Abstract
The stable effector functionLess (SEFL) antibody was designed as an IgG1 antibody with a constant region that lacks the ability to interact with Fcγ receptors. The engineering and stability and pharmacokinetic assessments of the SEFL scaffold is described in the accompanying article (Jacobsen, F. W., Stevenson, R., Li, C., Salimi-Moosavi, H., Liu, L., Wen, J., Luo, Q., Daris, K., Buck, L., Miller, S., Ho, S-Y., Wang, W., Chen, Q., Walker, K., Wypych, J., Narhi, L., and Gunasekaran, K. (2017) J. Biol. Chem. 292). The biological properties of these SEFL antibodies were assessed in a variety of human and cynomolgus monkey in vitro assays. Binding of parent molecules and their SEFL variants to human and cynomolgus monkey FcγRs were evaluated using flow cytometry-based binding assays. The SEFL variants tested showed decreased binding affinity to human and cynomolgus FcγRs compared with the wild-type IgG1 antibody. In addition, SEFL variants demonstrated no antibody-dependent cell-mediated cytotoxicity in vitro against Daudi cells with cynomolgus monkey peripheral blood mononuclear cells, and had minimal complement-dependent cytotoxicity activity similar to that of the negative control IgG2 in a CD20+ human Raji lymphoma cell line. SEFL mutations eliminated off-target antibody-dependent monocyte phagocytosis of cynomolgus monkey platelets, and cynomolgus platelet activation in vitro. These experiments demonstrate that the SEFL modifications successfully eliminated Fc-associated effector binding and functions.
Keywords: antibody engineering, Fc receptor, immunoglobulin G (IgG), phagocytosis, platelet, platelet depletion, antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, cynomolgus macaque
Introduction
Monoclonal antibodies (mAbs) are the largest class of biopharmaceuticals and have diverse clinical applications (1). The choice of therapeutic mAb isotype to develop (IgG1, IgG2, or IgG4) is dependent on the target (cell surface versus soluble), desired biology, safety (risk of immunogenicity and undesired immunological effects), and manufacturability (expression, formulation, and stability). More than 80% of approved therapeutic mAbs are IgG1 isotypes that target cell surface receptors and are effective for oncology indications (2). For these therapeutic approaches, mAb isotypes that can induce cell killing such as complement-dependent cytotoxicity (CDC)2 and antibody-dependent cell-mediated cytotoxicity (ADCC) are often desirable (3, 4). However, for non-oncologic indications, therapeutic mAbs without cytotoxic effector function may be more appropriate because cell killing may not be a goal of therapy.
The Fc portion of IgG has interaction sites for the effector ligands, including Fcγ receptors (FcγRI, FcγRII, and FcγRIII), C1q complement, and the neonatal Fc receptor (FcRn). IgG isotypes differentially engage Fcγ receptors and C1q binding to recruit immune effector functions and initiate cytotoxic effector functions, (either ADCC or CDC (5)). Historically, IgG2 or IgG4 isotypes were thought to have minimal cytotoxic effector function, and have been selected for applications where cytotoxic effector function is not required or desirable (5). However, recent evidence suggests that IgG2 is not completely devoid of cytotoxic effector function under certain culture conditions (6, 7). In addition, IgG2 can also bind to Fcγ receptors to induce non-cytotoxic effector functions. For example, IgG2 isotypes can mediate phagocytosis via monocytes/macrophages and neutrophils through interaction with the FcγRIIa on platelets (8), and binding to the FcγRIIa along with a cell surface antigen has been implicated in off-target effects of mAbs on platelets (9, 10).
IgG mAb isotypes differ in stability as well as effector functions. IgG2s have decreased conformational stability relative to IgG1s, which can result in a higher propensity to aggregate compared with IgG1 in the native state (11, 12). The three different disulfide bond arrangements possible in the hinge region of IgG2s enable disulfide shuffling, which can result in a more heterogeneous product (13). mAbs based on the IgG4 scaffold can disassociate and pair with endogenous IgG4 antibodies in vivo, leading to bispecific antibodies and variable efficacy of the therapeutic (14).
Improved mAb constructs would combine the best properties of the IgG1 and IgG2 molecules: the homogeneity and stability of an IgG1, a single disulfide bond, and virtually no effector functions. To eliminate effector functions, Fc modifications have been introduced that significantly reduce binding to FcγR (15, 16). Mutation of the glycosylation site (Asn297) in the CH2 domain of IgG1 generates aglycosylated mAbs, which have very low binding to FcγRs (11, 17). Mutations of Asn297 are advantageous in that the introduction of only a single mutation in the constant domain is likely to minimize the risk of immunogenicity (18). Several aglycosylated IgG1 therapeutic mAbs are currently in clinical trials (i.e. Millennium ACT2 mAb is an IgG1 with N297A mutations to remove ADCC (17)). However, the N297A mutation is less thermally stable compared with natural, unmutated IgG1s (19, 20). The Asn297 mutated to Gly results in an Fc constant region that lacks the ability to interact with the FcγRs (21) and shows improved thermal stability compared with other aglycosylated mAbs with good manufacturing and pharmacokinetic properties (22). These mAbs, termed stable effector functionless (SEFL) were tested for Fc functionality in a variety of biologic systems.
In this article, we describe the characterization of the biology of diverse therapeutic mAbs (16 variants of 5 mAbs) (22) to determine whether the SEFL modifications eliminated FcγR binding and Fc effector functions (Table 1). SEFL mAbs have been shown to retain FcRn binding and binding to the target antigen (22). Isotype control mAbs, mAbs that had previously been shown to have effector functions and SEFL variants of these mAbs were evaluated in a variety of assays, including binding strength to human and cynomolgus FcγRs in cell-based flow cytometry assays, binding to cynomolgus peripheral blood granulocytes, cynomolgus PBMC ADCC assay, and a human CDC assay. In addition to the assessment of target-mediated Fc effector function, off-target-mediated Fc effector function of SEFL mAb was assessed in platelet phagocytosis and platelet activation assays. These experiments demonstrated that the SEFL modification of mAbs successfully eliminated both on-target and off-target Fc-associated effector functions.
