Abstract
Transglutaminase 2 (TG2) catalyzes transamidation or deamidation of its substrates and is ordinarily maintained in a catalytically inactive state in the intestine and other organs. Aberrant TG2 activity is thought to play a role in celiac disease, suggesting that a better understanding of TG2 regulation could help to elucidate the mechanistic basis of this malady. Structural and biochemical analysis has led to the hypothesis that extracellular TG2 activation involves reduction of an allosteric disulfide bond by thioredoxin-1 (TRX), but cellular and in vivo evidence for this proposal is lacking. To test the physiological relevance of this hypothesis, we first showed that macrophages exposed to pro-inflammatory stimuli released TRX in sufficient quantities to activate their extracellular pools of TG2. By using the C35S mutant of TRX, which formed a metastable mixed disulfide bond with TG2, we demonstrated that these proteins specifically recognized each other in the extracellular matrix of fibroblasts. When injected into mice and visualized with antibodies, we observed the C35S TRX mutant bound to endogenous TG2 as its principal protein partner in the small intestine. Control experiments showed no labeling of TG2 knock-out mice. Intravenous administration of recombinant TRX in wild-type mice, but not TG2 knock-out mice, led to a rapid rise in intestinal transglutaminase activity in a manner that could be inhibited by small molecules targeting TG2 or TRX. Our findings support the potential pathophysiological relevance of TRX in celiac disease and establish the Cys370–Cys371 disulfide bond of TG2 as one of clearest examples of an allosteric disulfide bond in mammals.
Keywords: allosteric regulation, disulfide, intestine, thioredoxin, transglutaminase
Introduction
Transglutaminase 2 (TG2)2 is a ubiquitous member of the mammalian transglutaminase family that catalyzes transamidation or deamidation of its protein or peptide substrates. It is expressed in many cell types (1), and a considerable fraction of the expressed protein is released into the extracellular environment through an unconventional secretory mechanism whose details have not yet been elucidated (2, 3). Aberrant activity of extracellular TG2 has been implicated in several human diseases, including celiac disease, various cancers, and certain fibrotic disorders (4–6), yet the enzyme is dormant in the extracellular matrix (ECM) of virtually all organs under normal physiological conditions (7, 8). Whereas the enzymatic chemistry of TG2 has been extensively studied, our understanding of its function and regulation is still in its infancy.
The post-translational regulatory mechanisms of TG2 have been reviewed elsewhere (9). Here, we focus on the redox regulation of TG2, because it is believed to be a principal mechanism for controlling the activity of extracellular TG2. It has long been known that exposure to an oxidizing environment abolishes the enzymatic activity of TG2 (10, 11). The discovery of an unusual disulfide bond (between Cys370 and Cys371) located distal to the active site of human TG2 (12) was followed by extensive biochemical evidence for its reversible regulatory role (13). More recently, in vitro studies have shown that the redox protein cofactor thioredoxin-1 (TRX) is capable of reducing the Cys370–Cys371 disulfide bond in extracellular TG2 with dramatically higher specificity than typical disulfide bond reductants (8). However, the physiological relevance of this allosteric control mechanism has not yet been established.
TRX is a ubiquitous protein in virtually all cell types and is evolutionarily conserved from prokaryotes to mammals. Early work on TRX suggested it was primarily involved in controlling intracellular redox balance (14–16). Although subsequent studies have demonstrated that mammalian cells secrete TRX (17), only a few extracellular substrates have been identified. For example, a recent proteomic study revealed that several leukocyte cell surface proteins undergo reduction by TRX, but the functional consequences of this phenomenon remain largely unknown (18). Additionally, TRX activates the TRPC ion channel and the HIV-1 envelope protein gp120 via intramolecular disulfide bond reduction (19, 20). Elevated levels of extracellular TRX have been observed in the plasma of patients with several apparently unrelated diseases—including AIDS and sepsis—and are correlated with the clinical outcome (21, 22). Although pharmacological administration of TRX has been shown to have beneficial effects in several preclinical disease models, the molecular mechanisms underpinning these effects have remained elusive (23, 24).
Our interest in the relationship between extracellular TG2 and TRX is motivated by three related observations: (i) TRX activates TG2 with high specificity in vitro (kcat/Km = 1.6 μm−1 min−1) (8), (ii) IFN-γ is the principal pro-inflammatory cytokine secreted by T cells that drive celiac disease pathogenesis (25, 26), and (iii) IFN-γ promotes TRX secretion from monocytic cells (8). These observations are especially relevant to celiac disease pathogenesis because TG2-catalyzed regiospecific deamidation of gluten peptides is critical for rendering them into high affinity T cell antigens (27, 28). This has led to the hypothesis that extracellular TRX provides the missing link in a gluten-induced, self-amplificatory relationship between the activity of inflammatory T cells and TG2 in the small intestine of celiac disease patients.
Although TRX has been shown to recognize and activate TG2 in vitro, several important questions about the physiological relevance of this interaction remain. First, it has not yet been established whether cells capable of secreting TRX can do so in sufficient quantities to activate endogenous TG2 in their extracellular environments. Second, specific protein-protein recognition of TG2 by TRX has not been demonstrated in vivo. Finally, it is unknown whether TRX-promoted reduction is sufficient to activate extracellular TG2 in the small intestine or whether additional regulatory mechanisms might also be involved in suppressing enzyme activity. Here, we take a chemical biological approach to answer these questions.
Results
TG2 Activity in Macrophages Is Mediated by Endogenous TRX
Previously, we showed that basal enzymatic activity of extracellular TG2 was not detectable in WI-38 fibroblasts or T84 epithelial cells, but addition of exogenous TRX to both cell lines rapidly induced TG2 activity (8, 29). We therefore sought to extend these findings by asking whether endogenously produced TRX could be released by immune cells in sufficient quantities so as to activate extracellular TG2 that is associated with most adherent cells. To answer this question, we used an established cellular model of macrophage polarization derived from the THP-1 human monocytic cell line (30). Our choice of this model was prompted by earlier work showing that TG2 expression was induced in the monocyte to macrophage transition and that the resulting TG2 was important for clearance of apoptotic cells by macrophages (31, 32). Moreover, macrophages are also known to secrete TRX in response to inflammatory stimuli (33). We therefore hypothesized that THP-1-derived macrophages would secrete TRX and that the secreted protein would activate extracellular TG2.
