol. Microbiol. 43:823-834. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, A., and A. Laban. 1983. Plasmidic recombination in Escherichia coli K-12: the role of recF gene function. Mol. Gen. Genet. 189:471-474. [DOI] [PubMed] [Google Scholar]
- 11.Cox, M. M. 2001. Recombinational DNA repair of damaged replication forks in Escherichia coli. Annu. Rev. Genet. 35:53-82. [DOI] [PubMed] [Google Scholar]
- 12.Dillingham, M. S., M. Spies, and S. C. Kowalczykowski. 2003. RecBCD enzyme is a bipolar DNA helicase. Nature 423:893-897. [DOI] [PubMed] [Google Scholar]
- 13.Eisen, J. A., and P. C. Hanawalt. 1999. A phylogenomic study of DNA repair genes, proteins and process. Mutat. Res. 435:171-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Karoui, M., S. D. Ehrlich, and A. Gruss. 1998. Identification of the lactococcal exonuclease/recombinase and its modulation by the putative chi sequence. Proc. Natl. Acad. Sci. USA 95:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Karoui, M., V. Biaudet, S. Schbath, and A. Gruss. 1999. Characteristics of chi distribution on different bacterial genomes. Res. Microbiol. 150:579-587. [DOI] [PubMed] [Google Scholar]
- 16.Felsenstein, J. 1995. PHYLIP (Phylogeny Inference Package) version 3.57c. Department of Genetics, University of Washington, Seattle, Wash.
- 17.Fernández, S., S. Ayora, and J. C. Alonso. 2000. Bacillus subtilis homologous recombination: genes and products. Res. Microbiol. 151:481-486. [DOI] [PubMed] [Google Scholar]
- 18.Feschenko, V. V., L. A. Rajman, and S. T. Lovett. 2003. Stabilization of perfect and imperfect tandem repeats by single-strand DNA exonucleases. Proc. Natl. Acad. Sci. USA 100:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girard, M. L., M. Flores, S. Brom, D. Romero, R. Palacios, and G. Dávila. 1991. Structural complexity of the symbiotic plasmid of Rhizobium leguminosarum bv. phaseoli. J. Bacteriol. 173:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haijema, B. J., R. Meima, J. Kooistra, and G. Venema. 1996. Effects of lysine-to-glycine mutations in the ATP-binding consensus sequences in the AddA and AddB subunits on the Bacillus subtilis AddAB enzyme activities. J. Bacteriol. 178:5130-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horii, Z., and A. J. Clark. 1973. Genetic analysis of the RecF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J. Mol. Biol. 80:327-344. [DOI] [PubMed] [Google Scholar]
- 22.James, A. A., P. T. Morrison, and R. Kolodner. 1982. Genetic recombination of bacterial plasmid DNA: analysis of the effect of recombination-deficient mutations on plasmid recombination. J. Mol. Biol. 160:411-430. [DOI] [PubMed] [Google Scholar]
- 23.Kokotek, W., and W. Lotz. 1991. Construction of a mobilizable cloning vector for site-directed mutagenesis of gram-negative bacteria: application to Rhizobium leguminosarum. Gene 98:7-13. [DOI] [PubMed] [Google Scholar]
- 24.Kooistra, J., B. Vosman, and G. Venema. 1988. Cloning and characterization of a Bacillus subtilis transcription unit involved in ATP-dependent nuclease synthesis. J. Bacteriol. 170:4791-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooistra, J., and G. Venema. 1991. Cloning, sequencing, and expression of Bacillus subtilis genes involved in ATP-dependent nuclease synthesis. J. Bacteriol. 173:3644-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kooistra, J., B. J. Haijema, and G. Venema. 1993. The Bacillus subtilis addAB are functional in Escherichia coli. Mol. Microbiol. 7:915-923. [DOI] [PubMed] [Google Scholar]
- 27.Kooistra, J., B. A. Haijema, A. Hesseling-Meinders, and G. Venema. 1997. A conserved helicase motif of the AddA subunit of the Bacillus subtilis ATP-dependent nuclease (AddAB) is essential for DNA repair and recombination. Mol. Microbiol. 23:137-149. [DOI] [PubMed] [Google Scholar]