TABLE 1.
Therapeutic mAbs and variants tested for Fc binding and effector functions
| Molecule name | Type |
|---|---|
| mAbV.IgG2 | IgG2 isotype control |
| mAbX.IgG2a | IgG2 |
| mAbX.SEFL2.0b | IgG1 L242C,N297G,K334C |
| mAbY.IgG2c | IgG2 |
| mAbY.IgG1 | IgG1 |
| mAbY.SEFL2.0b | IgG1 L242C,N297G,K334C |
| RTX.IgG1 | IgG1 |
| RTX.IgG2 | IgG2 |
| RTX.SEFL1.1b | IgG1 N297G |
| RTX.SEFL2.0b | IgG1 L242C,N297G,K334C |
| mAbW.IgG1 | IgG1 |
| mAbW.IgG2 | IgG2 |
| mAbW.SEFL1.1b | IgG1 N297G |
| mAbW.SEFL2.0b | IgG1 L242C,N297G,K334C |
| mAbW.SEFL2.1b | IgG1 A287C,N297G,L306C |
| mAbW.SEFL2.2b | IgG1 R292C,N297G,V302C |
Results
Binding of parent mAb and their SEFL variants to human and cynomolgus monkey FcγRs were evaluated using flow-based binding assays.
Binding Affinity of IgG1 Variants to Human FcγRs
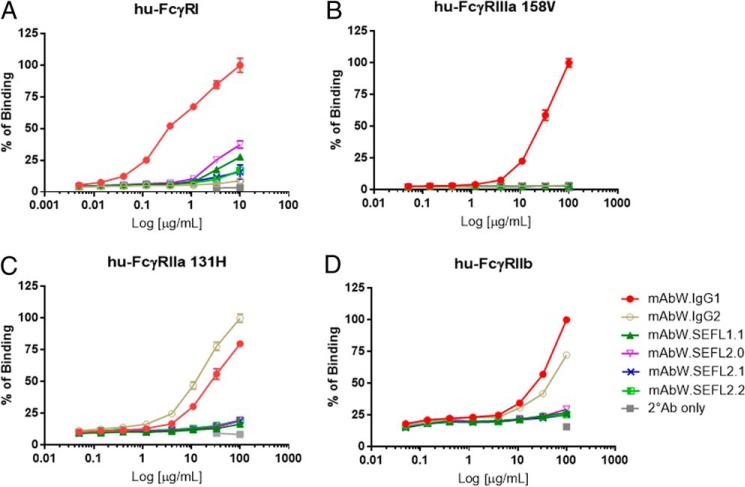
The binding affinity of IgG1 (mAbW.IgG1) and IgG2 (mAbW.IgG2), and modified IgG1 SEFL mAbs (mAbW.SEFL1.1, mAbW.SEFL2.0, mAbW.SEFL2.1, and mAbW.SEFL2.2; Table 1) to human FcγRI, FcγRIIa (isoform His131, which has higher binding affinity for both human IgG1 and IgG2 in comparison to other isoforms (7)), FcγRIIb, FcγRIIIa (isoform Val158), and FcγRIII (isoform Phe158) was assessed using flow cytometry. All mAbW SEFL variants showed decreased binding affinity to all the tested human FcγRs compared with the wild-type mAbW.IgG1 antibody (Fig. 1). For human FcγRI, the binding affinity of engineered mAbW SEFL variants decreased ∼70% to that of wild-type mAbW.IgG1 (Fig. 1A). For human FcγRIIIa (isoforms Val158 and Phe158), none of the engineered mAbW SEFL variants showed detectable binding, similar to the results obtained with mAbW.IgG2 (Fig. 1B) (data not shown for Phe158). For human FcγRIIb and FcγRIIa (isoform His131), the binding affinity showed minimal measurable binding of the SEFL variants compared with either wild-type mAbW.IgG1 or mAbW.IgG2 (Fig. 1, C and D).
FIGURE 1.
Binding of SEFL mAbW variants (mAbW.SEFL1.1, mAbW.SEFL2.0, mAbW.SEFL2.1, and mAbW.SEFL2.2) to human FcγRs. Dose-response binding curves of each mAb to human FcγRI (A), FcγRIIIa (Val158) (B), FcγRIIa (His131) (C), and FcγRIIb (D) were generated using FACS cell-based binding assay. mAbW IgG1 and IgG2 were used as controls. Data points were collected in duplicate and the normalized percentage of binding was calculated based on the mAbW.IgG1 for all except hu-FcγRIIa binding, which was based on mAbW.IgG2. The percentage of binding was plotted against antibody concentration.
Binding Affinity of IgG1 Variants to Cynomolgus Monkey FcγRs
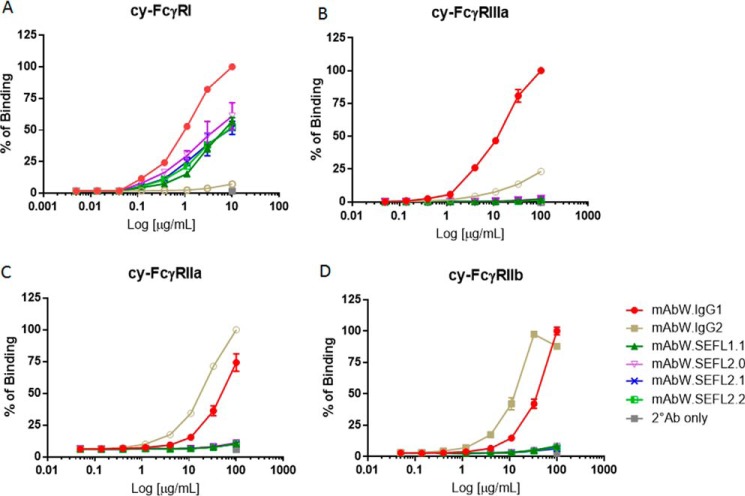
The binding affinity of mAbW.IgG1, mAbW.IgG2, mAbW.SEFL1.1, mAbW.SEFL2.0, mAbW.SEFL2.1, and mAbW.SEFL2.2 (Table 1) to cynomolgus monkey FcγRI, FcγRIIa, FcγRIIIa, and FcγRIIb was also assessed. Similar to the results of human FcγRs, all mAbW SEFL variants showed decreased binding affinity to all tested cynomolgus monkey FcγRs compared with wild-type mAbW.IgG1 (Fig. 2). For FcγRI, the binding affinity of mAbW.SEFL1.1 and mAbW.SEFL2.0 decreased 40–50% compared with that of wild-type IgG1 (Fig. 2A). For FcγRIIIa, mAb SEFL variants exhibited minimal or no measurable binding, similar to that of mAbW.IgG2 (Fig. 2B). For cynomolgus monkey FcγRIIa and FcγRIIb, the binding affinity of mAb SELF variants was not detectable, whereas mAbW.IgG2 demonstrated higher affinity than mAbW.IgG1 (Fig. 2, C and D).