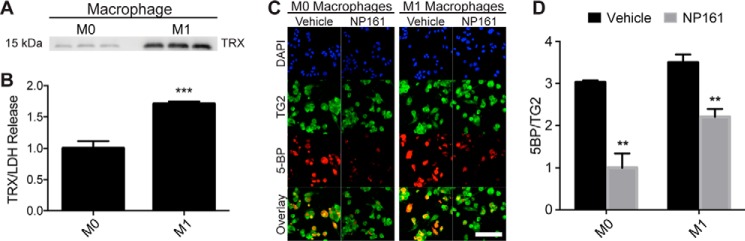
THP-1 cells were differentiated into macrophages by stimulation with phorbol 12-myristate 13-acetate, as described previously (30). The resulting adherent cells were either maintained in an unpolarized state (M0) or polarized to an inflammatory phenotype (M1) by the addition of LPS and IFN-γ. Western blotting revealed an ∼2-fold higher level of secreted TRX (normalized to lactate dehydrogenase release as a control for nonspecific cell lysis) in the culture medium of M1 cells compared with that of M0 cells (Fig. 1, A and B).
FIGURE 1.
Activation of extracellular TG2 is mediated by endogenous TRX in macrophage-like cells derived from the THP-1 monocytic cell line. A, Western blotting analysis of TRX in the culture medium of M0- and M1-like macrophages. The samples were analyzed in triplicate. B, densitometry analysis of the Western blot shown in A normalized to lactate dehydrogenase release. C, visualization of extracellular TG2 activity and its attenuation by a small molecule, NP161, that irreversibly inactivates extracellular TRX (29). TG2 protein was stained with a TG2-specific antibody (green), and its enzymatic activity was detected by incorporation of a biotinylated TG2 substrate, 5-BP (red). D, quantification of 5-BP incorporation, normalized to TG2 protein. The experiments were performed in biological triplicate, and the labeling of at least three images per biological replicate was quantified for each condition. The data are presented as the averages ± standard error. Statistical comparisons were performed using Student's t test. TRX secretion was significantly elevated in M1 relative to M0 macrophages (***, p < 0.001), and TRX inhibition by NP161 significantly attenuated 5-BP incorporation in both M0 and M1 macrophages (**, p < 0.01). There was a trend toward increased 5-BP incorporation in M1 relative to M0 macrophages (p = 0.079). Scale bar, 100 μm.
Next, we evaluated TG2 activity in M0 and M1 macrophages using an established assay to measure the incorporation of 5-biotinamidopentylamine (5-BP), a small molecule TG2 activity probe (7, 34). TG2 activity positively correlated with TRX secretion, because 5-BP incorporation was elevated in M1 macrophages relative to M0 macrophages (Fig. 1, C and D). To assess whether TG2 activity was due to secreted TRX, NP161, a small molecule inhibitor of extracellular TRX, was used (29). The addition of NP161 to the culture medium significantly reduced TG2 activity in both M0 and M1 macrophages (Fig. 1D) without significantly affecting cell viability, as judged by a lactate dehydrogenase release assay (data not shown). Overall, these results demonstrate that endogenously released TRX is capable of activating TG2 that is abundant in the extracellular matrix of cultured cells.
The C35S Mutant of Human TRX Forms a Metastable Covalent Adduct with TG2
To ascertain whether TRX can specifically recognize TG2 in vivo, we sought to trap the protein complex in a healthy animal and to visualize it in situ. Given the transient nature of the TRX-TG2 adduct, we had to devise a strategy that stabilized this complex. Previously, we proposed a mechanism in which TRX activates TG2 via thiol-disulfide exchange, where Cys32 of TRX attacks the Cys370–Cys371 disulfide bond of oxidized TG2, leading to a mixed disulfide intermediate (Fig. 2A) (8, 13). This TRX-TG2 adduct undergoes rapid resolution via attack by the Cys35 residue of TRX, resulting in the release of reduced, catalytically active TG2 and oxidized TRX. A corollary of this mechanism is that the C35S mutant TRX could trap the mixed disulfide adduct, as has been shown in previous studies with other TRX substrates (Fig. 2B) (19, 35, 36). To test this hypothesis, we incubated purified, oxidized recombinant TG2 with recombinant TRX or its C35S mutant for 2 h at room temperature. Reaction mixtures were quenched and resolved via SDS-PAGE under non-reducing conditions (Fig. 2C). Western blotting analysis revealed adduct formation in the presence of the C35S TRX mutant but not wild-type TRX (Fig. 2D). Notably, the steady-state abundance of the adduct was relatively low (as indicated by the relative intensities of the TG2 and TG2-TRX bands on SDS-PAGE), even in the presence of excess TRX. The mechanistic basis for adduct instability was not investigated and is considered under “Discussion.”
FIGURE 2.
The C35S mutant of TRX forms a metastable covalent adduct with TG2. A, proposed mechanism of TRX-mediated reduction of disulfide-bonded TG2 (8, 13). The mixed disulfide intermediate that covalently links TRX to TG2 is highly transient, because its formation is followed by rapid attack by C35 in the second half of the catalytic cycle. B, in contrast, the complex between C35S mutant of TRX and wild-type TG2 is more stable. C, SDS-PAGE analysis of purified wild-type TRX and its C35S mutant with or without oxidized TG2. The 12-kDa band corresponds to TRX, and the 80-kDa band corresponds to TG2. A weak but discernable 90–100-kDa band was observed when TG2 was mixed with the C35S mutant but not wild-type TRX. The other weak (30 kDa) band corresponds to dimeric TRX. D, Western blotting analysis of the gel shown in C using an anti-TRX antibody.
Extracellular TG2 Is a Preferred Substrate of TRX in Vitro and in Vivo
It is known that the extracellular environment of cultured WI-38 human fibroblasts contains large amounts of oxidized (i.e. catalytically inactive) TG2 (7, 8). To investigate whether TRX could also recognize TG2 bound to the ECM of primary cells, we obtained murine lung fibroblasts from TG2−/− mice and isogenic controls. The cells were exposed to either wild-type or C35S TRX, stained with antibodies against TG2 or His6 (to differentiate exogenous His6-tagged TRX from endogenous TRX), and visualized via fluorescence microscopy. Whereas wild-type TRX could not be observed in the ECM of TG2-expressing fibroblasts, the C35S TRX mutant bound to TG2 surrounding these cells (Fig. 3A). Strikingly, no C35S TRX localized with the ECM of TG2−/− fibroblasts, suggesting that TG2 is the preferred substrate of TRX in the ECM of these cells.
