Abstract
Mycoplasma pneumoniae is a pathogenic bacterium that is highly adapted to life on mucosal surfaces. This adaptation is reflected by the very compact genome and the small number of regulatory proteins. However, M. pneumoniae possesses the HPr kinase/phosphorylase (HPrK/P), the key regulator of carbon metabolism in the Firmicutes. In contrast to the enzymes of other bacteria, the HPrK/P of M. pneumoniae is already active at very low ATP concentrations, suggesting a different mode of regulation. In this work, we studied the ability of M. pneumoniae to utilize different carbohydrates and their effects on the activity of the different phosphotransferase system (PTS) components. Glucose served as the best carbon source, with a generation time of about 30 h. Fructose and glycerol were also used but at lower rates and with lower yields. In contrast, M. pneumoniae is unable to use mannitol even though the bacterium is apparently equipped with all the genes required for mannitol catabolism. This observation is probably a reflection of the continuing and ongoing reduction of the M. pneumoniae genome. The general enzymatic and regulatory components of the PTS, i.e., enzyme I, HPr, and HPrK/P, were present under all growth conditions tested in this study. However, HPrK/P activity is strongly increased if the medium contains glycerol. Thus, the control of HPrK/P in vivo differs strongly between M. pneumoniae and the other Firmicutes. This difference may relate to the specific conditions on lipid-rich cell surfaces.
Mycoplasma pneumoniae is a pathogen that lives on mucosal surfaces and causes diseases such as mild pneumonia and tracheobronchitis and also causes complications affecting the central nervous system, the skin, and mucosal surfaces (19, 24). The parasitic lifestyle of this bacterium is reflected by its small and highly compacted genome, its slow growth, and its reduced metabolic abilities. With only nine regulatory proteins, M. pneumoniae belongs to the organisms with the lowest number of regulators studied so far, suggesting a good adaptation to constant environments (5, 15, 37). In addition to regulatory proteins that are thought to act at the DNA level, we identified the key regulatory protein of carbon metabolism in gram-positive bacteria, HPr kinase/phosphorylase (HPrK/P), in M. pneumoniae (33, 42). Moreover, HPrK/P activity was detected in other Mollicutes such as Mycoplasma capricolum, Mycoplasma genitalium, and Acholeplasma laidlawii (17, 53).
HPrK/P controls the activity of the HPr protein of the bacterial phosphoenolpyruvate:sugar phosphotransferase system by phosphorylation at a regulatory site, Ser-46. In the gram-positive model organism Bacillus subtilis, this phosphorylation interferes with the phosphoenolpyruvate (PEP)- and enzyme I-dependent phosphorylation on His-15 of HPr, which is important for the phosphorylation of transported sugars (12, 38). In addition to its role in sugar transport, HPr is the major signal transducer in carbon metabolism in low-GC gram-positive bacteria (now referred to as Firmicutes [27]). In the absence of glucose, HPr is present, either nonphosphorylated or phosphorylated, at His-15. If glucose becomes available, a significant part of the cellular HPr pool is phosphorylated on Ser-46, and even some doubly phosphorylated HPr(His∼P)(Ser-P) was detected (25, 32; for a review, see reference 44). HPr(His∼P) is implicated in sugar transport and is moreover required for the activation of a class of transcription factors and of glycerol kinase in several bacteria (6, 43). In contrast, HPr(Ser-P) is not able to phosphorylate and thereby activate those enzymes and regulators but rather acts as a cofactor for the transcription regulator CcpA. The CcpA-HPr(Ser-P) complex binds to target sequences in the promoter regions of catabolic and certain anabolic operons to repress or activate their transcription (7, 26, 49). Thus, HPrK/P controls the phosphorylation state of HPr and thereby the regulatory activity of this protein. It is therefore crucial to study the activity of HPrK/P itself. In B. subtilis, the enzyme is active as a kinase under conditions of good nutrient supply which are indicated by high ATP and fructose-1,6-bisphosphate concentrations. In contrast, phosphorylase activity is triggered by high concentrations of inorganic phosphate, which indicate the absence of good carbon sources (12, 14, 21, 29, 38).
As stated above, several metabolic and regulatory features of the Mollicutes are in good agreement with their adaptation to their nutrient-rich mucosal habitats. This was also observed when we investigated the properties of M. pneumoniae HPrK/P. While the B. subtilis enzyme exhibits a phosphorylase activity by default, the M. pneumoniae protein is already active as a kinase at very low ATP concentrations and is barely regulated by fructose-1,6-bisphosphate (14, 42). These differences were attributed to the different affinities of B. subtilis and M. pneumoniae HPrK/P for ATP. While the former has a Kd value of about 100 to 300 μM, the latter has a Kd value of about 5 μM, indicating an at least 20-fold-increased affinity (21, 30, 36). The high affinity of M. pneumoniae HPrK/P for ATP results in a kinase activity as the apparent default state of this protein in vitro (42). Since the M. pneumoniae HPrK/P is the only known enzyme of its class with the inversed default activity, we wondered whether the aberrant regulation was reflected by the structure of the protein. The determination of the crystal structure revealed that the enzyme is composed of six identical subunits that are arranged as bilayered trimers. Each subunit is made up of a C-terminal domain that contains the ATP-binding P-loop motif and an N-terminal domain of so-far-unknown function (1, 41). The structure of the M. pneumoniae HPrK/P is very similar to those of HPrK/Ps from Lactobacillus casei and Staphylococcus xylosus, suggesting that subtle differences must be responsible for the differential activity patterns (9, 28, 33). Although HPrK/P is one of the very few regulatory proteins of M. pneumoniae, it is not essential as revealed by an analysis of randomly generated transposon mutants (18).
According to the genome sequence of M. pneumoniae and the biochemical evidence, these bacteria are able to utilize sugars as carbon sources by glycolysis (5, 15, 31). As observed for other Firmicutes, the concentration of fructose-1,6-bisphosphate is increased in glycolytically active cells of M. gallisepticum (8, 29). Moreover, enzymes of carbon metabolism seem to be important for other metabolic pathways as well. This is illustrated by the finding that the glycolytic kinases of several Mollicutes are moonlighting in nucleoside metabolism (35).