FIGURE 2.
Binding of SEFL mAbW variants to cynomolgus monkey FcγRs. Dose-response binding curves of each mAb to cynomolgus monkey FcγRI (A), FcγRIIIa (B), FcγRIIa (C), and FcγRIIb (D) were generated using FACS cell-based binding assay. mAbW.IgG1 and mAbW.IgG2 antibodies were used as controls. Data points were collected in duplicate and the normalized percentage of binding was calculated based on mAbW.IgG1 for all except cy-FcγRIIa binding. The percentage of binding was plotted against antibody concentration.
Cynomolgus Monkey Granulocyte Binding Assay
We have previously observed that some human IgG2 mAb clones will bind nonspecifically to cynomolgus granulocytes despite lack of target expression (for example, see Ref. 7). Therefore we compared the ability of the parental and SEFL forms of such mAbs to bind to cynomolgus peripheral blood granulocytes using flow cytometric analyses. SEFL variants of mAbW (Table 1) and an IgG2 control mAbV.IgG2 (Table 1) did not directly bind to cynomolgus monkey granulocytes (Fig. 3). In contrast, mAbW.IgG1 bound to cynomolgus monkey granulocytes demonstrating that off-target binding to cynomolgus monkey cells occurring with an IgG1 mAb can also be eliminated with the SEFL construct.
FIGURE 3.
Direct binding of mAbs to cynomolgus monkey granulocytes was assessed by flow cytometry. SEFL mAbW variants (mAbW.SEFL1.1, mAbW.SEFL2.0, mAbW.SEFL2.1, and mAbW.SEFL2.2), the IgG2 control mAbV.IgG2, and the IgG1 control mAbW.IgG1 were tested for direct binding to cynomolgus monkey granulocytes.
ADCC Assay
mAbW.IgG1 causes ADCC in vitro against Daudi cells in an assay using cynomolgus monkey PBMC effector cells (Fig. 4). To confirm that the ADCC activity of the SEFL mAbs was eliminated the mAbW antibody variants (mAbW.SEFL1.1 and mAbW.SEFL2.0; see Table 1) and control (mAbW.IgG2) were assessed in this assay. mAbW.IgG2, mAbW.SEFL1.1, and mAbW.SEFL2.0 did not mediate ADCC in vitro against Daudi cells with cynomolgus monkey PBMC. The slightly negative cytotoxicity seen for mAbW.SEFL1.1 is within the experimental variation due to the use of PBMC from cynomolgus monkey.
FIGURE 4.
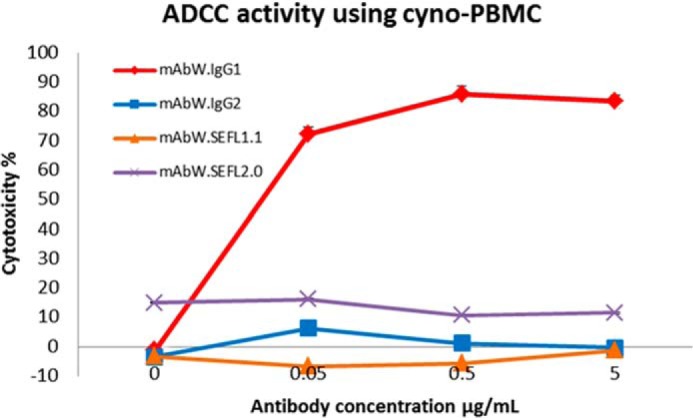
Evaluation of ADCC activity of the SEFL mAbW variants (mAbW.SEFL1.1 and mAbW.SEFL2.0). Only mAbW.IgG1 mediates in vitro ADCC on Daudi cells with cynomolgus monkey PBMC effector cells. Cyno, cynomolgus monkey. Each data point represents an average of triplicate measurements.
Rituximab-mediated CDC Assays
Rituximab causes CDC in a CD20+ human Raji lymphoma cell line (23). To further examine the effector functions of the SEFL mAb, the cytotoxic activities of two rituximab SEFL variants (RTX.SEFL1.1 and RTX.SEFL2.0; Table 1) were assessed for CDC activity in this assay (Fig. 5). Rituximab (RTX.IgG1) showed CDC activity of 27% cell death at a mAb concentration of 100 μg/ml, whereas both SEFL variants of rituximab (RTX.SEFL1.1 and RTX.SEFL2.0) at a concentration of 100 μg/ml were similar to that of the negative control RTX.IgG2.
FIGURE 5.
