Abstract
A Desulfovibrio vulgaris Hildenborough mutant lacking the nrfA gene for the catalytic subunit of periplasmic cytochrome c nitrite reductase (NrfHA) was constructed. In mid-log phase, growth of the wild type in medium containing lactate and sulfate was inhibited by 10 mM nitrite, whereas 0.6 mM nitrite inhibited the nrfA mutant. Lower concentrations (0.04 mM) inhibited the growth of both mutant and wild-type cells on plates. Macroarray hybridization indicated that nitrite upregulates the nrfHA genes and downregulates genes for sulfate reduction enzymes catalyzing steps preceding the reduction of sulfite to sulfide by dissimilatory sulfite reductase (DsrAB), for two membrane-bound electron transport complexes (qmoABC and dsrMKJOP) and for ATP synthase (atp). DsrAB is known to bind and slowly reduce nitrite. The data support a model in which nitrite inhibits DsrAB (apparent dissociation constant Km for nitrite = 0.03 mM), and in which NrfHA (Km for nitrite = 1.4 mM) limits nitrite entry by reducing it to ammonia when nitrite concentrations are at millimolar levels. The gene expression data and consideration of relative gene locations suggest that QmoABC and DsrMKJOP donate electrons to adenosine phosphosulfate reductase and DsrAB, respectively. Downregulation of atp genes, as well as the recorded cell death following addition of inhibitory nitrite concentrations, suggests that the proton gradient collapses when electrons are diverted from cytoplasmic sulfate to periplasmic nitrite reduction.
Sulfate-reducing bacteria are frequently exposed to nitrite by interaction with nitrate-reducing, sulfide-oxidizing bacteria in anoxic environments. Because sulfate is often the predominant electron acceptor in anoxic environments (e.g., marine sediments), sulfate-reducing bacteria are primarily responsible for organic carbon oxidation. The sulfide produced is then targeted by nitrate-reducing, sulfide-oxidizing bacteria, which generally use CO2 as their sole carbon source (4). Thus, sulfate-reducing bacteria and nitrate-reducing, sulfide-oxidizing bacteria symbiotically catalyze the oxidation of organic matter with nitrate through a sulfide intermediate. This symbiosis can potentially be stalled by production of nitrite by the nitrate-reducing, sulfide-oxidizing bacteria, which is a powerful inhibitor of sulfate-reducing bacteria. Some sulfate-reducing bacteria have a periplasmic nitrite reductase to prevent and/or overcome this inhibition. Cocultures of a Desulfovibrio sp. and the nitrate-reducing, sulfide-oxidizing bacterium Thiomicrospira sp. strain CVO were strongly or transiently inhibited, depending on the absence or presence of nitrite reductase activity in the Desulfovibrio sp. (6). Desulfovibrio vulgaris Hildenborough was very resistant to inhibition by either nitrite or strain CVO and nitrate and had high nitrite reductase activity through the presence of a periplasmic cytochrome c nitrite reductase (NrfHA) that reduces nitrite to ammonium. This reaction is purely detoxifying; no cell growth is associated with the use of nitrite as an electron acceptor (16).
We report here the construction of a D. vulgaris nrfA mutant and its physiological properties. The effects of nitrite on gene expression in the mutant and wild-type strains, as recorded by macroarray hybridization (7), were also recorded in order to determine the mechanism by which nitrite inhibits dissimilatory sulfate reduction.
MATERIALS AND METHODS
Materials.
Mixed gases, including 5% (vol/vol) H2, 10% (vol/vol) CO2, balance N2 and 10% (vol/vol) CO2, balance N2, were obtained from Praxair Products, Inc., Edmonton, Canada. Restriction enzymes, Taq polymerase, DNA ligase, and Hybond-N membranes were obtained from Amersham Pharmacia Biotech, Baie d'Urfe, Canada. Superscript II reverse transcriptase without RNase H was obtained from Invitrogen, Burlington, Canada. [α-32P]dCTP (10 mCi/ml; 3,000 mCi/mmol) was purchased from MP Biomedicals, Inc., Irvine, Calif. Reagent-grade chemicals were from Sigma, Fisher, or BDH. RNeasy kits, RNAprotect reagent, and RNase-free DNase were from Qiagen, Mississauga, Canada. Deoxyoligonucleotide primers were synthesized by University Core DNA Services, University of Calgary.
Media and culture conditions.
D. vulgaris was cultured in medium C (18), Widdel-Pfennig medium (WP-LS) (26), or modified Coleville synthetic brine (mCSB) (13). Growth in medium C, a rich medium containing 38 mM sodium lactate, 28 mM Na2SO4 and 1 g of yeast extract per liter, was in 5% (vol/vol) H2, 10% (vol/vol) CO2, balance N2 under conditions of gas exchange. WP-LS (23 mM sodium lactate, 28 mM Na2SO4) and mCSB (14 mM sodium lactate, 10 mM Na2SO4) were dispensed in 50-ml aliquots in 125-ml serum bottles, flushed with 10% (vol/vol) CO2, balance N2 and sealed with butyl rubber stoppers. A 2% (vol/vol) inoculum of a freshly grown D. vulgaris culture was used, and cultures were incubated at 30°C. To test the effects of nitrate on D. vulgaris growth and gene expression, 10 mM NaNO3 was added to WP-LS medium from a sterile, anaerobic stock solution at the time of inoculation. For nitrite inhibition of D. vulgaris, NaNO2 was added from a 1 M sterile, anaerobic stock solution to mid-log-phase cultures. Sulfate, sulfide, nitrate, and nitrite concentrations were monitored as described elsewhere (6, 13, 14). The optical density at 600 nm (OD600) of cultures in WP-LS was recorded with a Shimadzu UV-visible spectrophotometer, whereas that of cultures grown in medium C was recorded with a Klett meter.