So far, very few studies concerning the regulation of carbon metabolism in Mollicutes have been reported. However, this problem is important not only for a better understanding of the biology of these interesting bacteria but also to improve our knowledge of virulence mechanisms of the mycoplasmas. Recently, the implication of proteins of the phosphotransferase system in M. pneumoniae pathogenicity was demonstrated (54). While the regulatory output of the phosphotransferase system (PTS) is well understood in Escherichia coli and in the Firmicutes related to B. subtilis, nothing is known about regulatory pathways in M. pneumoniae. Among the proteins interacting with the different forms of HPr in B. subtilis, only the glycerol kinase is present in M. pneumoniae, whereas transcription regulators potentially phosphorylated by HPr(His∼P) are not found. Similarly, the transcription factor CcpA that interacts with HPr(Ser-P) has no counterpart in the Mollicutes (16). Thus, the mechanisms of carbon regulation, if present, must differ drastically from those studied in B. subtilis and its close relatives.
In this work, we studied the utilization of different carbohydrates by M. pneumoniae and found that glucose was the carbon source allowing the fastest growth. To address the relevance of the results obtained with M. pneumoniae HPrK/P in vitro, we analyzed the HPr phosphorylation state in vivo. Surprisingly, the enzyme did not exhibit constitutive kinase activity but required the presence of glycerol for HPr phosphorylation. The proteins acting on HPr, i.e., enzyme I of the PTS and HPrK/P, were constitutively present in cell extracts of M. pneumoniae. Thus, a novel mode of control seems to modulate the M. pneumoniae HPrK/P activity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli DH5α and BL21(DE3)/pLysS (40) were used for overexpression of recombinant proteins. The cells were grown in Luria Bertani medium containing ampicillin (100 μg ml−1).
The M. pneumoniae strain used in this study was M. pneumoniae M129 (ATCC 29342) in the 31st broth passage. M. pneumoniae was grown at 37°C in 150-cm2 tissue culture flasks containing 100 ml of modified Hayflick medium with the following composition. The basic medium consisted of 18.4 g of PPLO broth (Difco), 29.8 g of HEPES, 5 ml of 0.5% phenol red, and 35 ml of 2 N NaOH per liter. Horse serum (Gibco) and penicillin were included to a final concentration of 20% and 1,000 U/ml, respectively. Carbon sources were added as indicated. For each sugar, several individual culture flasks were inoculated with a biomass of 5 mg (wet weight), and one flask for each condition was harvested at the indicated time points and used to determine the fresh weight. For wet weight measurements, cells were washed twice with cold phosphate-buffered saline (PBS), scraped into 1.5 ml of PBS, and collected by centrifugation (5 min, 15,000 × g, 4°C) in a 2.0-ml microcentrifuge tube. Supernatants were discarded, and the pellets were recentrifuged to get rid of all excess liquid. The wet weight of the obtained cell pellet was determined by subtraction of the weight of the tube containing the pellet from that of the empty tube prior to cell collection.
Protein purification.
His6-tagged HPr (M. pneumoniae), His6-tagged enzyme I (B. subtilis), and Strep-tagged HPrK/P (M. pneumoniae) were overexpressed by using the expression plasmids pGP217 (42), pAG3 (11), and pGP611 (30), respectively. Expression was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) (final concentration, 1 mM) to exponentially growing cultures (optical density at 600 nm of 0.8). Cells were lysed by using a French press. After lysis, the crude extracts were centrifuged at 15,000 × g for 30 min. For purification of His-tagged proteins, the resulting supernatants were passed over an Ni2+ NTA superflow column (5-ml bed volume; QIAGEN) followed by elution with an imidazole gradient (from 0 to 500 mM imidazole in a buffer containing 10 mM Tris-HCl [pH 7.5], 600 mM NaCl, and 10 mM β-mercaptoethanol). For HPrK/P carrying an N-terminal Strep tag, the crude extract was passed over a Streptactin column (IBA, Göttingen, Germany). The recombinant protein was eluted with desthiobiotin (final concentration, 2.5 mM; Sigma). For the recombinant HPr protein, the overproduced protein was purified from the pellet fraction of the lysate by urea extraction and renatured as described previously (42).
After elution, the fractions were tested for the desired protein by using sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (PAGE). The relevant fractions were combined and dialysed overnight. Protein concentration was determined according to the method of Bradford (3) with a Bio-Rad dye-binding assay for which bovine serum albumin served as the standard.
Western blot analysis.
Purified His6-tagged HPr was used to generate rabbit polyclonal antibodies (SeqLab, Göttingen, Germany). For Western blot analysis, M. pneumoniae crude cell extracts were separated on sodium dodecyl sulfate-12.5% polyacrylamide gels. After electrophoresis, the proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad) by electroblotting. HPr was detected with polyclonal antibodies raised against HPr of M. pneumoniae. Antibodies were visualized by using anti-rabbit immunoglobulin G-alkaline phosphatase secondary antibodies (Promega) and the CDP* detection system (Roche Diagnostics).
In vivo HPr phosphorylation was assayed by Western blot analysis as follows. Bacteria were cultivated for 96 h. Cells were washed twice with cold PBS and harvested as described above for wet weight measurements. Subsequently, cells were resuspended in 500 μl of a solution containing 10 mM Tris-HCl (pH 7.5) and 600 mM NaCl and disrupted with sonication (3 × 10 s, 4°C, 50 W). Cell debris was pelleted by centrifugation (10 min, 15,000 × g, 4°C), and the obtained supernatant served as crude extract. Proteins were separated on nondenaturing 10% polyacrylamide gels. On these gels, phosphorylated HPr migrates faster than the nonphosphorylated protein. HPr(His∼P) was dephosphorylated by incubation of the crude extract for 10 min at 70°C. After electrophoresis, the proteins were blotted onto a polyvinylidene difluoride membrane. The different forms of HPr were detected by using antibodies directed against M. pneumoniae HPr.