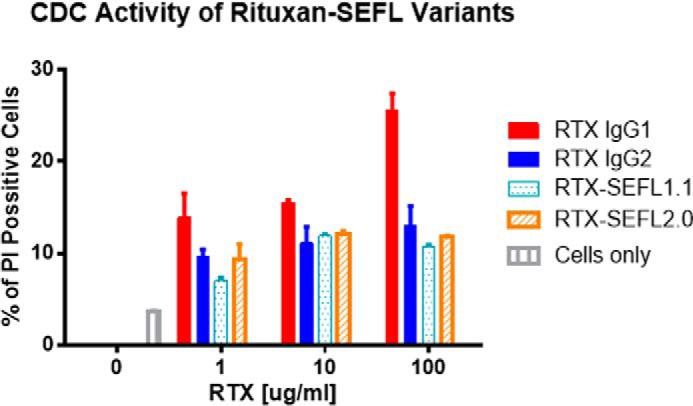
Rituximab variants RTX. SEFL1.1 or RTX.SEFL2.0 and negative control RTX.IgG2 have minimal CDC activity compared with the wild-type rituximab. Rituximab (RTX.IgG1), RTX.IgG2, RTX.SEFL1.1, and RTX.SEFL2.0 mAb at 1, 10, and 100 μg/ml were added to Raji lymphoma cells for 24 h and the percentage of cell killing measured by flow cytometric analysis after adding propidium iodide (5 μg/ml). Data points were collected in duplicate and percentage of propidium iodide-positive cells was measured against total gated cells.
Cynomolgus Monkey Platelet Phagocytosis
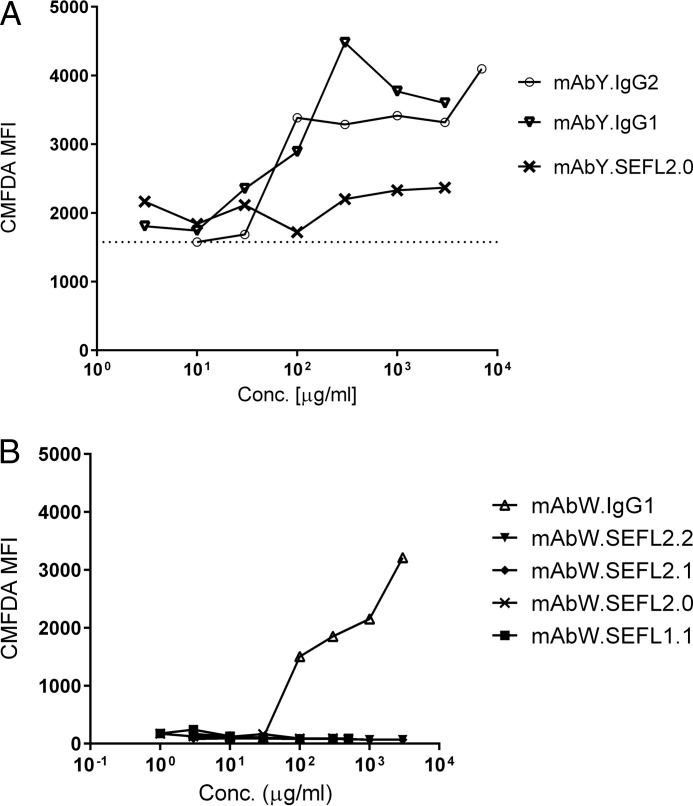
mAbY is an IgG2 mAb that has previously been shown to mediate non-target dependent phagocytosis of platelets by cynomolgus monocyte/macrophages both in vivo and in vitro in a process requiring Fc function (10). In vitro, parental mAbY.IgG2 and IgG1 constructs, mAbY.IgG1 and mAbW.IgG1, caused phagocytosis of cynomolgus monkey platelets at concentrations ≥0.1 mg/ml (Fig. 6). In contrast, SEFL variants of mAbY.IgG2 (mAbY.SEFL2.0) and mAbW.IgG1 (mAbW.SEFL1.1, mAbW.SEFL2.0, mAbW.SEFL2.1, and mAbW.SEFL2.2) did not induce cynomolgus monocytes to phagocytose platelets, demonstrating that the off-target effects are at least in part mediated by binding to FcγR.
FIGURE 6.
Phagocytosis of platelets by cynomolgus monocytes in vitro. Cynomolgus peripheral blood leukocytes and CMFDA-labeled platelets were co-incubated with a test article for 6 h and assessed for phagocytosis of platelets by monocytes by measuring CMFDA fluorescence in monocytes by flow cytometry. A, cynomolgus peripheral blood leukocytes and CMFDA-labeled platelets were co-incubated with mAbY.IgG2, mAbY.IgG1, or mAbY.SEFL2.0. Dotted line indicates the MFI of the IgG2 isotype control at the lowest concentration tested. B, cynomolgus peripheral blood leukocytes and CMFDA-labeled platelets were co-incubated with mAbW.IgG1, mAbW.SEFL1.1, mAbW.SEFL2.0, mAbW.SEFL2.1, or mAbW.SEFL2.2. Note that different antibodies and instruments were used in A and B and this results in the baseline variation. However, it is clear that SEFL antibodies do not induce phagocytosis unlike the IgG1 or IgG2 controls.
Platelet Activation Assays
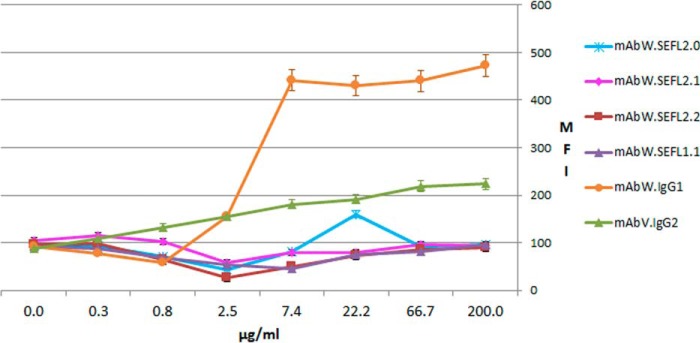
mAbX, is an IgG2 mAb that has previously been shown to cause cynomolgus, but not human, platelet activation in vitro through an Fc-dependent process (9). As shown in Fig. 7, parental mAbX.IgG2 (positive control) caused cynomolgus platelet activation at concentrations as low as 1 mg/ml. In contrast, a SEFL variant of mAbX (mAbX.SEFL2.0) caused no activation of cynomolgus platelets at concentrations up to 7.8 mg/ml.
FIGURE 7.