Construction of an nrfA mutant.
The 1,572-bp gene for Dvu0625 NrfA, the larger subunit of NrfHA nitrite reductase, flanked by 588 bp of upstream and downstream regions was PCR amplified with primers P208-f and P209-r (Table 1). The 2.8-kbp product was cut with XbaI and SacI and cloned in pNOT19 to give pNotNrfA. PCR amplification of this plasmid with primers P210-r and P211-f, cleavage with BamHI, and ligation gave plasmid pNotΔNrfA, in which bases +9 to +1581, containing the NrfA coding region, were deleted. Ligation of BamHI-cleaved pNotΔNrfA with the 1.4-kb BamHI fragment from pUC19Cm gave pNotΔNrfACm, and ligation of NotI-cleaved pNotΔNrfACm with the 4.2-kb NotI fragment from pMOB2 containing oriT and sacB gave pNotΔNrfACmMob.
TABLE 1.
Bacterial strains, primers, and plasmids
| Strain, plasmid, or primer | Characteristics or commentsa | Source or reference |
|---|---|---|
| Desulfovibrio vulgaris subsp. vulgaris | ||
| Hildenborough | NCIMB 8303; isolated from clay soil near Hildenborough, England | 18 |
| NrfA100 | ΔnrfA; Cmr | This study |
| NrfA11 | pNotΔNrfCmMob integrated into the chromosome; Sucs Cmr | This study |
| E. coli S17-1 | thi pro hsdR hsdM+ recA RP4-2 (Tc::Mu, Km::Tn7) | 22 |
| Plasmids | ||
| pUC19Cm | pUC19 containing the cat gene | 5 |
| pNOT19 | Cloning vector pUC19; NdeI site replaced by NotI site | 20 |
| pMOB2 | Contains oriT of plasmid RP4 and Bacillus subtilis sacBR genes on a 4.5-kb NotI fragment; Kmr Cmr | 20 |
| pNotNrfA, pNotΔNrfA, pNotΔNrfACm, pNotΔNrfACmMob | This study | |
| Primersa | ||
| P208-f | 5′-ccctctagaTGGCTGCTATGCTGCTCGC (−588 to −570) | |
| P209-r | 5′-cccgaGCTCCTGCCCTTCGACGGTG (+2151 to +2170) | |
| P210-r | 5′-cccggatccTTATTCATCGGCGACCTCTCT (−12 to +8) | |
| P211-f | 5′-cccggatccGCTCTTTCGCAAAGGTATG (+1582 to +1602) |
Positions are given relative to the nrfA coding region (bp +1 to +1572). Lowercase letters are bases added to create restriction endonuclease cleavage sites.
Mating of Escherichia coli S17-1 (pNotΔNrfACmMob) and D. vulgaris was performed on filter disks placed on medium E plates (18) containing 10 mM fumarate. The fumarate allows anaerobic respiration by E. coli and replaces the 15 mM nitrate added to mating plates in earlier recipes (5). We failed to obtain single-crossover integrants on medium E-nitrate plates, perhaps because E. coli produces nitrite, which induces transcription of the NrfHA genes. Two upstream and one downstream (D. vulgaris NrfA11) single-crossover integrants were obtained on medium E plates containing chloramphenicol and kanamycin (5), as determined by Southern blotting. Growth of D. vulgaris NrfA11 in the presence of sucrose and chloramphenicol followed by colony purification and screening with PCR primers P208 and P209 gave D. vulgaris Nrf100, which was again verified by Southern blotting (results not shown).
Macroarray hybridization.
As described elsewhere, 145 PCR products, representing open reading frames (ORFs) predicted to be involved in energy metabolism on the basis of the genome sequence (8), were spotted on nylon membranes (7). A list of these ORFs is provided in the supplementary material. All methods for array construction, total RNA extraction, reverse transcription to radiolabeled cDNA, hybridization with macroarrays, and calculations have been described in detail elsewhere (7). RNA was extracted from aliquots of uninhibited cultures at mid-log phase and from nitrite-inhibited cultures 15 to 270 min after nitrite addition, when the growth rate was still reduced. For each experimental condition, RNA was extracted from two separate cultures, and each RNA sample was split and hybridized with two arrays. The hybridization data resulting from these four arrays were averaged. Error was calculated as the average deviation of the signals for each spot on the four arrays. The ORFs of downregulated transmembrane electron transport complexes were identified, and motifs were further characterized by doing BLAST searches (1). Transmembrane helices were identified with the TMpred program at http://www.ch.embnet.org/software/TMPRED_form.html (9).
RESULTS
Phenotype of D. vulgaris nrfA.