In vitro activity assays of HPrK/P and enzyme I.
HPrK/P activity assays were carried out with 5 μg of freshly prepared cell extracts in 20 μl of assay buffer (25 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol) with purified His6-tagged HPr (final concentration, 20 μM). The concentration of ATP was 0.5 mM. The assay mixtures were incubated at 37°C for 120 min followed by thermal inactivation of the enzyme (10 min at 95°C).
For detection of enzyme I contained in mycoplasmal cell extracts, His6-tagged HPr (20 μM), PEP (0.5 mM), and 1 μg of cell extract were incubated in 20 μl of assay buffer for 30 min at 37°C. When indicated, the assay mixture was subjected to an additional incubation step at 70°C for 10 min to hydrolyze HPr(His∼P). The assay mixtures were analyzed on 10% native polyacrylamide gels as described previously (14). Proteins were visualized by Coomassie staining.
Northern blot analysis.
Preparation of total RNA of M. pneumoniae was carried out as described previously by Weiner et al. (51). Northern blot analysis was performed according to the protocol of Wetzstein et al. (52). The ptsH digoxigenin (DIG) RNA probe was obtained by in vitro transcription with T7 RNA polymerase (Roche Diagnostics) using a PCR-generated fragment obtained with the primer pair SH1 (5′-AGAAGATTCAAGTAGTCGTTAAAG)-SH2 (5′-CTAATACGACTCACTATAGGGAGATGCTTTAATAGCATTTAGTGCCTC). The reverse primer contained a T7 RNA polymerase recognition sequence (underlined in SH2). In vitro RNA labeling, hybridization, and signal detection were carried out according to the manufacturer's instructions (DIG RNA labeling kit and detection chemicals; Roche Diagnostics).
RESULTS
Utilization of different carbon sources by M. pneumoniae.
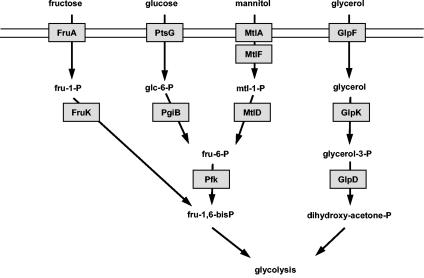
The inspection of the genome sequence of M. pneumoniae suggested that the bacteria are able to transport and utilize glucose, fructose, mannitol, and glycerol as sources of carbon and energy. The genes encoding the general components of the PTS, ptsI and ptsH, are present, as are the genes for permeases specific for glucose, fructose, and mannitol. The glucose and fructose permeases are three-domain enzymes with the domain order CBA and ABC, respectively. In contrast, the putative mannitol permease is composed of a CB and a separate A protein encoded by mtlA and mtlF, respectively. The GlpF protein is a glycerol facilitator. Moreover, M. pneumoniae possesses the enzymes to convert the primary phosphorylation products to intermediates of glycolysis (5, 15) (Fig. 1). Since the growth properties of M. pneumoniae in the presence of different carbon sources have not been studied previously, we decided to analyze whether M. pneumoniae can use these carbon sources.
FIG. 1.
Systems for the uptake and catabolism of carbohydrates in M. pneumoniae as predicted from the genome sequence (15). FruA (MPN078) is the EIIABC component specific for fructose, and PtsG (MPN207) is the EIICBA component for the uptake of glucose. MtlA (MPN651) and MtlF (MPN653) are the putative EIIBC and EIIA proteins for the transport of mannitol, whereas GlpF (MPN043) is the glycerol uptake facilitator. The glucose-6-phosphate isomerase PgiB (MPN250) and phosphofructokinase Pfk (MPN302) transform glucose-6-phosphate to fructose-1,6-bisphosphate. The 1-phosphofructokinase FruK (MPN079) and the mannitol-1-phosphate dehydrogenase MtlD (MPN652) are necessary for the conversion of fructose and mannitol to intermediates of glycolysis. The glycerol kinase GlpK (MPN050) and the glycerol-3-phosphate dehydrogenase GlpD (MPN051) metabolize glycerol to dihydroxyacetone phosphate.
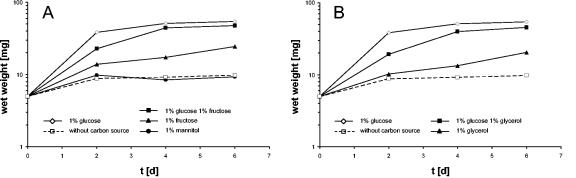
Precultures were obtained with modified Hayflick medium supplemented with glucose. Cells isolated from these cultures were used to inoculate fresh medium containing the different carbon sources. A culture without added sugar served as a control. As shown in Fig. 2, only slight initial growth resulting from residual glucose was observed in the control culture, and growth ceased after 2 days of incubation. In contrast, cultures incubated in the presence of glucose immediately started to grow, and growth continued until a biomass of about 50 mg (wet weight) per 100 ml of medium was reached on a surface of 150 cm2. The minimal generation time of M. pneumoniae in glucose-supplemented medium was determined to be about 30 h. With fructose, the bacteria grew as well; however, the yield was significantly lower (about 15 mg [wet weight] per 100 ml of medium on a surface of 150 cm2). In the presence of both glucose and fructose, the growth characteristics were similar to those observed with glucose. With mannitol, no growth was observed, suggesting that M. pneumoniae is not able to use this carbohydrate, at least under the conditions employed in this study (Fig. 2A). Glycerol was metabolized by M. pneumoniae, although it seems to be a poor substrate as observed for fructose. Again, the addition of glucose and glycerol resulted in higher biomass yields. Thus, among the candidate substrates, glucose was clearly the most efficient, and fructose and glycerol were utilized, whereas mannitol did not serve as a carbon source.
FIG. 2.
