Abstract
Since, like other osmolytes, proline can act as a protein stabilizer, we investigated the thermoprotectant properties of proline in vitro and in vivo. In vivo, elevated proline pools in Escherichia coli (obtained by altering the feedback inhibition by proline of γ-glutamylkinase, the first enzyme of the proline biosynthesis pathway) restore the viability of a dnaK-deficient mutant at 42°C, suggesting that proline can act as a thermoprotectant for E. coli cells. Furthermore, analysis of aggregated proteins in the dnaK-deficient strain at 42°C by two-dimensional gel electrophoresis shows that high proline pools reduce the protein aggregation defect of the dnaK-deficient strain. In vitro, like other “chemical chaperones,” and like the DnaK chaperone, proline protects citrate synthase against thermodenaturation and stimulates citrate synthase renaturation after urea denaturation. These results show that a protein aggregation defect can be compensated for by a single mutation in an amino acid biosynthetic pathway and that an ubiquitously producible chemical chaperone can compensate for a defect in one of the major chaperones involved in protein folding and aggregation.
Acclimation to osmotic stress involves intracellular accumulation (up to 0.5 M), by synthesis or uptake, of small organic molecules known as osmolytes, such as glycine betaine, proline, or trehalose (5, 6, 14). They serve as stabilizers of proteins and cell components against the denaturing effect of ionic strength. Furthermore, some of these osmolytes behave as “chemical chaperones” by promoting the correct refolding of proteins in vitro and in the cell and by protecting native proteins from heat denaturation (1, 4, 9, 13, 19).
Heat shock induces the massive production of heat shock proteins. In Escherichia coli, IbpA/IbpB (small heat shock proteins), DnaK/Hsp70, GroEL/Hsp60, HtpG/Hsp90, and ClpB/Hsp100 are the major chaperones that cooperate in preventing protein aggregation during heat stress and in promoting protein refolding after the stress (12). The DnaK system (DnaK/DnaJ/GrpE) appears to be the most effective chaperone system in preventing protein aggregation in vivo (15), and this activity explains the temperature-sensitive phenotype of ΔdnaK mutant cells (16). The major role of the DnaK system in preventing protein aggregation in vivo is supported by the lack of strong protein aggregation phenotypes of mutants lacking functions of other chaperones and by the inability of overproduction of GroEL/GroES, ClpB, HtpG, or IbpA/IbpB to restore the growth of ΔdnaK mutant cells at 42°C (15).
In enteric bacteria, proline can be transported from the medium by the ProP and ProU transporters and is catabolized to glutamate by the putA gene product (14, 22). Proline transport, however, does not result, even under osmotic stress conditions, in high intracellular proline pools (14). Proline is endogenously synthesized from glutamate by the proA, proB, and proC gene products, which are synthesized at similar levels regardless of the availability of proline (7). Proline synthesis is negatively regulated through feedback inhibition of the first biosynthetic enzyme of the pathway, the proB gene product γ-glutamylkinase (2). Proline-overproducing mutants, affected in the proB gene, have been isolated from Salmonella enterica serovar Typhimurium (5); these mutants possess a γ-glutamylkinase which is resistant to feedback inhibition by proline and have acquired osmotolerance. The most osmotolerant strain, carrying the proline-overproducing mutation proB74, has, depending on the osmolarity of the growth medium, a 30- to 400-fold-higher proline level than the parental strain. The proB74 mutation has been cloned in E. coli and has been shown to confer osmotolerance to the cells, a consequence of an increased proline pool (8).
In the present study, we show that proline overproduction (in an E. coli strain producing a desensitized γ-glutamylkinase) can restore the viability of a dnaK-deficient mutant at 42°C and partially reverses its protein aggregation defect in vivo; we also compare the effects of proline and DnaK on citrate synthase folding and aggregation in vitro.
Suppression of the thermosensitive phenotype of a dnaK deletion mutant at 42°C by increased proline pools.