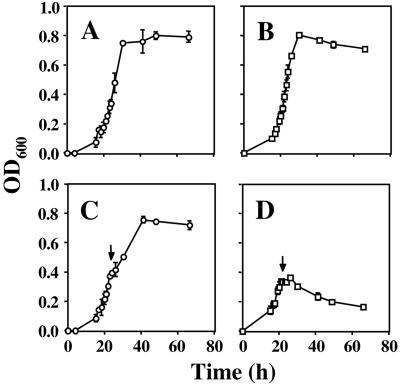
Growth of the D. vulgaris nrfA mutant in medium C was indistinguishable from that of the wild type (Fig. 1A and B). Addition of 5 mM nitrite to the wild type at mid-log phase resulted in a 1- to 2-h pause, after which growth resumed (Fig. 1C). In contrast, growth of the nrfA mutant stopped completely (Fig. 1D), and the OD600 dropped following addition of nitrite. Plating on medium E indicated that 70 h after addition of 5 mM nitrite, the wild type had a viable count of 1.6 x 108 ± 0.2 x 108 CFU/ml, whereas the nrfA mutant had only 1.2 x 103 ± 0.5 x 103 CFU/ml, indicating that cells had died as a result of the nitrite treatment.
FIG. 1.
Growth of wild-type (A) and nrfA mutant (B) D. vulgaris in medium C and effect of nitrite on wild-type (C) and nrfA mutant (D). The cell density (OD600) is plotted versus time. Nitrite (5 mM) was added at the indicated time (↓). Average data for two separate experiments are shown.
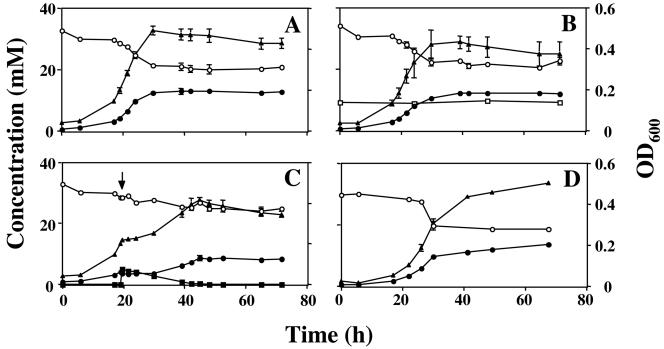
In WP-LS medium, the wild-type strain grew to a maximum OD600 of 0.472 ± 0.026 (average of six cultures) in both the presence and absence of nitrate, which was not used (Fig. 2A and B). The nrfA mutant had similar kinetics of growth, utilization of sulfate, and production of sulfide and grew to the same maximum cell density as the wild type (OD600 = 0.496 ± 0.010; average for six cultures), as shown in Fig. 2D. Addition of 5 mM nitrite to mid-log-phase cultures of the nrfA mutant led to complete cessation of growth, as in Fig. 1D, whereas growth of the wild type was only transiently inhibited, the recovery time being longer than in medium C. All nitrite was eventually removed from the culture by the wild-type strain (Fig. 2C). The average maximum OD600 for five wild-type cultures with 5 mM nitrite was 0.427 ± 0.029, which is 90% of the value in the absence of nitrite. Because growth in WP-LS medium is lactate limited, this indicates that lactate used for nitrite reduction does not contribute to cell growth. Addition of 0.1 mM nitrite to mid-log-phase D. vulgaris cultures in WP-LS had no effect on the rate of growth or sulfate reduction (not shown).
FIG. 2.
Comparison of growth of wild-type and nrfA mutant D. vulgaris in WP-LS. Cell density (▴, OD600) and the concentrations of sulfate (○), sulfide (•), nitrate (□), and nitrite (▪) are plotted as a function of time. (A) Wild type without nitrate or nitrite, (B) wild type with 10 mM nitrate, (C) wild type with 5 mM nitrite added at the indicated time (↓), (D) nrfA mutant without nitrate or nitrite.
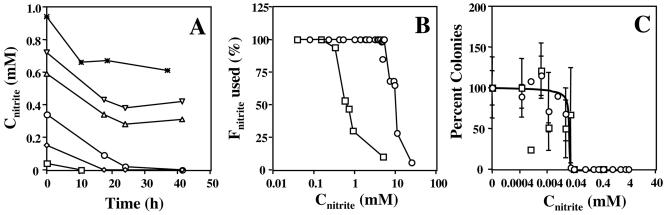
Despite the absence of NrfA, the mutant could reduce low nitrite concentrations (Fig. 3A). In view of the results of Wolfe et al. (27), this residual nitrite reductase activity may be assigned to DsrAB. At a mid-log-phase biomass concentration of 0.077 g of biomass dry weight liter−1, a residual specific nitrite reductase activity of 0.19 mmol of NO2− g−1 h−1 can be calculated. This value is 18-fold less than the 3.5 mmol of NO2− g−1 h−1 for the wild type reported previously (6), which is in agreement with a value of 3.3 mmol of NO2− g−1 h−1 that can be derived from Fig. 2C. Hence, at nitrite concentrations in the millimolar range, 95% of the nitrite reduction activity of wild-type D. vulgaris can be credited to NrfHA, with the remaining 5% being due to DsrAB. These different activities allow mid-log-phase nrfA mutant and wild-type cells to reduce 0.35 and 6.5 mM nitrite in 24 h, respectively (Fig. 3B). Addition of more nitrite (e.g., 0.6 and 10 mM, respectively) prevented recovery from nitrite inhibition and led to cell death (e.g., as in Fig. 1D), likely because this depleted the cells too long from ATP reserves.
