Abstract
Apelin-36 was discovered as the endogenous ligand for the previously orphan receptor APJ. Apelin-36 has been linked to two major types of biological activities: cardiovascular (stimulation of cardiac contractility and suppression of blood pressure) and metabolic (improving glucose homeostasis and lowering body weight). It has been assumed that both of these activities are modulated through APJ. Here, we demonstrate that the metabolic activity of apelin-36 can be separated from canonical APJ activation. We developed a series of apelin-36 variants in which evolutionarily conserved residues were mutated, and evaluated their ability to modulate glucose homeostasis and body weight in chronic mouse models. We found that apelin-36(L28A) retains full metabolic activity, but is 100-fold impaired in its ability to activate APJ. In contrast to its full metabolic activity, apelin-36(L28A) lost the ability to suppress blood pressure in spontaneously hypertensive rats (SHR). We took advantage of these findings to develop a longer-acting variant of apelin-36 that could modulate glucose homeostasis without impacting blood pressure (or activating APJ). Apelin-36-[L28C(30kDa-PEG)] is 10,000-fold less potent than apelin-36 at activating the APJ receptor but retains its ability to significantly lower blood glucose and improve glucose tolerance in diet-induced obese mice. Apelin-36-[L28C(30kDa-PEG)] provides a starting point for the development of diabetes therapeutics that are devoid of the blood pressure effects associated with canonical APJ activation.
Keywords: cardiovascular, diabetes, G protein-coupled receptor (GPCR), metabolic syndrome, obesity, signal transduction, apelin, APJ, ligand-receptor promiscuity, metabolic syndrome
Introduction
The apelin gene encodes a pre-pro-protein that is processed into a number of regulatory hormones. The best characterized of these peptide hormones are apelin-13, apelin-17, and apelin-36 (1). The 13 C-terminal amino acids of these peptides (comprising apelin-13) are shared, with apelin-17 extending an additional 4 amino acids from the N terminus, and apelin-36 extending a further 19 amino acids beyond the N terminus of apelin-17 (see Table 1). Apelin was discovered as an endogenous agonist of the G protein-coupled receptor APJ (2). Specifically, apelin-36 was purified from bovine stomach tissue extract based on its ability to stimulate signaling through APJ.
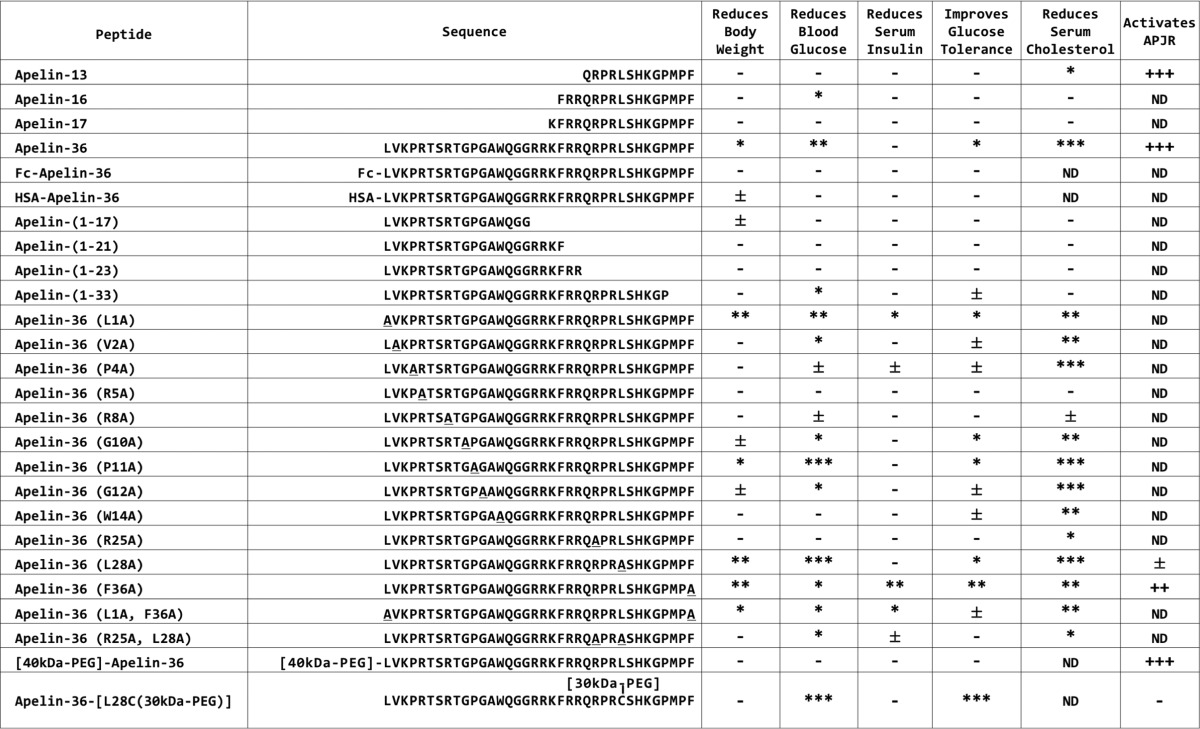
TABLE 1.
Properties of apelin peptides and variants
Body weight, blood glucose, insulin, glucose tolerance, cholesterol, and APJ activation were determined as described in Fig. 1. For metabolic parameters, asterisks indicate significant improvements versus control (control is AAV-GFP for all peptides except PEGylated apelins. For PEGylated apelins, control is PBS vehicle). ***, p <0.001; **, p <0.01; *, p <0.05; ±, p <0.1 (For body weight, blood glucose, and glucose tolerance, asterisks indicate significant improvements achieved during at least two time points. For serum insulin and cholesterol, asterisks indicate significant improvement during the single measurement). Dash (–) indicates that parameter was measured, but was not significantly different than control. ND, not determined. For APJ activation, plus signs indicate potency of receptor activation. +++, EC50<5×10−10 m; ++, EC50<5×10−9 m; +, EC50<5×10−8 m; ±, EC50<5×10−7 m; −, EC50>5×10−7 m.
