Abstract
A mutation was recovered in the slr0721 gene, which encodes the decarboxylating NADP+-dependent malic enzyme in the cyanobacterium Synechocystis sp. strain PCC 6803, yielding the mutant 3WEZ. Under continuous light, 3WEZ exhibits poor photoautotrophic growth while growing photoheterotrophically on glucose at rates nearly indistinguishable from wild-type rates. Interestingly, under diurnal light conditions (12 h of light and 12 h of dark), normal photoautotrophic growth of the mutant is completely restored.
Cyanobacteria are photoautotrophic gram-negative eubacteria that are capable of performing oxygenic photosynthesis. Synechocystis sp. strain PCC 6803 is a naturally transformable (7) unicellular cyanobacterium and has proven to be one of the best model organisms for studying the mechanism and regulation of oxygenic photosynthesis (14); it has also been used in a variety of global gene expression (6, 8, 13) and metabolomic (15, 16) studies. In addition to autotrophic growth, the presence of an unidentified mutation in the Williams strain of Synechocystis (14) confers glucose tolerance to this organism. With glucose as a carbon source, this strain can be grown under mixotrophic, photoheterotrophic (continuous photosynthetic illumination at 20 to 40 μmol of photons m−2 s−1 in the presence of the photosystem II inhibitor dichloromethylurea [DCMU]), and heterotrophic (nonphotosynthetic continuous illumination at <1 μmol of photons m−2 s−1) growth conditions (1). Synechocystis cells contain a single circular genome of ca. 3.6 Mbp, in 6 to 10 copies per cell, and can integrate exogenous DNA into the genome through active homologous recombination (7). The entire genome has been sequenced and is predicted to encode a total of 3,168 proteins (9). Here we describe the characterization of a mutant designated 3WEZ, which we had isolated previously (17), that bears a transposon insertional mutation in the NADP+-dependent malic enzyme. This enzyme catalyzes the oxidative decarboxylation of malate to pyruvate, concomitantly releasing CO2. This mutant exhibits extremely poor photoautotrophic growth characteristics under continuous light conditions. Propagation under a diurnal light regimen (12 h of light and 12 h of dark), however, fully restores photoautotrophy.
A glucose-tolerant strain of Synechocystis sp. strain PCC 6803 (14) was used as a control strain and as the DNA recipient strain in the present study. Cells of both the control strain and the 3WEZ mutant were maintained under photoheterotrophic growth conditions at 30°C with a light intensity of 40 μmol of photons m−2 s−1 on BG-11 growth medium (American Type Culture Collection medium 616) supplemented with 10 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid-KOH (pH 8.2), 5 mM glucose, 10 μM DCMU, 0.3% sodium thiosulfate, and 1.5% agar. Where appropriate, kanamycin was included in the medium at a final concentration of 10 μg/ml. For liquid cultures, the agar and thiosulfate were omitted and the cultures were continuously bubbled with sterile, humidified air.
Transposon mutagenesis (2) and semi-high-throughput screening (17) were performed as described previously. Genomic DNA of Synechocystis cells was prepared (14) and subjected to further purification with DNeasy tissue kits (Qiagen Corp.). The purified DNA was then used in restriction enzyme digestion, Southern blot hybridization, PCR, and DNA sequencing, all of which were performed by standard methods. Inverse PCR was performed as described previously (17). Complementation of the 3WEZ mutation by a cloned 2,089-bp PCR product containing the intact slr0721 gene was performed as described previously (5).