Activation of cynomolgus platelets as indicated by CD62P expression. Whole blood was incubated with isotype control antibody (mAbV.IgG2), mAbX.IgG2, mAbX.SEFL2.0, vehicle control, or 4-phorbol 12-myristate 13-acetate positive control. Platelet activation was assessed by CD62P expression in flow cytometry.
Discussion
Altering the Fc portion of a mAb has been conducted previously to either increase or decrease cytotoxic effector functions (ADCC and CDC) (5, 15, 24–30). Because IgGs bind similarly to both human and cynomolgus monkey FcγR (31), and cynomolgus monkeys are often used in the safety assessment of biotherapeutics, it is helpful to demonstrate that the modifications have similar functional consequences in humans and cynomolgus monkeys. The SEFL mAb variants tested showed decreased binding affinity to human and cynomolgus monkey FcγRs compared with the corresponding wild-type IgG1. The binding affinity of the SEFL IgG1 was decreased 40–50% for cynomolgus monkey FcγRI and decreased by ∼70% for human FcγRI in comparison to wild-type IgG1. In addition, cynomolgus monkey PBMC ADCC activity was eliminated using the SEFL mAb variants suggesting that reduced binding was sufficient to block this cytotoxic effector function. The SEFL modification of a mAb eliminated CDC activity in a human cell system, also indicating that the decreased FcγR binding correlated with a loss of cytotoxic effector function. However, under the constraints of the availability of cells for assays, ADCC and CDC activity was not tested in both cynomolgus monkey and human cells.
Several mAbs have now shown off-target effects (8), although the mechanisms involved are not well understood. Our results further support that some of these off-target effects require Fc-FcγR binding (10), and that the elimination of the effector function of a mAb may eliminate off-target reactions as well (as shown for mAbX and mAbY (9, 10)). The generation of a SEFL mAb construct offers a path forward for development of IgG1-based mAbs when cytotoxicity is not desired, by eliminating the Fc-mediated events. The use of SEFL constructs eliminated mAb binding to human and cynomolgus FcγRIIa (including high binding isoform His131) and FcγRIIb, whereas the non-mutated IgG1 and IgG2 show significant binding. Because FcγRII has been found on platelets and likely contributes to the non-target, non-cytotoxic effector functions seen with two IgG2 mAbs, mAbY and mAbX (9, 10), SEFL mAb constructs should eliminate these effects. Indeed, SEFL variants of mAbY and mAbX could not induce either platelet phagocytosis or platelet activation in contrast with their parental mAb constructs, and SEFL variants of mAbW did not bind to cynomolgus neutrophils. Thus, the change in FcγR binding also correlated with decreased off target effector function. Importantly, the SEFL mAb constructs do not impact FcRn binding such that the normal half-life of mAbs was maintained (22).
The SEFL mAb construct used in these studies also has superior manufacturability features compared with IgG2 mAbs, which are described in the accompanying article (22). This combination of stability, homogeneity (through elimination of disulfide variants and Asn297 glycosylation), and lack of effector functions make this a promising scaffolding on which to build protein therapeutics for diseases where effector functions are not desired, such as autoimmune diseases.
Conclusion
In these experiments, we have shown that novel SEFL mAb constructs can significantly decrease binding to human and cynomolgus monkey FcγR, and eliminate cytotoxic and non-cytotoxic effector functions in human and cynomolgus monkey in vitro systems. SEFL mutations show similar FcγR binding in humans and cynomolgus monkeys. The SEFL mAb variants attenuate ADCC and CDC activity in cynomolgus and human in vitro systems, respectively. Non-cytotoxic effector functions are also eliminated or reduced in a cynomolgus in vitro system (platelet activation, platelet phagocytosis, and nonspecific binding to cynomolgus neutrophils).
This article demonstrates that a mutation that eliminates effector function by decreasing binding to FcγR, acts similarly in humans and cynomolgus monkey immune cells in vitro, and is a promising scaffolding on which to build protein therapeutics for diseases where effector functions are not desired and where non-cytotoxic, non-target related effects on platelets may be an issue.
Experimental Procedures
Human FcγR Cell Lines
293T cells were stably transfected with single FcγR. There were different cell lines expressing human FcγRI, FcγRIIa-131H, FcγRIIa-131R, FcγRIIb, FcγRIIIa-158F, or FcγRIIIa-158V. Cells were maintained using Dulbecco's modified Eagle's medium (DMEM) (Gibco Grand Island, NY, number 11965) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 100 units/ml of penicillin and 100 μg/ml of streptomycin (Life Technologies, Grand Island, NY), and 2 mm l-glutamine (Gibco), 3–4 μg/ml of blasticidin (Life Technologies, A1113903), and 200 μg/ml of Zeocin (Invitrogen, catalog number ant-zn-1).
Cynomolgus Macaque FcγR Cell Lines
AM-1/D cells were also stably transfected with single FcγR. The different cell lines include expression of cyno FcγRI, FcγRIIa, FcγRIIb, or FcγRIIIa. Cells were maintained using DMEM (Gibco number 11965) supplemented with 10% FBS (HyClone), 100 units/ml of penicillin, and 100 μg/ml of streptomycin (Life Technologies), and 2 mm l-glutamine (Gibco), and 10 μg/ml of puromycin (Life Technologies, A1113902).
Fcγ Cell-based Binding Assay
The cell-based IgG1/FcγR flow cytometry assay measures the relative binding of IgG1 to 293T cells or AM-1D cells (Amgen Inc.) stably transfected to express a single type of FcγR. 293T or AM-1D-FcγR cells were incubated with the IgG1 samples at increasing concentrations in 200 μl of binding buffer containing 2% FBS in DPBS −Ca2+, −Mg2+ (Gibco). Incubation of the cells and antibody was for 1 h at 4 °C. The cells were washed twice with binding buffer and then the 2nd antibody anti-LC κ-FITC (Sigma, SAB4700605) was added to the cells. The final concentration of anti-LC κ-FITC for human FcγRs binding was 200 μg/ml; for cyno FcγRs binding was 40 μg/ml. The bound IgG1 was detected by labeling with an anti-LC κ-FITC. The complex was then fixed using a diluted formalin solution 10% neutral buffer (Sigma, HT5011) before detection using a flow cytometer (BD Bioscience FACSCaliburTM). Ten thousand events were captured and the mean fluorescence intensity (MFI) was plotted versus the antibody concentration.