Through a combination of pharmacological and genetic approaches, apelin has been linked to two major types of biological activities: cardiovascular (stimulation of cardiac contractility and suppression of blood pressure) and metabolic (improving glucose homeostasis and lowering body weight) (3, 4). Both of these activities have been assumed to be mediated solely through APJ, but there is substantial evidence to suggest that APJ may have apelin-independent activities (5, 6). Here, we provide evidence that this ligand-receptor promiscuity goes in both directions, and that apelin may have APJ-independent activities as well. Specifically, we demonstrate that the metabolic activity of apelin-36 can be dissociated from canonical APJ signaling.
Results
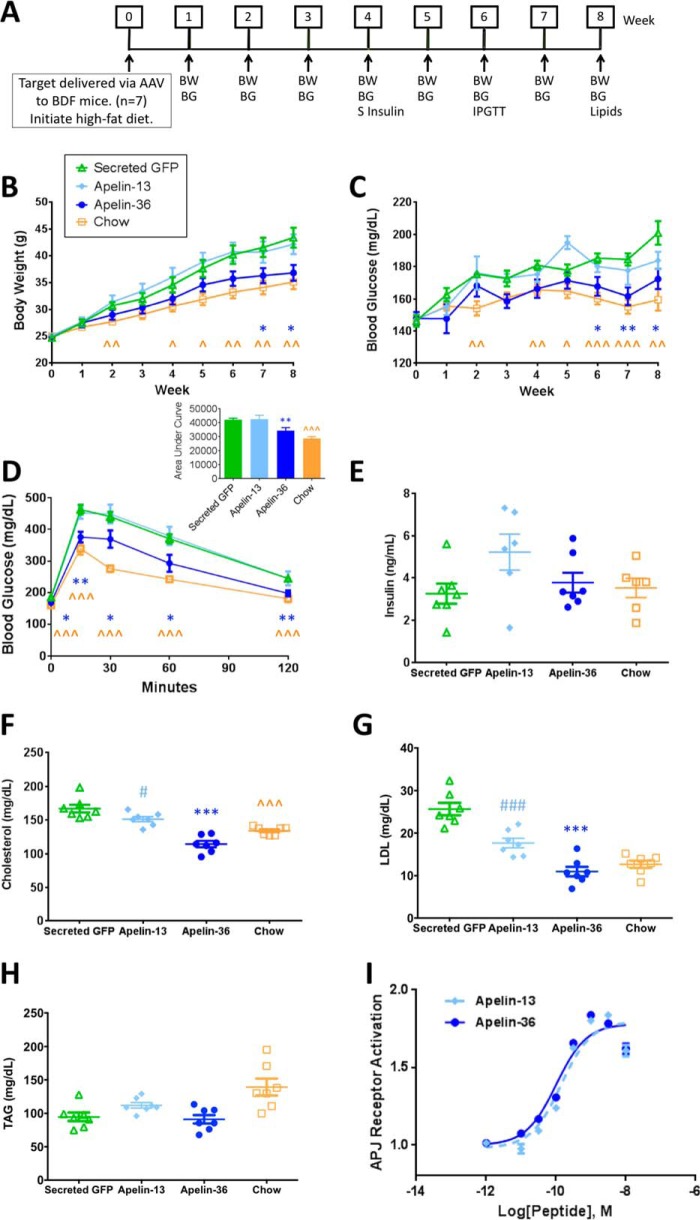
To evaluate the chronic metabolic activity of apelin peptides, we used an adeno-associated virus (AAV)3 “minigene” system to drive lasting systemic expression of apelin-13, apelin-36, or a negative control (secreted green fluorescent protein (GFP)) in a mouse diet-induced obese (DIO) prevention model. AAV expressing apelin-13, apelin-36, or GFP was injected through the tail vein into “BDF” (C57BL/6 × DBA/2 F1 hybrid) mice that were then immediately placed on a high-fat diet, followed by a series of metabolic assessments (Fig. 1A). Body weight and fasting blood glucose levels were measured weekly for 8 weeks. Fasting serum insulin levels were measured during the 4th week of the study, and to evaluate the impact of apelin-13 and apelin-36 on glucose clearance, intraperitoneal glucose tolerance tests were performed at week 6. Mice expressing apelin-36, but not apelin-13, gained significantly less weight on the high-fat diet than the GFP control mice (Fig. 1B), exhibited lower fasting blood glucose levels (Fig. 1C), and demonstrated improved glucose tolerance (Fig. 1D). Fasting serum insulin levels were not significantly different from controls (Fig. 1E). The metabolic benefits of apelin-36 are further exemplified by its impact on serum lipids, as 8 weeks after injection, apelin-36 significantly lowered serum total cholesterol (Fig. 1F) and LDL cholesterol (Fig. 1G). Triacylglyceride (TAG) levels were not significantly different from controls (Fig. 1H). Taken together, these data indicate that apelin-36, but not apelin-13, protects mice from the negative metabolic consequences of a high-fat diet.
FIGURE 1.
Apelin-36, but not apelin-13, delivery via an AAV minigene system prevents disease onset in a chronic mouse model of obesity and impaired glucose tolerance. A, paradigm for target evaluation. An 8-week DIO prevention model is shown. BW, body weight. BG, blood glucose. S Insulin, serum insulin. IPGTT, intraperitoneal glucose tolerance test. B, apelin-36 protects mice from the metabolic consequences of a high-fat diet. Chow refers to BDF mice on chow diet, Secreted GFP refers to BDF mice that were injected with 5 × 1011 genome copies (“5E+11 GC”) of rAAV expressing secreted GFP and placed on a high-fat diet, Apelin-13 refers to mice injected with 5E+11 GC of rAAV expressing apelin-13 and placed on a high-fat diet, and Apelin-36 refers to mice injected with 5E+11 GC of rAAV expressing apelin-36 and placed on a high-fat diet (n = 7 mice per group, data are from one experiment that is representative of three independent experiments). Body weight was measured weekly following AAV delivery. Recombinant AAV expressing murine apelin-36 significantly reduced body weight in mice on a high-fat diet. C, fasting blood glucose (4-h fast) was measured before AAV delivery, and then weekly thereafter. Recombinant AAV expressing murine apelin-36 significantly reduced blood glucose in mice on a high-fat diet. D, i.p. glucose tolerance was measured 6 weeks following AAV delivery. Following a 4-h fast, mice were administered 2 g/kg of glucose. Blood glucose levels were measured before glucose delivery (0 min) and 15, 30, 60, and 120 min following delivery. Apelin-36 significantly improved glucose tolerance. Inset, glucose area under the curve shown in column graph. E, fasting serum insulin (4-h fast) was measured 4 weeks following AAV delivery. F, serum cholesterol was measured 8 weeks following AAV delivery. G, serum LDL cholesterol (LDL) was measured 8 weeks following AAV delivery. H, serum triacylglycerides (TAG) were measured 8 weeks following AAV delivery. I, apelin-13 and apelin-36 potently activate APJ. Cells stably expressing human APJ were incubated for 30 min with 10 μm forskolin and apelin peptides at the indicated concentrations. (n = 8 replicates/concentration, error bars indicate standard deviation; data are from one experiment that is representative of three independent experiments). *, p < 0.05; **, p < 0.01 apelin-36 versus secreted GFP. ∧, p < 0.05; ∧∧, p < 0.01; ∧∧∧, p < 0.001 Chow versus Secreted GFP. Statistics were analyzed using Student's t test with two-tailed distribution.