FIG. 3.
(A) Nitrite utilization by the nrfA mutant. The concentration of nitrite added to mid-log-phase cultures in WP-LS is plotted versus time. (B) Fraction of nitrite utilized in 24 h by mid-log-phase cultures of wild-type (○) and nrfA mutant (□) D. vulgaris in WP-LS as a function of the added nitrite concentration. (C) Growth of wild-type (○) and nrfA mutant (□) D. vulgaris on plates with different nitrite concentrations. Approximately 100 CFU were plated, and the colonies appearing on the plates were counted after 5 days. Data are averages for two separate plates at nitrite concentrations below 0.04 mM, except where no error bars are shown. Values are percentages of the number of colonies on plates to which no nitrite was added.
The inhibitory concentrations depended on the biomass concentration at the time of nitrite addition. Cultures with a 2% (vol/vol) inoculum of the nrfA mutant and the wild type in WP-LS (i.e., approximately 25-fold fewer cells than in mid-log phase) were inhibited by 0.26 and 2.0 mM nitrite, respectively, added at time zero. The intrinsic nitrite resistance of D. vulgaris, defined as the inhibitory nitrite concentration at vanishing biomass concentration, was measured as the nitrite concentration inhibiting growth of single cells on plates into colonies. Approximately 100 CFU were plated on medium E with increasing nitrite concentrations. No colonies were formed at nitrite concentrations in excess of 0.04 mM by the wild type and nrfA mutant (Fig. 3C). This value is similar to the apparent nitrite dissociation constant reported for D. vulgaris DsrAB (Km = 0.03 mM) (27), confirming that DsrAB is the likely target of nitrite inhibition.
Effects of nitrate and nitrite on gene expression.
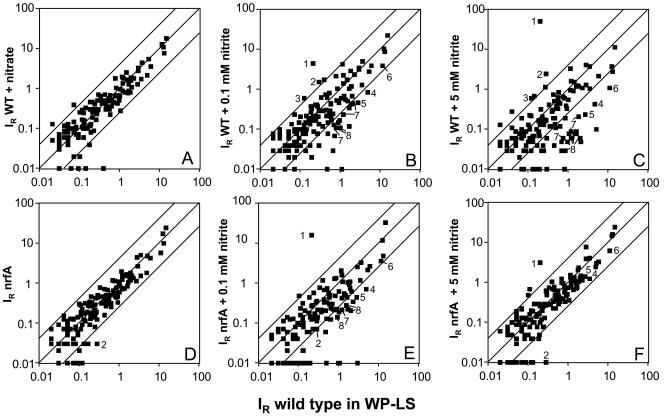
The expression of energy metabolism genes represented on the array was not affected by 10 mM nitrate (Fig. 4A), in agreement with the fact that D. vulgaris does not reduce nitrate. However, addition of 5 mM nitrite in mid-log phase gave upregulation of seven ORFs in five operons and downregulation of 39 ORFs in 17 operons (Fig. 4C). Results for 25 genes are given in Table 2; a complete listing of relative expression levels for all genes on the array is provided in the supplementary material. Genes for cytochrome c nitrite reductase (nrfHA) and hybrid cluster protein 2 (hcp2) were upregulated. Downregulated genes included the sulfate reduction genes sat, ppaC, and apsBA. Expression of the dissimilatory sulfite reductase operon dsrABD was not significantly affected by 5 mM nitrite. Two operons encoding transmembrane electron transport complexes, qmoABC and dsrMKJOP, as well as two operons for ATP synthase subunits were also downregulated (Table 2). Addition of 0.1 mM nitrite, which is rapidly reduced by log-phase cultures of wild-type D. vulgaris, gave a more moderate gene expression response (Fig. 4B, Table 2). The nrfHA and hcp2 genes were upregulated, whereas sat, ppaC, qmoABC, and some ATP synthase genes (Table 2, Dvu0774, -0775, -0776, -0777, -0778, -0779, -0780, -0917, and -0918) were downregulated.
FIG. 4.
Effect of nitrate and nitrite on gene expression in wild-type and nrfA mutant D. vulgaris grown in WP-LS. Relative hybridization intensities (IR, means of four array hybridizations) for 145 genes represented on the macroarray are compared with those for the wild type (x axis). The y axis represents (A) wild type with 10 mM nitrate included throughout the growth curve, (B) wild type with 0.1 mM nitrite added at mid-log phase, (C) wild type with 5 mM nitrite added at mid-log phase, (D) nrfA mutant, (E) nrfA mutant with 0.1 mM nitrite added at mid-log phase, (F) nrfA mutant with 5 mM nitrite added at mid-log phase. Upregulated genes outside the fourfold expression corridor include: 1, Dvu2543 hcp2; 2, Dvu0624 and Dvu0625 nrfHA; and 3, Dvu0173 phsA. Downregulated genes outside the fourfold expression corridor include genes involved in or postulated to be involved in sulfate reduction: 4, Dvu1295 sat; 5, Dvu1636 ppaC; 6, Dvu0846 and Dvu0847 apsBA; 7, Dvu0848, Dvu0849, and Dvu0850 qmoABC; and 8, Dvu1290, Dvu1289, Dvu1288, Dvu1287, and Dvu1286 dsrMKJOP.
TABLE 2.