Binding of SEFLs to Cynomolgus Peripheral Granulocytes
SEFL antibodies were assessed for surface binding to cynomolgus leukocytes as follows: heparinized cynomolgus blood was incubated with a dose titration of parental or SEFL antibodies or anti-streptavidin (negative control antibody) at final concentrations of 200 to 0.03 μg/ml for 30 min at room temperature. One microliter (1 μl) of anti-human IgG-FITC was added to each well (final concentration of 10 μg/ml), and incubated an additional 30 min at room temperature. Two ml of 1× ammonium chloride lysing solution (10× solution prepared as 1.5 m ammonium chloride, 100 mm sodium bicarbonate, 10 mm EDTA, pH 7.4) was added to each well to lyse red blood cells (RBC), and the mixture was incubated at room temperature in the dark for 15 min. Leukocytes were centrifuged at 300 × g for 5 min, and washed twice in FACS buffer (2% fetal bovine serum (FBS), 0.1% sodium azide, PBS, pH 7.4) and suspended in 200 μl of FACS buffer prior to acquisition on a FACSCantoIITM flow cytometer (BD Biosciences). The granulocyte population was gated using SSC versus forward scatter, and MFI was determined.
Antibody-dependent Cell-mediated Cytotoxicity Assay (ADCC) Assay
Cryopreserved cynomolgus monkey PBMCs were obtained from SNBL, USA, Ltd., and thawed just before testing in the ADCC assay according to the vendor's protocol. Daudi cells (1 × 104 cells/well) were seeded in a 96-well plate with or without serially diluted mAbW.IgG1, mAbW.IgG2, mAbW.SEFL1.1, and mAbW.SEFL2.0 in a total volume of 50 μl/well. After incubation at room temperature for 30 min, 25 μl of the cynomolgus PBMC (2 × 105 cells/well) were added to each well of the plate. All cells and antibody molecules were suspended in RPMI1640 medium containing 4% Ultra-low IgG FBS (Gibco). After incubation at 37 °C in 5% CO2 for 22 h, lactate dehydrogenase activities of cell culture supernatant were measured by using the Cytotoxicity Detection Kit from Roche Applied Science. The percentage of cytotoxicity was calculated as described in the manufacturer's protocol.
CDC Assay
Raji lymphoma cells were cultured in medium consisting of RPMI (Life Technologies), 10% FBS (Life Technologies), 100 units/ml of penicillin, 100 μg/ml of streptomycin (Life Technologies), and 2 mm l-glutamine (Life Technologies). The Raji cells were plated in RPMI medium at a density of 2.5 × 105 cells/well into a 96-well plate. Diluted antibody was added to final concentrations of 1, 10, and 100 μg/ml in a final volume of 125 μl. The suspension was kept at room temperature for 10 min before addition of the complement serum to the cells. 25 μl of human complement serum (QUIDEL, San Diego, CA) was added to the cell suspension in each well and the suspension was incubated overnight at 37 °C/5% CO2. The next day, propidium iodide (PI) was added to final concentration 5 μg/ml, 15 min before flow cytometry analysis. The dead cells that are PI positive were analyzed by FACSCaliburTM (BD Bioscience). Percentage of PI positive cells was measured against total gated cells.
Antibody Reagents for in Vitro Assays
The following detection antibodies used for flow cytometric analyses were purchased from BD Biosciences: CD14-PECy7 (number 557742), CD41a-APC (number 559777; mouse anti-human GPIIb), and CD62P-PE (number 550561; mouse anti-human P-selectin). Anti-human IgG-FITC (number A80-319F) was purchased from Bethyl Laboratories (Montgomery, TX). Antibody mAbV is an in-house generated human IgG2 that was used as an isotype control for SEFL antibodies. The mAbZ.IgG2 used as a positive control for surface binding studies on leukocytes is a proprietary human IgG2 antibody. Test articles (parental and modified antibodies) were supplied in vehicle solution at concentrations of 70 mg/ml (mAbX.IgG2, mAbY.IgG2, and mAbW.IgG1) or 30–40 mg/ml.
Sample Preparation
Blood from cynomolgus macaques and human donors was collected into acid citrate dextrose for platelet phagocytosis and activation, and into sodium heparin for flow cytometric analyses of leukocyte binding. Blood samples were used within 2 to 4 h of collection for most studies except when indicated otherwise. For studies involving platelets, blood was collected from subjects not exposed to aspirin, ibuprofen, or other anti-inflammatory analgesics for the previous 7 days. Whole blood samples from human donors or cynomolgus macaques were centrifuged at 170 × g for 15 min without braking. Platelet-rich plasma was collected from the top layer and used for platelet activation and phagocytosis experiments.
Phagocytosis Assay
The in vitro cynomolgus monocyte-platelet phagocytosis assay was performed as described elsewhere (10). In brief, peripheral blood leukocytes (PBL) and platelets were isolated from blood samples of cynomolgus macaques and the buffy coat layer was enriched for PBL. Platelets in platelet-rich plasma were labeled with Cell TrackerTM Green CMFDA (5-chloromethylfluorescein diacetate; Invitrogen, Eugene, OR) at a final concentration of 20 μm for 30 min at room temperature. After washing with PBS, labeled platelets were resuspended in RPMI1640, 10% FBS with a final concentration of 1 × 108 cells/ml. 100 μl of CMFDA-labeled platelets were then incubated with 100 μl of PBL at 5 × 106 cells/ml from an autologous cynomolgus donor with 20 μl of various concentrations of test articles in the dark at 37 °C. After 6 h, the reaction was stopped by transferring the plates to ice. Extracellular fluorescence was quenched by incubating 2 min with 100 μl of 0.1% trypan blue (Life Technologies, number 15250). After washing, cells were labeled with anti-CD14-PE-Cy7 and propidium iodide (Life Technologies, number P3566) for 30 min at 4 °C. CMFDA fluorescence in CD14-expressing monocytes was analyzed by a BD LSRIITM flow cytometer (BD Biosciences) and the data analyzed using FlowJo software (Treestar). The increase of CMFDA fluorescence intensity was used to estimate effects of various reagents on the phagocytic capacity of monocytes for platelets.