Apelin-36 was purified based on its ability to act as an agonist at the G protein-coupled receptor APJ (2). Numerous studies have demonstrated that apelin-13 is also a full agonist of APJ, exhibiting similar, if not superior, potency to apelin-36 (2, 7, 8). We corroborated these observations, finding that both apelin-13 and apelin-36 can potently activate APJ in an APJ stable cell line (apelin-13 EC50 = 1.40 × 10−10 m (1.02–1.91 × 10−10 m, 95% confidence interval (CI)), apelin-36 EC50 = 1.02 × 10−10 m (0.77–1.33 × 10−10 m, 95% CI), Fig. 1I). Our observations that apelin-13 and apelin-36 exhibit similar profiles of receptor activation, but that only apelin-36 can protect mice from the symptoms of metabolic syndrome, led us to examine the relationship between APJ activation and the metabolic activity of apelin peptides.
To address this relationship, we generated a series of apelin-36 variants that were expected to be impaired to various degrees in APJ activation (based on previous structure-activity relationship studies of apelin-13 and apelin-17 (2, 8–13)), and we evaluated their metabolic activity (using a 6-week version of the paradigm illustrated in Fig. 1A). We followed up the most interesting variants by studying their APJ activation profiles. The activity profiles of these variants are summarized in Table 1. One apelin-36 variant in particular, featuring a leucine to alanine substitution at amino acid 28 (apelin-36(L28A)), exhibited a striking relationship between metabolic activity and APJ activation. A corresponding variant of apelin-13 with this leucine to alanine substitution at the same location was reported to exhibit profoundly impaired APJ activation (8, 12), and indeed, as shown in Fig. 2A, although apelin-36 potently activated APJ, apelin-36(L28A) was over 100-fold less potent (apelin-36 EC50 = 2.03 × 10−11 m (1.64–2.51 × 10−11 m, 95% CI), apelin-36(L28A) EC50 = 1.90 × 10−8 m (1.47–2.45 × 10−8 m, 95% CI)). At concentrations below 10−8 m, apelin-36(L28A) did not substantially activate APJ. In contrast to its impaired APJ activation, apelin-36(L28A) remarkably retained full metabolic activity. Apelin-36(L28A) was able to protect mice from the negative metabolic consequences of prolonged high-fat diet, reducing body weight (Fig. 2B) and blood glucose (Fig. 2C), improving glucose tolerance (Fig. 2D), and correcting the lipid profile (Fig. 2, F and G) as effectively as wild-type apelin-36.
FIGURE 2.

Apelin-36(L28A) exhibits impaired APJ activation and blood pressure regulation, but retains full metabolic activity. A, apelin-36 potently activates APJ, but apelin-36(L28A) is >100-fold less potent. A U2OS cell line stably expressing human APJ was incubated with apelin peptides at the indicated concentrations. (n = 4 replicates/concentration, error bars indicate standard deviation; data are from one experiment that is representative of three independent experiments). B, apelin-36(L28A) retains full activity in protecting mice from the metabolic consequences of a high-fat diet. Chow refers to BDF mice on chow diet, Secreted GFP refers to BDF mice that were injected with 1 × 1012 genome copies (“1E+12 GC”) of rAAV expressing secreted GFP and placed on a high-fat diet, Apelin-36 refers to mice injected with 1E+12 GC of rAAV expressing apelin-36 and placed on a high-fat diet, and Apelin-36(L28A) refers to mice injected with 1E+12 GC of rAAV expressing apelin-36(L28A) and placed on a high-fat diet (n = 7 mice per group; data are from one experiment that is representative of two independent experiments). Body weight was measured weekly beginning 2 weeks following AAV delivery. Recombinant AAV expressing murine apelin-36 or apelin-36(L28A) significantly reduced body weight in mice on a high-fat diet. C, fasting blood glucose (4-h fast) was measured weekly beginning 2 weeks following AAV delivery. Recombinant AAV expressing murine apelin-36 or apelin-36(L28A) significantly reduced blood glucose in mice on a high-fat diet. (n = 7/group). D, i.p. glucose tolerance was measured 4 weeks following AAV delivery. Following a 4-h fast, mice were administered 2 g/kg of glucose. Blood glucose levels were measured before glucose delivery (0 min) and 15, 30, 60, and 120 min following delivery. Apelin-36 or apelin-36(L28A) significantly improved glucose tolerance. Inset, glucose area under the curve shown in column graph. (n = 7/group). E, fasting serum insulin (4-h fast) was measured 3 weeks following AAV delivery. (n = 7/group). F, G, and H, serum total cholesterol (cholesterol) (F), LDL cholesterol (LDL) (G), and triacylglycerides (TAG) (H) were measured 6 weeks following AAV delivery. (n = 4/group). *, p < 0.05; **, p < 0.01; ***, p < 0.001 apelin-36 versus secreted GFP. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 apelin-36(L28A) versus secreted GFP. ∧∧, p < 0.01; ∧∧∧, p < 0.001 Chow versus secreted GFP. I, apelin-36 lowers mean blood pressure (MBP) in SHR, whereas apelin-36(L28A) does not. Blood pressure was measured using a non-invasive tail cuff blood pressure monitor. *, p < 0.05; **, p < 0.01 apelin-36 versus apelin-36(L28A). Statistics were analyzed using Student's t test with two-tailed distribution.