Growth of M. pneumoniae in modified Hayflick medium containing different carbon sources. One hundred milliliters of medium was inoculated with 5 mg of cells and incubated for 2, 4, or 6 days at 37°C in 150-cm2 cell culture flasks. Glucose, fructose, mannitol (A), and glycerol (B) were added to a final concentration of 1% (wt/vol). Attached cells were collected by scraping, and growth was monitored by determination of the wet weight of the cell pellets. Medium without any additional carbon source served as a negative control. All measurements were done at least twice.
Detection of HPr in M. pneumoniae cells.
The growth assays demonstrated that M. pneumoniae is able to use sugars that are transported by the PTS. Moreover, the functionality and important role of PTS components for glucose and fructose utilization were already demonstrated in a global mutagenesis study (18). In gram-positive bacteria, the HPr protein links sugar transport and different regulatory pathways and is thus the key protein of the PTS. To study the regulation of HPr synthesis and its modifications in M. pneumoniae, we raised rabbit polyclonal antibodies against the His6-tagged M. pneumoniae HPr.
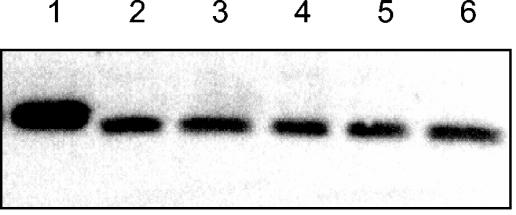
The amounts of HPr present in the cells after growth with different carbon sources were determined by Western blot analysis using crude cell extracts. The antibody reacted with a single protein band that corresponded to the size of the native HPr protein (9.5 kDa [Fig. 3 ]). The His6-tagged HPr used as a control was larger and migrated somewhat slower. As judged from these experiments, HPr is constitutively synthesized in M. pneumoniae. The cellular amount did not depend on the presence or absence of PTS substrates such as glucose or fructose. This finding suggests that HPr may be required not only for sugar transport but also for regulatory purposes.
FIG. 3.
Western blot analysis of HPr synthesis in M. pneumoniae. Antibodies raised against M. pneumoniae HPr were used to determine the total amounts of HPr in cells grown in the presence of glucose (lane 2), glucose and fructose (lane 3), fructose (lane 4), glucose and glycerol (lane 5), or glycerol (lane 6). The concentrations of the carbon sources were 1% (wt/vol). A total of 200 ng of recombinant His6-tagged HPr served as a control (lane 1). His6-tagged HPr is somewhat retarded due to its slightly higher molecular weight.
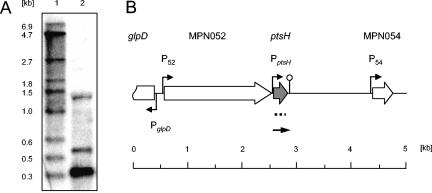
In contrast to most other bacteria, the ptsH and ptsI genes encoding HPr and enzyme I, respectively, are not clustered in M. pneumoniae. The transcription of ptsH was studied by Northern blot analysis (Fig. 4). The major transcript corresponded to a 0.32-kb mRNA. In addition, two larger minor signals were detected. The 0.32-kb mRNA has the size expected for the monocistronic ptsH gene, for which promoter and terminator sequences were predicted in silico (15, 50). The minor signals may result from cross-hybridization with 16S rRNA and a very abundant 550-bp RNA. The nature of this RNA is so far unknown.
FIG. 4.
Transcriptional organization of the ptsH locus (MPN053) of M. pneumoniae. (A) Northern blot. Ten micrograms of total RNA prepared from cells grown in modified Hayflick medium containing 1% (wt/vol) glucose was separated by using a 1.5% agarose gel containing 6% formaldehyde. After electrophoresis, the RNA was transferred onto a nylon membrane, and the ptsH mRNA was detected with a DIG-labeled riboprobe specific for ptsH (lane 2). DIG-labeled RNA molecular weight marker I (Roche Diagnostics) served as a standard (lane 1). (B) Genomic region surrounding the ptsH gene in M. pneumoniae. Indicated promoters are experimentally demonstrated (P52) or predicted in silico (50). The position of the riboprobe is indicated by the dotted line. The detected ptsH mRNA is schematically shown as a solid arrow.
Taken together, our results demonstrate that ptsH is a constitutively expressed monocistronic transcription unit. This finding is in good agreement with the previous observation that ptsH is one of the highly expressed M. pneumoniae genes (51).
In vivo phosphorylation pattern of M. pneumoniae HPr.
M. pneumoniae HPr is the target of two distinct phosphorylation events. However, the in vivo activity profile of the two phosphorylating enzymes, HPrK/P and enzyme I, has so far not been investigated in any Mollicute.
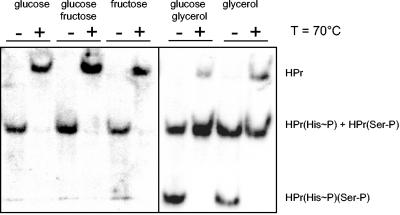
To study the in vivo phosphorylation pattern of HPr, we made use of the different migration behaviors of HPr carrying no phosphates or one or two phosphates in native acrylamide gels. Protein extracts from M. pneumoniae cells grown in modified Hayflick medium with different carbon sources were prepared as described in Materials and Methods and subjected to native gel electrophoresis. The different forms of HPr were detected by Western blot analysis, and the site of phosphorylation was determined by incubation of an aliquot of the cell extract at 70°C prior to electrophoresis. While phosphorylation on His-15 is heat labile, phosphorylation at Ser-46 is not (Fig. 5). In the presence of glucose, essentially all HPr was phosphorylated at His-15 as judged from the complete loss of phosphorylation upon heat exposure. Similar results were obtained with fructose and a mixture of glucose and fructose. Thus, HPr is exclusively phosphorylated by enzyme I in the presence of glucose or fructose, whereas HPrK/P has no kinase activity under these conditions. If glycerol was present as a carbon source, two phosphorylated forms of HPr, which correspond to singly and doubly phosphorylated forms of the protein, were observed. As expected, the doubly phosphorylated form disappeared completely after incubation at 70°C due to the heat lability of the His phosphate. Only a small fraction of total HPr was unphosphorylated after heat exposure. These observations indicate that HPr was present to about one third as HPr(His∼P), HPr(Ser-P), and HPr(His∼P)(Ser-P), respectively. The addition of glucose to glycerol-growing cells did not significantly affect the in vivo phosphorylation pattern of HPr (Fig. 5). Thus, we may conclude that HPrK/P kinase activity is triggered in the presence of glycerol in vivo and that it is not affected by glucose.