Platelet Activation Assay
Five μl of test articles mAbX.5 (0.2–7.6 mg/ml) and controls (5 mg/ml) mAbV.IgG2 (human IgG2 isotype control), 5 μm 4-phorbol 12-myristate 13-acetate (positive control), 5 mg/ml of mAbX.IgG2 (positive control for cynomolgus monkey), and 5 μl of vehicle control (A52SuT-acetate sucrose Tween buffer at pH 5.2) were incubated with 20 μl of acid citrate/dextrose anticoagulated whole blood at the above concentrations for 20 min at room temperature. After incubation, anti-CD41a-APC (for identification of platelets) and anti-CD62P-PE (for assessment of activation) were added and incubated for another 20 min at room temperature. Lyse/fixative solution was added to each sample as the last step and they were analyzed on a FACSCaliburTM (BD Biosciences) and data were analyzed using FlowJo software (Treestar). Activated platelets were identified by an increase in MFI of CD62P compared with resting platelets as previously described (10).
Generation of SEFL mAbs
The SEFL mAbs were generated at Amgen from unamplified Chinese hamster ovary (CHO) cell pools. The SEFL mAbs were recovered from clarified CHO cell condition media using a three-step process. First, the mAbs were affinity-captured using MabSelect SuRe (32) (GE Healthcare Life Sciences). This was then followed by cation exchange chromatography. Finally, the mAbs were dialyzed into sodium acetate buffer for long term stability. The engineering and characterization of these mAbs are presented in the accompanying article (22).
Author Contributions
L. L., F. W. J., N. E., Y. Z., Y. Y., N. L., D. C., M. P. N., M. F., P. N., K. K., L. N., K. G., and J. L. B. designed experiments and co-wrote the manuscript. J. L. B. wrote most of the paper. L. L., R. S., F. W. J., L. N., and K. G. coordinated data collection.
Acknowledgment
We thank Lindsay Martin for contributing to the cynomolgus monkey phagocytosis experiment.
This work was supported by Amgen. All authors work or have worked at Amgen.
- CDC
- complement-dependent cytotoxicity
- CMFDA
- 5-chloromethylfluorescein diacetate
- MFI
- mean fluorescence intensity
- PBMC
- peripheral blood mononuclear cells
- SEFL
- stable effector functionless
- PI
- propidium iodide
- PBL
- peripheral blood leukocyte
- ADCC
- antibody-dependent cell-mediated cytotoxicity.
References
- 1.Walsh G. (2003) Biopharmaceutical benchmarks: 2003. Nat. Biotechnol. 21, 865–870 [DOI] [PubMed] [Google Scholar]
- 2.Nelson A. L., Dhimolea E., and Reichert J. M. (2010) Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 9, 767–774 [DOI] [PubMed] [Google Scholar]
- 3.Musolino A., Naldi N., Bortesi B., Pezzuolo D., Capelletti M., Missale G., Laccabue D., Zerbini A., Camisa R., Bisagni G., Neri T. M., and Ardizzoni A. (2008) Immunoglobulin g fragment c receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 26, 1789–1796 [DOI] [PubMed] [Google Scholar]
- 4.Patel D., Guo X., Ng S., Melchior M., Balderes P., Burtrum D., Persaud K., Luna X., Ludwig D. L., and Kang X. (2010) IgG isotype, glycosylation, and EGFR expression determine the induction of antibody-dependent cellular cytotoxicity in vitro by cetuximab. Hum. Antibodies 19, 89–99 [DOI] [PubMed] [Google Scholar]
- 5.Jefferis R. (2009) Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol. Sci. 30, 356–362 [DOI] [PubMed] [Google Scholar]
- 6.Schneider-Merck T., Lammerts van Bueren J. J., Berger S., Rossen K., van Berkel P. H., Derer S., Beyer T., Lohse S., Bleeker W. K., Peipp M., Parren P. W., van de Winkel J. G., Valerius T., and Dechant M. (2010) Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J. Immunol. 184, 512–520 [DOI] [PubMed] [Google Scholar]
- 7.Warncke M., Calzascia T., Coulot M., Balke N., Touil R., Kolbinger F., and Heusser C. (2012) Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J. Immunol. 188, 4405–4411 [DOI] [PubMed] [Google Scholar]
- 8.Everds N. E., and Tarrant J. M. (2013) Unexpected hematologic effects of biotherapeutics in nonclinical species and in humans. Toxicol. Pathol. 41, 280–302 [DOI] [PubMed] [Google Scholar]
- 9.Santostefano M. J., Kirchner J., Vissinga C., Fort M., Lear S., Pan W.-J., Prince P. J., Hensley K. M., Tran D., Rock D., Vargas H. M., Narayanan P., Jawando R., Rees W., Reindel J. F., Reynhardt K., and Everds N. (2012) Off-target platelet activation in macaques unique to a therapeutic monoclonal antibody. Toxicol. Pathol. 40, 899–917 [DOI] [PubMed] [Google Scholar]
- 10.Everds N., Li N., Bailey K., Fort M., Stevenson R., Jawando R., Salyers K., Jawa V., Narayanan P., Stevens E., He C., Nguyen M. P., Tran S., Doyle N., Poitout-Belissent F., Jolette J., Xu C., and Sprugel K. (2013) Unexpected thrombocytopenia and anemia in cynomolgus monkeys induced by a therapeutic human monoclonal antibody. Toxicol. Pathol. 41, 951–969 [DOI] [PubMed] [Google Scholar]
- 11.Li C. H., Narhi L. O., Wen J., Dimitrova M., Wen Z. Q., Li J., Pollastrini J., Nguyen X., Tsuruda T., and Jiang Y. (2012) Effect of pH, temperature, and salt on the stability of Escherichia coli- and Chinese hamster ovary cell-derived IgG1 Fc. Biochemistry 51, 10056–10065 [DOI] [PubMed] [Google Scholar]