The dnaK deletion mutant GW 4813 (ΔdnaK52::Cmr, derived from strain AB1157) is deficient for growth and viability at 42°C (16), probably as a consequence of protein denaturation and aggregation (15). The dnaK mutant was transformed with plasmid pDU117, carrying proB74, the γ-glutamylkinase gene impaired in the allosteric repression by proline, or with plasmid pDU417, carrying the wild-type γ-glutamylkinase gene (both plasmids also contain the proA+ gene, coding for γ-glutamyl phosphate reductase, the second enzyme of the proline biosynthetic pathway); they were constructed as described previously (8) and introduced into the dnaK deletion mutant GW4813 by electroporation.
We first checked that the presence of a desensitized γ-glutamylkinase results in high intracellular proline pools in the dnaK mutant expressing the proB74 gene (analysis of free proline pools was carried out on a Beckman amino acid analyzer as described previously [5] on bacteria grown in Luria-Bertani medium, towards the end of the exponential phase). An intracellular proline pool of 295 nmol/mg of cell protein was measured in the proline overproducer, versus 12 nmol/mg of cell protein in the control strain (295 nmol of proline/mg of cell protein corresponds to a proline pool of around 90 mM, assuming an intracellular volume of 3.5 μl/mg of cell protein (17).
The colony-forming abilities of the two strains at 30 and 42°C were compared. At 30°C, the colony-forming abilities of the two strains were similar (the dnaK proB74 strain formed smaller colonies than the dnaK proB+ control strain) (not shown). At 42°C, the dnaK proB+ strain displayed less than 0.2% plating efficiency (of that at 30°C); in contrast, the dnaK proB74 strain displayed 52% plating efficiency (of that at 30°C) (not shown). These results suggest that a high intracellular proline pool can partially compensate for the deficiency of the dnaK mutant at 42°C, probably by reducing its known protein aggregation defect at elevated temperatures (see below).
Effects of proline pool on protein aggregation in the dnaK mutant.
We investigated the extent of heat-induced protein aggregation in the wild-type strain AB1157 and in the isogenic dnaK-deficient mutant transformed either with plasmid pDU117, carrying proB74, the γ-glutamylkinase gene impaired in the allosteric repression by proline, or with plasmid pDU417 carrying the wild-type γ-glutamylkinase gene. Cells were grown at 30°C and then subjected to heat shock treatment for 60 min at 42°C (within the time course of the experiment, none of the strains showed growth defects as judged by increases in turbidity of the cultures). After lysis of cells, the insoluble cell fraction was isolated by centrifugation at 15,000 × g as described previously (15), and the pellets were analyzed by two-dimensional gel electrophoresis (10).
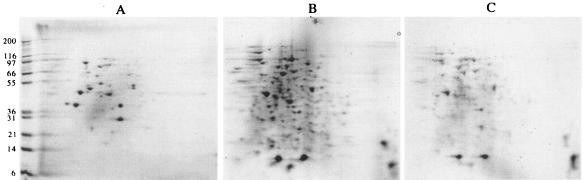
Heat shock treatment caused an increased protein aggregation in the dnaK-deficient strain transformed with the wild-type proB gene (Fig. 1B, displaying approximately 30 major spots and 60 minor spots) compared with the wild-type strain (Fig. 1A, displaying approximately 10 major spots and 20 minor spots) (similar increased protein aggregation was observed in the dnaK-deficient strain without any plasmid [reference 15 and data not shown]). In contrast, the dnaK-deficient strain transformed with the proB74-producing plasmid was much less prone to protein aggregation (Fig. 1C, showing approximately 10 major spots and 40 minor spots) than the dnaK deficient strain transformed with the control proB+ plasmid. The antiaggregative effect of proline overproduction did not seem to be related to the size or pI of the proteins. The aggregation of most aggregated proteins was dramatically reduced by high proline pools, in accordance with a general effect of chemical chaperones on protein aggregation resulting from a modification of the water-protein interactions (1). However, the pattern of aggregated proteins in the dnaK-deficient strain overproducing proline was more similar to the pattern of the dnaK-deficient strain than to that of the wild-type strain, suggesting that the DnaK deficiency is still apparent but attenuated. The results described in this section suggest that high proline pools can significantly reduce the protein aggregation defect at 42°C resulting from dnaK deficiency.
FIG. 1.