FIG. 5.
Western blot for the detection of the different phosphorylation forms of HPr. Crude extracts of M. pneumoniae grown in the presence of different carbon sources (1% final concentration) were separated by using native gels. For each condition tested, a parallel aliquot was incubated for 10 min at 70°C to hydrolyze the heat-labile HPr(His∼P). The different HPr species [HPr, HPr(His∼P), HPr(Ser-P), and HPr(His∼P)(Ser-P)] were detected by using polyclonal rabbit antibodies raised against M. pneumoniae His6-tagged HPr. Ten micrograms of extract was applied to each lane.
Detection of HPr phosphorylating enzymes in cell extracts of M. pneumoniae.
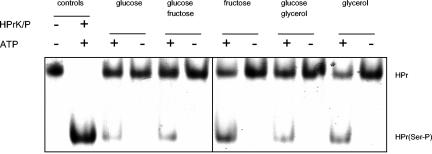
The in vivo phosphorylation experiment suggests that enzyme I was active under all conditions studied here, whereas HPrK/P kinase activity was detectable only in glycerol-grown cells. Therefore, the synthesis or the activity of HPrK/P might be controlled by carbon source availability. To differentiate between these two possibilities, we investigated the presence of enzymatic activity of HPrK/P in M. pneumoniae cells after growth in modified Hayflick medium with different carbon sources. Crude extracts were incubated with HPr and with or without ATP, and the reaction mixture was analyzed by native gel electrophoresis (Fig. 6). None of the extracts was able to phosphorylate HPr in the absence of ATP. In contrast, all extracts contained HPrK/P resulting in the formation of HPr(Ser-P). Judging from these results, HPrK/P was present under all conditions. Thus, enzymatic activity rather than expression seems to be regulated.
FIG. 6.
In vitro phosphorylation assay to detect HPrK/P (MPN223) in M. pneumoniae crude extracts. M. pneumoniae His6-tagged HPr (20 μM) was incubated with 5 μg of crude extract and 0.5 mM ATP in assay buffer in a final volume of 20 μl at 37°C for 120 min. Subsequently, the HPrK/P was heat inactivated by boiling for 10 min. The proteins were analyzed by using 10% native PAGE. M. pneumoniae crude extracts were from cells that had been cultivated in the presence of different sugars as indicated. The first lanes are positive controls with M. pneumoniae His6-tagged HPr (first lane) and His6-tagged HPr that had been phosphorylated at Ser-46 in vitro (second lane).
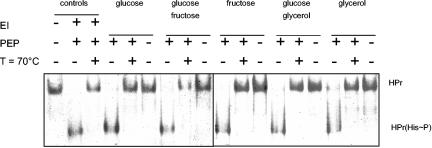
If M. pneumoniae cells grow on glucose or fructose, HPr is quantitatively phosphorylated by enzyme I. In contrast, only a portion of HPr is phosphorylated on His-15 if glycerol is present in the medium (Fig. 5). We asked, therefore, whether enzyme I was present in lower amounts in glycerol-grown cells. To address this question, we used the cell extracts from cultures grown with the different carbohydrates and studied the presence of enzyme I. This was performed by incubating the cell extracts with HPr and PEP as the phosphate donor. To control the reaction, we incubated enzyme I of B. subtilis with M. pneumoniae HPr. As shown in Fig. 7, this process resulted in heat-labile phosphorylation of HPr. All cell extracts converted HPr to HPr(His∼P) in the presence of PEP. In contrast, no HPr phosphorylation occurred in the absence of the phosphate donor (Fig. 7). Thus, enzyme I was present in all cell extracts tested. We may therefore conclude that the partial phosphorylation of HPr by enzyme I in glycerol-grown cells might result from the competition of HPrK/P and enzyme I for their common target, HPr.
FIG. 7.
In vitro phosphorylation assay to detect enzyme I (MPN627) in M. pneumoniae crude extracts. M. pneumoniae His6-tagged HPr (20 μM) was incubated with 1 μg of crude extract and 0.5 mM PEP in assay buffer in a final volume of 20 μl at 37°C for 30 min. Assay mixtures that had been incubated for an additional 10 min at 70°C to hydrolyze the heat-labile HPr(His∼P) and samples where PEP had been omitted served as negative controls. The proteins were analyzed by using 10% native PAGE. The crude extracts were prepared from cells that had been cultivated in the presence of different sugars as indicated. The first three lanes are positive controls with M. pneumoniae His6-tagged HPr (first lane), in vitro phosphorylated His6-tagged HPr(His∼P) using B. subtilis enzyme I (second lane), and the same assay mixture after 10 min at 70°C (third lane).
DISCUSSION
For growth in artificial medium, M. pneumoniae requires the presence of an added carbohydrate. Among the carbohydrates tested in this study, glucose allowed the most rapid growth. In contrast, fructose and glycerol are poor carbon sources for M. pneumoniae. Interestingly, mannitol did not serve as a single carbon source even though the genetic information to use this carbohydrate is complete (Fig. 1). Two possible explanations for this finding can be envisioned: the mtlA, mtlF, or mtlD gene required for mannitol transport and conversion to fructose-6-phosphate might be poorly expressed. This argument is supported by the observation that MtlA, the mannitol-specific protein IIBC of the PTS, is not expressed in growing cultures of M. pneumoniae (20). Alternatively, one of the genes necessary for mannitol utilization might harbor a mutation that results in the loss of the pathway. However, the loss of both expression and enzymatic function would result from a mutation(s) that may affect the promoter and the structural genes, respectively. Thus, we may be witnesses of a further step in the reductive evolution of the M. pneumoniae genome. There are several indications supporting this idea: M. pneumoniae possesses the genes for a putative ABC transporter for glycerol-3-phosphate with the notable exception of a binding protein. This finding may explain the inability of M. pneumoniae to use glycerol-3-phosphate as the single carbon source (data not shown). Moreover, in M. genitalium, the genes for mannitol transport are completely lost. With only 580 kb, M. genitalium may be a step ahead in the path of genome reduction (10, 16). Genes that are not expressed or encode nonfunctional proteins are also found in other bacteria. In E. coli, the bgl operon encoding the genes for the transport and utilization of aromatic β-glucosides is cryptic and requires mutations that activate the promoter (13, 39). The B. subtilis gudB gene encoding a cryptic glutamate dehydrogenase is an example of an enzyme that is inactive due to a mutation of the structural gene (2). Massive gene decay is also observed in the obligately pathogenic bacterium Mycobacterium leprae (48).