Apelin peptides have been linked to two major types of biological activities: cardiovascular and metabolic. Having examined the relationship between metabolic activity and APJ activation, we set out to understand the relationship of receptor activation with cardiovascular activity (specifically blood pressure). We evaluated the impact of wild-type apelin-36 and apelin-36(L28A) on blood pressure in SHR, and found that wild-type apelin-36 caused a sharp drop in blood pressure as compared with vehicle-treated animals, but apelin-36(L28A) did not (Fig. 2I). These data are consistent with apelin-36 acting through canonical APJ signaling to modulate blood pressure, but not metabolic activity.
Apelin peptides are being pursued therapeutically for the treatment of heart failure (3), and have also been proposed as candidate therapeutics for diabetes and obesity (4). However, the cardiovascular activity of apelin peptides could be a liability in obese diabetic patients, as these peptides would be expected to increase the energy demands on the heart (14). Thus, our discovery of an apelin variant that uncouples cardiovascular from metabolic activity suggests the possibility of developing apelin peptides with the ability to treat metabolic disease without impacting cardiovascular function. In addition to lacking cardiovascular activity, such a candidate therapeutic would also need to exhibit a longer half-life than wild-type apelin-36 to allow for a once daily or less frequent dosing interval (15).
Appending polyethylene glycol (PEG) polymer chains to peptides has been demonstrated to increase in vivo half-life of peptides by increasing hydrodynamic size (thus reducing renal clearance) and through protection from proteolytic degradation (16). [40kDa-PEG]-apelin-36, in which a PEG moiety was appended to the N terminus of apelin-36, has been shown to exhibit enhanced cardiovascular activity and extended half-life as compared with unmodified apelin-36 (17). However, we have found that attaching PEG molecules at either the N terminus or the C terminus of apelin-36 disrupted the metabolic activity of the peptide (discussed further below and see Fig. 3). The impaired metabolic activity of these molecules may be due to steric hindrance of the N or C termini by the PEG moiety. Therefore, to prevent such steric hindrance, we inserted a PEG moiety at the Leu-28 position of apelin-36, a position that is distant from either terminus of the peptide, and which we demonstrated above is tolerant to substitutions (with respect to metabolic activity). Additionally, given the requirement of Leu-28 for APJ activation and blood pressure regulation (Fig. 2), we inferred that PEGylation at this position might disrupt these activities.
FIGURE 3.
Apelin-36-[L28C(30kDa-PEG)] is >10,000-fold less potent than apelin-36 at activating APJ and does not lower blood pressure, but powerfully lowers blood glucose and improves glucose tolerance in DIO mice. A and B, apelin-36 and [40kDa-PEG]-apelin-36 potently activate APJ, but apelin-36-[L28C(30kDa-PEG)] is >10,000-fold less potent. A U2OS cell line stably expressing human APJ was incubated with apelin peptides at the indicated concentrations. (n = 4 replicates/concentration, error bars indicate standard deviation; data are from one experiment that is representative of three independent experiments). C, 16-week-old C57BL/6 DIO mice (12 weeks on high-fat diet) were administered a single subcutaneous dose of apelin-36-[L28C(30kDa-PEG)] or [40kDa-PEG]-apelin-36 at the indicated dosage level, and fed blood glucose levels were measured before dosing (0 h), and 16, 40, and 64 h after dosing (n = 6 mice/group; data are from one experiment that is representative of three independent experiments). The 3 and 10 mg/kg doses of apelin-36-[L28C(30kDa-PEG)] significantly lowered blood glucose levels 16 h after dosing: glucose levels returned to baseline by 40 h after dose. D, C57BL/6 DIO mice were administered a single subcutaneous dose of apelin-36-[L28C(30kDa-PEG)] or [40kDa-PEG]-apelin-36 at the indicated dosage level, and 16 h after dosing, the mice were fasted for 4 h. Following the fast (20 h after injection), an oral glucose tolerance test (1 g/kg) was performed. Apelin-36-[L28C(30kDa-PEG)] treatment caused a dose-dependent reduction in fasting blood glucose levels (see the 0-min time point) and improvement in glucose tolerance. Inset, glucose area under the curve shown in column graph. E, C57BL/6 DIO mice were administered a single subcutaneous dose of apelin-36-[L28C(30kDa-PEG)] or [40kDa-PEG]-apelin-36 at the indicated dosage level, and overnight food intake was measured (0–16 h after dosing). Apelin-36-[L28C(30kDa-PEG)] treatment caused a dose-dependent reduction in food intake. F, C57BL/6 DIO mice were administered a single subcutaneous dose of apelin-36-[L28C(30kDa-PEG)] or [40kDa-PEG]-apelin-36 at the indicated dosage level, and body weight was measured before dosing (0 h), and 16, 40, and 64 h after dosing. Neither of the peptides impacted body weight during the course of the study. **, p < 0.01 apelin-36-[L28C(30kDa-PEG)] 1 mg/kg versus vehicle. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 apelin-36-[L28C(30kDa-PEG)] 3 mg/kg versus vehicle. ∧∧, p < 0.01; ∧∧∧, p < 0.001 apelin-36-[L28C(30kDa-PEG)] 10 mg/kg versus vehicle. G, apelin-36 lowers mean blood pressure (MBP) in SHR, whereas apelin-36-[L28C(30kDa-PEG)] does not. Blood pressure was measured using a non-invasive tail cuff blood pressure monitor. *, p < 0.05; **, p < 0.01 apelin-36 versus apelin-36-[L28C(30kDa-PEG)]. Statistics were analyzed using Student's t test with two-tailed distribution.
We generated apelin-36-[L28C(30kDa-PEG)] by substituting leucine 28 with a cysteine and appending a 30-kDa PEG polymer at this position (Table 1, bottom row). We evaluated the ability of this peptide to activate APJ as compared with wild-type apelin-36. We evaluated N-terminally PEGylated [40kDa-PEG]-apelin-36 as a control. [40kDa-PEG]-apelin-36 activated APJ with similar efficacy and potency to wild-type apelin-36 ([40kDa-PEG]-apelin-36 EC50 = 5.84 × 10−11 m (4.26–8.01 × 10−11 m, 95% CI), apelin-36 EC50 = 2.03 × 10−11 m (1.64–2.51 × 10−11 m, 95% CI), Fig. 3A). In contrast, apelin-36-[L28C(30kDa-PEG)] had profoundly impaired APJ activation, with a potency >10,000-fold lower than PEGylated wild-type apelin-36 ([40kDa-PEG]-apelin-36 EC50 = 1.23 × 10−10 m (0.89–1.69 × 10−10 m, 95% CI), apelin-36-[L28C(30kDa-PEG)] EC50 = 3.05 × 10−6 m (0.94–9.90 × 10−6 m, 95% CI), Fig. 3B).