For measuring growth rates, the cells of both the control strain and the 3WEZ mutant were inoculated into 150 ml of liquid medium at an initial optical density at 730 nm of ca. 0.01 and grown at 30°C with continuous light at an intensity of 40 μmol of photons m−2 s−1. In all instances, the cultures were bubbled continuously with sterile, humidified air. For autotrophic growth BG-11 medium was used, and for photoheterotrophic growth the BG-11 medium was supplemented with 5 mM glucose and 10 μM DCMU (for mixotrophic growth, the DCMU was omitted). To determine if a pyruvate limitation was responsible for the slow growth observed for the 3WEZ mutant, a mixotrophic experiment was performed by supplementing BG-11 with 5 mM pyruvate. Pyruvate-dependent photoheterotrophic growth experiments were performed with BG-11 medium supplemented with 5 mM pyruvate and 10 μM DCMU. For experiments designed to test the effects of diurnal growth conditions, a cycle of 12 h of light and 12 h of dark was used. The growth of cultures grown under these different conditions was measured daily by monitoring the optical density of the cultures at 730 nm. All of the measurements were repeated at least three times, and the averages of these rates were taken for comparisons. The sizes of the control Synechocystis cells and cells of the 3WEZ mutant were examined by differential interference microscopy. Both cell types were approximately the same size (±10%) (data not shown).
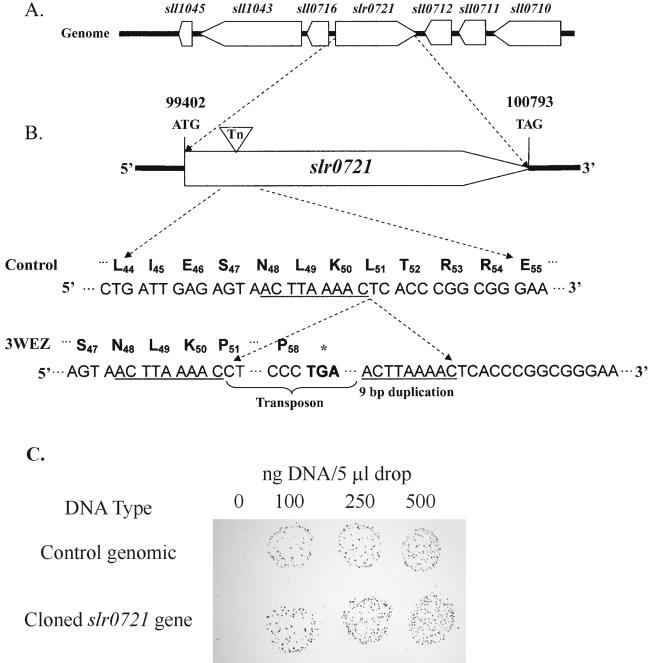
With genomic DNA from the 3WEZ mutant as a template, PCR analysis with transposon-specific primers demonstrated the presence of the transposon in the genome of 3WEZ, while restriction analysis followed by Southern blot hybridization with a transposon-specific probe verified that the mutant contained only a single transposon insertion (data not shown). To identify the site of transposon insertion in the 3WEZ mutant, inverse PCR was performed. The results of this experiment are shown in Fig. 1. The transposon was determined to be inserted into the slr0721 gene, which encodes the NADP+-dependent decarboxylating malic enzyme (12). No other identifiable malic enzyme genes are present in the Synechocystis genome. The intact malic enzyme gene encodes a protein of 463 amino acid residues. The transposon insertion at codon 51 generated a premature translational stop after amino acid 58.
FIG. 1.
Genomic structure of the control strain and the mutant 3WEZ. A. Gene organization in the vicinity of the slr0721 gene. The flanking genes (sll0716 and sll0712) are both transcribed in opposite directions from slr0721. B. Location of the transposon (Tn) insertion in slr0721. Insertion of the transposon in codon 51 of slr0721 leads to the introduction of a premature stop codon in the mutant. Note the 9-bp duplication flanking the transposon insertion site. C. Complementation of the 3WEZ mutation by control genomic DNA and the cloned wild-type slr0721 gene. Dot transformations were performed on BG-11 medium as described previously (5).
3WEZ exhibits extremely poor photoautotrophic growth on agar plates which contain only BG-11 medium (17). Complementation analysis was performed with either control strain genomic DNA or a cloned 2,089-bp PCR product containing the wild-type slr0721 gene (Fig. 1C). Both types of DNA restored photoautotrophic growth to 3WEZ mutant cells. This result demonstrates that the transposon insertion into the slr0721 gene was responsible for the observed phenotype of the mutant.