Two-dimensional gel electrophoresis of insoluble fractions. The wild-type strain AB1167 (A) and the dnaK deletion mutant GW4813 transformed with the control plasmid pDU417 (B) or with the proline-overproducing plasmid pDU117 (C) were grown at 30°C in Luria-Bertani medium to logarithmic phase and shifted to 42°C for 60 min. Insoluble proteins were analyzed by 2-dimensional gel electrophoresis. Pellets suspended in water were treated with methanol-chloroform (3:1) to remove salt and lipids (20). The chloroform-treated proteins were precipitated with 60% (vol/vol) methanol, and protein pellets were resuspended in isoelectric focusing buffer (7 M urea, 2 M thiourea, 4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 100 mM dithiothreitol, and 0.5% pH 3 to 10 immobilized pH gradient buffer). Protein samples were loaded on 7-cm pH 3 to 10 immobilized pH gradient strips (Amersham Biosciences, Uppsala, Sweden) which were passively rehydrated overnight, and isoelectric focusing was carried out for 1,800 V · h at a maximum of 2,000 V in the ZOOM IPGRunner system (Invitrogen, Carlsbad, Calif.) with the Protean II isoelectric focusing cell (Bio-Rad, Hercules, Calif.). Immobilized pH gradient strips were positioned onto precast 4 to 12% bis-Tris ZOOM sodium dodecyl sulfate-polyacrylamide gels (Invitrogen), and electrophoresis was run at 200 V for 50 min. The amounts of protein loaded in panels A, B, and C correspond to identical amounts of bacteria. The pH 3 to 10 gradient is from left to right. Staining was done with Coomassie blue. Molecular mass markers (in kilodaltons) are indicated at the left.
Proline protects citrate synthase from denaturation and irreversible aggregation during thermal stress.
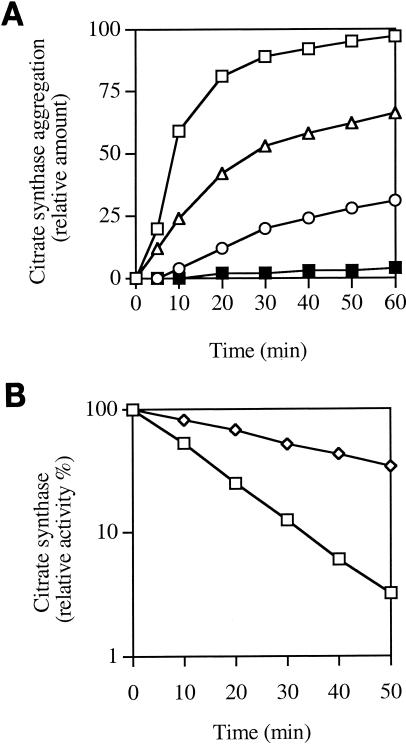
Citrate synthase loses its native conformation and undergoes aggregation during incubation at 43°C (3, 18). Molecular chaperones (DnaK, GroEL, and small heat shock proteins) and chemical chaperones (glycine betaine and choline) protect citrate synthase against thermal aggregation (3, 4, 9, 18). We investigated whether proline also protects citrate synthase (from porcine heart [Sigma]) against thermal aggregation in vitro. The native enzyme was incubated at 43°C in the absence or in the presence of proline or DnaK. Citrate synthase aggregation was monitored by measuring the absorbance at 650 nm as described previously (18). Proline concentrations of between 150 and 500 mM efficiently reduced citrate synthase aggregation (Fig. 2A). Under similar conditions, 5 μM DnaK completely suppressed citrate synthase aggregation (Fig. 2A) (18). Thus, like a classical chaperone, although at a much higher concentration (a characteristic of other chemical chaperones), proline efficiently protects citrate synthase against thermal aggregation (4).
FIG. 2.
Thermoprotection of citrate synthase by proline. (A) Thermal aggregation of citrate synthase in the presence of proline. The kinetics of citrate synthase aggregation was determined by light scattering at 650 nm. Native citrate synthase was diluted to a final concentration of 0.8 μM in 40 mM HEPES-50 mM KCl-10 mM (NH4)2SO4-2 mM potassium acetate (pH 8.0) at 43°C alone (open squares) or in the presence of 150 mM (triangles) or 500 mM (circles) proline or 5 μM DnaK (filled squares). The absorbance at 650 nm (×1,000) is represented in ordinate. (B) Thermodenaturation of citrate synthase. Citrate synthase (80 nM) was incubated at 45°C in the same buffer as for panel A in the absence (squares) or presence (diamonds) of 500 mM proline. Samples were removed at intervals and cooled, and enzymatic activity was subsequently determined at 25°C.
