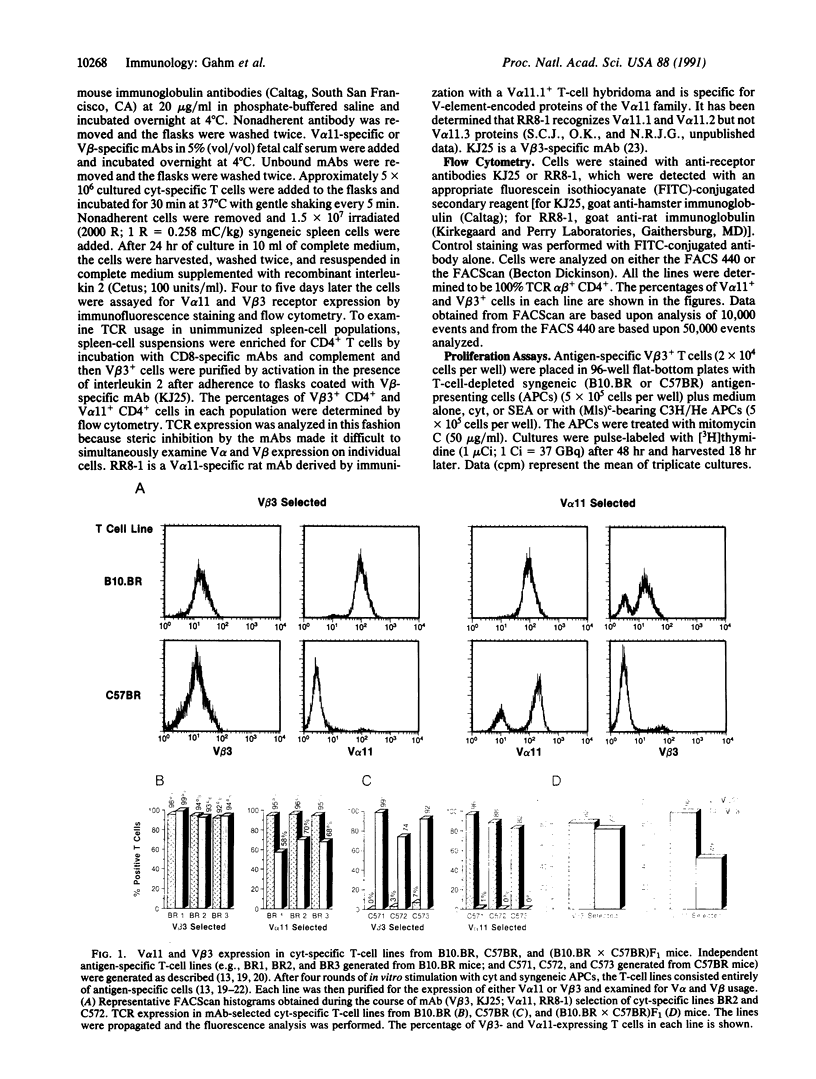
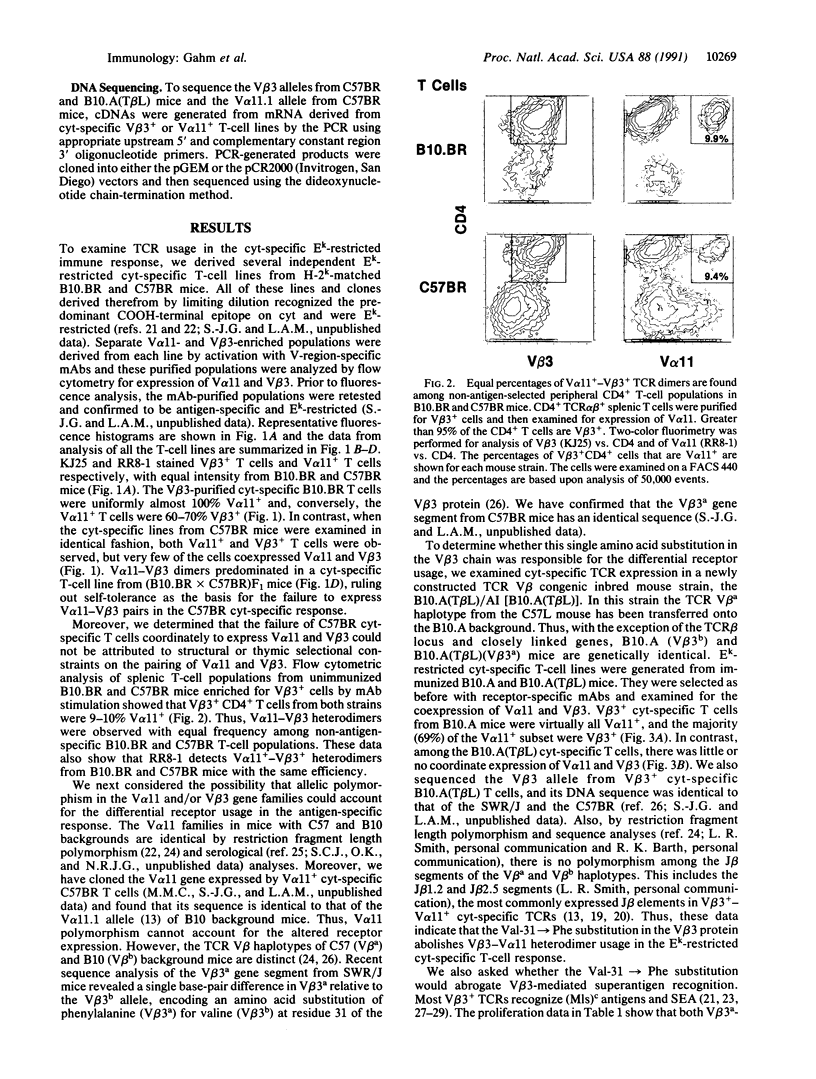
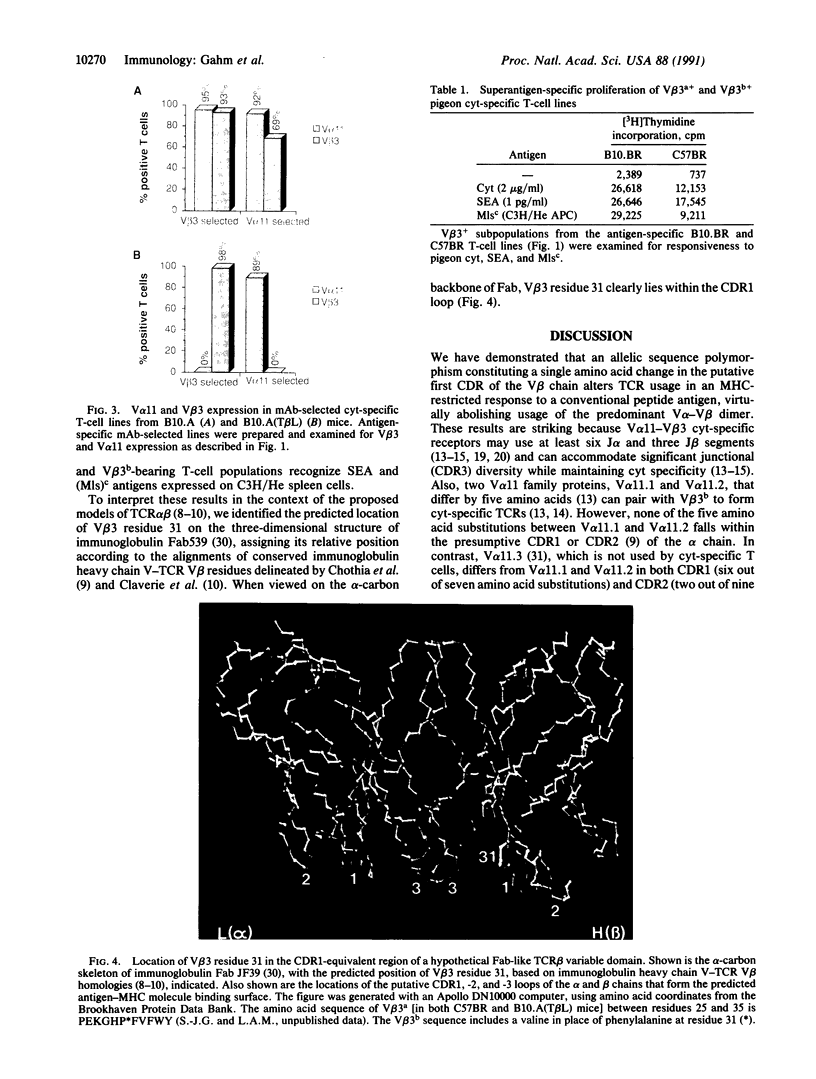
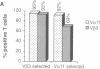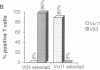Abstract
Amino acid residues that are critical in maintaining the framework structure of immunoglobulin heavy- and light-chain variable (V) regions are strongly conserved in the V alpha and V beta proteins of the alpha beta T-cell antigen receptor (TCR alpha beta). Consequently, it has been proposed that TCR alpha beta has a conformation similar to that of an immunoglobulin Fab fragment and that the regions of the TCR homologous to the three immunoglobulin complementarity-determining regions (CDRs 1, 2, and 3) bind to the peptide antigen-major histocompatibility complex (MHC) molecule ligand. A single amino acid substitution in the predicted CDR1 of the V beta 3 protein of certain mouse strains dramatically altered TCR alpha beta usage in an antigen-specific MHC-restricted immune response but did not abrogate V beta 3 specificity for the superantigens minor lymphocyte stimulatory locus (Mls)c and staphylococcal enterotoxin A (SEA). The results confirm the importance of the V beta CDR1 in antigen-MHC molecule recognition, supporting the Fab-like structural model of TCR alpha beta, and provide further evidence that conventional antigen-MHC recognition and superantigen recognition are mediated by distinct regions of the TCR beta chain. They also suggest that allelic polymorphism may be a significant source of diversity in the TCR repertoire.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Vacchio M. S., Fox B., Hodes R. J. Preferential expression of the T-cell receptor V beta 3 gene by Mlsc reactive T cells. Nature. 1988 Oct 27;335(6193):827–830. doi: 10.1038/335827a0. [DOI] [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Pattern P., Chien Y., Yokota T., Eshhar Z., Giedlin M., Gascoigne N. R., Goodnow C., Wolf R., Arai K. Variability and repertoire size of T-cell receptor V alpha gene segments. Nature. 1985 Oct 3;317(6036):430–434. doi: 10.1038/317430a0. [DOI] [PubMed] [Google Scholar]
