Abstract
Wall teichoic acids are anionic, phosphate-rich polymers linked to the peptidoglycan of gram-positive bacteria. In Bacillus subtilis, the predominant wall teichoic acid types are poly(glycerol phosphate) in strain 168 and poly(ribitol phosphate) in strain W23, and they are synthesized by the tag and tar gene products, respectively. Growing evidence suggests that wall teichoic acids are essential in B. subtilis; however, it is widely believed that teichoic acids are dispensable under phosphate-limiting conditions. In the work reported here, we carefully studied the dispensability of teichoic acid under phosphate-limiting conditions by constructing three new mutants. These strains, having precise deletions in tagB, tagF, and tarD, were dependent on xylose-inducible complementation from a distal locus (amyE) for growth. The tarD deletion interrupted poly(ribitol phosphate) synthesis in B. subtilis and represents a unique deletion of a tar gene. When teichoic acid biosynthetic proteins were depleted, the mutants showed a coccoid morphology and cell wall thickening. The new wall teichoic acid biogenesis mutants generated in this work and a previously reported tagD mutant were not viable under phosphate-limiting conditions in the absence of complementation. Cell wall analysis of B. subtilis grown under phosphate-limited conditions showed that teichoic acid contributed approximately one-third of the wall anionic content. These data suggest that wall teichoic acid has an essential function in B. subtilis that cannot be replaced by teichuronic acid.
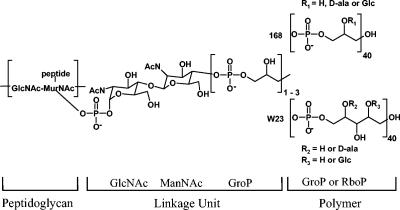
Wall teichoic acids represent a diverse group of peptidoglycan-linked, phosphate-rich polymers found in gram-positive bacteria. The model gram-positive organism Bacillus subtilis has been studied to elucidate the genetic organization, transcriptional regulation, and function of teichoic acid biosynthetic enzymes. Much of this work has been completed in B. subtilis strain 168, which produces poly(glycerol phosphate) as its major teichoic acid, and, to a lesser extent, in strain W23, which produces poly(ribitol phosphate). In B. subtilis teichoic acid (Fig. 1), the main chain contains approximately 40 repeating units and is attached to peptidoglycan via a linkage unit consisting of N-acetylglucosamine-1-phosphate-N-acetylmannosamine and one to three glycerol phosphate residues (2, 44). The tag genes (tagABDEFGHO) in strain 168 (31) and the tar genes (tarABIJKLDF) in strain W23 (27) encode the teichoic acid biosynthetic machinery. The protein products of the tagD, tagF, tagA, and tagO genes have been assigned functions based on biochemical data (35, 38) and sequence similarity to characterized enzymes (27, 31).
FIG. 1.
Chemical structures of the two types of predominant teichoic acid in B. subtilis. Poly(glycerol phosphate) is found in strain 168, and poly(ribitol phosphate) is found in strain W23. Covalent attachment of the polymer to peptidoglycan occurs via a highly conserved linkage unit consisting of a disaccharide and one to three glycerol phosphate monomers. Hydroxyl groups on the main chain are often glucosylated or d-alanylated. GroP, glycerol phosphate; RboP, ribitol phosphate; GlcNAc, N-acetylglucosamine; ManNAc, N-acetylmannosamine; Glc, glucose; MurNAc, N-acetylmuramic acid.
Although wall teichoic acid was considered dispensable for decades, the isolation of temperature-sensitive mutants derived from random mutagenesis of strain 168 (7, 9, 37) suggested that wall teichoic acid may be essential. At the restrictive temperature, these mutants exhibited extremely poor growth and an abnormal coccoid morphology characteristic of cell wall mutants (7, 21). The quantity of teichoic acid was shown to be greatly reduced in the walls of these mutant strains. More recently, our group unequivocally confirmed the indispensability of tagD at a physiologically relevant temperature in a defined genetic background by construction of a tagD deletion strain (4). tagD was precisely replaced by an antibiotic resistance cassette, while a complementing copy of the gene, under xylose-inducible control, was inserted at the amyE locus. In the absence of xylose, this deletion strain displayed the mutant phenotype described above, but it was rescued by induction of the complementing copy.
Various functions of wall teichoic acid have been proposed, and many of them are related to its anionic character, such as cell shape maintenance by charge repulsion (34) or buffering of magnesium ions (19). However, the ability of teichoic acid to act as a phosphate reservoir appears to present an exception to its indispensability. Ellwood and Tempest and other workers have reported that teichoic acid is replaced by teichuronic acid when B. subtilis is grown under phosphate-limited conditions (15, 26). Teichuronic acid is a phosphate-free carbohydrate polymer containing glucuronic acid (43) and is synthesized by enzymes encoded in the tua operon (40). When phosphate is limited, the PhoP/PhoR system induces transcription of the tua operon (29) and represses transcription of the tag divergon (28, 32). Grant demonstrated that B. subtilis releases its existing teichoic acid into the medium and takes in phosphate from the medium (16). It is thought that the anionic glucuronic acid components of teichuronic acid substitute for the anionic phosphate groups of teichoic acid, thus satisfying the cellular requirement for an anionic polymer; meanwhile, phosphate is liberated for more urgent uses, such as nucleic acid synthesis. A number of groups, however, have produced results that are at odds with this accepted paradigm. Namely, these groups found significant quantities of phosphate in the cell walls of strain 168 grown under phosphate-limited conditions (25, 40). These findings raised the possibility that teichoic acid may still be essential for growth during phosphate limitation.
Another challenge to our understanding of the importance of wall teichoic acid to gram-positive bacteria arose from a report that teichoic acid is not essential in Staphylococcus aureus (42). Like B. subtilis strain W23, this gram-positive pathogen produces poly(ribitol phosphate). When the putative N-acetylglucosamine-1-phosphate transferase gene tagO was deleted from S. aureus, the organism was ineffective at colonizing nasal passages in cotton rats, indicating that teichoic acid is a virulence factor. However, the tagO null strain remained viable under laboratory conditions despite the teichoic acid deficiency. The surprisingly dissimilar requirements for teichoic acid in different species call for further investigation into this polymer and its functions.