Proline also stabilizes the enzymatic activity of citrate synthase during thermal stress. Citrate synthase inactivation at 45°C followed first-order kinetics, with a half-life ranging from 10 min in the absence of proline to 35 min in the presence of 500 mM proline (Fig. 2B). Thus, like glycine betaine and choline (4), proline stabilizes the activity of citrate synthase during thermal stress.
Proline increases the amount of correctly folded citrate synthase.
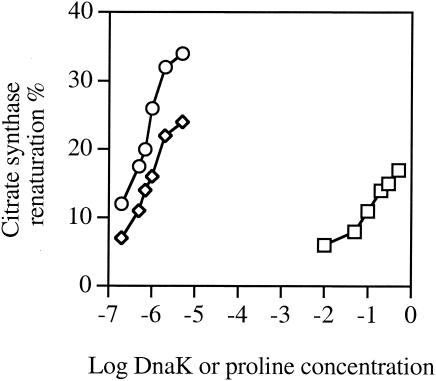
Chaperones not only protect proteins against thermal stress but also stimulate protein renaturation. We investigated whether proline can stimulate the folding of citrate synthase, a protein whose refolding is facilitated by several chaperones, such as GroEL, DnaK, Hsp90, and small heat shock proteins (3, 18). Citrate synthase was unfolded in the presence of urea and allowed to refold upon dilution of the denaturant in the absence or in the presence of proline or of the DnaK/DnaJ/GrpE/ATP chaperone machine. Under our experimental conditions, the refolding yield of 0.1 μM citrate synthase was increased from 7% in the absence of proline to 12% in the presence of 200 mM proline and to 17% in the presence of 500 mM proline (Fig. 3). Under similar conditions, the maximal renaturation of citrate synthase in the presence of DnaK alone was 23%, and the maximal renaturation of citrate synthase in the presence of DnaK/DnaJ/GrpE/ATP was 33%, which was obtained in the presence of 1 μM DnaK, 0.3 μM DnaJ, and 0.3 μM GrpE (Fig. 3). Thus, proline, like classical chaperones, stimulates the renaturation of an unfolded protein, although at much higher concentrations and with a lower efficiency.
FIG. 3.
Influence of proline on the refolding of urea-denatured citrate synthase. Citrate synthase was unfolded in the presence of urea (8 M urea, 50 mM Tris-HCl, 2 mM EDTA, 20 mM dithiothreitol [pH 8.0]) for 50 min and allowed to refold for 20 min at a concentration of 0.1 μM by 100-fold dilution in 40 mM HEPES-50 mM KCl-10 mM (NH4)2SO4-2 mM potassium acetate-1 mM MgCl2 (pH 8.0) in the presence of proline (squares), DnaK (diamonds), or DnaK-DnaJ-GrpE at a ratio of 1:0.3:0.3 in the presence of 0.5 mM ATP (open circles) at the indicated concentrations of proline or DnaK.
Proline as an antiaggregation agent.
Our results suggest that proline, in addition to being an osmoprotectant, is involved in thermoprotection both in vitro and in vivo. In vitro, proline protects citrate synthase against thermodenaturation and stimulates its renaturation, like protein chaperones, although at much higher concentrations. High concentrations of chemical chaperones (100 mM to 1 M), which are easily attained under several stress conditions, are usually required for their effect during osmotic or thermal stress (4, 5, 8, 9, 19).
Proline restores the viability of the dnaK deletion mutant at 42°C and decreases its protein aggregation defect. This supports the hypothesis that the temperature sensitivity of dnaK-deficient mutants is due to a protein aggregation defect (15). It has been recently reported that aggregation of a single enzyme (homoserine transsuccinylase, the first enzyme of the methionine biosynthesis pathway) limits growth of E. coli at elevated temperatures (11). Such protection is interesting, since protein chaperones, including GroEL/GroES, ClpB, HtpG, and IbpAB, do not significantly prevent the protein aggregation defect of the dnaK-deficient strain (15).
