Abstract
This study shows that the Vibrio cholerae RTX toxin is secreted by a four-component type I secretion system (TISS) encoded by rtxB, rtxD, rtxE, and tolC. ATP-binding site mutations in both RtxB and RtxE blocked secretion, demonstrating that this atypical TISS requires two transport ATPases that may function as a heterodimer.
Vibrio cholerae, the causative agent of the potentially life-threatening disease cholera, expresses several factors to establish and cause disease in the host, including toxin-coregulated pili and the secreted ADP-ribosylating cholera toxin, which causes the profuse diarrhea that is a hallmark of cholera disease. Other secreted “accessory” toxins that also contribute to disease pathogenesis have been identified, including a zinc-metalloprotease, a pore-forming hemolysin/cytotoxin, and the RTX toxin (12). The zinc-metalloprotease and cholera toxin are exported by a type II secretion system encoded by the eps genes (23). Hemolysin export is dependent upon the hlyB gene (2). However, the mechanism of secretion of the RTX toxin has not yet been characterized.
At 484 kDa, the V. cholerae RTX toxin is the second largest single-polypeptide toxin known and causes cell rounding and depolymerization of the actin cytoskeleton in a broad range of cell types. Concurrent with actin stress fiber disassembly, actin monomers are covalently cross-linked into dimers, trimers, and higher multimers, which can be visualized by Western blotting (15, 20, 26).
The RTX toxin is a member of the RTX family of bacterial protein toxins, which includes Escherichia coli hemolysin, Bordetella pertussis adenylate cylcase-hemolysin toxin, and Pasteurella haemolytica and Actinobacillus actinomycetemcomitans leukotoxins. These toxins all share common features: posttranslational maturation, a C-terminal calcium-binding domain of acidic glycine-rich nonapeptide repeats (responsible for the RTX [repeats-in-toxin] nomenclature), and export out of the cell by type I secretion systems (TISS) (31). In this study, we examined a putative TISS apparatus encoded by the operon divergently transcribed from the RTX toxin gene rtxA to assess whether this operon is essential for RTX toxin secretion.
Strains.
The strains used in this study are listed in Table 1.
TABLE 1.
V. cholerae strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| KFV43 | E1 Tor O1 biotype N16961; ΔhapA Smr | 14 |
| BBV1 | KFV43 rtxD-ΔLRER Smr | This study |
| BBV2 | KFV43 rtxE-K522A Smr | This study |
| BBV3 | KFV43 rtxB-K496A Smr | This study |
| BBV16 | KFV43 rtxB::Km: Smr Kmr | This study |
| BBV21 | KFV43 ΔrtxB Smr | This study |
| CW128 | KFV43 ΔrtxA Smr | 15 |
| CW149 | KFV43 ΔrtxE Smr | This study |
| KFV80 | KFV43 ΔrtxACBD Smr | This study |
| KFV131 | ΔtolC ΔhapA Smr | 4; this study |
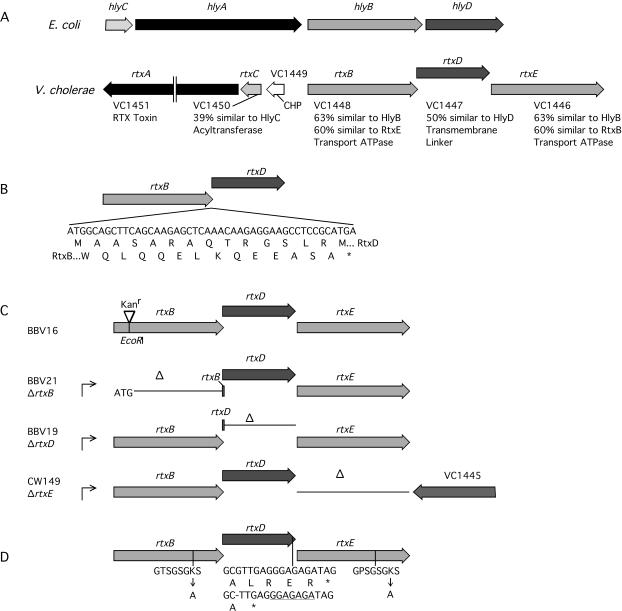
The operon divergently transcribed from the rtxA operon contains three putative TISS genes.