FIGURE 3.

TG2 is the preferred extracellular substrate of TRX in vitro and in the small intestine. A, primary lung fibroblasts derived from either TG2-positive or TG2-null mice were incubated with 10 μm recombinant TRX (wild-type or the C35S mutant) or vehicle for 1 h. Immunofluorescence analysis of TG2 (green) and His6-tagged TRX (red) showed evidence for adduct formation between endogenous TG2 and the C35S mutant but not wild-type TRX. Neither wild-type TRX nor the C35S mutant bound to the ECM of the TG2-null fibroblasts. Scale bar, 50 μm. B, C57BL/6 or TG2−/− mice were injected intraperitoneally with wild-type or C35S TRX, and sacrificed after 30 min. Small intestinal tissue sections were stained with DAPI (blue) and antibodies against TG2 (green) and His6-tagged TRX (red). The adduct derived from C35S TRX colocalized with TG2 in the small intestine. The analogous adduct could not be visualized in mice treated with wild-type TRX or in TG2-null mice treated with either TRX protein. Three mice were included in each cohort, and at least three images/mouse were analyzed, giving least nine images per condition, of which a representative image is shown. All images were taken under the same gain settings. In each panel, all images were processed identically using ImageJ. Scale bar, 100 μm.
Further evidence for this protein-protein interaction was obtained in vivo. C57BL/6 or TG2−/− mice were treated with a single dose of TRX (500 mg/kg) or the C35S mutant injected intraperitoneally and sacrificed 30 min later. Thinly sliced small intestinal sections were stained with antibodies against TG2 and His6 (to detect the injected TRX) and analyzed by fluorescence microscopy. Whereas adduct formation was not observed in animals dosed with wild-type TRX, the C35S mutant was found to colocalize with TG2 in the lamina propria from wild-type but not TG2−/− mice (Fig. 3B).
TRX Activates TG2 in Vivo
Previous studies have established that extracellular TG2 shows undetectable catalytic activity under basal physiological conditions in mice (7, 37). To determine whether this inactivity is due to oxidative inactivation, we dosed recombinant wild-type TRX to healthy C57BL/6 or TG2−/− mice.
A single dose of recombinant TRX (500 mg/kg) was injected intravenously along with 5-BP (100 mg/kg, intraperitoneal) at various time points, as detailed under “Experimental Procedures.” The mice were sacrificed 2 h after TRX administration, and 5-BP incorporation was measured in the small intestine via a fluorescence microscopy assay reported previously (7, 34, 37). No TG2-dependent 5-BP incorporation was detectable in control wild-type mice. However, mice that received wild-type TRX showed a significant increase in 5-BP incorporation (Fig. 4, A and D). Enhanced magnification demonstrated that the 5-BP colocalized with TG2 in the lamina propria of the small intestine (Fig. 4, B and C). To verify that 5-BP incorporation into the extracellular matrix was catalyzed by TG2, TRX administration was performed in combination with the widely used small molecule TG2 inhibitor, ERW1041E (38), or in TG2−/− mice (39). 5-BP incorporation was abrogated by both pharmacological and genetic ablation of TG2, implying that TRX had indeed activated small intestinal TG2 (Fig. 4, A and D).
FIGURE 4.
TRX induces small intestinal TG2 activity in mice. A, TG2 protein (green) was detected by an anti-TG2 antibody, whereas its catalytic activity (red) was detected by the intensity of 5-BP incorporation into the ECM. In the absence of TRX, no 5-BP incorporation was observed in wild-type mice. Wild-type TRX activated TG2; the observed enzymatic activity was fully inhibited by ERW1041E. No 5-BP incorporation was observed in TG2−/− mice. Scale bar, 200 μm. B, higher magnification image of a representative villus from a wild-type mouse injected with wild-type TRX in A. Scale bar, 50 μm. C, analogous high resolution image from a representative mouse exposed to both wild-type TRX and ERW1041E. Scale bar, 50 μm. All images were processed identically using ImageJ. D, quantitative analysis of TG2 activity in the small intestines of all mice from this experiment. For each experimental condition, three independent biological replicates (i.e. separate mice) were used, and at least three images were collected per mouse, giving at least nine images per biological condition, of which representative images are shown in A. 5-BP incorporation was significantly elevated in the TRX + 5-BP cohort compared with all control cohorts (***, p < 0.0001). No significant differences were found between the means of any of the control cohorts (p > 0.05). Analyses were performed using a one-way analysis of variance followed by Tukey's multiple comparison test.
Given our overriding interest in the role of TG2 activity in the context of celiac disease, our analysis of 5-BP incorporation mainly focused on the small intestine. However, we parenthetically note that TRX-mediated TG2 activity was not observed in other organs, including the lungs and the liver (data not shown). Possible reasons for this unexpected observation were not pursued, but are considered under “Discussion.”
To demonstrate that disulfide bond exchange is required to activate small intestinal TG2, the mice were dosed intravenously with wild-type TRX along with the small molecule TRX inhibitor, NP161 (29), or alternatively with the C32S/C35S TRX double mutant. By virtue of its mode of action, NP161 is expected to irreversibly inactivate extracellular TRX without significant loss of intracellular activity of this protein cofactor (29). Pharmacological inhibition of TRX activity resulted in attenuated 5-BP incorporation (Fig. 4D). Furthermore, 5-BP incorporation was not detected when the C32S/C35S mutant of TRX was administered instead of the wild-type protein. Thus, recognition and activation of TG2 by TRX in vivo requires disulfide isomerase activity of the latter protein.
Discussion
Although disulfide bonds are ubiquitous in mammalian biology, a vast majority of these post-translational modifications have evolved to stabilize the active conformations of extracellular proteins such as cytokines and cell surface receptors. However, there is growing recognition that certain disulfide bonds in a few extracellular proteins undergo facile reduction under physiological or pathophysiological conditions, leading to the activation or inactivation of the function of that protein. In most cases, these disulfide bonds are not an integral part of the protein active site, leading to their description as “allosteric disulfide bonds” (40, 41). Examples of proteins likely to harbor allosteric disulfide bonds include CD4 (42, 43), gp120 (19, 44), von Willebrand factor (45, 46), tissue factor (47), and factor XI (48). TG2 is another such protein.