In light of the issues described above, we sought to resolve the indispensability of teichoic acid in the well-characterized model organism B. subtilis, which allows straightforward genetic manipulation and phenotypic analysis. We describe here the construction of three conditionally complemented deletion strains. We generated tagB and tagF deletion strains that verified the essentiality of these genes and of teichoic acid in strain 168. Moreover, we report the construction of a tarD deletion strain from a 168-W23 hybrid strain, in which the tag genes were replaced by the tar genes in a 168 background (22). This strain produces predominantly the poly(ribitol phosphate) teichoic acid polymer found in other gram-positive bacteria, including S. aureus. tarD, which putatively encodes the CDP-glycerol cytidylyltransferase in W23, was found to be essential; this represents the first demonstration of the indispensability of a poly(ribitol phosphate) biosynthetic enzyme. Furthermore, when we imposed termination of teichoic acid biosynthesis in our four deletion strains under phosphate-limited conditions, they were not viable. These results, combined with analyses that detected considerable quantities of phosphate in the walls of phosphate-limited cultures, indicate that teichuronic acid does not completely replace teichoic acid during phosphate limitation. The data presented here substantiate the essentiality of teichoic acid in B. subtilis strains that produce poly(glycerol phosphate) and poly(ribitol phosphate).
MATERIALS AND METHODS
B. subtilis strains, plasmids, and cultures.
Bacterial strains and plasmids are described in Table 1. The sequences of the primers used in this study are provided in Table S1 in the supplemental material. Unless otherwise indicated, strains were grown at 30°C in Luria-Bertani (LB) liquid medium with aeration at 250 rpm or on solid LB agar supplemented with the following compounds: 150 μg of spectinomycin per ml, 10 μg of chloramphenicol per ml, and 2% (wt/vol) xylose. Phosphate limitation medium (PL medium) was made according to the specifications of Grant (16). The general cloning methods used for B. subtilis were those described by Cutting and Youngman (14).
TABLE 1.
B. subtilis strains and plasmids used in this work
| B. subtilis strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| L5087 | hisA1 argC4 metC3 | 9 |
| L6602 | hisA1 argC4 metC3 tag-12 | 9 |
| EB124 | hisA1 argC4 metC3 amyE::xylR PxylA tagD cat86 | 4 |
| EB240 | hisA1 argC4 metC3 amyE::xylR PxylA tagD cat86 tagD::Specr | 4 |
| EB312a | hisA1 argC4 metC3 amyE::xylR PxylA tagF cat86 | This study |
| EB313b | hisA1 argC4 metC3 amyE::xylR PxylA tagF cat86 | This study |
| EB521 | hisA1 argC4 metC3 amyE::xylR PxylA tagB cat86 | This study |
| EB633 | hisA1 argC4 metC3 amyE::xylR PxylA tagB cat86 tagB::Specr | This study |
| EB669 | hisA1 argC4 metC3 amyE::xylR PxylA tagF cat86 tagF::Specr | This study |
| EB771 | L6602-suppressor | This study |
| L5706 | metB5 tagABDEF::tarABIJKLDF | 45 |
| EB842 | metB5 tagABDEF::tarABIJKLDF amyE::xylR PxylA tagD cat86 | This study |
| EB856 | metB5 tagABDEF::tarABIJKLDF amyE::xylR PxylA tagD cat86 tarD::Specr | This study |
| Plasmids | ||
| pSWEET-tagB | pSWEET with wild-type tagB from B. subtilis 168 | This study |
| pSWEET-tagD | pSWEET with wild-type tagD from B. subtilis 168 | 5 |
| pSWEET-tagF | pSWEET with wild-type tagF from B. subtilis 168 | 38 |
| pUS19 | pUC19 derivative containing spectinomycin resistance cassette | 3 |
The tagF gene is downstream of an optimal ribosome binding site (41).
The tagF gene is downstream of its endogenous ribosome binding site.
Construction of tagB, tagF, and tarD deletion strains.
To generate the pSWEET-tagB derivative, tagB was amplified by PCR from B. subtilis 168 chromosomal DNA with primers tagBoptrbsfor and tagBrev3 and cloned into pSWEET-bgaB (5). The resulting plasmid, pSWEET-tagB, was transformed into L5087. pSWEET-tagF (38) and pSWEET-tagD (5) were transformed into L5087 and L5706, respectively. L5087 was made competent by the protocol of Cutting and Youngman (14), and L5706 was made competent by the method of Karamata and Gross (20). Integration of pSWEET derivatives at the amyE locus was verified by a starch utilization assay (18) and by PCR. The resulting strains were designated EB521, EB313, and EB842.
To precisely replace each gene at its native locus, 1 kb of sequence upstream of the gene and 1 kb of sequence downstream of the gene were amplified by PCR from the B. subtilis chromosome by using Expand High Fidelity DNA polymerase (Roche Applied Science, Laval, Quebec, Canada) and the primer pairs upflanka-upflankb and downflankc-downflankd, respectively. A spectinomycin resistance cassette was amplified by PCR with Expand DNA polymerase from plasmid pUS19 by using primers specfor and specrev. Primers upflankb and downflankc contained sequences complementary to sequences in primers specfor and specrev, respectively. The resulting PCR fragments annealed so that the spectinomycin resistance cassette was situated between the flanking sequences, forming in essence a 3-kb template that was PCR amplified with primers upflanka and downflankd. The 3-kb fragment was used to transform each parent strain (EB521, EB313, and EB842) with selection on spectinomycin, and the transformants (designated EB633, EB669, and EB856, respectively) were verified by PCR.
Characterization of tagB, tagF, and tarD deletion strains.