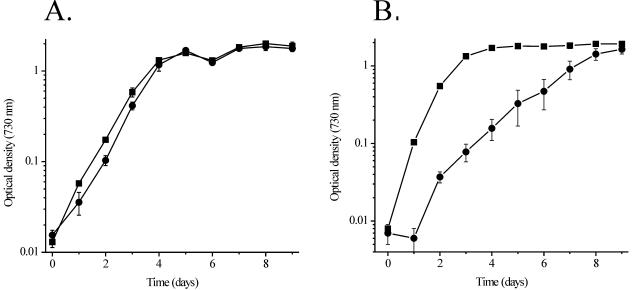
Figure 2 demonstrates the growth of the control strain and the mutant 3WEZ with continuous illumination under photoheterotrophic and photoautotrophic conditions. Under photoheterotrophic growth conditions, the photosystem II inhibitor DCMU was provided to abolish whole-chain photosynthetic electron transport, and glucose was supplied as a carbon source. The control and mutant strains were observed to grow at very similar rates (Fig. 2A). Under photoautotrophic conditions, however, a marked difference in the growth of these two strains was observed. The 3WEZ mutant grew nearly 15 times slower than the control strain. These results indicated that the transposon insertion in the slr0721 gene led to a loss of optimal photoautotrophy in the mutant 3WEZ.
FIG. 2.
Glucose-dependent photoheterotrophic (A) and photoautotrophic (B) growth of the control strain and mutant 3WEZ. Symbols: ▪, control strain; •, 3WEZ. Error bars are ±1.0 standard deviation. In some instances the error bars are smaller than the symbols used. For both the photoheterotrophic and photoautotrophic growth experiments, n = 3 for the control and n = 6 for 3WEZ.
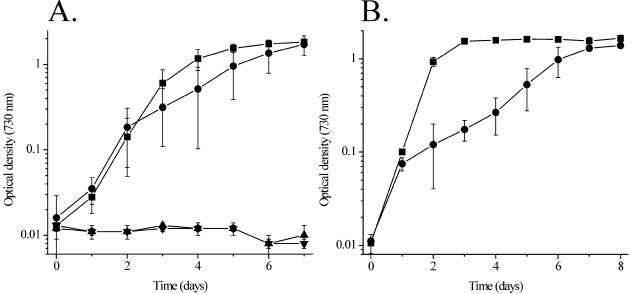
Disruption of the gene encoding the malic enzyme could lead to an increased concentration of malic acid and possibly a decreased concentration of pyruvate in the cell. Either of these conditions might be detrimental to cell growth. The increased malate concentration could be toxic to Synechocystis cells, or a pyruvate deficiency could lead to defects in cellular respiratory energy production and anabolic metabolism. The latter appears to be the case. Figure 3A illustrates growth experiments in which the BG-11 growth medium was supplemented with either 5 mM pyruvate alone (mixotrophic growth conditions) or 5 mM pyruvate plus 10 μM DCMU (pyruvate-dependent photoheterotrophic growth conditions). Under mixotrophic conditions, the inclusion of pyruvate restored growth of the 3WEZ mutant to levels near those for the control strain. This result supports the hypothesis that pyruvate, produced by the decarboxylation of malate, is required for optimal photoautotrophy under continuous illumination conditions. Apparently, the pyruvate produced by other metabolic pathways is insufficient to support optimal growth under continuous illumination conditions. Interestingly, neither the control strain nor 3WEZ could grow under pyruvate-dependent photoheterotrophic growth conditions (Fig. 3A). During heterotrophic growth (and presumably photoheterotrophic growth) the principal mode of glucose catabolism is via the pentose phosphate pathway (16). The reductive phase of this pathway generates large quantities of NADPH. Such NADPH production would not be generated with pyruvate as a carbon source. This may explain the inability of either strain to grow under pyruvate-dependent photoheterotrophic conditions. Additionally, under mixotrophic conditions, the 3WEZ mutant grows relatively poorly with glucose as a carbon source (Fig. 3B). This may be due to possible down regulation of pyruvate kinase under continuous light conditions (see below).
FIG. 3.