Previously characterized TISS consist of three components: a homodimer of an inner membrane transport ATPase, a trimer of a periplasmic linker protein, and a trimer of an outer membrane porin, exemplified by the Escherichia coli hemolysin TISS, consisting of HlyB, HlyD, and TolC, respectively (3). As shown in Fig. 1A, the hly operon of E. coli is arranged in a single operon, hlyCABD.
FIG. 1.
(A) The gene clusters of V. cholerae rtx and E. coli hly vary significantly. (B) rtxB and rtxD overlap by 46 nt in different reading frames, as shown by amino acid sequences of RtxB and RtxD. (C) Genetic organization of rtxB::Km, ΔrtxB, ΔrtxD, and ΔrtxE strains. In order to accommodate the 46-nt overlap of rtxB and rtxD, the ATG of the rtxD gene was moved to the position of the ATG of rtxB downstream of the promoter for the creation of strain BBV21. For ΔrtxD, the stop codon of rtxD was moved to the position of the stop codon of rtxB to create strain BBV19. For the creation of the ΔrtxE strain, the stop codon of rtxE was moved to the position of the stop codon of rtxD to create strain CW149. (D) Locations of point mutations introduced to create strains RtxBK496A, RtxDΔLRER, and RtxEK522A. The Shine-Dalgarno sequence of rtxE is underlined. The figure was generated in MacVector 7.0. (Oxford Biosystems) with sequences from E. coli (accession numbers M10133 and M12863 [10, 11]) and V. cholerae (accession number NC_002505 [16]). CHP, conserved hypothetical protein; *, stop codon.
By contrast, the rtx locus of the V. cholerae genome is composed of two operons containing a total of six open reading frames (ORFs) (16). The first ORF in the left-oriented operon (as shown in Fig. 1A) is 360 nucleotides (nt) in length and encodes a 120-amino-acid conserved hypothetical protein. The second ORF is the putative RTX toxin-activating acyltransferase gene rtxC followed by the toxin structural gene rtxA, as previously detailed by Lin et al. (20).
The adjacent operon encodes a putative TISS and is divergently transcribed. The first ORF in the right-oriented operon is a putative transport ATPase gene, rtxB, and the second ORF is a putative periplasmic linker protein gene, rtxD, as previously described (20). rtxB and rtxD overlap by 46 nt in different reading frames and encode proteins that are 63 and 50% similar to HlyB and HlyD, respectively (Fig. 1B). Downstream of rtxD is another open reading frame, termed rtxE, which encodes a protein that is 63% similar to HlyB and 60% similar to RtxB. Thus, there are two potential TISS transport ATPases located within this operon. The deduced amino acid sequences for both RtxB and RtxE include conserved Walker box A mononucleotide-binding motifs (30), indicating that both are potentially capable of binding and hydrolyzing ATP.
Genetic manipulation of the putative TISS.
In order to fully characterize these putative TISS components, a genetic approach of insertions, deletions, and point mutations was adopted. All mutants represented in Fig. 1C and D were created directly on the V. cholerae chromosome by double homologous recombination. For construction of recombination plasmids, all flanking DNA was generated by PCR and two flanking regions were fused by crossover PCR or by cloning in standard cloning vectors. The inserts were moved to sacB-counterselectable plasmid pWM91 (21), and inserts were sequenced to detect any mutations arising during DNA amplification and other DNA manipulations. KFV80 (ΔrtxACBD) was created as previously described for KFV82 (13). The resulting plasmids were delivered to V. cholerae by use of SM10λpir, and sucrose counterselection was done as previously detailed (14).
Assay for toxin secretion.
In order to determine whether RTX toxin was secreted, supernatant fluids and cell lysates were collected and assayed for the RTX toxin-associated activities of cell rounding and actin cross-linking. For small-scale preparations, 25-ml cultures were grown to an optical density at 600 nm of ∼0.4 at 30°C. Bacteria were pelleted by centrifugation, and the supernatant fluid was filtered over a 0.2-μm-pore-size Corning Spin-X microfilter. The pellets were then washed in 20 ml of 2 mM CaCl2, resuspended in 6.4 ml of ice-cold distilled water, and sonicated with a Bronson digital Sonifier 450 with a 3-mm tapered microtip at 30% amplitude and 50% duty for 40 s. After sonication, 0.8 ml of 200 mM Tris-10 mM EDTA (pH 8.0) was added, and the lysate was centrifuged first at 5,000 × g to pellet unlysed bacteria and then at 20,000 × g to pellet debris.