- 12.Ejima D., Tsumoto K., Fukada H., Yumioka R., Nagase K., Arakawa T., and Philo J. S. (2007) Effects of acid exposure on the conformation, stability, and aggregation of monoclonal antibodies. Proteins Struct. Funct. Genet. 66, 954–962 [DOI] [PubMed] [Google Scholar]
- 13.Franey H., Brych S. R., Kolvenbach C. G., and Rajan R. S. (2010) Increased aggregation propensity of IgG2 subclass over IgG1: role of conformational changes and covalent character in isolated aggregates. Protein Sci. 19, 1601–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Neut Kolfschoten M., Schuurman J., Losen M., Bleeker W. K., Martínez-Martínez P., Vermeulen E., den Bleker T. H., Wiegman L., Vink T., Aarden L. A., De Baets M. H., van de Winkel J. G., Aalberse R. C., and Parren P. W. (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 [DOI] [PubMed] [Google Scholar]
- 15.Raju T. S. (2008) Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 20, 471–478 [DOI] [PubMed] [Google Scholar]
- 16.Fernández L. Á., and Muyldermans S. (2011) Recent developments in engineering and delivery of protein and antibody therapeutics. Curr. Opin. Biotechnol. 22, 839–842 [DOI] [PubMed] [Google Scholar]
- 17.Jung S. T., Kang T. H., Kelton W., and Georgiou G. (2011) Bypassing glycosylation: Engineering aglycosylated full-length IgG antibodies for human therapy. Curr. Opin. Biotechnol. 22, 858–867 [DOI] [PubMed] [Google Scholar]
- 18.Koren E., Zuckerman L. A., and Mire-Sluis A. R. (2002) Immune responses to therapeutic proteins in humans: clinical significance, assessment and prediction. Curr. Pharm. Biotechnol. 3, 349–360 [DOI] [PubMed] [Google Scholar]
- 19.Mimura Y., Church S., Ghirlando R., Ashton P. R., Dong S., Goodall M., Lund J., and Jefferis R. (2000) The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol. Immunol. 37, 697–706 [DOI] [PubMed] [Google Scholar]
- 20.Liu H., Bulseco G. G., and Sun J. (2006) Effect of posttranslational modifications on the thermal stability of a recombinant monoclonal antibody. Immunol. Lett. 106, 144–153 [DOI] [PubMed] [Google Scholar]
- 21.Leabman M. K., Meng Y. G., Kelley R. F., DeForge L. E., Cowan K. J., and Iyer S. (2013) Effects of altered FcγR binding on antibody pharmacokinetics in cynomolgus monkeys. MAbs. 5, 896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen F., Stevenson R., Li C., Salimi-Moosavi H., Liu L., Wen J., Luo Q., Daris K., Buck L., Miller S., Ho J., Wang W., Chen Q., Walker K., Wypych J., Narhi L., and Gunasekaran K. (2017) Engineering an IgG scaffold lacking effector function with optimized developability. J. Biol. Chem. 292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Meerten T., van Rijn R. S., Hol S., Hagenbeek A., and Ebeling S. B. (2006) Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin. Cancer Res. 12, 4027–4035 [DOI] [PubMed] [Google Scholar]
- 24.Ishiguro T., Kawai S., Habu K., Sugimoto M., Shiraiwa H., Iijima S., Ozaki S., Matsumoto T., and Yamada-Okabe H. (2010) A defucosylated anti-CD317 antibody exhibited enhanced antibody-dependent cellular cytotoxicity against primary myeloma cells in the presence of effectors from patients. Cancer Sci. 101, 2227–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awan F. T., Lapalombella R., Trotta R., Butchar J. P., Yu B., Benson D. M. Jr., Roda J. M., Cheney C., Mo X., Lehman A., Jones J., Flynn J., Jarjoura D., Desjarlais J. R., Tridandapani S., Caligiuri M. A., Muthusamy N., and Byrd J. C. (2010) CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood 115, 1204–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara C., Brünker P., Suter T., Moser S., Püntener U., and Umaña P. (2006) Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of golgi enzyme localization domain and co-expression of heterologous β1,4-N-acetylglucosaminyltransferase III and Golgi α-mannosidase II. Biotechnol. Bioeng. 93, 851–861 [DOI] [PubMed] [Google Scholar]
- 27.Ha S., Ou Y., Vlasak J., Li Y., Wang S., Vo K., Du Y., Mach A., Fang Y., and Zhang N. (2011) Isolation and characterization of IgG1 with asymmetrical Fc glycosylation. Glycobiology 21, 1087–1096 [DOI] [PubMed] [Google Scholar]
- 28.Ju M. S., and Jung S. T. (2014) Aglycosylated full-length IgG antibodies: steps toward next-generation immunotherapeutics. Curr. Opin. Biotechnol. 30, 128–139 [DOI] [PubMed] [Google Scholar]
- 29.Natsume A., Shimizu-Yokoyama Y., Satoh M., Shitara K., and Niwa R. (2009) Engineered anti-CD20 antibodies with enhanced complement-activating capacity mediate potent anti-lymphoma activity. Cancer Sci. 100, 2411–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., Hanai N., and Shitara K. (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen F. W., Padaki R., Morris A. E., Aldrich T. L., Armitage R. J., Allen M. J., Lavallee J. C., and Arora T. (2011) Molecular and functional characterization of cynomolgus monkey IgG subclasses. J. Immunol. 186, 341–349 [DOI] [PubMed] [Google Scholar]
- 32.Ishihara T., Nakajima N., and Kadoya T. (2010) Evaluation of new affinity chromatography resins for polyclonal, oligoclonal and monoclonal antibody pharmaceuticals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878, 2141–2144 [DOI] [PubMed] [Google Scholar]