Relative expression levels of ORFs up- and downregulated >4-fold in D. vulgaris and NrfA100 mutant cultures in WP-LS, inhibited with 5 and 0.1 mM nitritea
| Gene | Dvu | Descriptionb | Relative expression, wild type, WP-LS | Change in expressionc (fold)
|
||||
|---|---|---|---|---|---|---|---|---|
| Wild type, 5 mM NO2− | Wild type, 0.1 mM NO2− | NrfA100, WP-LS | NrfA100, 5 mM NO2− | NrfA100, 0.1 mM NO2− | ||||
| phsA | 0173 | Thiosulfate reductase | 0.12 ± 0.01 | 5.1 | 5.0 | 1.8 | 1.6 | 2.6 |
| nrfHA | 0624-5 | Cytochrome c nitrite reductase | 0.28 ± 0.02 | 8.7 | 5.6 | −11.2d | −28.3e | −3.8d |
| hcp2 | 2543 | Hybrid cluster protein | 0.20 ± 0.03 | 254.8 | 22.6 | 2.3 | 15.9 | 76.1 |
| atpC | 0774 | ATP synthase F1 subunit epsilon | 0.63 ± 0.21 | −5.3 | −20.4 | −2.5 | −1.5 | 1.0 |
| atpD | 0775 | ATP synthase F1 subunit beta | 5.14 ± 0.50 | −52.7 | −1.8 | −1.1 | −1.7 | −2.4 |
| atpG | 0776 | ATP synthase F1 subunit gamma | 0.68 ± 0.30 | −21.1 | −5.2 | 1.7 | 1.5 | −2.3 |
| atpA | 0777 | ATP synthase F1 subunit alpha | 2.07 ± 0.05 | −42.9 | −4.6 | −1.4 | −1.4 | −3.4 |
| atpH | 0778 | ATP synthase F1 subunit delta | 0.45 ± 0.06 | −10.1 | −44.8e | −1.3 | 1.7 | −7.2 |
| atpF2 | 0779 | ATP synthase F0 subunit B | 0.70 ± 0.07 | −14.5 | −10.2 | −1.1 | 1.8 | −3.2 |
| atpF1 | 0780 | ATP synthase F0 subunit B | 1.05 ± 0.50 | −13.2 | −104.6e | −1.2 | −2.8 | −104.6e |
| apsBA | 0846-7 | APS reductase | 11.04 ± 0.35 | −10.2 | −2.8 | −1.9 | −1.7 | −3.1 |
| qmoA | 0848 | Qmo complex, FAD, FeS protein | 1.18 ± 0.03 | −12.6 | −4.9 | −1.7 | −1.5 | −5.7 |
| qmoB | 0849 | Qmo complex, FAD, FeS protein | 0.76 ± 0.15 | −12.0 | −7.0 | −1.5 | −1.9 | −3.6 |
| qmoC | 0850 | Qmo complex, membrane cytochrome b, FeS protein | 1.86 ± 0.55 | −9.0 | −4.3 | −1.6 | −1.7 | −2.6 |
| atpE | 0917 | ATP synthase F0 subunit C | 1.61 ± 0.19 | −20.9 | −13.6 | −1.9 | −1.7 | −3.8 |
| atpB | 0918 | ATP synthase F0 subunit A | 1.64 ± 0.18 | −18.3 | −4.7 | −1.6 | −2.3 | −7.5 |
| dsrP | 1286 | Dsr complex, integral membrane protein | 1.19 ± 0.03 | −21.2 | 1.2 | 1.3 | 1.0 | −2.8 |
| dsrO | 1287 | Dsr complex, periplasmic FeS protein | 0.85 ± 0.12 | −31.2 | −7.8 | −1.1 | −1.2 | −4.0 |
| dsrJ | 1288 | Dsr complex, triheme cytochrome c | 0.98 ± 0.22 | −21.8 | −8.9 | −1.7 | −1.9 | −5.1 |
| dsrK | 1289 | Dsr complex, cytoplasmic FeS protein | 1.51 ± 0.10 | −19.7 | −2.9 | 1.1 | −1.4 | −5.5 |
| dsrM | 1290 | Dsr complex, membrane cytochrome b protein | 1.97 ± 0.09 | −31.1 | −2.9 | −1.3 | −1.5 | −9.9 |
| sat | 1295 | Sulfate adenylyl transferase | 4.79 ± 0.17 | −11.2 | −5.6 | −2.6 | −1.9 | −7.0 |
| ppaC | 1636 | Pyrophosphatase | 2.71 ± 0.16 | −11.3 | −5.7 | −2.5 | −1.9 | −6.2 |
Data for all 145 hybridizations are plotted in Fig. 4.
APS, adenosine phosphosulfatase; FAD, flavin adenine dinucleotide.
Relative to the wild type in WP-LS. mRNA was extracted at mid-log phase, 4.5 h (wild type, 5 mM), 0.25 h (wild type, 0.1 mM), or 0.5 h (NrfA 100, 5 mM and 0.1 mM) following addition of nitrite. Values in bold represent up- or downregulation greater than fourfold.
Residual signal for nrfH-containing mRNA; the complete nrfA gene was deleted.
Expression was below the detection limit under the changed conditions.