Large-scale preparations of concentrated supernatant fluids were prepared from 1.5-liter cultures grown to an optical density at 600 nm of ∼0.4 at 30°C by ultrafiltration across both 100- and 300-kDa-cutoff filters as previously described (15). After collection of the supernatant fluid, the pellets were combined, washed twice in 2 mM CaCl2, resuspended in 17 ml of ice-cold distilled water, and sonicated with a Bronson digital Sonifier 450 with a 13-mm disruptor horn at 20% amplitude for 2 min. After sonication, Roche Biochemicals complete protease inhibitor was added, and the lysate was adjusted to 20 mM Tris-100 mM NaCl-1 mM EDTA (pH 8.0) and centrifuged first at 5,000 × g to pellet unlysed bacteria and then at 20,000 × g to pellet debris. Both supernatant fluid and clarified cell lysate preparations were adjusted to 10% glycerol, and samples were frozen in aliquots and stored at −80°C. The protein concentration was quantified with the bicinchoninic acid assay (Pierce).
Either 0.5 ml of small-scale preparations of supernatant fluid or cell lysate, 7.5 μg of concentrated supernatant fluids, or 2.5 mg of large-scale preparations of cell lysate was added to 2.5 × 105 HEp-2 cells. After 90 min, the cells were either fixed with 4% paraformaldehyde and photographed for rounding or collected for detection of actin cross-linking by Western blot analysis with a 1:2,000 polyclonal antiactin antibody (Sigma, St. Louis, Mo.). Images of rounded cells were captured by phase microscopy with an inverted DMIRE2 microscope (Leica, Wetzlar, Germany) with a C4742-95-12ERG digital charge-coupled device camera (Hamamatsu Photonics, Tokyo, Japan) in conjunction with the Openlab software (Improvision, Coventry, United Kingdom) for image processing.
A polar TISS mutant fails to secrete RTX toxin, which accumulates intracellularly and remains active.
To begin characterization of the TISS of V. cholerae, a polar mutation in rtxB was constructed to inactivate the entire operon and to establish whether this putative TISS is required for the secretion of RTX toxin. The kanamycin resistance cassette Kanπ (Pharmacia) was cloned into the EcoRI site found 407 nt into rtxB (Fig. 1C), and the cassette was transferred onto the V. cholerae chromosome.
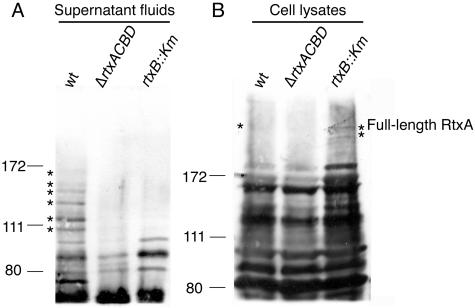
To determine whether rtxB::Km strain BBV16 has a defect in toxin secretion, concentrated supernatant fluids were prepared for Western blotting and RTX toxin was detected by using a rabbit polyclonal antibody raised against an internal 138-kDa fragment of the RTX toxin (15). Consistent with toxin secretion (Fig. 2A, lane 1), RTX toxin breakdown products were detected in supernatant fluids of wild-type strain KFV43. However, RTX toxin breakdown products were not detected in concentrated supernatant fluids prepared from either the rtxB::Km strain BBV16 or the rtxACBD deletion strain KFV80 (Fig. 2A, lanes 2 and 3). In addition, RTX toxin-associated activities were absent in BBV16 supernatant fluids. Neither cell rounding nor actin cross-linking activity was detected when supernatant fluid from BBV16 was added to HEp-2 cells (Fig. 3).
FIG. 2.
RTX toxin is not secreted by TISS mutant BBV16. (A) Thirty-seven micrograms of concentrated supernatant fluids or (B) 119 μg of cell lysate from strains KFV43 (wild type [wt]), KFV80 (ΔrtxACBD), and BBV16 (rtxB::Km) was separated on a sodium dodecyl sulfate-6% polyacrylamide gel, and RTX toxin was detected by Western blotting with polyclonal anti-RtxA antibody. Asterisks mark breakdown products of the RTX toxin in concentrated supernatant fluids and possible full-length toxin in cell lysates. Numbers indicate molecular masses in kilodaltons.
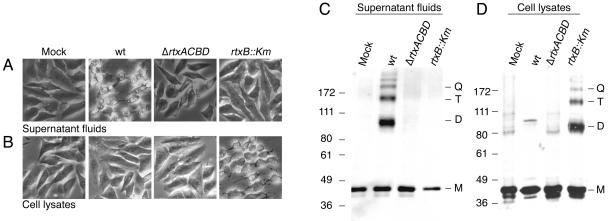
FIG. 3.