Deletion and parent strains were grown overnight on plates containing LB medium supplemented with chloramphenicol, spectinomycin, and 2% xylose and on plates containing LB medium supplemented with chloramphenicol, respectively. Both strains were subsequently streaked onto plates containing LB medium supplemented with chloramphenicol with or without 2% xylose and grown for 36 h at 30°C. For liquid culture experiments, strains EB521 and EB633 from LB medium plates (supplemented with 2% xylose in the case of EB633) were suspended in sterile saline and used to inoculate fresh LB medium supplemented with chloramphenicol without xylose or with 0.006, 0.02, 0.06, or 2% xylose in a 96-well sterile microtiter plate. The plate was incubated at 30°C with shaking at 250 rpm, and growth was monitored every hour by determining the optical density at 600 nm (OD600).
Microscopy.
Deletion and parent strains were grown overnight on solid LB medium at 30°C. Cells were suspended in saline, fixed by the procedure of Harry et al. (17), and visualized by phase-contrast microscopy by using an Olympus CX41 microscope (Carsen Group, Markham, Ontario, Canada) with an oil immersion ×100 objective. Images were obtained with an Olympus Q-Color3 camera, and the images were processed by using the MediaCybernetics Image-Pro Express software (Carsen Group). For scanning electron microscopy, EB521 and EB633 were grown overnight on solid LB medium in the absence of xylose. Cells were suspended in sterile saline, spotted onto a coverslip, and gold coated to a thickness of approximately 7 nm by using a Polaron SEM coating system (Polaron Instruments Inc., Hatfield, Pa.). Specimens were visualized by using an environmental scanning electron microscope (ESEM model 2020; ElectroScan Corporation, Wilmington, Mass.). For transmission electron microscopy, EB521 and EB633 cells from overnight cultures on solid LB medium were initially suspended in sterile saline. The cells were fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Samples were postfixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.4). Dehydration was performed by using a graded ethanol series, followed by treatment with propylene oxide and imbedding in Spurr's resin. Sections (70 nm) were cut with a Reichert Ultracut E ultramicrotome (Leica Inc., Vienna, Austria). Samples were stained for 5 min with saturated uranyl acetate and for 2 min with lead citrate. Samples were visualized and photographed with a JEOL 1200 EX Biosystem electron microscope (JEOL Ltd., Tokyo, Japan).
Phosphate limitation of deletion strains.
Deletion strains, parent strains, and L5087 were acclimatized to phosphate-limited conditions by plating them on PL medium containing 2.5 mM phosphate (phosphate-replete conditions) for 48 h and twice on PL medium containing 0.25 mM phosphate (phosphate-limited conditions) for 48 h each. The deletion strains were grown in the presence of 2% xylose. The strains were then plated on solid PL medium supplemented with 0.25 and 2.5 mM phosphate in the presence or absence of 2% xylose. To obtain growth profiles in liquid medium, overnight cultures of each strain were grown in PL medium containing 0.25 mM phosphate, and xylose was withheld in order to deplete cells of existing protein. PL medium supplemented with various levels of phosphate (no phosphate and 0.0313, 0.0625, 0.125, 0.25, and 2.5 mM phosphate) in the presence or absence of 2% xylose were inoculated to an OD600 of approximately 0.015, and the organisms were grown in 96-well microtiter plates. The plates were incubated with shaking at 250 rpm, and growth was monitored hourly. Growth profiles were determined at least in triplicate.
Cell wall isolation and analysis.
L5087 was acclimatized to three different phosphate concentrations (0.0625, 0.25, and 2.5 mM) on solid PL medium. L5087 from each of the three plates was used to inoculate overnight cultures in media containing phosphate at the corresponding concentrations; these cultures were subsequently used to inoculate 200-ml portions of PL medium containing phosphate at the corresponding concentrations. The resulting 200-ml cultures were grown overnight to saturation, and cell walls were extracted as described by MacDonald (30), with the following changes: 25 mM citrate buffer (pH 6.0) was used instead of phosphate buffer; a Beckman MLA 80 rotor was used for ultracentrifugation steps; and samples were treated with DNase, RNase, and trypsin (24) prior to the second round of boiling in sodium dodecyl sulfate. The wall phosphate content was assayed by the protocol of Chen et al. (12) by using KH2PO4 as a standard, after mineralization as previously described (1). The wall uronic acid content was assayed by the method of Blumenkrantz and Asboe-Hansen (6) by using glucuronic acid as a standard.
Random mutagenesis of the temperature-sensitive tagD mutant (L6602).
Five-milliliter cultures of L6602 were grown in LB medium until the mid-exponential phase (OD600, ∼0.8). Then 1.5 ml of each culture was pelleted, resuspended in 150 μl of sterile saline, and diluted 1,000-fold in sterile saline. Aliquots (5 ml) were placed into petri dishes and UV irradiated at 254 nm by using a model UVGL-25 lamp (UVP Inc., San Gabriel, Calif.) at a distance of 12 cm. The dishes were irradiated for 15, 30, and 90 s under dim light (to minimize the action of DNA photolyases) and simultaneously agitated. Suppressors were selected on solid LB medium at the nonpermissive temperature (47°C). This protocol was repeated multiple times, irradiating approximately 1.1 × 109 cells. tagD was amplified from EB771 chromosomal DNA by PCR by using primers tagDfor and tagDrev and sequenced. EB771 chromosomal DNA was congressed into L6602; transformants were selected on solid LB medium at 47°C, and their tagD genes were sequenced as described above.
RESULTS
Deletion of tagB and tagF in B. subtilis 168 and deletion of tarD in the 168-W23 hybrid strain.
To date, the only characterized mutants with mutations in tagB and tagF are temperature-sensitive B. subtilis strains (7, 8, 21, 36). However, the high temperature required for manifestation of the lethal phenotype and the undefined nature of the strain genotype have left some ambiguity regarding the essentiality of TagB and TagF in B. subtilis. To clearly resolve the question of tagB and tagF dispensability, we constructed strains of B. subtilis 168 that had precise deletions of these genes. First, a complementing copy of the gene subject to xylose-inducible expression was introduced at the amyE locus of B. subtilis, creating new strains designated EB521, EB312, and EB313 (the last two strains differ only in the ribosome binding site upstream of tagF). The native copy of each tag gene was subsequently replaced by a spectinomycin resistance cassette at the tag locus, yielding strains EB633 and EB669.