- Berg L. J., Frank G. D., Davis M. M. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell. 1990 Mar 23;60(6):1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Danska J. S., Livingstone A. M., Paragas V., Ishihara T., Fathman C. G. The presumptive CDR3 regions of both T cell receptor alpha and beta chains determine T cell specificity for myoglobin peptides. J Exp Med. 1990 Jul 1;172(1):27–33. doi: 10.1084/jem.172.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Engel I., Hedrick S. M. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988 Aug 12;54(4):473–484. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Matis L. A., McElligott D. L., Bookman M., Hedrick S. M. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986 May 15;321(6067):219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Fry A. M., Cotterman M. M., Matis L. A. The influence of self-MHC and non-MHC antigens on the selection of an antigen-specific T cell receptor repertoire. J Immunol. 1989 Oct 15;143(8):2723–2729. [PubMed] [Google Scholar]
- Fry A. M., Jones L. A., Kruisbeek A. M., Matis L. A. Thymic requirement for clonal deletion during T cell development. Science. 1989 Nov 24;246(4933):1044–1046. doi: 10.1126/science.2511630. [DOI] [PubMed] [Google Scholar]
- Fry A. M., Matis L. A. Self-tolerance alters T-cell receptor expression in an antigen-specific MHC restricted immune response. Nature. 1988 Oct 27;335(6193):830–832. doi: 10.1038/335830a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Engel I., McElligott D. L., Fink P. J., Hsu M. L., Hansburg D., Matis L. A. Selection of amino acid sequences in the beta chain of the T cell antigen receptor. Science. 1988 Mar 25;239(4847):1541–1544. doi: 10.1126/science.2832942. [DOI] [PubMed] [Google Scholar]
- Jameson S. C., Kaye J., Gascoigne N. R. A T cell receptor V alpha region selectively expressed in CD4+ cells. J Immunol. 1990 Sep 1;145(5):1324–1331. [PubMed] [Google Scholar]