A. Pyruvate-dependent mixotrophic and pyruvate-dependent photoheterotrophic growth of the control strain and mutant 3WEZ. Symbols: ▪, control strain (mixotrophic); •, 3WEZ (mixotrophic); ▴, control strain (photoheterotrophic); ▾, 3WEZ (photoheterotrophic). Error bars are ±1.0 standard deviation. In some instances the error bars are smaller than the symbols used. For the pyruvate-dependent mixotrophic experiment, n = 6 for both strains; for the pyruvate-dependent photoheterotrophic experiment, n = 3 for both strains. B. Glucose-dependent mixotrophic growth of the control strain and mutant 3WEZ. For comparison to glucose-dependent photoheterotrophic growth, see Fig. 2. Symbols: ▪, control strain; •, 3WEZ. Error bars are ±1.0 standard deviation. In some instances the error bars are smaller than the symbols used. For both strains, n = 4.
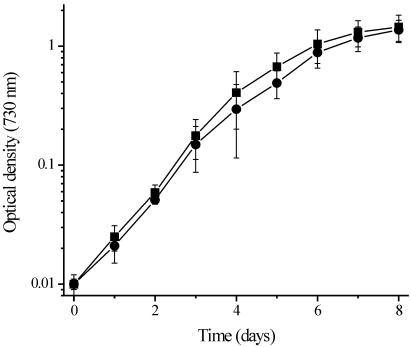
All of the preceding experiments were performed under continuous light conditions. A markedly different autotrophic growth result, however, was observed (Fig. 4) when the mutant was grown under a diurnal light regimen (12 h of light and 12 h of dark). Under these conditions, the autotrophic growth of 3WEZ is completely restored to control levels. This result indicates that other pathways for pyruvate production exist in addition to that for production of pyruvate by the malic enzyme, and these other pathways appear to be inactive during continuous illumination but are active during a diurnal illumination regimen.
FIG. 4.
Photoautotrophic growth of the control strain and 3WEZ under diurnal (12 h of light and 12 h of dark) illumination conditions. Symbols: ▪, control strain; •, 3WEZ. Error bars are ±1.0 standard deviation. In some instances the error bars are smaller than the symbols used. For both strains, n = 7.
That pyruvate is required for optimal photoautotrophic growth is not surprising given the central position of this metabolite in a variety of catabolic and anabolic metabolic pathways. What was quite interesting, however, was the observation that under continuous light growth conditions, pyruvate production appeared, in large measure, to require the NADP+-dependent malic enzyme. While we have not examined the source of the malate used as a substrate for this enzyme, one obvious candidate is a pathway involving phosphoenolpyruvate (PEP) carboxylase and malate dehydrogenase. Genes encoding both of these enzymes (sll0920 and sll0891, respectively) are present in Synechocystis. Additionally, metabolomic studies have indicated that there is a significant flux of carbon flowing through PEP carboxylase and the NADP+-dependent malic enzyme under mixotrophic conditions (16). Those authors found that about 25% of the CO2 sequestered under mixotrophic growth conditions is fixed via PEP carboxylase. This result was consistent with the observation that cyanobacteria incorporate a significant amount of CO2 into aspartate and malate in the light (3, 11).
While it is clear that the malic enzyme is required for optimal photoautotrophy under continuous illumination, it does not appear to be required under diurnal light conditions (Fig. 4). This surprising result indicates that other pathways, which appear to be diurnally controlled, can provide the required pyruvate. One candidate for such a control point is the terminal enzyme of the glycolytic pathway, pyruvate kinase. Two pyruvate kinase genes (sll0587 and sll1275) are present in Synechocystis. A pyruvate kinase from Synechococcus sp. strain PCC 6301, which appears to be homologous to the Sll1275 protein (pyruvate kinase-2) from Synechocystis, has been extensively characterized (10). This pyruvate kinase appeared to be more active in the dark than in the light and was activated by AMP and inhibited by ATP. Additionally, it was suggested that a drop in the intercellular pH upon cessation of active photosynthetic electron transport at the dark transition (4) would favor increased pyruvate kinase activity (10).