The concept of a tarD deletion strain is novel in that none of the tar genes have been tested yet for indispensability, largely due to the difficulties of genetic manipulation of B. subtilis strain W23. Karamata et al. generated 168-W23 hybrid strains, in which the tar divergon replaced the tag divergon in strain 168, by congressing W23 DNA into the temperature-sensitive tagD strain and selecting organisms at the nonpermissive temperature (22). This replacement was a result of a pseudoallelic relationship between these teichoic acid biosynthetic genes (45). These hybrid strains produce mainly poly(ribitol phosphate) while maintaining normal cellular envelope functions, and thus analysis of the tar genes is facilitated. The tarD deletion strain was obtained by integration of pSWEET-tagD at the amyE locus of hybrid strain L5706, and tarD was precisely replaced as described above for the tag deletion strains. The pSWEET-tagD derivative was used since TagD and TarD exhibit 76% identity and have been predicted to catalyze the same reaction (27).
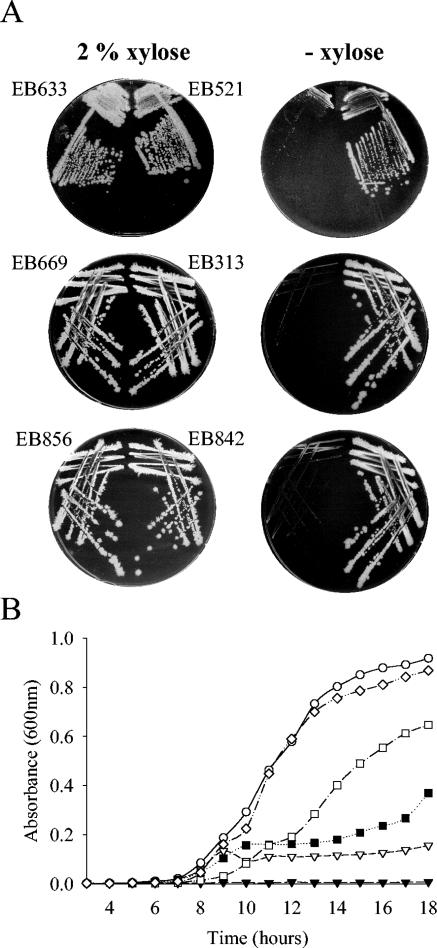
The dispensability of TagB, TagF, and TarD was tested by growing the deletion strains and their parent strains on solid medium at a physiological temperature in the presence or absence of 2% xylose. As shown in Fig. 2A, parent strains EB521, EB313, and EB842 showed robust growth with normal colony morphology. In contrast, the corresponding deletion strains, strains EB633, EB669, and EB856, were unable to form single colonies in the absence of expression of the complementing copy, and they showed only poor growth on the highest-inoculum area of the plate. This growth phenotype for the deletion strains was entirely reversible when the organisms were grown in the presence of the inducer xylose.
FIG. 2.
Xylose-dependent growth of tagB, tagF, and tarD deletion strains. (A) Analysis of growth of tag and tar deletion strains on solid medium. Parent strains EB313, EB521, and EB842 and deletion strains EB633, EB669, and EB856 were grown on solid LB medium containing chloramphenicol in the presence or absence of inducer (2% xylose). (B) Analysis of growth of tagB deletion strain in liquid medium. Strain EB521 was inoculated into LB medium containing chloramphenicol supplemented with 2% xylose (○), and EB633 was inoculated into LB medium containing chloramphenicol supplemented with 2% xylose (⋄), 0.06% xylose (□), 0.02% xylose (▪), 0.006% xylose (▿), or no xylose (▾). Growth was monitored by determining the optical density.
Figure 2B shows that the growth kinetics of the parent strain EB521 and the fully complemented deletion strain EB633 were virtually identical. This contrasted with the findings for the deletion strain grown in the absence of inducer, which showed no growth in liquid medium. We also noted that when EB633 cultures were supplemented with intermediate concentrations of the inducer, the extent of growth and the growth rate decreased as the level of the inducer decreased. We do not attribute the change in growth to a metabolic effect of xylose since EB521 grown in the absence of xylose showed only a slight decrease in growth (less than 20% difference [data not shown]).
Characterization of deletion strains by microscopy.
All deletion strains and the corresponding parent strains were examined by phase-contrast microscopy after growth in the absence of the inducer. Strains EB633 and EB521 were examined further by environmental scanning electron microscopy and transmission electron microscopy. While the parent strains exhibited normal rod-shaped morphology (see Fig. S1A to C in the supplemental material), phase-contrast microscopy revealed a loss of the rod-shaped integrity in the deletion strains (see Fig. S1D to F in the supplemental material), which led to cell rounding as well as a pronounced increase in the cell width. Examination of the cell surface of EB633 by environmental scanning electron microscopy revealed that cells from which TagB was depleted had conspicuous bulges and multiple furrows, perhaps indicative of multiple septa, in addition to a notable thickening of the cell (see Fig. S1H in the supplemental material). The overall curvature of the cells was in clear contrast to the cells of the parent strain (see Fig. S1G in the supplemental material), which were straight with only a single furrow, presumably a cell septum, and no noticeable bulging. Examination of thin sections revealed the expected morphology for the parent strain (see Fig. S1I in the supplemental material), with clear visualization of the nucleoid region and the relatively thin cell wall. In contrast to the parent strain, the tagB deletion strain (see Fig. S1J in the supplemental material) had an aberrant cell shape and pronounced thickening of the cell wall. This mutant strain also appeared to shed cell wall. A low-magnification field of this strain (see Fig. S1K in the supplemental material) showed multicompartmentalized cells possessing atypical cell septa with regard to number and curvature. There was also evidence of cell lysis. The striking morphological phenotype observed upon depletion of TagB is reminiscent of that observed upon depletion of TagD in a previously reported tagD deletion mutant (4).
Phosphate limitation of tagB, tagD, tagF, and tarD deletion strains.