Active RTX toxin is detected in cell lysates of a polar TISS mutant, BBV16. HEp-2 cells were treated with equal total protein concentrations of (A and C) concentrated supernatant fluids or (B and D) cell lysate from strains KFV43 (wild type [wt]), KFV80 (ΔrtxACBD), and BBV16 (rtxB::Km) as described in the text. Cells were (A and B) photographed for rounding or (C and D) collected for detection of actin cross-linking by Western blot analysis; 20 mM Tris-1 mM EDTA (pH 8.0) with 10% glycerol was used as a mock treatment. Monomer (M), dimer (D), trimer (T), and quatramer (Q) forms of actin are indicated at the right of the gels. Recovery of actin cross-linking activity in cell lysates of KFV43 varied between experiments. Numbers indicate molecular masses in kilodaltons.
To show that the loss of the RTX toxin in supernatant fluids is due to a defect in secretion, large-scale preparations of clarified cell lysates were assayed for the presence of the RTX toxin. Full-length RTX toxin as well as breakdown products were detected in cell lysates from BBV16 (Fig. 2B, lane 3), demonstrating that full-length toxin remains in the bacterial cell if there is a mutation in rtxB. A faint band could also be seen in samples from KFV43 (Fig. 2B, lane 1), suggesting that some RTX toxin is found intracellularly prior to secretion in the wild-type strain.
Surprisingly, toxin present in cell lysates of BBV16 was biologically active. BBV16 cell lysates rounded HEp-2 cells and cross-linked cellular actin, whereas cells treated with lysates from rtxA null strain KFV80 did not have RTX-associated activity (Fig. 3). As suggested by RTX toxin detection in cell lysates from KFV43, a small amount of dimeric actin was found in KFV43-treated cells, consistent with some RTX activity, although the activity is insufficient to cause visible cell rounding (Fig. 3).
The ability of the rtxB::Km strain to secrete cell-rounding and actin cross-linking activities was not restored in trans by rtxB under arabinose-inducible control, thus indicating that strain BBV16 has a polar mutation in rtxB disrupting other genes within the operon (data not shown).
The TISS of V. cholerae requires two transport ATPases.
As shown in Fig. 1A, the TISS contains two putative transport ATPase genes, in contrast to the case for other RTX toxin TISS, which have only a single ATPase gene. To determine whether one or both of the transport ATPases is necessary for the TISS of the RTX toxin, nonpolar deletions of the rtxB and rtxE genes were created as shown in Fig. 1C. The resulting ΔrtxB strain BBV21 and ΔrtxE strain CW149 lacked RTX toxin activities in supernatant fluids but retained cell-rounding and actin cross-linking activities within the bacterium (Table 2). BBV21 and CW149 could both be complemented by wild-type copies of their corresponding gene presented in trans under control of an arabinose-inducible promoter (data not shown), demonstrating that both genes are essential for RTX toxin secretion.
TABLE 2.
RTX toxin activity in supernatant fluids and cell lysates of TISS deletion strains
| Strain | Genetic characteristics | Actin cross-linking activitya in:
|
|
|---|---|---|---|
| Supernatant fluid | Cell lysate | ||
| Mock | − | − | |
| KFV43 | E1 Tor O1 biotype N16961; ΔhapA | +++ | + |
| CW128 | KFV43 ΔrtxA | − | − |
| KFV80 | KFV43 ΔrtxACBD | − | − |
| BBV21 | KFV43 ΔrtxB | − | +++ |
| CW149 | KFV43 ΔrtxE | − | +++ |
| KFV131 | KFV43 ΔtolC | − | +++ |
Supernatant fluid or clarified cell lysate was added to 2.5 × 105 HEp-2 cells, and cells were collected for detection of actin cross-linking by Western blot analysis as described in the text. Luria broth and 20 mM Tris-1 mM EDTA (pH 8.0) were used as mock controls for supernatant fluid- and cell lysate-treated cells, respectively. −, actin monomer only; +, dimer only; ++, cross-link to quatramer; +++, cross-link to pentamer and higher.