- 28.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. N. Peterson. 1995. Four derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 29.Kowalczykowski, S. C., D. A. Dixon, A. K. Eggelston, S. D. Lauder, and W. M. Rehrauer. 1994. Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 58:401-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalczykowski, S. C. 2000. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 25:156-165. [DOI] [PubMed] [Google Scholar]
- 31.Kuzminov, A. 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 63:751-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laban, A., and A. Cohen. 1981. Interplasmidic and intraplasmidic recombination in Escherichia coli K-12. Mol. Gen. Genet. 184:200-207. [DOI] [PubMed] [Google Scholar]
- 33.Lam, S. T., M. M. Stahl, K. D. McMilin, and F. W. Stahl. 1974. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics 77:425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovett, S. T., P. T. Drapkin, V. A. Sutera, Jr., and T. J. Gluckman-Peskin. 1993. A sister-strand exchange mechanism for recA-independent deletion of repeated DNA sequences in Escherichia coli. Genetics 135:631-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovett, S. T., T. J. Gluckman, P. J. Simon, V. A. Sutera, Jr., and P. T. Drapkin. 1994. Recombination between repeats in Escherichia coli by a recA-independent, proximity-sensitive mechanism. Mol. Gen. Genet. 245:294-300. [DOI] [PubMed] [Google Scholar]
- 36.Lovett, S. T., R. L. Hurley, V. A. Sutera, Jr., R. H. Aubuchon, and M. A. Lebedeva. 2002. Crossing over between regions of limited homology in Escherichia coli: RecA-dependent and RecA-independent pathways. Genetics 160:851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luisi-DeLuca, C., S. T. Lovett, and R. D. Kolodner. 1989. Genetic and physical analysis of plasmid recombination in recB recC sbcB and recB recC sbcA Escherichia coli K-12. Genetics 122:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez-Salazar, J. M., and C. Gómez-Eichelmann. 1987. Molecular rearrangements between two different plasmids of the FII incompatibility group in different recombination-deficient Escherichia coli strains. Plasmid 18:237-245. [DOI] [PubMed] [Google Scholar]
- 39.Martínez-Salazar, J. M., D. Romero, M. L. Girard, and G. Dávila. 1991. Molecular cloning and characterization of the recA gene of Rhizobium phaseoli and construction of recA mutants. J. Bacteriol. 173:3035-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Salazar, J. M., and D. Romero. 2000. Role of ruvB gene in homologous and homeologous recombination in Rhizobium etli. Gene 243:125-131. [DOI] [PubMed] [Google Scholar]
- 41.Mavingui, P., M. Flores, X. Guo, G. Dávila, X. Perret, W. J. Broughton, and R. Palacios. 2002. Dynamic of genome architecture in Rhizobium sp. strain NGR234. J. Bacteriol. 184:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miranda, A., and A. Kuzminov. 2003. Chromosomal lesion suppression and removal in Escherichia coli via linear DNA degradation. Genetics 163:1255-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morimatsu, K., and S. C. Kowalczykowski. 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 11:1337-1347. [DOI] [PubMed] [Google Scholar]
- 44.Motamedi, M. R., K. S. Szigety, and M. Rosenberg. 1999. Double-strand-break repair recombination in Escherichia coli: physical evidence for a DNA replication mechanism in vivo. Genes Dev. 13:2889-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers, R. S., and F. W. Stahl. 1994. Chi and the RecBCD enzyme of Escherichia coli. Annu. Rev. Genet. 28:49-70. [DOI] [PubMed] [Google Scholar]