Our findings indicate that the decarboxylating NADP+-dependent malic enzyme encoded by the slr0721 gene in Synechocystis is required for optimal photoautotrophic growth under continuous illumination conditions but not under a diurnal cycle. We hypothesize that this enzyme is involved in a novel metabolic pathway for the generation of pyruvate in the light. This pathway, involving PEP carboxylase, malate dehydrogenase, and the malic enzyme, may be required due to the down regulation of pyruvate kinase under photosynthetic conditions.
Acknowledgments
This work was supported by grants from the Department of Energy and the National Science Foundation to T.M.B and L.K.F. Additional support was provided by a grant from the National Science Foundation to J.V.M.
REFERENCES
- 1.Anderson, S. L., and L. McIntosh. 1991. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: a blue-light-requiring process. J. Bacteriol. 173:2761-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaya, D., A. Takahashi, P. Shahi, and A. R. Grossman. 2001. Novel motility mutants of Synechocystis strain PCC 6803 generated by in vitro transposon mutagenesis. J. Bacteriol. 183:6140-6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman, J. R., and B. Colman. 1980. Demonstration of C3 photosynthesis in the blue-green alga, Coccochloris peniocystis. Planta 149:318-320. [DOI] [PubMed] [Google Scholar]
- 4.Coleman, J. R., and B. Colman. 1981. Inorganic carbon accumulation and photosynthesis in a blue-green alga as a function of external pH. Plant Physiol. 67:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzelzkalns, V. A., and L. Bogorad. 1988. Mutational analysis of a mutant defective in photosynthetic oxygen evolution and isolation of a complementing clone by a novel screening procedure. EMBO J. 7:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill, R. T., E. Katsoulakis, W. Schmitt, G. Taroncher-Oldenburg, J. Misra, and G. Stephanopoulos. 2002. Genome-wide dynamic transcriptional profiling of the light-to-dark transition in Synechocystis sp. strain PCC 6803. J. Bacteriol. 184:3671-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigorieva, G., and S. Shestokov. 1982. Transformation in the cyanobacterium Synechocystis SP 6803. FEMS Microbiol Lett. 13:367-370. [Google Scholar]
- 8.Hihara, Y., K. Sonoike, M. Kanehisa, and M. Ikeuchi. 2003. DNA microarray analysis of redox-responsive genes in the genome of the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 185:1719-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakmura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakzaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 10.Knowles, V. L., C. S. Smith, C. R. Smith, and W. C. Plaxton. 2001. Structural and regulatory properties of pyruvate kinase from the cyanobacterium Synechococcus PCC 6301. J. Biol. Chem. 276:20966-20972. [DOI] [PubMed] [Google Scholar]
- 11.Owittrim, G. W., and B. Colman. 1988. Phosphoenolpyruvate carboxylase mediated carbon flow in a cyanobacterium. Biochem. Cell Biol. 66:93-99. [Google Scholar]
- 12.Pearce, J., C. K. Leach, and N. G. Carr. 1969. The incomplete tricarboxylic acid cycle in the blue-green alga Anabaena variabilis. Gen. Microbiol. 49:301-313. [DOI] [PubMed] [Google Scholar]
- 13.Singh, A. K., L. M. McIntyre, and L. A. Sherman. 2003. Microarray analysis of the genome-wide response to iron deficiency and iron reconstitution in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 132:1825-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams, J. G. K. 1988. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 167:766-778. [Google Scholar]
- 15.Yang, C., Q. Hua, and K. Shimizu. 2002. Integration of the information from gene expression and metabolic fluxes for the analysis of the regulatory mechanisms in Synechocystis. Appl. Microbiol. Biotechnol. 58:813-822. [DOI] [PubMed] [Google Scholar]
- 16.Yang, C., Q. Hua, and K. Shimizu. 2002. Metabolic flux analysis in Synechocystis using isotope distribution from 13C-labeled glucose. Metab. Eng. 4:202-216. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, S., S. M. Laborde, L. K. Frankel, and T. M. Bricker. 2004. Identification of four novel genes required for efficient photoautotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803 by in vitro transposon mutagenesis. J. Bacteriol. 186:875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]