The activity of transport ATPases depends on an intact nucleotide-binding site (NBS) for hydrolysis of ATP (18). To determine whether ATP hydrolysis at both RtxB and RtxE is necessary for secretion of RtxA, point mutations were made in the NBSs of RtxB and RtxE. The Walker box A motif GXXGXGK(S/T) is a conserved motif that is essential for ATP hydrolysis (30). Thus, the RtxB codon K496 of the Walker box A motif GTSGSGKS and the RtxE codon K522 of the Walker box A motif GPSGSGKS were changed to alanine as shown in Fig. 1D. The Stratagene Quick-Change kit was used for site-directed mutagenesis of plasmids which were then used to transfer the mutations directly onto the V. cholerae chromosome.
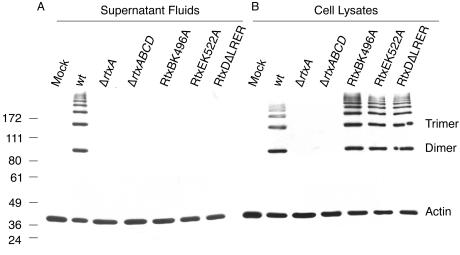
Supernatant fluids from the RtxBK496A strain BBV3 and the RtxEK522A strain BBV2 failed to round HEp-2 cells and cause actin cross-linking, thus demonstrating that both RtxB and RtxE require an intact NBS for RTX toxin secretion (Fig. 4 and data not shown). Both BBV3 and BBV2 retained active RTX toxin intracellularly, as indicated by cell rounding and actin cross-linking caused by cell lysates (Fig. 4 and data not shown). The RtxBK496A and RtxEK522A strains could not be complemented by wild-type copies of the corresponding gene in trans, which suggests that the point mutation may have a dominant negative effect (data not shown). The RtxB and RtxE homologue HlyB acts as a dimer, and therefore this result was not unexpected (19).
FIG. 4.
TISS point mutants accumulate toxin intracellularly. HEp-2 cells were treated with (A) supernatant fluids or (B) clarified lysates prepared from strains KFV43 (wild type [wt]), CW128 (ΔrtxA), KFV80 (ΔrtxACBD), BBV3 (RtxBK496A), BBV2 (RtxEK522A), and BBV1(RtxDΔLRER). Luria broth and 20 mM Tris-1 mM EDTA (pH 8.0) were used as mock controls for supernatant- and cell lysate-treated cells, respectively. Recovery of actin cross-linking activity in cell lysates of KFV43 varied between experiments. Numbers indicate molecular masses in kilodaltons.
A deletion of the last four amino acids of RtxD eliminates secretion of the RTX toxin.
To determine whether rtxD is essential for toxin secretion, we attempted to create a nonpolar deletion. However, mutants arose at a frequency of 1%, compared to the expected 50% recovery of mutants versus wild type after sucrose selection. When strains with the desired ΔrtxD genetic arrangement were isolated, the mutants, such as BBV19 (Fig. 1C), either could not be complemented for secretion defects or failed to synthesize the RTX toxin. These observations suggested that rtxD mutants arose only in the context of other rtx gene cluster mutations.
Due to the inability to delete rtxD, the question of whether rtxD is essential for RTX toxin secretion was approached in an alternative manner. For several membrane protein complexes of V. cholerae, it has been shown that deletion of a single component can be detrimental to growth while a mutant with a mutation affecting function, but not inclusion in the overall complex, grows normally (8, 23). It has been previously proposed that several C-terminal amino acids of HlyD (Leu475, Glu477, and Arg478) interact directly with TolC, creating a bridge across the periplasm (25). Loss of these C-terminal residues resulted in a loss of secretion of E. coli hemolysin. Therefore, the corresponding C-terminal amino acids of RtxD were deleted to create a nonfunctional mutant that would still be incorporated into the TISS. As shown in Fig. 1D, the Stratagene Quick-Change kit was used to delete nucleotide 1388 of rtxD, thus generating a stop codon that eliminates the last four codons of rtxD (residues 464 to 467) and creating a HindIII site for easy screening of V. cholerae recombinants. This small mutation does not affect the ribosome-binding site of downstream rtxE and should produce a protein able to form a complete, albeit inactive, TISS complex. After sucrose selection, the point mutation was recovered at near 50% frequency and the mutants grew similarly to the wild type, consistent with our speculation that this type of mutant would be viable even though a deletion mutant may arise only in the context of other rtx gene mutations.