The need for an external carbon source seen in this study is in good agreement with the results of a global transposon mutagenesis approach with M. pneumoniae and M. genitalium; mutants affecting the fructose permease of the PTS were obtained only if glucose was provided. On the other hand, no mutations affecting the general components of the PTS, i.e., enzyme I and HPr, were observed (18). The general importance of the PTS for M. pneumoniae is also underlined by the observation that enzyme I and HPr are synthesized under all tested conditions (this study and reference 20). Constitutive expression of the general PTS components was also detected in E. coli and B. subtilis. This expression allows the general PTS proteins to fulfill their different regulatory functions in both the absence and the presence of PTS sugars (46).
The finding that glucose is the best carbon source for M. pneumoniae is in agreement with the fact that this sugar is preferred in many bacteria, including E. coli and B. subtilis. Moreover, glucose was detected in nasal secretions of compromised patients but was not detected in those of healthy patients (34). Thus, this sugar is available for M. pneumoniae in its natural habitats. Similarly, we would expect that glycerol resulting from the degradation of phospholipids is present on mucosal surfaces. Indeed, our results indicate that both glucose and glycerol are of special importance for M. pneumoniae. Glucose is the best carbon source, but glycerol is the one that provoked a regulatory output as determined by in vivo HPr phosphorylation assays.
In all organisms studied so far, the kinase activity of HPrK/P is maximal if the bacteria grow in the presence of glucose, i.e., under conditions that cause carbon catabolite repression (22, 25, 32, 45). Moreover, with the exception of the M. pneumoniae HPrK/P, the kinase activity of all these enzymes requires high ATP concentrations due to a low affinity for ATP (21, 33, 42). The results presented in this work indicate that the M. pneumoniae HPrK/P is unique not only in its high affinity for ATP (30) but also in its unusual mode of in vivo activity. The enzyme does not respond to the presence of the best carbon source, glucose, but its kinase activity is highest if the cells grow in the presence of glycerol. Interestingly, this activity is not affected by glucose as long as glycerol is available. This finding suggests a specific need for regulation in the presence of glycerol. The availability of glycerol might be an indication for the bacteria that they found their preferred ecological niche, the lipid-rich mucosal surface. If this was the case, one would expect significant changes in the global gene expression pattern in M. pneumoniae in response to the presence or absence of glycerol. In Mycoplasma mycoides, induction of cytotoxic H2O2 production requires the availability of glycerol (47). The use of a sugar as an indication of the nature of the habitat is not unprecedented in bacteria; in Listeria monocytogenes, the availability of the β-glucoside salicin is an indication that the bacteria are living in soil rather than in the human body. Accordingly, the activity of the regulatory protein BvrA, which responds to salicin, is mutually exclusive with that of the key activator of L. monocytogenes virulence gene expression, PrfA (4). Moreover, HPr phosphorylation by HPrK/P might be important for triggering glycerol catabolism; in Firmicutes such as Enterococcus faecalis, Enterococcus casseliflavus, and B. subtilis, glycerol utilization requires a functional PTS even though this substrate is not transported by the PTS. The glycerol kinases of these organisms require HPr-dependent phosphorylation for activity (6). It has been previously demonstrated that the doubly phosphorylated HPr(His∼P)(Ser-P) can serve as a phosphate donor for the lactose permease of Streptococcus salivarius (23). Thus, it is possible that double phosphorylation of HPr in the presence of glycerol is required for phosphorylation and concomitant activation of the glycerol kinase of M. pneumoniae.
It will be interesting to study the global changes of gene expression in M. pneumoniae in response to the carbohydrate availability and the mechanisms that control the utilization of individual substrates such as glycerol. This work will undoubtedly be helpful in understanding the biology of M. pneumoniae as well as the role of carbon metabolism in virulence and pathogenicity.
Acknowledgments
We are grateful to Elsbeth Pirkl, Carl-Ulrich Zimmerman, and Richard Herrmann for introducing us to the handling of M. pneumoniae. M. Schmalisch is acknowledged for helpful discussions.
This work was supported by the Fonds der Chemischen Industrie. S.H. was supported by a personal grant from the Fonds der Chemischen Industrie.