The existence of allosteric disulfide bonds in extracellular proteins has also prompted a search for mechanisms triggering their reduction. In at least some of the above cases, the well known cytosolic protein cofactor TRX has emerged as a promising candidate (8, 19, 20, 49); in other cases, TRX homologs such as protein-disulfide isomerase have been proposed to play this role (44, 48). However, in all cases two major caveats remain unresolved. First, in no case has TRX been shown to reduce an allosteric disulfide bond in vivo. Second, the reversibility of this allosteric regulatory mechanism has not been established. This work addresses the first of these unresolved issues.
Prior to this work, our hypothesis that TG2 is activated by TRX-promoted reduction of an allosteric disulfide bond was principally supported by biochemical assays utilizing recombinant TG2 and TRX, as well as assays in which cultured cells or harvested mammalian tissues that were exposed to recombinant TRX (8). Here, we sought to address key physiological shortcomings of the above models, particularly with an eye toward testing the role of this post-translational regulatory mechanism in celiac disease pathogenesis.
First, we showed that inflammatory signals prompt macrophage-like cells to release adequate TRX to activate endogenous TG2 in their own extracellular environments. This finding, along with a recent report showing that TRX is partially responsible for activating extracellular TG2 in endothelial cells stimulated with anti-TG2 autoantibodies from celiac disease patients (50), provides the most compelling evidence to date that TRX flux outside the cell can be sufficient to regulate TG2 activity. Notably, other cell types, such as dendritic cells and monocytes (8, 52), have also been reported to secrete TRX during the immune response, suggesting that elevated TG2 activity may be a general consequence of inflammation in celiac disease and associated conditions.
Second, we demonstrated for the first time that TRX has the specificity to recognize extracellular TG2 in a living animal. This was a challenging proposition, because both TG2 and TRX are abundant proteins in most organs and because TG2-TRX complexes are inherently unstable. Using C35S TRX as a reagent to trap TRX to its protein substrates, we demonstrated that recombinant C35S TRX not only recognizes purified TG2 but also the same target protein in the extracellular environment of cultured fibroblasts. Taking advantage of a prior observation that injectable recombinant human TRX is well tolerated in mice with a t½ of ∼1 h (23), we also showed that injected C35S TRX distributed into the intestinal mucosa and recognized TG2 in the extracellular matrix of the small intestine. Together, these results highlighted the specificity of this protein-protein interaction.
Finally, we showed that recognition of extracellular TG2 by wild-type TRX was sufficient for inducing transamidation activity of this enzyme in the mouse small intestine. Although elevated TG2 activity has been implicated in many inflammatory conditions including celiac disease, the molecular mechanism by which the enzyme is post-translationally activated has remained elusive (1, 4–6). At a minimum, our finding that TRX can rapidly and specifically activate TG2 provides a facile pharmacological rheostat for modulating the activity of this enigmatic enzyme (Fig. 5), thereby opening the door to interrogate its biological function.
FIGURE 5.
TRX-promoted regulation of TG2 activity in the small intestine. Activation of TG2 in the lamina propria of the small intestine can be accomplished through systemic dosing of exogenous TRX. Subsequent administration of a selective TG2 inhibitor, ERW1041E, attenuates TG2 activity.
Parenthetically, two notable but somewhat tangential observations were made in the course of our studies. First, although the mixed disulfide complex between TG2 and the C35S mutant of TRX was considerably more stable than the corresponding complex involving wild-type TRX, it was nonetheless fairly labile (Fig. 2). Perhaps this reflects the exceptionally high reactivity of the Cys32 residue in mammalian TRX. Comparative analysis of the reactivity of the C35S mutant with other TRX substrates along with analogous studies involving other disulfide isomerases should be insightful in this regard. Second, although systemically administered TRX readily activated extracellular TG2 in the small intestine, it was not nearly as effective in recognizing TG2 in other organs. Although this may simply reflect our limited understanding of the pharmacokinetic properties of TRX or 5-BP, it may also forebode more complex regulation of extracellular TG2 activity in organs such as the heart, lungs, and liver.
Experimental Procedures
Chemicals and Other Reagents
Unless otherwise noted, chemicals were from Sigma-Aldrich. SDS-PAGE gradient gels (4–20%) were from Bio-Rad, nickel-nitrilotriacetic acid resin was from Qiagen, the HiTrap-Q anion exchange column and PD-10 desalting columns were from GE Healthcare, and 7K MWCO spin columns were from Pierce. For histological analyses, Vectashield mounting medium with DAPI was purchased from Vector Laboratories. Cell culture medium, fetal bovine serum, antibiotics, trypsin-EDTA, and sterile PBS were from Invitrogen.
Preparation of Recombinant Proteins
C35S TRX was engineered using QuikChange site-directed mutagenesis with 5′-acgtggtgtgggccttccaaaatgatcaagcct-3′ (forward) and 5′-aggcttgatcattttggaaggcccacaccacgt-3′ (reverse) primers and plasmid pCK11 encoding full-length thioredoxin-1 as a template (8), to generate the resulting plasmid pNP1. C32S/C35S was generated from pNP1 using the same method to generate plasmid pNP20, with 5′-gacttctcagccacgtggtctgggccttccaaaatgatc-3′ (forward) and 5′-gatcattttggaaggcccagaccacgtggctgagaagtc-3′ (reverse) primers. Wild-type TRX, C35S TRX, C32S/C35S TRX, and the V224 variant of human TG2 were expressed in and purified from Escherichia coli Rosetta 2, essentially as previously described (8, 51). Briefly, 1-liter cultures of LB medium, supplemented with 50 μg/liter kanamycin and 33 μg/liter chloramphenicol, were grown to A600 = 0.6 in a shaking incubator at 37 °C, and expression was induced by addition of isopropyl β-d-thiogalactopyranoside to a final concentration of 0.2 mm. Following overnight cultivation at 18 °C, the recombinant proteins were purified by nickel affinity chromatography and anion exchange chromatography on a fast protein liquid chromatograph at 4 °C. DTT was present in anion exchange buffers at a concentration of 1 mm to maintain both proteins in a reduced, active state. Purified protein was flash frozen and stored at −80 °C. TG2 concentration was determined using the Bradford assay with bovine serum albumin as the reference, whereas TRX concentration was determined by absorbance at 280 nm (ϵ = 7570 m−1 cm−1).
Oxidized TG2 was prepared by incubating the purified protein with 10 mm oxidized glutathione at room temperature for 6 h in 5 ml of 20 mm Tris-HCl, pH 7.6, containing 1 mm EDTA. Following oxidation, TG2 was repurified by anion exchange chromatography.