Deletion of the residues LRER from the C terminus of RtxD eliminated both cell rounding and actin cross-linking activities from culture supernatant fluids (Fig. 4 and data not shown). Cell lysates from the RtxDΔLRER strain BBV1 retained cell rounding and actin cross-linking activities (Fig. 4 and data not shown). The RtxDΔLRER strain BBV1 could not be complemented by rtxD in trans, suggesting that this mutation may have a dominant negative effect. The RtxD homologue HlyD acts as a trimer, and therefore this result was not unexpected (27). As the single-nucleotide deletion is unlikely to create polarity on rtxE, it was concluded that RtxD is essential for toxin secretion and that the last four amino acids of RtxD may function to contact outer membrane porin TolC as is proposed for E. coli HlyD.
The outer membrane porin TolC is required for secretion of the RTX toxin.
Previously, tolC (VC2436) was shown to be required for the detection of cell-rounding activity in V. cholerae-inoculated cells (4). To complete the characterization of the RTX toxin TISS, the tolC mutant M150, obtained from Bina and Mekalanos (4), was made isogenic with other strains used in this study by deletion of the gene for the exported zinc-metalloprotease hapA as previously described for construction of KFV43 (14). Supernatant fluids from ΔtolC strain KFV131 did not contain either cell-rounding or actin cross-linking activity, but cell lysates from KFV131 possessed RTX toxin activities (Table 2). These data confirm work that previously showed that TolC is required for cell rounding by V. cholerae (4) and further demonstrate that the RTX toxin accumulates intracellularly in this mutant.
Model for the type I secretion of the RTX toxin by V. cholerae.
Taken together, our results show that the V. cholerae RTX toxin TISS is distinct in that four components, including an additional transport ATPase, are required for secretion. Similar to the case for other TISS, a periplasmic linker protein, RtxD, is essential for secretion, and the last four amino acids, LRER, likely interact with the outer membrane porin TolC to create a channel through the periplasm and the outer membrane.
Unlike in other TISS, the transport ATPase found in the inner membrane may be a heterodimer rather than a homodimer. We have shown that mutation of the NBSs of both HlyB-like proteins, RtxB and RtxE, results in the failure of V. cholerae to secrete the RTX toxin, thus showing that the TISS of V. cholerae requires two distinct transport ATPases. Indeed, it is possible that RtxB and RtxE could form a heterodimer, since both proteins contain the highly conserved ATP-binding cassette (ABC) family signature sequence or LSGGQ motif that is important in the formation of ABC dimer interfaces (7, 24). It has been proposed that the conserved LSGGQ motif of one subunit of the DNA repair enzyme Rad50 completes the active site in the second subunit, thus placing the dimer in a head-to-tail orientation (17). Individual point mutations in several of the residues of the LSGGQ motif have been shown to be critical for protein export function of HlyB (18).
Although heterodimeric transport ATPases have not been previously described for bacterial TISS, examples of heterodimeric nucleotide-binding domains (NBDs) have been described for other ABC transporters, including the transporter associated with antigen processing (TAP). TAP consists of two polypeptides, TAP1 and TAP2, each of which contains a single NBD and which together form a heterodimeric ABC transporter. Both TAP NBDs are proposed to bind and utilize ATP in an alternating and interdependent manner, so that only one NBD binds and utilizes ATP at any given time (1). Additionally, it has been proposed that the TAP peptide translocation cycle is regulated by the C-terminal tails of TAP1 and TAP2 and conformational cross talk between NBDs (5). Thus, the RtxB-RtxE heterodimer could work in a similar fashion. The two RTX transport ATPases could also act by the two-cylinder engine model, as suggested for the two NBDs of the multidrug-resistant transporters LmrA and MDR1 (22, 28). In this model, the transport protein is proposed to alternate between two configurations, each of which contains a high-affinity substrate-binding site and a low-affinity substrate release site, and the conversion of one configuration to the other is ATP dependent (22, 28).