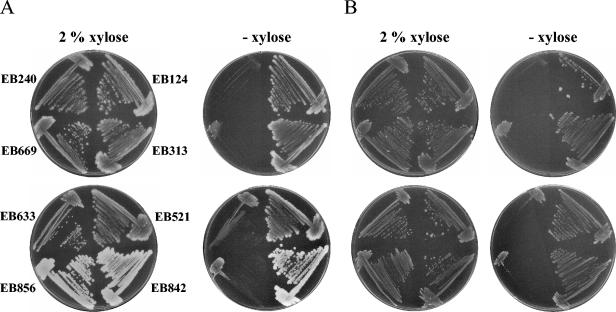
The four deletion strains harboring inducible complementing gene copies were used to assess teichoic acid dispensability during phosphate limitation. The deletion strains and the corresponding parent strains were acclimatized to 0.25 mM phosphate in PL medium (see Materials and Methods) and subsequently plated on solid PL medium containing either 2.5 mM phosphate (phosphate-replete condition) or 0.25 mM phosphate (phosphate-limiting condition) in the presence and absence of 2% xylose. These phosphate concentrations were chosen to allow comparison of our observations with those reported by other workers who also used 2.5 mM phosphate as the phosphate-replete condition and 0.25 mM phosphate as the phosphate-limiting condition (16, 25, 26, 33, 39, 40). As shown in Fig. 3, all parent strains grew normally on all types of media. As observed by other workers (16, 26), growth on phosphate-limited PL medium was less robust than growth on complete medium, presumably due to decreased availability of phosphate. Intriguingly, the deletion strains exhibited clear xylose-dependent growth irrespective of the concentration of phosphate in the PL medium. While the growth in the presence of xylose matched the growth of the parent strains, the growth on phosphate-limited PL medium lacking xylose was severely impaired and comparable to the growth of the deletion strains grown on rich LB medium lacking xylose (Fig. 2). EB633 showed somewhat more growth, although it was not nearly as robust as parent strain EB521; this was likely due to a transcriptional leak from the xylA promoter.
FIG. 3.
Xylose dependence of tag and tar gene deletion strains under phosphate-limited conditions. Parent strains EB124, EB313, EB521, and EB842 and deletion strains EB240, EB633, EB669, and EB856 were acclimatized to phosphate-limiting conditions (see Materials and Methods) and subsequently grown on solid PL medium supplemented with 2.5 mM phosphate (A) or 0.25 mM phosphate (B) in the presence and absence of an inducer (2% xylose).
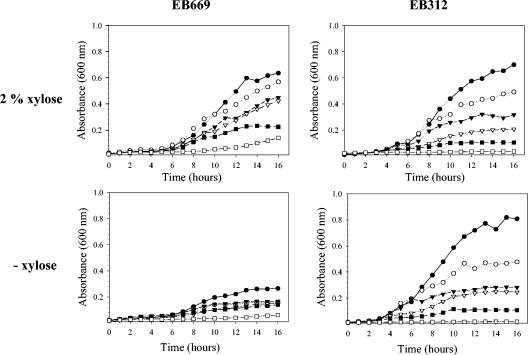
The essentiality of the teichoic acid biosynthetic enzymes during phosphate limitation suggests that teichoic acid remains an important cell wall component under these conditions. However, since teichuronic acid production has been shown to be affected by the phosphate concentration (26), we wanted to ensure that our observations did not stem from insufficiently low phosphate levels. Thus, the deletion strains and parent strains were grown in liquid cultures containing serially diluted phosphate in the presence or absence of xylose. The resulting growth profiles for tagF deletion strain EB669 and control strain EB312 are shown in Fig. 4. As expected, the maximum cell density decreased as the phosphate concentration decreased, and growth was negligible in the absence of phosphate. However, while the profiles for EB312 remained fairly constant despite the addition of xylose, the profiles for EB669 were significantly affected by xylose. In accordance with the xylose dependence of the deletion strains in phosphate-replete media, EB669 cultured in medium containing 2.5 mM phosphate and 2% xylose grew to a cell density that was 2.5 times greater than the density in the same medium without xylose. A comparable reduction in growth in the absence of xylose was obtained in media containing 0.25 mM phosphate, thereby confirming the results obtained with solid media (Fig. 3). Although a pronounced difference in growth in the presence and absence of xylose was evident at the highest phosphate concentrations used in this experiment (2.5, 0.25, and 0.125 mM), this effect was reduced at the lower phosphate concentrations and was in fact negligible in medium containing 0.031 mM phosphate. This was likely due to low cell densities associated with phosphate-limited cultures in the presence of the inducer and basal cell densities attributable to a transcriptional leak in the absence of the inducer. The results were confirmed on solid media containing 0.0625 mM phosphate, which showed that deletion strains were not viable in the absence of the inducer (data not shown).
FIG. 4.
Effects of phosphate and xylose levels on tagF deletion strain. EB669 and control strain EB312 were grown in liquid PL media in the absence of phosphate (□) or in the presence of 0.0313 mM phosphate (▪), 0.0625 mM phosphate (▿), 0.125 mM phosphate (▾), 0.25 mM phosphate (○), or 2.5 mM phosphate (•) and in the presence or absence of an inducer (2% xylose). Growth was monitored by determining the optical density. The experiment was performed in triplicate, and representative profiles are shown.
Overall, these growth profiles demonstrate that in the absence of the TagF enzyme, production of teichuronic acid induced by extreme phosphate limitation is insufficient to rescue the cells. The growth profiles of the other deletion strains and their parent strains resulted in patterns similar to those described here (data not shown). The exception, as before, was EB633; in comparison to the other deletion strains, this strain exhibited small reductions in growth even in phosphate-replete media, suggesting that a transcriptional leak from pSWEET was responsible. The extent of the transcriptional leak may be especially significant given that TagB is predicted to have a low copy number in vivo (31).
Cell wall analysis of wild-type B. subtilis grown in phosphate-replete and phosphate-limited media.