The present study shows that proline, one of the so-called chemical chaperones (19), can partially assume the function of a bona fide chaperone, the dnaK gene product, and emphasizes the role played by small molecules such as polyols (13, 21), trimethylamines (19, 21), and amino acids (21) in the protection of cells against stress. Finally, this study suggests that desensitization of γ-glutamylkinase to proline inhibition, by genetic or chemical means, might help prevent protein aggregation in other cell types.
Acknowledgments
We thank R. d'Ari for critical reading of the manuscript and A. Kropfinger for correction of the English language.
This work was supported by grant CR 521090 from the DGA to G.R.
REFERENCES
- 1.Arakawa, T., and S. N. Timasheff. 1985. The stabilization of proteins by osmolytes. Biophys. J. 47:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baich, A. 1969. Proline synthesis in Escherichia coli. A proline-inhibitable glutamic acid kinase. Biochim. Biophys. Acta 192:462-467. [DOI] [PubMed] [Google Scholar]
- 3.Buchner, J., M. Schmidt, M. Fuchs, R. Jaenicke, R. Rudolph, F. X. Schmid, and T. Kiefhaber. 1991. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry 30:1586-1591. [DOI] [PubMed] [Google Scholar]
- 4.Caldas, T., N. Demont-Caulet, A. Ghazi, and G. Richarme. 1999. Thermoprotection by glycine betaine and choline. Microbiology 145:2543-2548. [DOI] [PubMed] [Google Scholar]
- 5.Csonka, L. N. 1981. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol. Gen. Genet. 182:82-86. [DOI] [PubMed] [Google Scholar]
- 6.Csonka, L. N. 1982. A third l-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J. Bacteriol. 151:1433-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csonka, L. N. 1988. Regulation of cytoplasmic proline levels in Salmonella typhimurium: effect of osmotic stress on synthesis, degradation, and cellular retention of proline. J. Bacteriol. 170:2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandekar, A. M., and S. L. Uratsu. 1988. A single base pair change in proline biosynthesis genes causes osmotic stress tolerance. J. Bacteriol. 170:5943-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamant, S., N. Eliahu, D. Rosenthal, and P. Goloubinoff. 2001. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276:39586-39591. [DOI] [PubMed] [Google Scholar]
- 10.Guillot, A., C. Gitton, P. Anglade, and M. Y. Mistou. 2003. Proteomic analysis of Lactococcus lactis, a lactic acid bacterium. Proteomics 3:337-354. [DOI] [PubMed] [Google Scholar]
- 11.Gur, E., D. Biran, E. Gazit, and E. Z. Ron. 2002. In vivo aggregation of a single enzyme limits growth of Escherichia coli at elevated temperatures. Mol. Microbiol. 46:1391-1397. [DOI] [PubMed] [Google Scholar]
- 12.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 13.Hengge-Aronis, R., W. Klein, R. Lange, M. Rimmele, and W. Boos. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173:7918-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:310-330. [DOI] [PubMed] [Google Scholar]
- 15.Mogk, A., T. Tomoyasu, P. Goloubinoff, S. Rüdiger, D. Röder, H. Langen, and B. Bukau. 1999. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18:6934-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paek, K. H., and G. C. Walker. 1987. Escherichia coli dnaK null mutants are inviable at high temperature. J. Bacteriol. 169:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perroud, B., and D. Le Rudulier. 1985. Glycine betaine transport in Escherichia coli: osmotic modulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richarme, G., and T. D. Caldas. 1997. Chaperone properties of the bacterial periplasmic substrate-binding proteins. J. Biol. Chem. 272:15607-15612. [DOI] [PubMed] [Google Scholar]
- 19.Tatzelt, J., S. B. Prusiner, and W. J. Welch. 1996. Chemical chaperones interfere with the formation of scrapie prion protein. EMBO J. 15:6363-6373. [PMC free article] [PubMed] [Google Scholar]
- 20.Wessel, D., and U. I. Flugge. 1984. A method for the quantitative recovery of proteins in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]
- 21.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]
- 22.Zhu, W., and D. F. Becker. 2003. Flavin redox state triggers conformational changes in the PutA protein from Escherichia coli. Biochemistry 42:5469-5477. [DOI] [PubMed] [Google Scholar]