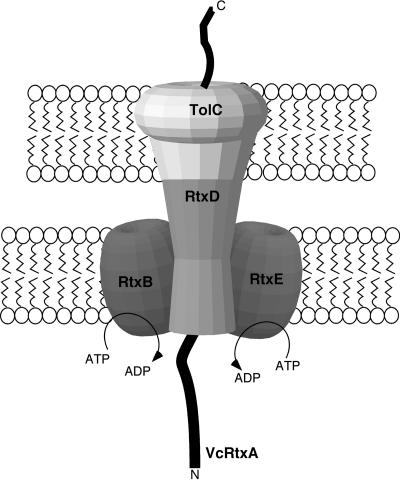
Based on these previous findings and the results of our characterization of the TISS of V. cholerae, we put forth a model for the type I secretion of the RTX toxin by V. cholerae (Fig. 5). First, the RTX toxin comes into contact with RtxB and/or RtxE. RtxD recruits TolC to the TISS complex, which occurs simultaneously with a conformational change in all four components, thus opening the cavity to the extracellular milieu and allowing the RTX toxin to exit the cell. The translocation process would be driven by the two-cylinder engine model proposed for the multidrug-resistant transporters LmrA and MDR1. We speculate that the large size of the V. cholerae RTX toxin may require multiple contact points within the structurally diverse regions of RtxB and RtxE to enhance specificity, thereby necessitating two distinct transport ATPases.
FIG. 5.
Model of RTX toxin TISS.
A four-component TISS is conserved in genomes with toxins related to the RTX toxin.
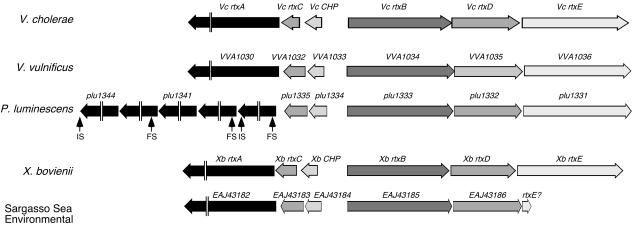
V. cholerae RTX toxin has unique features that distinguish it from other RTX toxins, including the presence of glycine-rich repeats at the N terminus of the protein and an atypical 18-bp RTX repeat at the C terminus (20). Since the discovery of the RTX toxin (20), seven related toxins in four different species have been identified by genome sequence analysis (6, 9, 29). All of these rtxA-like genes encode toxins that are larger than 3,500 amino acids and share the N-terminal and 18-amino-acid RTX repeats. However, these toxins differ in the central portions, suggesting that they may carry different cytotoxic activities. An analysis of the genomic structure upstream of the rtxA-like toxin genes in these genomes reveals that all share synteny with the V. cholerae rtx gene cluster (Fig. 6). All of the genomes have two divergent operons with the toxin gene found as the third gene downstream of an rtxC homologue and a conserved hypothetical gene found only in these RTX gene clusters. The divergent operon always contains three genes that encode homologues of RtxB, RtxD, and RtxE. Thus, it seems as if a four-component TISS is a conserved feature of this subclass of the RTX toxin family.
FIG. 6.
rtx gene clusters in five species share a similar genomic structure that includes the atypical TISS. The figure was generated in MacVector 7.0 (Oxford Biosystems) with sequences from V. cholerae (accession number NC_002505 [16]), Vibrio vulnificus (accession number NC_005140 [6]), Photorhabdus luminescens (accession number NC_005126 [9]), and Environmental Sargasso Sea sequence assembly CH006444 (29). The Xenorhabdus boviennii rtx cluster was downloaded from the publicly available database at www.xenorhabdus.org provided by B. Goldman of The Monsanto Company and collaborators. Frameshift (FS), insertion sequence (IS), conserved hypothetical protein (CHP).
Acknowledgments
We thank J. Mekalanos for providing previously unpublished strain CW149. We thank B. Meehan and M. Feda for development of procedures to isolate active intracellular RTX toxin, S. Antinone and T. Kouo for plasmid construction, and K. Sheahan for microscopy assistance.
This work was supported by a Biomedical Research Support Program award from the Howard Hughes Medical Institute and by Public Health Service grant AI051490 from the National Institute of Allergy and Infectious Diseases (to K.J.F.S).