To determine whether the requirement for teichoic acid under phosphate-limited conditions stems from the continued presence of this compound in the cell wall, L5087 was grown in batch cultures in PL medium with three phosphate concentrations, and the cell wall phosphate and uronic acid contents were assayed. L5087 was previously acclimatized to each phosphate concentration for numerous generations in order to achieve a stable wall composition. The three concentrations included phosphate-replete conditions (2.5 mM) and two phosphate-limited conditions that have been used previously, 0.25 mM phosphate used in chemostat cultures and 0.0625 mM phosphate used in batch cultures (25).
The results of the assays are shown in Table 2. Consistent with theory and essentially all previously described analyses, a negligible quantity of uronic acid was found in the walls of cells grown in phosphate-replete medium. However, after growth in medium containing 0.25 mM phosphate, the walls contained twice as much phosphate as uronic acid. This ratio was inverted in the walls of cells grown in media containing 0.0625 mM phosphate, but phosphate still accounted for approximately 30% of the anionic content. We do not attribute this relatively high phosphate content to a difference between growth in batch cultures and growth in chemostat cultures. Lahooti and Harwood determined that the difference in nutrient levels between batch and chemostat cultures is approximately 15% (25), implying that the phosphate accounts for approximately 25% of the anionic wall polymer in chemostat cultures. It was also observed that the overall anionic content of the wall declined as the phosphate concentration in the medium decreased.
TABLE 2.
Cell wall analysis of wild-type B. subtilis to determine the teichoic and teichuronic acid contents under phosphate-replete and phosphate-limited conditions
| Phosphate concn in medium (mM) | Amt of phosphate in cell wall (μmol/mg)c | Amt of uronate in cell wall (μmol/mg)c | Phosphate/uronate ratio | Total amt of anionic polymers (μmol/mg) |
|---|---|---|---|---|
| 2.5a | 1.40 ± 0.08 | 0.010 ± 0.006 | 130 ± 78 | 1.41 ± 0.08 |
| 0.25b | 0.67 ± 0.05 | 0.34 ± 0.05 | 2.0 ± 0.3 | 1.01 ± 0.07 |
| 0.0625b | 0.25 ± 0.03 | 0.55 ± 0.06 | 0.45 ± 0.07 | 0.81 ± 0.07 |
Phosphate-replete conditions.
Phosphate-limited conditions. See text for details.
Averages and standard deviations were calculated from three independent assays by using the same cell wall preparation.
Isolation of a suppressor mutation in the tagD temperature-sensitive strain (L6602).
To isolate suppressor mutations that compensate for the lack of functional TagD and, more generally, for the obstruction of teichoic acid biosynthesis, L6602 was randomly mutagenized (see Materials and Methods) and grown at the nonpermissive temperature (47°C) to select for life. This produced one suppressor strain, designated EB771, which was generated by treatment of L6602 with 30 s of UV irradiation. The tagD gene of EB771 was sequenced to determine if an intragenic mutation had reversed the effects of the temperature-sensitive mutation. The tagD gene of EB771 had a cytosine at position 242, the central nucleotide of codon 81 and the precise location of the T-to-A transition responsible for temperature sensitivity. Codon 81 encodes isoleucine in the wild-type tagD gene, asparagine in the temperature-sensitive tagD gene, and threonine in the tagD gene of the suppressor strain. To confirm that this base pair disparity was responsible for the suppressor phenotype, EB771 chromosomal DNA was congressed into L6602, and selection at 47°C induced incorporation of the suppressor mutation into the L6602 chromosome. The tagD gene of one transformant was sequenced. This revealed a cytosine at bp 242 (data not shown), indicating that this mutation, in all likelihood, suppressed the temperature-sensitive phenotype. The finding that the only mutant in this hunt was an intragenic suppressor supports the hypothesis that teichoic acid cannot be functionally replaced by teichuronic acid.
DISCUSSION
In this paper we report on three conditionally complemented deletions of teichoic acid biosynthetic genes in B. subtilis. Complementation was dependent on the expression of a xylose-inducible copy of the gene at an alternate locus (amyE). We clearly showed that tagB and tagF are essential to the viability of B. subtilis 168, which is consistent with the involvement of each gene product in a critical step of teichoic acid biosynthesis. The indispensable nature of tagB and tagF is in good agreement with previous studies in which the workers utilized either thermosensitive mutant strains (7, 37) or disruptions of these genes obtained via insertion of a nonreplicating plasmid (23). The B. subtilis 168 tagB and tagF deletion strains used in this work are not restricted to studies performed at an elevated temperature and have well-defined genetic backgrounds, unlike the thermosensitive mutants that arose from chemical mutagenesis (7, 9, 37). Furthermore, conditional complementation of a precise deletion rules out the possibility of a polar effect, a problem that can arise when plasmid-based disruption strategies are employed. We were especially mindful of this fact given that tagB and tagF are situated in a divergently transcribed operon containing other essential genes (31).
The precise deletion and conditional complementation of tarD in a 168-W23 hybrid strain mark the first time that a poly(ribitol phosphate) biosynthetic enzyme has been shown to be essential. This suggests that poly(ribitol phosphate) is indispensable for the viability of B. subtilis W23. The essentiality of tarD also produces another interesting implication: namely, that the glycerol phosphate monomers in the linkage unit are essential. Studies with strain 168 could not have yielded this conclusion, since the CDP-glycerol cytidylyltransferase TagD synthesizes activated glycerol phosphate for both the linkage unit and the main chain polymer.
Our microscopic examination of the deletion strains correlated well with the morphological phenotype of characterized teichoic acid mutants. Examination of strains grown in the absence of an inducer revealed the irregular coccoid shape observed for the temperature-sensitive tagB mutant at the restrictive temperature (8). Further microscopic characterization of the tagB deletion strain showed wall thickening, septal aberrations, and cell lysis, which we are coming to regard as hallmarks of teichoic acid biogenesis mutants (4, 13). The conservation of teichoic acid biosynthetic proteins and the striking phenotype associated with depletion of the B. subtilis tag gene products suggest that teichoic acid biogenesis is a vital and conserved process in gram-positive bacteria.