Fluorescent Microscopy
All fluorescent microscopy images were taken at room temperature using a Zeiss LSM 780 Meta confocal microscope with ZEN Black acquisition software. The images were collected using either a 10×/0.3NA Zeiss EC Plan-Neofluar, a 20×/0.8NA Zeiss Plan-Apochromat, or a 63×/1.4NA oil Zeiss NA Plan-Apochromat lens. For a given experiment, all images were generated under identical gain settings, which were chosen to avoid detector saturation.
Generation of a Polyclonal Antibody against TG2
A custom polyclonal antibody against was raised by Pacific Immunology (Ramona, CA) in rabbits against TG2 using full-length recombinant murine TG2 as the antigen. Serum was affinity-purified using recombinant murine TG2 as the bait. Western blotting analysis showed approximately equal reactivity with recombinant human and murine TG2, with minimal cross-reactivity with recombinant human TG1, TG3, TG4, or FXIII. Control staining experiments revealed strong immunoreactivity in TG2-positive murine fibroblasts and intestinal tissue that was absent in TG2-null controls.
TRX Secretion and TG2 Activity in Macrophages
Human monocytic THP-1 cells (ATCC) were maintained in culture in RPMI 1640 (Invitrogen catalog, no. 61870-036) culture medium containing fetal bovine serum (ThermoFisher, catalog no. 16140071) and penicillin/streptomycin (ThermoFisher, catalog no. 15140122). The cells were seeded at 106 cells/ml on 24-well glass cell culture plates (0.5 ml, CellVis catalog no. P24-1.5H-N) or 96-well plastic cell culture plates (0.1 ml, Costar, catalog no. 3596) and were differentiated into M0 macrophages by exposure to 100 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma, catalog no. P8139) for 48 h, followed by a 24-h recovery in PMA-free RPMI medium. Then macrophages were polarized into M1 macrophages by incubation with 20 ng/ml of IFN-γ (R&D Systems, catalog no. 285-IF) and 1 ng/ml LPS (InvivoGen, catalog no. tlrl-pb5lps) for 36 h in reduced serum (1%) medium. Control (M0) macrophages were maintained in PMA-free RPMI lacking LPS and IFN-γ. After polarization, the cell culture supernatants were concentrated 10-fold using Amicon Ultra centrifugal filters with a 3-kDa molecular mass cutoff (EMD Millipore, catalog no. UFC500396). The samples were diluted with 2× Laemmli sample buffer (Bio-Rad, catalog no. 1610737) and applied to a reducing 4–20% SDS-polyacrylamide gel (Bio-Rad, catalog no. 4561903). Proteins were transferred to a polyvinylidene difluoride membrane using a TransBlot Turbo system (Bio-Rad). The exported TRX were detected and quantified by immunoblotting with anti-TRX antibody (Santa Cruz, catalog no. 58440, 1:200 dilution). The blot was developed using ECL2 substrate (Pierce, catalog no. 32132) and was visualized using a Typhoon fluorescence imager (GE Healthcare). Cell viability was measured with the lactate dehydrogenase cytotoxicity assay kit (Thermo Fisher, catalog no. 88953) according to the manufacturer's instructions.
To evaluate TG2 activity, M0 macrophages grown in glass-bottomed plates were polarized or maintained in a M0 state for 36 h, as previously described. During polarization, the cells were incubated with 50 μm NP161 or vehicle in the absence or presence of 200 μm 5-biotinamidopentylamine, which was synthesized as described previously (34). The cells were washed in PBS, fixed for 5 min in 2% paraformaldehyde, and blocked overnight with 5% bovine serum albumin in PBS, pH 7.4, at 4 °C. The cells were then incubated in anti-TG2 pAb (1.1 μg/ml in blocking buffer) overnight at 4 °C, washed three times in PBS, and then incubated for 1 h at room temperature with chicken anti-rabbit Alexa Fluor 488 (2 μg/ml, Invitrogen, catalog no. A21441) and streptavidin Alexa Fluor 647 (1 μg/ml, Invitrogen, catalog no. S32357), washed three times with PBS, and counterstained with DAPI (ThermoFisher, catalog no. D1306). Fluorescence microscopy images were collected as derived above.
Detection of C35S TRX and TG2 in Vitro
Oxidized TG2 (0.3 mg, 15 μm) was alkylated with iodoacetamide (54 μg, 5 mm) for 1 h at room temperature, and buffer-exchanged into 20 mm Tris-HCl, 1 mm EDTA, pH 7.6, using a Zeba spin desalting column with a 7-kDa cutoff (Thermo Scientific, catalog no. 89882). Oxidized TG2 (8 μg, 2.5 μm) was incubated with either wild-type or C35S TRX (9.5 μg, 20 μm) for 2 h at room temperature. Samples were applied to a non-reducing 4–20% SDS-polyacrylamide gel (Bio-Rad, catalog no. 4561903), followed by electrophoretic transfer to a nitrocellulose membrane (GE Healthcare, catalog no. RPN203D). Thioredoxin was visualized with rabbit anti-thioredoxin mAb (Thermo H.753.8, 1:2000 dilution) followed by horseradish peroxidase-conjugated donkey anti-rabbit IgG (Biolegend Poly4064, 1:2000 dilution). Signal was produced with chemiluminescence reagent (PerkinElmer Life Sciences) and detected by exposure to BioMax MR film.
For immunofluorescence experiments, primary fibroblasts derived from TG2-expressing (C57BL/6) and knock-out mice (39) were isolated as described previously (53) and grown to maturity in 12-well glass-bottomed culture dishes (CellVis, catalog no. P12-1.5H-N). Wild-type or C35S TRX (10 μm) that had been prereduced in a 10-fold molar excess of DTT for 1 h at room temperature, followed by buffer exchange using Zeba spin desalting columns into PBS, pH 7.4, was diluted into fresh medium and incubated with fibroblasts for 1 h. Fibroblasts were then washed with warm PBS three times and fixed with 4% (w/v) paraformaldehyde in PBS for 15 min. The cells were thoroughly washed and blocked overnight in 5% bovine serum albumin in PBS at 4 °C. Primary antibodies, rabbit anti-TG2 pAb (1.1 μg/ml), and sheep anti-His6 IgG pAb (Abcam, catalog no. ab84162, 1:250 dilution) were used to label TG2 and TRX, respectively, in an overnight incubation at 4 °C. The cells were washed three times with PBS + 0.1% Tween 20 (PBS-T) and incubated once again overnight at 4 °C with secondary antibodies, donkey anti-rabbit Alexa Fluor 488 (ThermoFisher, catalog no. A21206, 2 μg/ml), and donkey anti-sheep IgG Alexa Fluor 647 (Abcam, catalog no. ab150179, 1:500 dilution). The cells were washed in PBS-T and counterstained with DAPI, and culture plates were imaged as described above.