Comparison of the gene expression pattern of the wild type and nrfA mutant indicated a reduced nrfHA hybridization signal. The residual signal is due to expression of nrfH-containing mRNA as the entire nrfA gene has been deleted (Fig. 4D; Table 2). Addition of 0.1 mM nitrite gave upregulation of the hcp2 gene and downregulation of some of the same genes for sulfate reduction enzymes and membrane-bound electron transport complexes that are downregulated in the wild type (Fig. 4E; Table 2). Genes for carbon monoxide hydrogenase (Dvu2286, -2287, and -2288, cooMKL) and the high-molecular-weight cytochrome (Hmc) complex (Dvu0531, -0532, -0534, and -0535, hmcFECB) were uniquely downregulated in the nrfA mutant. Quite different from the wild type, addition of 5 mM nitrite, which is highly inhibitory and causes cell death (Fig. 1D), gave less extensive gene expression changes than observed for 0.1 mM nitrite (Fig. 4F; Table 2).
DISCUSSION
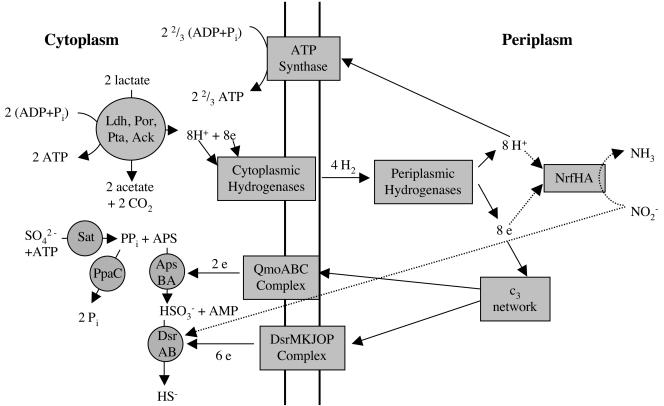
Sulfate-reducing bacteria couple the oxidation of hydrogen or organic compounds (e.g., lactate) to the reduction of sulfate to sulfide. Cytoplasmic oxidation of two lactate molecules yields two ATP molecules, which are required for sulfate activation, through substrate-level phosphorylation (Fig. 5). Additional ATP is formed by ATP synthase-driven phosphorylation, which consumes the electrochemical gradient formed by hydrogen cycling (15) or equivalent electrochemical processes (24). As indicated in Fig. 5, nitrite-induced inhibition of D. vulgaris growth is thought to result from (i) inhibition of DsrAB by nitrite and (ii) collapse of the electrochemical gradient when protons and electrons are diverted from cytoplasmic sulfate to periplasmic nitrite reduction.
FIG. 5.
Model for sulfate reduction by D. vulgaris derived from the effects of nitrite on gene expression in the wild type. Transmembrane electron transport complexes qmoABC and dsrMKJOP donate electrons to the ApsBA- and DsrAB-catalyzed reactions, which consume two and six protons, respectively (not indicated). Periplasmic protons resulting from hydrogen cycling or other proton translocation mechanisms reenter the cell through ATP synthase, leading to additional ATP synthesis. The 22/3 ATP indicated is the theoretically maximal value. When nitrite is present, periplasmic protons and electrons are used by NrfHA to reduce nitrite to ammonia. The model suggests that this lowers the transmembrane proton gradient driving ATP synthase as well as transmembrane electron transport through qmoABC and dsrMKJOP. Nitrite inhibits the reduction of sulfite to sulfide by DsrAB, which slowly reduces nitrite to ammonia. Enzyme abbreviations: Sat, sulfate adenylyltransferase; PpaC, pyrophosphatase; ApsBA, adenosine phosphosulfate reductase; DsrAB, dissimilatory sulfite reductase; QmoABC, quinone-interacting membrane-bound oxidoreductase; DsrMKJOP, triheme cytochrome c-containing membrane-bound oxidoreductase; Ldh, lactate dehydrogenase; Por, pyruvate:ferredoxin oxidoreductase; Pta, phosphate transacetylase; Ack, acetate kinase; c3 network, network of periplasmic, tetrahemic c-type cytochromes (8). APS, adenosine phosphosulfate.
D. vulgaris DsrAB, which catalyzes the six-electron reduction of sulfite to sulfide as the final step in sulfate reduction, slowly reduces nitrite to ammonia (27), allowing nitrite to serve as a competitive inhibitor. Blockage of DsrAB may cause accumulation of sulfite (Fig. 5, HSO3−), and this likely explains the downregulation of upstream genes in the sulfate reduction pathway (sat, ppaC, and apsBA) during nitrite inhibition (Table 2). We were initially surprised to find that the wild-type and mutant strains have the same intrinsic nitrite resistance (Fig. 3C). However, given a Km of 1.4 mM of Desulfovibrio nitrite reductase for nitrite (12), no contribution of NrfHA to nitrite reduction is expected at nitrite concentrations below 0.1 mM. The physiological function of NrfHA is thus to allow Desulfovibrio spp. to survive in environments containing millimolar concentrations of nitrite. Such concentrations are readily generated when nitrate-reducing, sulfide-oxidizing bacteria reduce nitrate (6, 13). Under these conditions, nrf-containing sulfate-reducing bacteria are often only transiently inhibited, whereas inhibition of sulfate-reducing bacteria lacking nrf is long-lasting (6).