The strict requirement for wall teichoic acid, moreover, appears to extend to phosphate limitation. The notion that Bacillus species completely replace teichoic acid with teichuronic acid under phosphate-limited conditions has long been entrenched in the literature. Contrary evidence has surfaced, but arbitrating between conflicting cell wall analyses is difficult, especially when the methodologies of groups differ slightly. The results of our cell wall analysis, in accordance with those of Lang et al. (26), show that the proportion of phosphate in the walls is reduced as the phosphate concentration in the medium decreases. However, in contrast to Lang et al. and in agreement with Soldo et al. (40) and Lahooti and Harwood (25), we found that phosphate comprises a significant fraction of the anionic component in the walls of phosphate-limited cells. Our values are consistent with observations that the level of tag gene expression is one-third or one-half of the normal level during phosphate limitation (28, 32). Together, these data support a model in which substantial quantities of teichoic acid continue to be incorporated into the wall.
What makes this model convincing is the essentiality of various teichoic acid biosynthetic genes when phosphate is limited. The response of tagB, tagD, and tagF deletion strains [notably, a strain with a deletion in tagF, which encodes the poly(glycerol phosphate) polymerase] suggests that the polymer itself is indispensable. Indeed, this phosphate-independent essentiality appears to extend also to poly(ribitol phosphate), since tarD was also shown here to be essential under phosphate-limiting conditions. It appears that teichoic acid remains a major cell wall polymer when phosphate is scarce, so that a forced genetic halt of teichoic acid biosynthesis has severe consequences.
The simplest interpretation of our data is that the failure of teichuronic acid to rescue the teichoic acid synthesis mutants is rooted in functional differences between teichuronic acid and teichoic acid. The two polymers have been shown to bind magnesium ions with nearly identical affinities (19) and to regulate autolysins by a proton motive force (10). However, teichoic acid may play a unique role that teichuronic acid cannot play. Indeed, as phosphate becomes increasingly limited, B. subtilis 168 sacrifices culture density rather than fully depleting its teichoic acid (26). Also indicative of a unique function is our inability to generate an extragenic suppressor mutation in the tagD temperature-sensitive strain. Intragenic reversion might be considered less likely than upregulation of teichuronic acid synthesis by mutation of phoP, phoR, or the tua promoter. For example, Muller et al. (33) isolated a phoR mutant that induced and hyperinduced teichuronic acid production under phosphate-replete and phosphate-limited conditions, respectively (although in neither of these cases was replacement very extensive). The failure to identify extragenic suppressors does not preclude their potential generation, but it suggests that teichoic acid is essential in B. subtilis 168 and cannot be easily replaced by increased teichuronic acid synthesis or alteration of other pathways.
While our results have cast doubt on the paradigm of total replacement of teichoic acid by teichuronic acid during phosphate limitation, conflicts in the literature persist. As mentioned above, our cell wall analyses differ from those of other groups. Moreover, Cheah et al. reported that they could not detect significant activity of TarD and other Tar proteins in phosphate-limited cultures of strain W23 (11). It is difficult to reconcile the indispensability and inactivity of the TarD enzyme under identical conditions. New approaches are needed to clarify the mechanics of anionic polymer synthesis in phosphate-limited B. subtilis.
Supplementary Material
Acknowledgments
We acknowledge the generosity of Vladimir Lazarevic (Université de Lausanne), who provided L5706; Dmitri Karamata (Université de Lausanne), who provided L6602; and Petra Levin (Washington University), who provided pUS19.
A.P.B. was supported by an Ontario Graduate Scholarship from the Ontario Ministry of Training, Colleges, and Universities and by a Canada Graduate Scholarship from the Canadian Institutes of Health Research. J.W.S. was supported by an Ontario Graduate Scholarship awarded by the Ontario Ministry of Training, Colleges, and Universities. E.D.B. holds a Canada Research Chair in Microbial Biochemistry. This work was supported by operating grant MOP-15496 from the Canadian Institutes of Health Research.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ames, B. N. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8:115-118. [Google Scholar]
- 2.Araki, Y., and E. Ito. 1989. Linkage units in cell walls of gram-positive bacteria. Crit. Rev. Microbiol. 17:121-135. [DOI] [PubMed] [Google Scholar]
- 3.Benson, A. K., and W. G. Haldenwang. 1993. Regulation of sigma B levels and activity in Bacillus subtilis. J. Bacteriol. 175:2347-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhavsar, A. P., T. J. Beveridge, and E. D. Brown. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol 3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J. Bacteriol. 183:6688-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhavsar, A. P., X. Zhao, and E. D. Brown. 2001. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl. Environ. Microbiol. 67:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenkrantz, N., and G. Asboe-Hansen. 1973. New method for quantitative determination of uronic acids. Anal. Biochem. 54:484-489. [DOI] [PubMed] [Google Scholar]
- 7.Boylan, R. J., and N. H. Mendelson. 1969. Initial characterization of a temperature-sensitive rod− mutant of Bacillus subtilis. J. Bacteriol. 100:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan, R. J., N. H. Mendelson, D. Brooks, and F. E. Young. 1972. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J. Bacteriol. 110:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briehl, M., H. M. Pooley, and D. Karamata. 1989. Mutants of Bacillus subtilis 168 thermosensitive for growth and wall teichoic acid biosynthesis. J. Gen. Microbiol. 135:1325-1334. [Google Scholar]
- 10.Calamita, H. G., and R. J. Doyle. 2002. Regulation of autolysins in teichuronic acid-containing Bacillus subtilis cells. Mol. Microbiol. 44:601-606. [DOI] [PubMed] [Google Scholar]
- 11.Cheah, S. C., H. Hussey, and J. Baddiley. 1981. Control of synthesis of wall teichoic acid in phosphate-starved cultures of Bacillus subtilis W23. Eur. J. Biochem. 118:497-500. [DOI] [PubMed] [Google Scholar]
- 12.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 18:1756-1758. [Google Scholar]