Detection of Colocalized C35S TRX and TG2 in Vivo
The Stanford IACUC approved all animal studies, and animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility. Purified recombinant TRX and C35S TRX were freshly reduced in a 10-fold molar excess of DTT and buffer-exchanged into PBS, pH 7.4, using a PD-10 desalting column. The proteins were passed through a disposable 0.2-μm polyethersulfone sterile filter prior to injection. Male C57BL/6 (Charles River Laboratories, Boston, MA) or TG2−/− mice (39) ∼6–8 weeks old (∼20 g in weight) were administered wild-type TRX or C35S TRX in a single intraperitoneal injection of 500 mg/kg. Following injection, the mice were euthanized 30 min later by carbon dioxide inhalation, and tissues were collected, embedded in optimal cutting temperature medium inside biopsy cryomolds, and frozen on dry ice. Frozen cryomolds were transferred to a Leica CM3050S cryostat already equilibrated to −20 °C. Tissue blocks were removed from the cryomolds, cut into 10-μm thin sections, and transferred to room temperature Superfrost Plus microslides. Tissue mounted microslides were fixed in 4% (w/v) paraformaldehyde for 15 min at room temperature, thoroughly washed with PBS, and blocked with 5% (w/v) BSA in PBS-T overnight at 4 °C. Tissue mounted microslides were then treated with rabbit anti-TG2 pAb (1.1 μg/liter) and a 1:250 dilution of sheep anti-His6 pAb (Abcam, catalog no. ab84162) in blocking buffer overnight at 4 °C. The slides were washed three times with PBS-T for 5 min at room temperature. The slides were then treated with secondary antibodies, donkey anti-rabbit Alexa Fluor 488 (ThermoFisher A21206, 2 μg/ml), and donkey anti-sheep IgG Alexa Fluor 647 (Abcam, catalog no. ab150179, 1:500 dilution) in blocking buffer overnight at 4 °C. The slides were washed five times for 5 min each with PBS-T. The coverslips were mounted using Vectashield + DAPI (VectorLabs, catalog no. H-1200) mounting medium and sealed onto the slides with clear nail polish. Sealed slides were stored in the dark at 4 °C and imaged as described. Brightness and contrast was adjusted identically for all images using ImageJ. The experiment was performed three times with three mice/cohort.
Thioredoxin-mediated TG2 Activation in Vivo
All reagents were freshly formulated and used on the same day. ERW1041E was dissolved to 100 mg/ml in DMSO and diluted 10-fold (final concentration, 10 mg/ml) in a vehicle consisting of 2.5% (2-hydroxypropyl)-β-cyclodextrin, 2.0% Tween-80, and sterile PBS, pH 7.4. NP161 was prepared in 25% (w/v) (2-hydroxypropyl)-β-cyclodextrin, 15% (v/v) ethanol (200 proof) in PBS, pH 7.4, to 4 mg ml−1. 5-BP was synthesized as described previously (34) and dissolved in PBS to 20 mg ml−1. Recombinant human TRX was concentrated to 100 mg ml−1, reduced using a 10-fold molar excess of DTT, buffer-exchanged into PBS using a PD-10 desalting column, and used within 1.5 h. All injectables were passed through a disposable 0.2-μm sterile PVDF membrane filter prior to injection. C57BL/6 mice were from Charles River Laboratories (Boston, MA). Male C57BL/6 mice or TG2−/− mice 6–8 weeks old (∼20 g body weight) were injected with 100 mg/kg 5-BP as an i.p. injection. 30 min later, the mice were injected with a second i.p. injection of 5-BP concurrently with a dose of 500 mg/kg TRX intravenously. 5-BP was dosed again at 60 and 120 min. To ensure that 5-BP incorporation depended on the TG2 activity stemming from TRX dosing, we contemporaneously administered either 50 mg/kg i.p. of a selective TG2 inhibitor, ERW1041E, or 20 mg/kg of a selective TRX inhibitor, NP161, along with each 5-BP dose. Alternatively, the C32S/C35S double mutant TRX was injected instead of WT TRX. The mice were sacrificed after 150 min by carbon dioxide inhalation, and small intestine, heart, spleen, kidney, liver, and lung tissues were collected and directly embedded in optimal cutting temperature medium. Tissue sections were obtained as described above. Tissue mounted microslides were treated with anti-TG2 pAb (1.1 μg/ml) in blocking buffer overnight at 4 °C. The slides were washed three times in PBS for 5 min at room temperature. The slides were then treated with goat anti-rabbit Alexa Fluor 488 (ThermoFisher, catalog no. A11008, 2 μg/ml) and streptavidin Alexa Fluor 647 conjugates (Invitrogen, catalog no. S32357, 1 μg/ml) overnight at 4 °C. The slides were thoroughly washed four times for 5 min in PBS-T. The coverslips were mounted using Vectashield mounting medium and sealed onto the slides using clear nail polish. The sealed slides were stored in the dark at 4 °C, and imaged using a Zeiss LSM 780 Meta confocal microscope under identical gain settings. Brightness and contrast were adjusted identically for all images using ImageJ. Quantification of 5-BP was performed by extracting the mean signal intensities of 5-BP for each image and then normalizing to the total tissue area, as determined by a thresholding algorithm implemented in the MatLab BioFormats Toolbox.
Statistical Analyses
Statistical tests (Student's t test or one-way analysis of variance with Tukey's multiple comparison test) were used as described in the figure legends. All statistical analyses were performed using GraphPad Prism software.
Author Contributions
N. M. P. and B. A. P. contributed equally to this work. C. K., N. M. P., and B. A. P. designed the study and wrote the manuscript. C.-H. W. performed and analyzed experiments relating to TRX-mediated activation of TG2 in macrophages. B. A. P. and N. M. P. cloned and expressed the recombinant proteins, synthesized the chemical tools, and performed the experiments in cultured fibroblasts. M. A. advised the experimental design of experiments conducted in mice. N. M. P., B. A. P., and M. A. performed the in vivo colocalization and activation studies in mice. N. M. P. and B. A. P. processed the tissues and did the immunohistochemistry and microscopy. All authors analyzed the results and approved the final version of the manuscript.