The D. vulgaris genes upregulated by nitrite include those for NrfHA and Hcp2 (Table 2). D. vulgaris NrfHA has been reported to be inducible (11) and constitutive (16). The properties and distribution of NrfHA have recently been reviewed (21). Nitrate induces nrfHA in E. coli (25) but not in D. vulgaris, which lacks the nitrate reductase that converts nitrate to nitrite (Fig. 2B). The D. vulgaris genome sequence indicated the presence of hcp2 in addition to hcp1, encoding hybrid cluster protein 1, previously referred to as prismane protein (23). Hcps are induced by nitrate, nitrite, nitric oxide, and/or hydrogen peroxide, suggesting a role in nitrogen metabolism and/or oxidative stress defense (2, 3, 10). The hcp2 gene was strongly upregulated with nitrite in both the wild-type and nrfA mutant strains (Table 2); hcp1 was constitutive. The hcp2 gene was also upregulated with ethanol as the sole electron donor and thiosulfate as the electron acceptor, during inhibition with biocides, and in D. vulgaris hmc and hyd mutants (Haveman et al., unpublished data). Whatever the exact function of Hcps, these results suggest that Hcp1 functions during normal cellular metabolism, whereas Hcp2 augments this function by induction under stress conditions. Lack of hcp2 induction in the nrfA mutant in the absence of nitrite (Table 2) indicates that, contrary to the hmc and hyd mutations, which lead to altered patterns of electron flow (24), the nrfA mutation is not stressful to D. vulgaris in the absence of nitrite. Hence, nitrite reduction appears to be the only physiological function of NrfHA.
Downregulation of two operons for putative electron transport complexes suggests their involvement in the sulfate reduction pathway. The Dvu0848-0849-0850 operon, located 148 bp downstream of the apsBA genes, encodes the quinone-interacting membrane-bound oxidoreductase (Qmo) complex recently isolated from Desulfovibrio desulfuricans (17). Because the Qmo complex does not appear to have a periplasmic component, Pires et al. (17) suggested that it transports electrons from the quinone pool to adenosine phosphosulfate reductase. The close proximity of apsBA and qmoABC genes in the genome as well as the downregulation of both sets of genes with nitrite (Table 2) support this suggestion.
The gene products of the Dvu1290-1289-1288-1287-1286 operon are homologous to subunits DsrMKJOP, encoded by the dsrABEFHCMKLJOPNRS locus for dissimilatory sulfite reductase of Allochromatium vinosum (19). The colocalization of the dsrMKJOP and dsrAB genes in A. vinosum indicates that both operons are involved in sulfide oxidation in this organism. In sulfate-reducing bacteria, DsrMKJOP likely transports electrons to DsrAB for reduction of sulfite to sulfide either from the periplasmic cytochrome c3 network (8) or from the membrane-bound quinone pool. The observation of reduced expression of dsrMKJOP when sulfite reduction by DsrAB is blocked with nitrite (Table 2) is in agreement with this suggestion.
In summary, it appears that the effects of nitrite on D. vulgaris, inhibition of the sulfate reduction pathway at DsrAB and periplasmic consumption of protons and electrons, can be identified from the gene expression response. The former explains downregulation of sat, ppaC, and apsBA, while the latter explains downregulation of qmoABC, dsrMKJOP, and the two atp operons. Nitrite inhibition does not cause downregulation of genes for enzymes involved in lactate oxidation or for cytoplasmic or periplasmic hydrogenases because the electron and proton flow catalyzed by these enzymes is now directed towards nitrite reduction (Fig. 5). The presence of nitrite reductase allows the organism to survive in environments containing millimolar concentrations of nitrite (i.e., as generated by nitrate-reducing, sulfide-oxidizing bacteria), whereas in its absence such concentrations are lethal.
Supplementary Material
Acknowledgments
This research was supported by Strategic and Discovery Grants from the Natural Science and Engineering Research Council of Canada to G.V.
We thank Veronique Brunelle for experimental assistance.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipmann. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Beliaev, A. S., D. K. Thompson, T. Khare, H. Lim, C. C. Brandt, G. Li, A. E. Murray, J. F. Heidelberg, C. S. Giometti, J. Yates III, K. H. Nealson, J. M. Tiedje, and J. Zhou. 2002. Gene and protein expression profiles of Shewanella oneidensis during anaerobic growth with different electron acceptors. OMICS 6:39-60. [DOI] [PubMed] [Google Scholar]
- 3.Briolat, V., and G. Reysset. 2002. Identification of the Clostridium perfringens genes involved in the adaptive response to oxidative stress. J. Bacteriol. 184:2333-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fossing, H., V. A. Gallardo, B. B. Jørgensen, M. Hüttel, L. P. Nielsen, H. Schulz, D. E. Canfield, S. Forster, R. N. Glud, J. K. Gundersen, J. Küver, N. B. Ramsing, A. Teske, B. Thamdrup, and O. Ulloa. 1995. Concentration and transport of nitrate by the mat-forming sulphur bacterium Thioploca. Nature 374:713-715. [Google Scholar]