The acute metabolic impact of [40kDa-PEG]-apelin-36 and apelin-36-[L28C(30kDa-PEG)] was evaluated in C57BL/6 DIO mice. A single administration of apelin-36-[L28C(30kDa-PEG)], but not [40kDa-PEG]-apelin-36, reduced fed blood glucose levels in a dose-dependent manner (Fig. 3C). Furthermore, apelin-36-[L28C(30kDa-PEG)] treatment caused a dose-dependent reduction in fasting blood glucose levels (see the 0-min time point in Fig. 3D), an improvement in glucose tolerance (Fig. 3D), and a reduction in overnight food intake (Fig. 3E). [40kDa-PEG]-apelin-36 had no impact on fasting blood glucose levels, glucose tolerance, or food intake. Neither of the peptides impacted body weight during the acute study (Fig. 3F). Thus, the metabolic activity profile of these two PEGylated apelin-36 peptides is reversed as compared with APJ activation.
Having established that apelin-36-[L28C(30kDa-PEG)] has beneficial metabolic activity, but essentially no ability to activate canonical APJ signaling, we next set out to determine whether the lack of APJ activation would translate to a lack of blood pressure regulatory activity. We evaluated the impact of wild-type apelin-36 and apelin-36-[L28C(30kDa-PEG)] on blood pressure in SHR and found that wild-type apelin-36 caused a sharp drop in blood pressure as compared with vehicle-treated animals, but apelin-36-[L28C(30kDa-PEG)] did not (Fig. 3G). Taken together, these data identify apelin-36-[L28C(30kDa-PEG)] as a starting point for the development of therapeutics with beneficial metabolic activity, but no impact on blood pressure and no ability to activate canonical APJ signaling.
Discussion
In the present study, we demonstrate that the metabolic activity of apelin-36 can be separated from canonical APJ activation. We provide the following evidence to support this conclusion: 1) we demonstrate that although apelin-13 and apelin-36 are equivalent in activating APJ, apelin-36 lowers body weight and blood glucose, and improves glucose tolerance and lipid profile, whereas apelin-13 does not; 2) we identify an apelin variant, apelin-36(L28A), that retains full metabolic activity, but is >100-fold impaired in its ability to activate APJ (and unlike wild-type apelin-36, is unable to lower blood pressure); and 3) we develop a longer-acting variant of apelin-36, apelin-36-[L28C(30kDa-PEG)], that is >10,000-fold less potent than apelin-36 at activating APJ and does not lower blood pressure, but is able to significantly lower blood glucose and improve glucose tolerance in DIO mice.
Our data are consistent with a model in which APJ mediates the hypotensive activity of apelin-36, whereas the metabolic activity is mediated by a distinct receptor or receptor signaling complex. This “metabolic receptor” may involve a distinct APJ complex, in which APJ is associated with another receptor or with distinct downstream effectors that mediate the metabolic activity. Signaling through heterodimeric receptor complexes is well established in the G protein-coupled receptor field (18), as is biased signaling downstream of a monomeric G protein-coupled receptor (19). In fact, both of these mechanisms have been described for APJ (20). APJ has been shown to dimerize with the AT1 receptor to inhibit the effects of angiotensin II signaling in a model of atherosclerosis (21), with the bradykinin 1 receptor to mediate apelin-13-dependent PKC activation and endothelial NOS (eNOS) phosphorylation (22), and with neurotensin receptor 1 to mediate apelin-13- and neurotensin-dependent phosphorylation of ERK1/2 and cell proliferation (23). Additionally, Ceraudo et al. (24) reported that apelin-17 signals in a β-arrestin-dependent and G protein-dependent manner, whereas apelin-17 missing the C-terminal phenylalanine signals only through G proteins.
Alternatively, there may be a second, as yet unidentified receptor for apelin-36. This type of ligand/receptor promiscuity would not be unprecedented in the apelin/APJ system. A peptide alternatively named ELABELA, Toddler, or Apela has recently been identified in zebrafish as a second endogenous ligand for APJ, and this peptide acts through APJ to control cell migration during gastrulation and to mediate normal heart development (6, 25). Further evidence of ligand/receptor promiscuity in the apelin/APJ system is provided by mouse knock-out studies. APJ knock-out mice display a distinct phenotype as compared with apelin knock-out mice with respect to cardiac development and the sensitivity of cardiomyocytes to hypertrophy (26). APJ knock-out mice exhibit embryonic lethality due to defects in heart development, whereas apelin knock-out mice do not (26, 27). These data provide additional support for the existence of apelin-independent APJ signaling. Similarly, apelin may have the capability of functioning in an APJ-independent manner.
Determining the molecular identity of the receptor complex mediating the metabolic activity of apelin-36 should also provide insight regarding the tissues responsible for these effects. The peripheral tissues most widely implicated in the metabolic activities of apelin peptides are adipose, skeletal muscle, and pancreas (28). The effects we observed of apelin-36 on serum lipids are consistent with action on adipose tissue. Additionally, we observed improved glucose tolerance with apelin-36 and apelin-36[L28C(30kDa-PEG)] treatment, which could be explained by enhanced glucose uptake in skeletal muscle. We detected relatively little effect of apelin-36 administration on serum insulin levels. This result does not rule out the pancreas as a target tissue, but we do not have evidence that the pancreas is a major driver of the improved glucose metabolism. Follow-up studies using hyperglycemic and hyperinsulinemic-euglycemic clamps, along with glucose tracers, could be used to more definitively identify the target tissues mediating improved glucose homeostasis following apelin-36 and apelin-36[L28C(30kDa-PEG)] treatment. Together with identification of the metabolic receptor complex and the tissues in which the receptor is expressed, these studies would provide a more thorough understanding of the mechanism of action through which apelin-36-derived peptides modulate metabolic function.