Acknowledgments
We thank Prof. Bana Jabri, Prof. Ludvig Sollid, and Sangman Kim for helpful discussions. We also acknowledge Arek Melkonian, Ruize Zhuang, Andrew Hilmer, and Michael Yi for assistance with experiments.
This work was supported by National Institutes of Health Grant R01 DK063158 (to C. K.). C. K. is an advisor to and shareholder of Sitari Pharmaceuticals, a company that is developing drugs that target transglutaminase 2. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TG2
- transglutaminase 2
- TRX
- thioredoxin-1
- 5-BP
- 5-biotinamidopentylamine
- ECM
- extracellular matrix
- PMA
- phorbol 12-myristate 13-acetate.
References
- 1.Gundemir S., Colak G., Tucholski J., and Johnson G. V. (2012) Transglutaminase 2: a molecular Swiss army knife. Biochim. Biophys. Acta 1823, 406–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamczyk M., Griffiths R., Dewitt S., Knäuper V., and Aeschlimann D. (2015) P2X7 receptor activation regulates rapid unconventional export of transglutaminase-2. J. Cell Sci. 128, 4615–4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zemskov E. A., Mikhailenko I., Hsia R.-C., Zaritskaya L., and Belkin A. M. (2011) Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One 6, e19414-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klöck C., Diraimondo T. R., and Khosla C. (2012) Role of transglutaminase 2 in celiac disease pathogenesis. Semin. Immunopathol. 34, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S., and Mehta K. (2013) Tissue transglutaminase, inflammation, and cancer: how intimate is the relationship? Amino Acids. 44, 81–88 [DOI] [PubMed] [Google Scholar]
- 6.Collighan R. J., and Griffin M. (2009) Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids 36, 659–670 [DOI] [PubMed] [Google Scholar]
- 7.Siegel M., Strnad P., Watts R. E., Choi K., Jabri B., Omary M. B., and Khosla C. (2008) Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One 3, e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin X., Stamnaes J., Klöck C., DiRaimondo T. R., Sollid L. M., and Khosla C. (2011) Activation of extracellular transglutaminase 2 by thioredoxin. J. Biol. Chem. 286, 37866–37873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klöck C., and Khosla C. (2012) Regulation of the activities of the mammalian transglutaminase family of enzymes. Protein Sci. 21, 1781–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boothe R. L., and Folk J. E. (1969) A reversible, calcium-dependent, copper-catalyzed inactivation of guinea pig liver transglutaminase. J. Biol. Chem. 244, 399–405 [PubMed] [Google Scholar]
- 11.Connellan J. M., and Folk J. E. (1969) Mechanism of the inactivation of guinea pig liver transglutaminase by 5,5′-dithiobis-(2-nitrobenzoic acid). J. Biol. Chem. 244, 3173–3181 [PubMed] [Google Scholar]
- 12.Pinkas D. M., Strop P., Brunger A. T., and Khosla C. (2007) Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 5, e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamnaes J., Pinkas D. M., Fleckenstein B., Khosla C., and Sollid L. M. (2010) Redox regulation of transglutaminase 2 activity. J. Biol. Chem. 285, 25402–25409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmgren A., and Lu J. (2010) Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem. Biophys. Res. Commun. 396, 120–124 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H. (2004) Thioredoxin as a key molecule in redox signaling. Antioxid. Redox Signal. 6, 15–17 [DOI] [PubMed] [Google Scholar]
- 16.Lillig C. H., and Holmgren A. (2007) Thioredoxin and related molecules: from biology to health and disease. Antioxid. Redox Signal. 9, 25–47 [DOI] [PubMed] [Google Scholar]
- 17.Rubartelli A., Bajetto A., Allavena G., Wollman E., and Sitia R. (1992) Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 267, 24161–24164 [PubMed] [Google Scholar]
- 18.Metcalfe C., Cresswell P., Ciaccia L., Thomas B., and Barclay A. N. (2011) Labile disulfide bonds are common at the leucocyte cell surface. Open Biol. 1, 10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azimi I., Matthias L. J., Center R. J., Wong J. W., and Hogg P. J. (2010) Disulfide bond that constrains the HIV-1 gp120 V3 domain is cleaved by thioredoxin. J. Biol. Chem. 285, 40072–40080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S.-Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C. J., Bahnasi Y. M., Al-Shawaf E., Porter K. E., Jiang L.-H., Emery P., et al. (2008) TRPC channel activation by extracellular thioredoxin. Nature 451, 69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura H., De Rosa S. C., Yodoi J., Holmgren A., Ghezzi P., and Herzenberg L. A. (2001) Chronic elevation of plasma thioredoxin: inhibition of chemotaxis and curtailment of life expectancy in AIDS. Proc. Natl. Acad. Sci. U.S.A. 98, 2688–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer S., Rosenhagen C., Nakamura H., Yodoi J., Bopp C., Zimmermann J. B., Goebel M., Schemmer P., Hoffmann K., Schulze-Osthoff K., Breitkreutz R., and Weigand M. A. (2009) Thioredoxin in human and experimental sepsis. Crit. Care Med. 37, 2155–2159 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H., Hoshino Y., Okuyama H., Matsuo Y., and Yodoi J. (2009) Thioredoxin 1 delivery as new therapeutics. Adv. Drug Deliv. Rev. 61, 303–309 [DOI] [PubMed] [Google Scholar]
- 24.Matsuo Y., and Yodoi J. (2013) Extracellular thioredoxin: a therapeutic tool to combat inflammation. Cytokine Growth Factor Rev. 24, 345–353 [DOI] [PubMed] [Google Scholar]
- 25.Nilsen E. M., Jahnsen F. L., Lundin K. E., Johansen F. E., Fausa O., Sollid L. M., Jahnsen J., Scott H., and Brandtzaeg P. (1998) Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115, 551–563 [DOI] [PubMed] [Google Scholar]
- 26.Nilsen E. M., Lundin K. E., Krajci P., Scott H., Sollid L. M., and Brandtzaeg P. (1995) Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut 37, 766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molberg O., Mcadam S. N., Körner R., Quarsten H., Kristiansen C., Madsen L., Fugger L., Scott H., Norén O., Roepstorff P., Lundin K. E., Sjöström H., and Sollid L. M. (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 [DOI] [PubMed] [Google Scholar]