REFERENCES
- 1.Allen, G. S., K. Steinhauer, W. Hillen, J. Stülke, and R. G. Brennan. 2003. Crystal structure of HPr kinase/phosphatase from Mycoplasma pneumoniae. J. Mol. Biol. 326:1203-1217. [DOI] [PubMed] [Google Scholar]
- 2.Belitsky, B. R., and A. L. Sonenshein. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180:6298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brehm, K., M.-T. Ripio, J. Kreft, and J.-A. Vázquez-Boland. 1999. The bvr locus of Listeria monocytogenes mediates virulence gene repression by β-glucosides. J. Bacteriol. 181:5024-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandekar, T., M. Huynen, J. T. Regula, B. Ueberle, C. U. Zimmermann, M. A. Andrade, T. Doerks, L. Sánchez-Pulido, B. Snel, M. Suyama, Y. P. Yuan, R. Herrmann, and P. Bork. 2000. Re-annotating the Mycoplasma pneumoniae genome sequence: adding value, function and reading frames. Nucleic Acids Res. 28:3278-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darbon, E., P. Servant, S. Poncet, and J. Deutscher. 2002. Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P∼GlpK dephosphorylation control Bacillus subtilis glpFK expression. Mol. Microbiol. 43:1039-1052. [DOI] [PubMed] [Google Scholar]
- 7.Deutscher, J., E. Küster, U. Bergstedt, V. Charrier, and W. Hillen. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 15:1049-1053. [DOI] [PubMed] [Google Scholar]
- 8.Egan, W., M. Barile, and S. Rottem. 1986. 31P-NMR studies of Mycoplasma gallisepticum cells using a continuous perfusion technique. FEBS Lett. 204:373-376. [DOI] [PubMed] [Google Scholar]
- 9.Fieulaine, S., S. Morera, S. Poncet, V. Monedero, V. Gueguen-Chaignon, A. Galinier, J. Janin, J. Deutscher, and S. Nessler. 2001. X-ray structure of HPr kinase: a bacterial protein kinase with a P-loop nucleotide-binding domain. EMBO J. 20:3917-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, J. L. Fritchman, J. F. Weidman, K. V. Small, M. Sandusky, J. Fuhrmann, D. Nguyen, T. R. Utterback, D. M. Saudek, C. A. Phillips, J. M. Merrick, J. F. Tomb, B. A. Dougherty, K. F. Bott, P. C. Hu, T. S. Lucier, S. N. Peterson, H. O. Smith, C. A. Hutchinson, and J. C. Venter. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 11.Galinier, A., J. Haiech, M.-C. Kilhoffer, M. Jaquinod, J. Stülke, J. Deutscher, and I. Martin-Verstraete. 1997. The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression. Proc. Natl. Acad. Sci. USA 94:8439-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galinier, A., M. Kravanja, R. Engelmann, W. Hengstenberg, M.-C. Kilhoffer, J. Deutscher, and J. Haiech. 1998. New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc. Natl. Acad. Sci. USA 95:1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, B. 1998. Activation of the bgl operon by adaptive mutation. Mol. Biol. Evol. 15:1-5. [DOI] [PubMed] [Google Scholar]
- 14.Hanson, K. G., K. Steinhauer, J. Reizer, W. Hillen, and J. Stülke. 2002. HPr kinase/phosphatase of Bacillus subtilis: expression of the gene and effects of mutations on enzyme activity, growth and carbon catabolite repression. Microbiology 148:1805-1811. [DOI] [PubMed] [Google Scholar]
- 15.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B.-C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24:4420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himmelreich, R., H. Plagens, H. Hilbert, B. Reiner, and R. Herrmann. 1997. Comparative analysis of the genomes of the bacteria Mycoplasma pneumoniae and Mycoplasma genitalium. Nucleic Acids Res. 25:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoischen, C., A. Dijkstra, S. Rottem, J. Reizer, and M. H. Saier, Jr. 1993. Presence of protein constituents of the gram-positive bacterial phosphotransferase regulatory system in Acholeplasma laidlawii. J. Bacteriol. 175:6599-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson, C. A., III, S. C. Peterson, S. R. Gill, R. T. Cline, O. White, C. M. Fraser, H. O. Smith, and J. C. Venter. 1999. Global transposon mutagenesis and a minimal mycoplasma genome. Science 286:2165-2169. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs, E. 1997. Mycoplasma infections of the human respiratory tract. Wien. Klin. Wochenschr. 109:574-577. [PubMed] [Google Scholar]
- 20.Jaffe, J. D., H. C. Berg, and G. M. Church. 2004. Proteogenomic mapping as a complementary method to perform genome annotation. Proteomics 4:59-77. [DOI] [PubMed] [Google Scholar]
- 21.Jault, J. M., S. Fieulaine, S. Nessler, P. Gonzalo, A. Di Pietro, J. Deutscher, and A. Galinier. 2000. The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding. J. Biol. Chem. 275:1773-1780. [DOI] [PubMed] [Google Scholar]
- 22.Leboeuf, C., L. Leblanc, Y. Auffray, and A. Hartke. 2000. Characterization of the ccpA gene of Enterococcus faecalis: identification of starvation-inducible proteins regulated by ccpA. J. Bacteriol. 182:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lessard, C., A. Cochu, J. D. Lemay, D. Roy, K. Vaillancourt, M. Frenette, S. Moineau, and C. Vadeboncoeur. 2003. Phosphorylation of Streptococcus salivarius lactose permease (LacS) by HPr(His∼P) and HPr(Ser-P)(His∼P) and effects on growth. J. Bacteriol. 185:6764-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lind, K. 1983. Manifestations and complications of Mycoplasma pneumoniae disease: a review. Yale J. Biol. Med. 56:461-468. [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwig, H., N. Rebhan, H.-M. Blencke, M. Merzbacher, and J. Stülke. 2002. Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol. Microbiol. 45:543-553. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig, H., H.-M. Blencke, M. Schmalisch, C. Detsch, M. Merzbacher, and J. Stülke. 2003. Positive regulation of gene expression by the catabolite control protein CcpA in Bacillus subtilis, p. 181-186. In P. Dürre and B. Friedrich (ed.), Regulatory networks in prokaryotes. Horizon Scientific Press, Norfolk, United Kingdom.