- Klotz J. L., Barth R. K., Kiser G. L., Hood L. E., Kronenberg M. Restriction fragment length polymorphisms of the mouse T-cell receptor gene families. Immunogenetics. 1989;29(3):191–201. doi: 10.1007/BF00373645. [DOI] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Letourneur F., Rebaï N., Dunn D. E., Fitch F. W., Hood L., Malissen B. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988 Oct 7;55(1):49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Sorger S. B., McElligott D. L., Fink P. J., Hedrick S. M. The molecular basis of alloreactivity in antigen-specific, major histocompatibility complex-restricted T cell clones. Cell. 1987 Oct 9;51(1):59–69. doi: 10.1016/0092-8674(87)90010-9. [DOI] [PubMed] [Google Scholar]
- Matis L. A. The molecular basis of T-cell specificity. Annu Rev Immunol. 1990;8:65–82. doi: 10.1146/annurev.iy.08.040190.000433. [DOI] [PubMed] [Google Scholar]
- Nalefski E. A., Wong J. G., Rao A. Amino acid substitutions in the first complementarity-determining region of a murine T-cell receptor alpha chain affect antigen-major histocompatibility complex recognition. J Biol Chem. 1990 May 25;265(15):8842–8846. [PubMed] [Google Scholar]
- Nikolić-Zugić J., Bevan M. J. Role of self-peptides in positively selecting the T-cell repertoire. Nature. 1990 Mar 1;344(6261):65–67. doi: 10.1038/344065a0. [DOI] [PubMed] [Google Scholar]
- Novotný J., Tonegawa S., Saito H., Kranz D. M., Eisen H. N. Secondary, tertiary, and quaternary structure of T-cell-specific immunoglobulin-like polypeptide chains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):742–746. doi: 10.1073/pnas.83.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N. Allelic variations of human TCR V gene products. Immunol Today. 1990 Oct;11(10):368–373. doi: 10.1016/0167-5699(90)90143-w. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Robinson M. A. Allelic sequence variations in the hypervariable region of a T-cell receptor beta chain: correlation with restriction fragment length polymorphism in human families and populations. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9422–9426. doi: 10.1073/pnas.86.23.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seboun E., Robinson M. A., Doolittle T. H., Ciulla T. A., Kindt T. J., Hauser S. L. A susceptibility locus for multiple sclerosis is linked to the T cell receptor beta chain complex. Cell. 1989 Jun 30;57(7):1095–1100. doi: 10.1016/0092-8674(89)90046-9. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Pullen J. K., Pease L. R., Russell J. H., Loh D. Y. Positive selection of transgenic receptor-bearing thymocytes by Kb antigen is altered by Kb mutations that involve peptide binding. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6186–6190. doi: 10.1073/pnas.87.16.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff S., Silverton E. W., Padlan E. A., Cohen G. H., Smith-Gill S. J., Finzel B. C., Davies D. R. Three-dimensional structure of an antibody-antigen complex. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8075–8079. doi: 10.1073/pnas.84.22.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. R., Plaza A., Singer P. A., Theofilopoulos A. N. Coding sequence polymorphisms among V beta T cell receptor genes. J Immunol. 1990 Apr 15;144(8):3234–3237. [PubMed] [Google Scholar]
- Sorger S. B., Hedrick S. M., Fink P. J., Bookman M. A., Matis L. A. Generation of diversity in T cell receptor repertoire specific for pigeon cytochrome c. J Exp Med. 1987 Feb 1;165(2):279–301. doi: 10.1084/jem.165.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S. W., Bhat T. N., Navia M. A., Cohen G. H., Rao D. N., Rudikoff S., Davies D. R. The galactan-binding immunoglobulin Fab J539: an X-ray diffraction study at 2.6-A resolution. Proteins. 1986 Sep;1(1):74–80. doi: 10.1002/prot.340010112. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Winoto A., Urban J. L., Lan N. C., Goverman J., Hood L., Hansburg D. Predominant use of a V alpha gene segment in mouse T-cell receptors for cytochrome c. Nature. 1986 Dec 18;324(6098):679–682. doi: 10.1038/324679a0. [DOI] [PubMed] [Google Scholar]