- 13.Cole, R. M., T. J. Popkin, R. J. Boylan, and N. H. Mendelson. 1970. Ultrastructure of a temperature-sensitive rod− mutant of Bacillus subtilis. J. Bacteriol. 103:793-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutting, S., and P. Youngman. 1994. Gene transfer in gram-positive bacteria, p. 348-364. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 15.Ellwood, D. C., and D. W. Tempest. 1969. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. niger. Biochem. J. 111:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant, W. D. 1979. Cell wall teichoic acid as a reserve phosphate source in Bacillus subtilis. J. Bacteriol. 137:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harry, E. J., K. Pogliano, and R. Losick. 1995. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol. 177:3386-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harwood, C., and S. Cutting (ed.). 1990. Modern microbiological methods: molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 19.Heckels, J. E., P. A. Lambert, and J. Baddiley. 1977. Binding of magnesium ions to cell walls of Bacillus subtilis W23 containing teichoic acid or teichuronic acid. Biochem. J. 162:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karamata, D., and J. D. Gross. 1970. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol. Gen. Genet. 108:277-287. [DOI] [PubMed] [Google Scholar]
- 21.Karamata, D., M. McConnell, and H. J. Rogers. 1972. Mapping of rod mutants of Bacillus subtilis. J. Bacteriol. 111:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karamata, D., H. M. Pooley, and M. Monod. 1987. Expression of heterologous genes for wall teichoic acid in Bacillus subtilis 168. Mol. Gen. Genet. 207:73-81. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Debarbouille, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M. F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauel, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. Ohanan, M. O'Reilly, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. Seegers, J. Sekiguchi, A. Sekowska, S. J. Seror, M. Simon, P. Stragier, R. Studer, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, U. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruyssen, F. J., W. R. de Boer, and J. T. Wouters. 1980. Effects of carbon source and growth rate on cell wall composition of Bacillus subtilis subsp. niger. J. Bacteriol. 144:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahooti, M., and C. R. Harwood. 1999. Transcriptional analysis of the Bacillus subtilis teichuronic acid operon. Microbiology 145:3409-3417. [DOI] [PubMed] [Google Scholar]
- 26.Lang, W. K., K. Glassey, and A. R. Archibald. 1982. Influence of phosphate supply on teichoic acid and teichuronic acid content of Bacillus subtilis cell walls. J. Bacteriol. 151:367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarevic, V., F. X. Abellan, S. B. Moller, D. Karamata, and C. Mauel. 2002. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology 148:815-824. [DOI] [PubMed] [Google Scholar]
- 28.Liu, W., S. Eder, and F. M. Hulett. 1998. Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP-P. J. Bacteriol. 180:753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, W., and F. M. Hulett. 1998. Comparison of PhoP binding to the tuaA promoter with PhoP binding to other Pho-regulon promoters establishes a Bacillus subtilis Pho core binding site. Microbiology 144:1443-1450. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald, K. 2001. Bactericidal effect of Pseudomonas aeruginosa PA01 gentamicin-induced membrane vesicles on Gram-positive bacteria. M.S. thesis. University of Guelph, Guelph, Ontario, Canada. [Online.] http://wwwlib.umi.com/cr/uoguelph/fullcit?pMQ61923.
- 31.Mauel, C., M. Young, and D. Karamata. 1991. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J. Gen. Microbiol 137:929-941. [DOI] [PubMed] [Google Scholar]
- 32.Mauel, C., M. Young, A. Monsutti-Grecescu, S. A. Marriott, and D. Karamata. 1994. Analysis of Bacillus subtilis tag gene expression using transcriptional fusions. Microbiology 140:2279-2288. [DOI] [PubMed] [Google Scholar]
- 33.Muller, J. P., Z. An, T. Merad, I. C. Hancock, and C. R. Harwood. 1997. Influence of Bacillus subtilis phoR on cell wall anionic polymers. Microbiology 143:947-956. [DOI] [PubMed] [Google Scholar]
- 34.Pal, M. K., T. C. Ghosh, and J. K. Ghosh. 1990. Studies on the conformation of and metal ion binding by teichoic acid of Staphylococcus aureus. Biopolymers 30:273-277. [DOI] [PubMed] [Google Scholar]
- 35.Park, Y. S., T. D. Sweitzer, J. E. Dixon, and C. Kent. 1993. Expression, purification, and characterization of CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. J. Biol. Chem. 268:16648-16654. [PubMed] [Google Scholar]
- 36.Pooley, H. M., F. X. Abellan, and D. Karamata. 1992. CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase, which is involved in the synthesis of the major wall teichoic acid in Bacillus subtilis 168, is encoded by tagF (rodC). J. Bacteriol. 174:646-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers, H. J., M. McConnell, and I. D. Burdett. 1970. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J. Gen. Microbiol. 61:155-171. [DOI] [PubMed] [Google Scholar]
- 38.Schertzer, J. W., and E. D. Brown. 2003. Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro. J. Biol. Chem. 278:18002-18007. [DOI] [PubMed] [Google Scholar]
- 39.Soldo, B., V. Lazarevic, and D. Karamata. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148:2079-2087. [DOI] [PubMed] [Google Scholar]
- 40.Soldo, B., V. Lazarevic, M. Pagni, and D. Karamata. 1999. Teichuronic acid operon of Bacillus subtilis 168. Mol. Microbiol. 31:795-805. [DOI] [PubMed] [Google Scholar]
- 41.Vellanoweth, R. L., and J. C. Rabinowitz. 1992. The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol. Microbiol. 6:1105-1114. [DOI] [PubMed] [Google Scholar]
- 42.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 43.Wright, J., and J. E. Heckels. 1975. The teichuronic acid of cell walls of Bacillus subtilis W23 grown in a chemostat under phosphate limitation. Biochem. J. 147:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama, K., T. Miyashita, Y. Araki, and E. Ito. 1986. Structure and functions of linkage unit intermediates in the biosynthesis of ribitol teichoic acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur. J. Biochem. 161:479-489. [DOI] [PubMed] [Google Scholar]
- 45.Young, M., C. Mauel, P. Margot, and D. Karamata. 1989. Pseudo-allelic relationship between non-homologous genes concerned with biosynthesis of polyglycerol phosphate and polyribitol phosphate teichoic acids in Bacillus subtilis strains 168 and W23. Mol. Microbiol. 3:1805-1812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.