- 27.Ludwig, W., and H.-P. Klenk. 2001. Overview: a phylogenetic backbone and taxonomic framework for prokaryotic systematics, p. 49-65. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer, New York, N.Y. [Google Scholar]
- 28.Márquez, J. A., S. Hasenbein, B. Koch, S. Fieulaine, S. Nessler, R. B. Russell, W. Hengstenberg, and K. Scheffzek. 2002. Structure of the full-length HPr kinase/phosphatase from Staphylococcus xylosus at 1.95 Å resolution: mimicking the product/substrate of the phosphotransfer reactions. Proc. Natl. Acad. Sci. USA 99:3458-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason, P. W., D. P. Carbone, R. A. Cushman, and A. S. Waggoner. 1981. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J. Biol. Chem. 256:1861-1866. [PubMed] [Google Scholar]
- 30.Merzbacher, M., C. Detsch, W. Hillen, and J. Stülke. 2004. Mycoplasma pneumoniae HPr kinase/phosphorylase: assigning functional roles to the P-loop and the HPrK/P signature sequence motif. Eur. J. Biochem. 271:367-374. [DOI] [PubMed] [Google Scholar]
- 31.Miles, R. J. 1992. Catabolism in mollicutes. J. Gen. Microbiol. 138:1773-1783. [DOI] [PubMed] [Google Scholar]
- 32.Monedero, V., S. Poncet, I. Mijakovic, S. Fieulaine, V. Dossonnet, I. Martin-Verstraete, S. Nessler, and J. Deutscher. 2001. Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. EMBO J. 20:3928-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nessler, S., S. Fieulaine, S. Poncet, A. Galinier, J. Deutscher, and J. Janin. 2003. HPr kinase/phosphorylase, the sensor enzyme of catabolite repression in gram-positive bacteria: structural aspects of the enzyme and the complex with its protein substrate. J. Bacteriol. 185:4003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philips, B. J., J.-X. Meguer, J. Redman, and E. H. Baker. 2003. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 29:2204-2210. [DOI] [PubMed] [Google Scholar]
- 35.Pollack, J. D., M. A. Myers, T. Dandekar, and R. Herrmann. 2002. Suspected utility of enzymes with multiple activities in the small genome Mycoplasma species: the replacement of the missing “household” nucleoside diphosphate kinase gene and activity by glycolytic kinases. OMICS 6:247-257. [DOI] [PubMed] [Google Scholar]
- 36.Pompeo, F., Y. Granet, J.-P. Lavergne, C. Grangeasse, S. Nessler, J.-M. Jault, and A. Galinier. 2003. Regulation and mutational analysis of the HPr kinase/phosphorylase from Bacillus subtilis. Biochemistry 42:6762-6771. [DOI] [PubMed] [Google Scholar]
- 37.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reizer, J., C. Hoischen, F. Titgemeyer, C. Rivolta, R. Rabus, J. Stülke, D. Karamata, M. H. Saier, Jr., and W. Hillen. 1998. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol. Microbiol. 27:1157-1169. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds, A. E., J. Felton, and A. Wright. 1981. Insertion of DNA activates the cryptic bgl operon of E. coli K12. Nature 293:625-629. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Steinhauer, K., G. S. Allen, W. Hillen, J. Stülke, and R. G. Brennan. 2002. Crystallization, preliminary X-ray analysis and biophysical characterization of HPr kinase/phosphatase of Mycoplasma pneumoniae. Acta Crystallogr. Sect. D Biol. Crystallogr. 58:515-518. [DOI] [PubMed] [Google Scholar]
- 42.Steinhauer, K., T. Jepp, W. Hillen, and J. Stülke. 2002. A novel mode of control of Mycoplasma pneumoniae HPr kinase/phosphatase activity reflects its parasitic life style. Microbiology 148:3277-3284. [DOI] [PubMed] [Google Scholar]
- 43.Stülke, J., M. Arnaud, G. Rapoport, and I. Martin-Verstraete. 1998. PRD—a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol. Microbiol. 28:865-874. [DOI] [PubMed] [Google Scholar]
- 44.Stülke, J., and M. Schmalisch. 2004. The bacterial phosphotransferase system: a perfect link of sugar transport and signal transduction. Topics Curr. Genet. 9:179-205. [Google Scholar]
- 45.Vadeboncoeur, C., D. Brochu, and J. Reizer. 1991. Quantitative determination of the intracellular concentration of the various forms of HPr, a phosphocarrier protein of the phosphoenolpyruvate:sugar phosphotransferase system in growing cells of oral streptococci. Anal. Biochem. 196:24-30. [DOI] [PubMed] [Google Scholar]
- 46.Vadeboncoeur, C., M. Frenette, and L. A. Lortie. 2000. Regulation of the pts operon in low G+C Gram-positive bacteria. J. Mol. Microbiol. Biotechnol. 2:483-490. [PubMed] [Google Scholar]
- 47.Vilei, E. M., and J. Frey. 2001. Genetic and biochemical characterization of glycerol uptake in Mycoplasma mycoides subsp. mycoides SC: its impact on H2O2 production and virulence. Clin. Diagn. Lab. Immunol. 8:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vissa, V. D., and P. J. Brennan. 2001. The genome of Mycobacterium leprae: a minimal mycobacterial gene set. Genome Biol. 2:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner, J., III, R. Herrmann, and G. F. Browning. 2000. Transcription in Mycoplasma pneumoniae. Nucleic Acids Res. 28:4488-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner, J., III, C. U. Zimmerman, H. W. Göhlmann, and R. Herrmann. 2003. Transcription profiles of the bacterium Mycoplasma pneumoniae grown at different temperatures. Nucleic Acids Res. 31:6306-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wetzstein, M., U. Völker, J. Dedio, S. Löbau, U. Zuber, M. Schiesswohl, C. Herget, M. Hecker, and W. Schumann. 1992. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J. Bacteriol. 174:3300-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, P.-P., N. Nosworthy, A. Ginsburg, M. Miyata, Y.-J. Seok, and A. Peterkofsky. 1997. Expression, purification, and characterization of enzyme IIAglc of the phosphoenolpyruvate:sugar phosphotransferase system of Mycoplasma capricolum. Biochemistry 36:6947-6953. [DOI] [PubMed] [Google Scholar]
- 54.Zigangirova, N. A., V. N. Gershanovich, I. V. Rakovskaya, O. I. Barchatova, and A. L. Gintsburg. 2003. Correlation between activity of the phosphoenol-pyruvate-dependable phosphotransferase system (PTS) and synthesis of adhesion P1 protein in Mycoplasma pneumoniae. Mol. Gen. Mikrobiol. Virusol. 2:10-13. (In Russian.) [PubMed] [Google Scholar]