- 28.van de Wal Y., Kooy Y., van Veelen P., Peña S., Mearin L., Papadopoulos G., and Koning F. (1998) Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161, 1585–1588 [PubMed] [Google Scholar]
- 29.DiRaimondo T. R., Plugis N. M., Jin X., and Khosla C. (2013) Selective inhibition of extracellular thioredoxin by asymmetric disulfides. J. Med. Chem. 56, 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genin M., Clement F., Fattaccioli A., Raes M., and Michiels C. (2015) M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15, 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez F. O., Helming L., Milde R., Varin A., Melgert B. N., Draijer C., Thomas B., Fabbri M., Crawshaw A., Ho L. P., Ten Hacken N. H., Cobos Jiménez V., Kootstra N. A., Hamann J., Greaves D. R., et al. (2013) Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 121, e57–e69 [DOI] [PubMed] [Google Scholar]
- 32.Nadella V., Wang Z., Johnson T. S., Griffin M., and Devitt A. (2015) Transglutaminase 2 interacts with syndecan-4 and CD44 at the surface of human macrophages to promote removal of apoptotic cells. Biochim. Biophys. Acta 1853, 201–212 [DOI] [PubMed] [Google Scholar]
- 33.Checconi P., Salzano S., Bowler L., Mullen L., Mengozzi M., Hanschmann E.-M., Lillig C. H., Sgarbanti R., Panella S., Nencioni L., Palamara A. T., and Ghezzi P. (2015) Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PLoS One 10, e0127086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiRaimondo T. R., Klöck C., Warburton R., Herrera Z., Penumatsa K., Toksoz D., Hill N., Khosla C., and Fanburg B. (2014) Elevated transglutaminase 2 activity is associated with hypoxia-induced experimental pulmonary hypertension in mice. ACS Chem. Biol. 9, 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwertassek U., Balmer Y., Gutscher M., Weingarten L., Preuss M., Engelhard J., Winkler M., and Dick T. P. (2007) Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 26, 3086–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C., Jain M. R., Li Q., Oka S., Li W., Kong A.-N., Nagarajan N., Sadoshima J., Simmons W. J., and Li H. (2014) Identification of novel nuclear targets of human thioredoxin 1. Mol. Cell. Proteomics 13, 3507–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dafik L., Albertelli M., Stamnaes J., Sollid L. M., and Khosla C. (2012) Activation and inhibition of transglutaminase 2 in mice. PLoS One 7, e30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klöck C., Herrera Z., Albertelli M., and Khosla C. (2014) Discovery of potent and specific dihydroisoxazole inhibitors of human transglutaminase 2. J. Med. Chem. 57, 9042–9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Laurenzi V., and Melino G. (2001) Gene disruption of tissue transglutaminase. Mol. Cell. Biol. 21, 148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt B., Ho L., and Hogg P. J. (2006) Allosteric disulfide bonds. Biochemistry 45, 7429–7433 [DOI] [PubMed] [Google Scholar]
- 41.Azimi I., Wong J. W., and Hogg P. J. (2011) Control of mature protein function by allosteric disulfide bonds. Antioxid. Redox Signal. 14, 113–126 [DOI] [PubMed] [Google Scholar]
- 42.Owen G. R., Channell J. A., Forsyth V. T., Haertlein M., Mitchell E. P., Capovilla A., Papathanasopoulos M., and Cerutti N. M. (2016) Human CD4 metastability is a function of the allosteric disulfide bond in domain 2. Biochemistry 55, 2227–2237 [DOI] [PubMed] [Google Scholar]
- 43.Matthias L. J., Yam P. T., Jiang X.-M., Vandegraaff N., Li P., Poumbourios P., Donoghue N., and Hogg P. J. (2002) Disulfide exchange in domain 2 of CD4 is required for entry of HIV-1. Nat. Immunol. 3, 727–732 [DOI] [PubMed] [Google Scholar]
- 44.Barbouche R., Miquelis R., Jones I. M., and Fenouillet E. (2003) Protein-disulfide isomerase-mediated reduction of two disulfide bonds of HIV envelope glycoprotein 120 cccurs post-CXCR4 binding and is required for fusion. J. Biol. Chem. 278, 3131–3136 [DOI] [PubMed] [Google Scholar]
- 45.Ganderton T., Berndt M. C., Chesterman C. N., and Hogg P. J. (2007) Hypothesis for control of von Willebrand factor multimer size by intra-molecular thiol-disulphide exchange. J. Thromb. Haemost. 5, 204–206 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q., Zhou Y.-F., Zhang C.-Z., Zhang X., Lu C., and Springer T. A. (2009) Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc. Natl. Acad. Sci. U.S.A. 106, 9226–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krudysz-Amblo J., Jennings M. E. 2nd, Knight T., Matthews D. E., Mann K. G., and Butenas S. (2013) Disulfide reduction abolishes tissue factor cofactor function. Biochim. Biophys. Acta 1830, 3489–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zucker M., Seligsohn U., Yeheskel A., and Mor-Cohen R. (2016) An allosteric disulfide bond is involved in enhanced activation of factor XI by protein disulfide isomerase. J. Thromb. Haemost. 14, 2202–2211 [DOI] [PubMed] [Google Scholar]
- 49.Wang P., Wu Y., Li X., Ma X., and Zhong L. (2013) Thioredoxin and thioredoxin reductase control tissue factor activity by thiol redox-dependent mechanism. J. Biol. Chem. 288, 3346–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadalutti A. C., Korponay-Szabó I. R., Kaukinen K., Wang Z., Griffin M., Mäki M., and Lindfors K. (2013) Thioredoxin is involved in endothelial cell extracellular transglutaminase 2 activation mediated by celiac disease patient IgA. PLoS One 8, e77277-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi M. C., Palanski B. A., Quintero S. A., Plugis N. M., and Khosla C. (2015) An unprecedented dual antagonist and agonist of human transglutaminase 2. Bioorg. Med. Chem. Lett. 25, 4922–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelini G., Gardella S., Ardy M., Ciriolo M. R., Filomeni G., Di Trapani G., Clarke F., Sitia R., and Rubartelli A. (2002) Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. U.S.A. 99, 1491–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seluanov A., Vaidya A., and Gorbunova V. (2010) Establishing primary adult fibroblast cultures from rodents. J. Vis. Exp. pii, 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]