- 5.Fu, R., and G. Voordouw. 1997. Targeted gene replacement mutagenesis of dcrA, encoding an oxygen sensor of the sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Microbiology 143:1815-1826. [DOI] [PubMed] [Google Scholar]
- 6.Greene, E. A., C. Hubert, M. Nemati, G. E. Jenneman, and G. Voordouw. 2003. Nitrite reductase activity of sulfate-reducing bacteria prevents their inhibition by nitrate-reducing, sulfide-oxidizing bacteria. Environ. Microbiol. 5:607-617. [DOI] [PubMed] [Google Scholar]
- 7.Haveman, S. A., V. Brunelle, J. K. Voordouw, G. Voordouw, J. F. Heidelberg, and R. Rabus. 2003. Gene expression analysis of energy metabolism mutants of Desulfovibrio vulgaris Hildenborough indicates an important role for alcohol dehydrogenase. J. Bacteriol. 185:4345-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidelberg, J. F., R. Seshadri, S. A. Haveman, C. J. Hemme, I. A. Paulsen, J. F. Kolonay, J. A. Eisen, N. Ward, B. Methe, L. M. Brinkac, S. C. Daugherty, R. T. Deboy, R. J. Dodson, A. S. Durkin, R. Madupu, W. C. Nelson, S. A. Sullivan, D. Fouts, D. H. Haft, J. Selengut, J. D. Peterson, T. M. Davidsen, N. Zafar, L. Zhou, R. Radune, G. Dimitrov, M. Hance, K. Tran, H. Khouri, J. Gill, T. R. Utterback, T. V. Feldblyum, J. D. Wall, G. Voordouw, and C. M. Fraser. 2004. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough: consequences for its energy metabolism, biocorrosion and reductive metal bioremediation. Nat. Biotechnol. 22:554-559. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann, K., and W. Stoffel. 1993. TMbase-A database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 374:166. [Google Scholar]
- 10.Kim, C. C., D. Monack, and S. Falkow. 2003. Modulation of virulence by two acidified nitrite-responsive loci of Salmonella enterica serovar Typhimurium. Infect. Immun. 71:3196-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell, G. J., J. G. Jones, and J. A. Cole. 1986. Distribution and regulation of nitrate and nitrite reduction by Desulfovibrio and Desulfotomaculum species. Arch. Microbiol. 144:35-40. [Google Scholar]
- 12.Moura, I., S. Bursakov, C. Costa, and J. J. G. Moura. 1997. Nitrate and nitrite utilization in sulfate-reducing bacteria. Anaerobe 3:279-290. [DOI] [PubMed] [Google Scholar]
- 13.Nemati, M., G. E. Jenneman, and G. Voordouw. 2001. Mechanistic study of microbial control of hydrogen sulfide production in oil reservoirs. Biotechnol. Bioeng. 74:424-434. [DOI] [PubMed] [Google Scholar]
- 14.Nemati, M., T. J. Mazutinec, G. E. Jenneman, and G. Voordouw. 2001. Control of biogenic H2S production with nitrite and molybdate. J. Ind. Microbiol. Biotechnol. 26:350-355. [DOI] [PubMed] [Google Scholar]
- 15.Odom, J. M., and H. D. Peck, Jr. 1981. Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria, Desulfovibrio sp. FEMS Microbiol. Lett. 12:47-50. [Google Scholar]
- 16.Pereira, I. A. C., J. LeGall, A. V. Xavier, and M. Teixeira. 2000. Characterization of heme c nitrite reductase from a non-ammonifying microorganism, Desulfovibrio vulgaris Hildenborough. Biochim. Biophys. Acta 1481:119-130. [DOI] [PubMed] [Google Scholar]
- 17.Pires, R. H., A. I. Lourenco, F. Morais, M. Teixeira, A. V. Xavier, L. M. Saraiva, and I. A. C. Pereira. 2003. A novel membrane-bound respiratory complex from Desulfovibrio desulfuricans ATCC 27774. Biochim. Biophys. Acta 1605:67-82. [DOI] [PubMed] [Google Scholar]
- 18.Postgate, J. R. 1984. The sulphate-reducing bacteria, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 19.Pott, A. S., and C. Dahl. 1998. Sirohaem sulfite reductase and other proteins encoded by genes at the dsr locus of Chromatium vinosum are involved in the oxidation of intracellular sulfur. Microbiology 144:1881-1894. [DOI] [PubMed] [Google Scholar]
- 20.Schweizer, H. P. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol. Microbiol. 6:1195-1204. [DOI] [PubMed] [Google Scholar]
- 21.Simon, J. 2002. Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol. Rev. 26:285-309. [DOI] [PubMed] [Google Scholar]
- 22.Simon, R., U. Priefer, and A. Pühler. 1983. A broad-host-range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 23.Stokkermans, J. P. W. G., A. J. Pierik, R. B. G. Wolbert, W. R. Hagen, W.M.A.M. van Dongen, and C. Veeger. 1992. The primary structure of a protein containing a putative [6Fe-6S] prismane cluster from Desulfovibrio vulgaris (Hildenborough). Eur. J. Biochem. 208:435-442. [DOI] [PubMed] [Google Scholar]
- 24.Voordouw, G. 2002. Carbon monoxide cycling by Desulfovibrio vulgaris Hildenborough. J. Bacteriol. 184:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, H., and R. P. Gunsalus. 2000. The nrfA and nirB reductase operons in Escherichia coli are expressed differently in response to nitrate than to nitrite. J. Bacteriol. 182:5813-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3370. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 27.Wolfe, B. M., S. M. Lui, and J. A. Cowan. 1994. Desulfoviridin, a multimeric-dissimilatory sulfite reductase from Desulfovibrio vulgaris (Hildenborough) purification, characterization, kinetics and EPR studies. Eur. J. Biochem. 223:79-89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.