Only a handful of diabetes therapeutics with novel mechanisms of action have been developed in recent decades (29). There is an acute need for such medicines, as 415 million people worldwide suffer from diabetes and current treatments do little to alter the course of disease progression (30, 31). Our development of apelin-36-[L28C(30kDa-PEG)], a longer-acting apelin variant with full metabolic activity, but impaired APJ activation and no effect on blood pressure, highlights the possibility of targeting the apelinergic system for the treatment of diabetes. Apelin-36-[L28C(30kDa-PEG)] exhibits attractive antidiabetic activity without the potential cardiovascular safety concerns associated with wild-type apelin-36 (lowering of blood pressure and increasing cardiac contractility, which would increase the energy demand on the heart). This discovery provides novel insight into the metabolic actions of apelin-36 that could serve as a starting point for the development of new antidiabetic agents.
Experimental Procedures
Animals
BDF mice were purchased from Harlan Laboratories (Indianapolis, IN). C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). SHR were purchased from Charles River Laboratories (Wilmington, MA). Animals were kept in accordance with welfare guidelines under controlled light (12-h light and 12-h dark cycle, dark 6:30 p.m. to 6:30 a.m.), temperature (22 ± 4 °C), and humidity (50 ± 20%) conditions. They had free access to water (autoclaved distilled water) and were fed ad libitum on a commercial diet (Harlan Laboratories, Irradiated 2018 Teklad Global 18% Protein Rodent Diet) containing 18 kcal % fat, 24 kcal % protein, and 58 kcal % carbohydrate. Alternatively, mice were maintained on a high-fat diet (D12492, Research Diets, New Brunswick, NJ) containing 60 kcal % fat, 20 kcal % protein, and 20 kcal % carbohydrate. All animal studies were approved by the NGM Institutional Animal Care and Use Committee for NGM-5-2008 entitled “Characterization of Biologics, Compounds and Viral Vectors for Treatment of Diabetes Using Rodent Models.”
Synthetic Peptides
Synthetic apelin peptides were produced by American Peptide Company (Sunnyvale, CA) or Peptides International (Louisville, KY) using solid-phase synthesis, and then purified using reverse-phase liquid chromatography.
cDNA Sequences
The following cDNA sequences were used: mouse/human (m/h)Apelin-13: 5′-cagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; m/hApelin16: 5′-tttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; m/hApelin17: 5′-aaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36: 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin36-hFc: 5′-atggcgaccgatagccgcaccagctggctgctgaccgtgagcctgctgtgcctgctgtggccgcaggaagcgagcgcgtttccggcgatgccgctgagcagcctgtttagcaacgcggtgctgcgcgcgcgcggcaaacgcctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgtttggtggtggtggtggtactcacacatgcccaccgtgcccagcacctgaactcctggggggaccgtcagtcttcctcttccccccaaaacccaaggacaccctcatgatctcccggacccctgaggtcacatgcgtggtggtggacgtgagccacgaagaccctgaggtcaagttcaactggtacgtggacggcgtggaggtgcataatgccaagacaaagccgcgggaggagcagtacaacagcacgtaccgtgtggtcagcgtcctcaccgtcctgcaccaggactggctgaatggcaaggagtacaagtgcaaggtctccaacaaagccctcccagcccccatcgagaaaaccatctccaaagccaaagggcagccccgagaaccacaggtgtacaccctgcccccatcccgggatgagctgaccaagaaccaggtcagcctgacctgcctagtcaaaggcttctatcccagcgacatcgccgtggagtgggagagcaatgggcagccggagaacaactacaagaccacgcctcccgtgctggactccgacggctccttcttcctctacagcaagctcaccgtggacaagagcaggtggcagcaggggaacgtcttctcatgctccgtgatgcatgaggctctgcacaaccactacacgcagaagagcctctccctgtctccgggtaaa-3′; mApelin-(1–17): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggcggc-3′; mApelin-(1–21): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggtggacgccgcaaattt-3′; mApelin-(1–23): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggtggacgccgcaaatttcgccgc-3′; mApelin-36ΔMPF (1–33): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggtggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccg-3′; mApelin-36 (L1A): 5′-gcggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (V2A): 5′-ctggcgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (P4A): 5′-ctggtgaaagcgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (R5A): 5′-ctggtgaaaccggccaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (R8A): 5′-ctggtgaaaccgcgcaccagcgccaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (G10A): 5′-ctggtgaaaccgcgcaccagccgcaccgccccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (P11A): 5′-ctggtgaaaccgcgcaccagccgcaccggcgcgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (G12A): 5′-ctggtgaaaccgcgcaccagccgcaccggcccggccgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (W14A): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcggcgcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (R25A): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccaggccccgcgcctgagccataaaggcccgatgccgttt-3′; mApelin-36 (L28A): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcgcgagccataaaggcccgatgccgttt-3′; mApelin-36 (F36A): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccggct-3′; mApelin-36 (L1A, F36A): 5′-gcggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccagcgcccgcgcctgagccataaaggcccgatgccggct-3′; mApelin-36 (R25A, L28A): 5′-ctggtgaaaccgcgcaccagccgcaccggcccgggcgcgtggcagggaggacgccgcaaatttcgccgccaggccccgcgcgcgagccataaaggcccgatgccgttt-3′.
cDNA Cloning
Polymerase chain reactions were set up using the iProofTM High-Fidelity PCR Kit (Bio-Rad) according to the manufacturer's instructions. Amplified DNA fragments were digested with restriction enzymes SpeI and NotI (New England Biolabs, Ipswich, MA) (the restriction sites were included in the 5′ or 3′ PCR primers, respectively) and were then ligated with AAV transgene vectors that had been digested with the same restriction enzymes. The vector used for expression contained a selectable marker and an expression cassette composed of a strong eukaryotic promoter 5′ of a site for insertion of the cloned coding sequence, followed by a 3′-untranslated region and bovine growth hormone polyadenylation tail. The expression construct is also flanked by internal terminal repeats at the 5′ and 3′ ends.