REFERENCES
- 1.Alberts, P., O. Daumke, E. V. Deverson, J. C. Howard, and M. R. Knittler. 2001. Distinct functional properties of the TAP subunits coordinate the nucleotide-dependent transport cycle. Curr. Biol. 11:242-251. [DOI] [PubMed] [Google Scholar]
- 2.Alm, R. A., and P. A. Manning. 1990. Characterization of the hlyB gene and its role in the production of the El Tor haemolysin of Vibrio cholerae O1. Mol. Microbiol. 4:413-425. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, C., C. Hughes, and V. Koronakis. 2000. Chunnel vision. Export and efflux through bacterial channel-tunnels. EMBO Rep. 1:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bina, J. E., and J. J. Mekalanos. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 69:4681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouabe, H., and M. R. Knittler. 2003. The distinct nucleotide binding states of the transporter associated with antigen processing (TAP) are regulated by the nonhomologous C-terminal tails of TAP1 and TAP2. Eur. J. Biochem. 270:4531-4546. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y. C., K. M. Wu, C. H. Chang, H. C. Tsai, T. L. Liao, Y. M. Liu, H. J. Chen, A. B. Shen, J. C. Li, T. L. Su, C. P. Shao, C. T. Lee, L. I. Hor, S. F. Tsai, and C. Y. Chen. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 13:2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, A. L. 2002. Mechanism of coupling of transport to hydrolysis in bacterial ATP-binding cassette transporters. J. Bacteriol. 184:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, B. M., E. H. Lawson, M. Sandkvist, A. Ali, S. Sozhamannan, and M. K. Waldor. 2000. Convergence of the secretory pathways for cholera toxin and the filamentous phage, CTXphi. Science 288:333-335. [DOI] [PubMed] [Google Scholar]
- 9.Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin, and F. Kunst. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 21:1307-1313. [DOI] [PubMed] [Google Scholar]
- 10.Felmlee, T., S. Pellett, E. Y. Lee, and R. A. Welch. 1985. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J. Bacteriol. 163:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felmlee, T., S. Pellett, and R. A. Welch. 1985. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J. Bacteriol. 163:94-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fullner, K. J. 2003. Toxins of Vibrio cholerae: consensus and controversy, p. 481-502. In G. Hecht (ed.), Microbial pathogenesis and the intestinal epithelial cell. ASM Press, Washington, D.C.
- 13.Fullner, K. J., W. I. Lencer, and J. J. Mekalanos. 2001. Vibrio cholerae-induced cellular responses of polarized T84 intestinal epithelial cells are dependent on production of cholera toxin and the RTX toxin. Infect. Immun. 69:6310-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fullner, K. J., and J. J. Mekalanos. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 67:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fullner, K. J., and J. J. Mekalanos. 2000. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 19:5315-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopfner, K. P., A. Karcher, D. S. Shin, L. Craig, L. M. Arthur, J. P. Carney, and J. A. Tainer. 2000. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101:789-800. [DOI] [PubMed] [Google Scholar]
- 18.Koronakis, E., C. Hughes, I. Milisav, and V. Koronakis. 1995. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol. Microbiol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 19.Koronakis, V., C. Hughes, and E. Koronakis. 1993. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol. Microbiol. 8:1163-1175. [DOI] [PubMed] [Google Scholar]
- 20.Lin, W., K. J. Fullner, R. Clayton, J. A. Sexton, M. B. Rogers, K. E. Calia, S. B. Calderwood, C. Fraser, and J. J. Mekalanos. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 96:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg, M. F., G. Velarde, R. C. Ford, C. Martin, G. Berridge, I. D. Kerr, R. Callaghan, A. Schmidlin, C. Wooding, K. J. Linton, and C. F. Higgins. 2001. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J. 20:5615-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, and V. J. DiRita. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt, L., and R. Tampe. 2002. Structure and mechanism of ABC transporters. Curr. Opin. Struct. Biol. 12:754-760. [DOI] [PubMed] [Google Scholar]
- 25.Schulein, R., I. Gentschev, S. Schlor, R. Gross, and W. Goebel. 1994. Identification and characterization of two functional domains of the hemolysin translocator protein HlyD. Mol. Gen. Genet. 245:203-211. [DOI] [PubMed] [Google Scholar]
- 26.Sheahan, K. L., C. L. Cordero, and K. J. Satchell. 2004. Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc. Natl. Acad. Sci. USA 101:9798-9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Veen, H. W., A. Margolles, M. Muller, C. F. Higgins, and W. N. Konings. 2000. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 19:2503-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venter, J. C., K. Remington, J. F. Heidelberg, A. L. Halpern, D. Rusch, J. A. Eisen, D. Wu, I. Paulsen, K. E. Nelson, W. Nelson, D. E. Fouts, S. Levy, A. H. Knap, L. M. W., K. Nealson, O. White, J. Peterson, J. Hoffman, R. Parsons, H. Baden-Tillson, C. Pfannkoch, Y. H. Rogers, and H. O. Smith. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Nature 304:58-60. [DOI] [PubMed] [Google Scholar]
- 30.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases, and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch, R. A. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85-111. [DOI] [PubMed] [Google Scholar]