- 46.Page, R. D. M. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 47.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 48.Quiberoni, A., I. Biswas, M. El Karoui, L. Rezaïki, P. Tailliez, and A. Gruss. 2001. In vivo evidence of two active nuclease motifs in the double-strand break repair enzyme RexAB of Lactococcus lactis. J. Bacteriol. 183:4071-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quiberoni, A., L. Rezaïki, M. El Karoui, I. Biswas, P. Tailliez, and A. Gruss. 2001. Distinctive features of homologous recombination in an “old” microorganism, Lactococcus lactis. Res. Microbiol. 152:131-139. [DOI] [PubMed] [Google Scholar]
- 50.Quinto, C., H. De la Vega, M. Flores, J. Leemans, M. A. Cevallos, M. A. Pardo, R. Azpiroz, M. L. Girard, E. Calva, and R. Palacios. 1985. Nitrogenase reductase: a functional multigene family in Rhizobium phaseoli. Proc. Natl. Acad. Sci. USA 82:1170-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez, C., and D. Romero. 1998. Multiple recombination events maintain sequence identity among members of the nitrogenase multigene family in Rhizobium etli. Genetics 149:785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero, D., S. Brom, J. M. Martínez-Salazar, M. L. Girard, R. Palacios, and G. Dávila. 1991. Amplification and deletion of a nod-nif region in the symbiotic plasmid of Rhizobium phaseoli. J. Bacteriol. 173:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romero, D., J. M. Martínez-Salazar, M. L. Girard, S. Brom, G. Dávila, R. Palacios, M. Flores, and C. Rodríguez. 1995. Discrete amplifiable regions (amplicons) in the symbiotic plasmid of Rhizobium etli CFN42. J. Bacteriol. 177:973-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romero, D., and R. Palacios. 1997. Gene amplification and genomic plasticity in prokaryotes. Annu. Rev. Genet. 31:91-111. [DOI] [PubMed] [Google Scholar]
- 55.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 56.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology. 1:784-791. [Google Scholar]
- 57.Sourice, S., V. Biaudet, M. El Karoui, S. D. Erlich, and A. Gruss. 1998. Identification of the Chi site of Haemophilus influenzae as several sequences related to the Escherichia coli Chi site. Mol. Microbiol. 27:1021-1029. [DOI] [PubMed] [Google Scholar]
- 58.Sugino, A., C. L. Peebles, K. N. Kreuzer, and N. R. Cozzarelli. 1977. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA 74:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor, F. A., and G. R. Smith. 2003. RecBCD enzyme is a DNA helicase with fast and slow motors of opposite polarity. Nature 423:889-893. [DOI] [PubMed] [Google Scholar]
- 60.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umezu, K., N. W. Chi, and R. Kolodner. 1993. Biochemical interaction of the Escherichia coli RecF, RecO, and RecR proteins with RecA protein and single-stranded DNA binding protein. Proc. Natl. Acad. Sci. USA 90:3875-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valencia-Morales, E., and D. Romero. 2000. Recombination enhancement by replication (RER) in Rhizobium etli. Genetics 154:971-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb, B. L., M. M. Cox, and R. B. Inman. 1997. Recombinational DNA repair: the RecF and RecR proteins limit the extension of RecA filaments beyond single-strand DNA gaps. Cell 91:347-356. [DOI] [PubMed] [Google Scholar]
- 64.Webb, B. L., M. M. Cox, and R. B. Inman. 1999. ATP hydrolysis and DNA binding by the Escherichia coli RecF protein. J. Biol. Chem. 274:15367-15374. [DOI] [PubMed] [Google Scholar]
- 65.Xu, B., C. Paszty, and P. F. Lurquin. 1988. A plasmid-based method to quantitate homologous recombination frequencies in gram-negative bacteria. BioTechniques 6:752-760. [PubMed] [Google Scholar]
- 66.Zaman, M. M., and T. C. Boles. 1996. Plasmid recombination by the RecBCD pathway of Escherichia coli. J. Bacteriol. 178:3840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]