Production and Purification of AAV
AAV 293 cells (obtained from Agilent Technologies, Santa Clara, CA) were cultured in DMEM (Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum and 1× antibiotic-antimycotic solution (Mediatech). The cells were plated at 50% density on day 1 in 150-mm cell culture plates and transfected after day 2, using the calcium phosphate precipitation method, with the following three plasmids (20 μg/plate of each): AAV transgene plasmid, pHelper plasmids (Agilent Technologies), and AAV2/9 plasmid (32). 48 h after transfection, the cells were scraped off the plates, pelleted by centrifugation at 3000 × g, and resuspended in buffer containing 20 mm Tris, pH 8.5, 100 mm NaCl, and 1 mm MgCl2. The suspension was frozen in an alcohol dry ice bath and was then thawed in a 37 °C water bath. The freeze and thaw cycles were repeated for a total of three times; Benzonase (Sigma-Aldrich) was added to 50 units/ml; and deoxycholate was added to a final concentration of 0.25%. After an incubation at 37 °C for 30 min, cell debris was pelleted by centrifugation at 5000 × g for 20 min. Viral particles in the supernatant were purified using a discontinuous iodixanol (Sigma-Aldrich) gradient as described previously (33). The viral stock was concentrated using Vivaspin 20 (molecular mass cutoff 100,000 Da, Sartorius Stedim Biotech, Aubagne, France), resuspended in PBS with 10% glycerol, and stored at −80 °C. To determine the viral genome copy number, 2 μl of viral stock was incubated in 6 μl of solution containing 50 units/ml Benzonase, 50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, and 10 mm CaCl2 for at 37 °C for 30 min.
Afterward, 15 μl of the solution containing 2 mg/ml proteinase K, 0.5% SDS, and 25 mm EDTA was added, and the mixture was incubated for an additional 20 min at 55 °C to release viral DNA. Viral DNA was cleaned up using the mini DNeasy Kit (Qiagen, Valencia, CA) and eluted with 40 μl of water. Viral genome copy (GC) was determined by using quantitative PCR. Viral stocks were diluted with PBS to the desired concentration (GC/ml). 200 μl of viral working solution was delivered into mice via tail vein injection.
APJ Activation
CHO-K1 cells (PerkinElmer) and U2OS cells (Invitrogen) stably expressing APJ were used to measure APJ activation. These cell lines were treated with apelin peptides at the indicated concentrations. The ability of the peptides to inhibit cAMP accumulation in APJ CHO-K1 cells was measured using the Lance Ultra cAMP time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay (PerkinElmer) according to the manufacturer's instructions. The ability of peptides to stimulate an APJ reporter assay in APJ U2OS cells was measured using the TangoTM β-lactamase reporter assay (Invitrogen) according to the manufacturer's instructions.
Blood Glucose Assays
Blood was sampled in mice by nicking the tail vein, and blood glucose levels were measured using ACCU-CHEK Active test strips read by an ACCU-CHEK Active meter (Roche Diagnostics) following the manufacturer's instructions. All readings were performed in duplicate and averaged.
Serum Insulin Assays
Whole blood (about 50 μl/mouse) from mouse tail snips was collected into plain capillary tubes (BD Clay Adams SurePrep, BD Diagnostics). Serum and blood cells were separated by spinning the tubes in an Autocrit Ultra 3 (BD Diagnostics). Insulin levels in serum were determined using insulin EIA kits (80-Insms-E01, Alpco Diagnostics, Salem, NH) by following the manufacturer's instructions.
Glucose Tolerance Test
Mice were assigned into groups based on their blood glucose and body weight. BDF mice fasted for 4 h received glucose (2 g/kg) in PBS via intraperitoneal injection (data in Figs. 1 and 2). C57BL/6 DIO mice fasted for 4 h received glucose (1 g/kg) in PBS via oral gavage (data in Fig. 3). Blood glucose levels were determined as described above at the time points indicated (0, 15, 30, 60, and 120 min after administration of glucose).
Blood Pressure
Blood pressure was measured in anesthetized SHR using a CODA non-invasive tail cuff blood pressure monitoring system according to the manufacturer's instructions (Kent Scientific, Torrington, CT).
Clinical Chemistry
Serum levels of total cholesterol, LDL cholesterol, and triacylglycerides were measured using enzymatic end point assays on a COBAS INTEGRA 400 clinical analyzer (Roche Diagnostics, Rotkreuz, Switzerland).
Author Contributions
H. G. T., H. Y., S. M. S., K. J. P., B. K., and C. T. performed experiments, analyzed data, and reviewed the manuscript. D. D. K. designed experiments, performed data analysis and interpretation, and wrote the manuscript. J. L. designed experiments, performed data analysis, and reviewed the manuscript. M. A. B., H. T., L. J., J. G., C. M. R., and A. K. contributed with discussion and data interpretation, and reviewed the manuscript.
Acknowledgments
We thank Christopher Rhodes (MedImmune) and Jin-Long Chen (NGM Biopharmaceuticals) for constructive feedback on the manuscript.
H. G. T., H. Y., S. M. S., K. J. P., B. K., C. T., J. L., H. T., and D. D. K. are employees of NGM Biopharmaceuticals, Inc. M. A. B., L. J., J. G., C. M. R., and A. K. are employees of MedImmune, LLC.
- AAV
- adeno-associated virus
- rAAV
- recombinant AAV
- DIO
- diet-induced obese
- TAG
- triacylglyceride
- CI
- confidence interval
- SHR
- spontaneously hypertensive rats
- GC
- genome copy
- m
- mouse
- h
- human.
References
- 1.Kleinz M. J., and Davenport A. P. (2005) Emerging roles of apelin in biology and medicine. Pharmacol. Ther. 107, 198–211 [DOI] [PubMed] [Google Scholar]
- 2.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M. X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C., Kurokawa T., Onda H., and Fujino M. (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 251, 471–476 [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekaran B., Dar O., and McDonagh T. (2008) The role of apelin in cardiovascular function and heart failure. Eur. J. Heart Fail. 10, 725–732 [DOI] [PubMed] [Google Scholar]
- 4.Castan-Laurell I., Dray C., Attané C., Duparc T., Knauf C., and Valet P. (2011) Apelin, diabetes, and obesity. Endocrine 40, 1–9 [DOI] [PubMed] [Google Scholar]
- 5.Scimia M. C., Hurtado C., Ray S., Metzler S., Wei K., Wang J., Woods C. E., Purcell N. H., Catalucci D., Akasaka T., Bueno O. F., Vlasuk G. P., Kaliman P., Bodmer R., Smith L. H., Ashley E., et al. (2012) APJ acts as a dual receptor in cardiac hypertrophy. Nature 488, 394–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chng S. C., Ho L., Tian J., and Reversade B. (2013) ELABELA: A hormone essential for heart development signals via the apelin receptor. Dev. Cell 27, 672–680 [DOI] [PubMed] [Google Scholar]
- 7.Kawamata Y., Habata Y., Fukusumi S., Hosoya M., Fujii R., Hinuma S., Nishizawa N., Kitada C., Onda H., Nishimura O., and Fujino M. (2001) Molecular properties of apelin: tissue distribution and receptor binding. Biochim. Biophys. Acta 1538, 162–171 [DOI] [PubMed] [Google Scholar]
- 8.Medhurst A. D., Jennings C. A., Robbins M. J., Davis R. P., Ellis C., Winborn K. Y., Lawrie K. W. M., Hervieu G., Riley G., Bolaky J. E., Herrity N. C., Murdock P., and Darker J. G. (2003) Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 84, 1162–1172 [DOI] [PubMed] [Google Scholar]
- 9.Lee D. K., Saldivia V. R., Nguyen T., Cheng R., George S. R., and O'Dowd B. F. (2005) Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology 146, 231–236 [DOI] [PubMed] [Google Scholar]
- 10.De Mota N., Lenkei Z., and Llorens-Cortès C. (2000) Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 72, 400–407 [DOI] [PubMed] [Google Scholar]
- 11.El Messari S., Iturrioz X., Fassot C., De Mota N., Roesch D., and Llorens-Cortes C. (2004) Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J. Neurochem. 90, 1290–1301 [DOI] [PubMed] [Google Scholar]
- 12.Fan X., Zhou N., Zhang X., Mukhtar M., Lu Z., Fang J., DuBois G. C., and Pomerantz R. J. (2003) Structural and functional study of the apelin-13 peptide, an endogenous ligand of the HIV-1 coreceptor, APJ. Biochemistry 42, 10163–10168 [DOI] [PubMed] [Google Scholar]
- 13.Murza A., Parent A., Besserer-Offroy E., Tremblay H., Karadereye F., Beaudet N., Leduc R., Sarret P., and Marsault É. (2012) Elucidation of the structure-activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem 7, 318–325 [DOI] [PubMed] [Google Scholar]
- 14.Huss J. M., and Kelly D. P. (2005) Mitochondrial energy metabolism in heart failure: a question of balance. J. Clin. Invest. 115, 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Japp A. G., Cruden N. L., Barnes G., van Gemeren N., Mathews J., Adamson J., Johnston N. R., Denvir M. A., Megson I. L., Flapan A. D., and Newby D. E. (2010) Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 121, 1818–1827 [DOI] [PubMed] [Google Scholar]
- 16.Harris J. M., and Chess R. B. (2003) Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 [DOI] [PubMed] [Google Scholar]
- 17.Jia Z. Q., Hou L., Leger A., Wu I., Kudej A. B., Stefano J., Jiang C., Pan C. Q., and Akita G. Y. (2012) Cardiovascular effects of a PEGylated apelin. Peptides 38, 181–188 [DOI] [PubMed] [Google Scholar]
- 18.Terrillon S., and Bouvier M. (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep. 5, 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajagopal S., Rajagopal K., and Lefkowitz R. J. (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang P., Maguire J. J., and Davenport A. P. (2015) Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol. Sci. 36, 560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun H. J., Ali Z. A., Kojima Y., Kundu R. K., Sheikh A. Y., Agrawal R., Zheng L., Leeper N. J., Pearl N. E., Patterson A. J., Anderson J. P., Tsao P. S., Lenardo M. J., Ashley E. A., and Quertermous T. (2008) Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J. Clin. Invest. 118, 3343–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai B., Liu L., Zhang N., Wang C., Jiang Y., and Chen J. (2014) Heterodimerization of human apelin and bradykinin 1 receptors: Novel signal transduction characteristics. Cell. Signal. 26, 1549–1559 [DOI] [PubMed] [Google Scholar]
- 23.Bai B., Cai X., Jiang Y., Karteris E., and Chen J. (2014) Heterodimerization of apelin receptor and neurotensin receptor 1 induces phosphorylation of ERK1/2 and cell proliferation via Gαq-mediated mechanism. J. Cell. Mol. Med. 18, 2071–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceraudo E., Galanth C., Carpentier E., Banegas-Font I., Schonegge A. M., Alvear-Perez R., Iturrioz X., Bouvier M., and Llorens-Cortes C. (2014) Biased signaling favoring Gi over β-arrestin promoted by an apelin fragment lacking the C-terminal phenylalanine. J. Biol. Chem. 289, 24599–24610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauli A., Norris M. L., Valen E., Chew G.-L., Gagnon J. A., Zimmerman S., Mitchell A., Ma J., Dubrulle J., Reyon D., Tsai S. Q., Joung J. K., Saghatelian A., and Schier A. F. (2014) Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 343, 1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charo D. N., Ho M., Fajardo G., Kawana M., Kundu R. K., Sheikh A. Y., Finsterbach T. P., Leeper N. J., Ernst K. V., Chen M. M., Ho Y. D., Chun H. J., Bernstein D., Ashley E. A., and Quertermous T. (2009) Endogenous regulation of cardiovascular function by apelin-APJ. Am. J. Physiol. Heart Circ. Physiol. 297, H1904–H1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuba K., Zhang L., Imai Y., Arab S., Chen M., Maekawa Y., Leschnik M., Leibbrandt A., Markovic M., Schwaighofer J., Beetz N., Musialek R., Neely G. G., Komnenovic V., Kolm U., et al. (2007) Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 101, e32–e42 [DOI] [PubMed] [Google Scholar]
- 28.Castan-Laurell I., Dray C., Knauf C., Kunduzova O., and Valet P. (2012) Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol. Metab. 23, 234–241 [DOI] [PubMed] [Google Scholar]
- 29.Kahn S. E., Cooper M. E., and Del Prato S. (2014) Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 383, 1068–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Diabetes Federation (2015) IDF Diabetes Atlas, Seventh Ed., p. 50, International Diabetes Federation, Brussels, Belgium [Google Scholar]
- 31.Fonseca V. A. (2009) Defining and characterizing the progression of type 2 diabetes. Diabetes Care 32, Suppl. 2, S151–S156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao G., Vandenberghe L. H., Alvira M. R., Lu Y., Calcedo R., Zhou X., and Wilson J. M. (2004) Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zolotukhin S., Byrne B. J., Mason E., Zolotukhin I., Potter M., Chesnut K., Summerford C., Samulski R. J., and Muzyczka N. (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6, 973–985 [DOI] [PubMed] [Google Scholar]