Abstract
FLT3-mutated acute myeloid leukemia (AML), despite not being recognized as a distinct entity in the World Health Organization (WHO) classification system, is readily recognized as a particular challenge by clinical specialists who treat acute leukemia. This is especially true with regards to the patients harboring the most common type of FLT3 mutation, the internal tandem duplication (FLT3-ITD) mutation. Here we present 4 patient cases from our institution and discuss how our management reflects what we have learned about this subtype of the disease. We also reflect on how we anticipate the management might change in the near future, with the emergence of clinically useful tyrosine kinase inhibitors.
Patient 1
A 48-year-old woman with an unremarkable past medical history noticed that she began bruising very easily. A complete blood count revealed a white blood cell (WBC) count of 66 000, virtually all of which were blasts. She was diagnosed with monocytic AML, and initial workup revealed normal cytogenetics. A mutation panel performed by a commercial diagnostics laboratory revealed that her blasts were NPM1 wild-type (WT) and harbored a 27 bp FLT3 internal tandem duplication (FLT3-ITD) mutation. No mutant-to-wild-type allelic ratio was reported back to the oncologist by the commercial laboratory. The patient received a conventional 7+3 induction chemotherapy regimen with intravenous idarubicin at 12 mg/m2 daily on days 1 to 3 and IV cytarabine by continuous infusion at 100 mg/m2 on days 1 to 7. She achieved a complete remission by the International Working Group (IWG) criteria1 and proceeded with consolidation therapy using high-dose cytarabine (“HiDAc”-3000 mg/m2 IV twice daily on days 1, 3, and 5). Although she was eligible for allogeneic transplant, her oncologist did not refer her to a transplant center. No further bone marrow biopsies or molecular assessments were performed during 4 cycles of consolidation. While her blood counts still were recovering after the fourth cycle of HiDAc, circulating blasts were noted, and the total WBC count quickly rose to 120 000, mostly blasts. Relapsed AML was confirmed by flow cytometry, and she was referred to our institution for a clinical trial. Her first remission had lasted <5 months. An assay for the FLT3-ITD mutation performed at our institution revealed the 357-bp mutant fragment present with a mutant-to-wild type allelic ratio of 6.46:1 (ie, ∼6.5 times as many mutant alleles as WT alleles). The patient was enrolled on a cooperative group protocol (E1906; NCT00634244) in which patients were randomized to receive 1 of 3 salvage chemotherapy regimens. She was randomly selected to receive carboplatin and topotecan, and she tolerated this well. However, by day 24 she had circulating peripheral blood blasts readily discernable, and was labeled relapsed and refractory to salvage.
The above case demonstrates the typical clinical course of an AML patient with a FLT3-ITD mutation treated with conventional chemotherapy, and serves as a useful springboard for a review about what we have learned about this subtype of acute leukemia. What we hope to accomplish in this article is to present our best and most current grasp of the nature of FLT3-mutated AML, and to offer our current approach to managing it, as well as what we might project as a future approach.
The biology of the disease
FLT3 is a receptor tyrosine kinase (RTK). It dimerizes on binding its cognate ligand, the cytokine FLT3 ligand (FL), undergoes autophosphorylation, and transduces signals promoting proliferation and survival via proteins such as STAT5, AKT, and ERK.2-4 In hematopoietic tissues, FLT3 is expressed in a stem/progenitor population that is not pluripotent but rather one that is already lineage-restricted,5 and it plays important roles in the function of early T-cell precursors and dendritic cells.6,7 Not surprisingly, therefore, transgenic mice lacking either FLT3 or FL are viable but have subtle defects in dendritic cell and T-cell function. Its ligand, FL, is a cytokine that acts in synergy with other cytokines to promote the expansion of hematopoietic precursors. FL can exist in membrane-bound or soluble form.8 At baseline, the concentrations of soluble FL are very low, but rise dramatically in response to chemotherapy-induced aplasia.9
FLT3 is expressed on blasts in a majority of cases of AML.10 It was because of this that a group in Japan thought to investigate mRNA levels of FLT3 as a potential marker for minimal residual disease, and in doing so discovered the existence of FLT3-ITD mutations.11 These mutations consist of duplicated coding sequence derived from the juxtamembrane domain inserted in tandem. They are in-frame, range from 3 to >200 bp in length (although most are <100 bp), and result in a disruption of the autoinhibitory function of this domain. Kiyoi and colleagues subsequently characterized the ITD mutations as causing constitutive activation of the tyrosine kinase function and were the first to report their prognostic impact in a large cohort of patients with AML.12,13 The increased relapse rate and reduced overall survival of FLT3-ITD AML patients was quickly confirmed in several large retrospective European studies.14-17 For example, the German AML Cooperative Group found FLT3-ITD patients to have an event-free survival (EFS) of 7.4 months vs 12.9 months (P = .0072) in WT counterparts.16 Point mutations in the activation loop of the kinase domain, most commonly at residue aspartate 835 (D835) and referred to as tyrosine kinase domain (TKD) mutations, were also identified as constitutively activating FLT3,18,19 although signaling from FLT3-TKD receptors is not as aberrant and their prognostic impact is not as consistently found to be as negative as that associated with the ITD mutations—particularly in young patients.20 FLT3-ITD mutations are found in ∼23% of AML cases, whereas TKD mutations are found in 7%.21
One final, potentially important aspect of FLT3 biology is that the ITD-mutated receptors are still highly responsive to FL.22 Autophosphorylation of FLT3-ITD receptors, as well as their ability to stimulate growth, are both significantly augmented by the addition of FL (although there are no data regarding the effect of FL on FLT3-TKD receptors).23 FL is expressed by the leukemia cells themselves as well as by a broad array of cell types throughout the body, and FL levels in plasma, normally present at barely detectable levels, rise by two- to three-log–fold during chemotherapy-induced aplasia. In other words, after chemotherapy, any residual AML cells are bathed in high levels of the very cytokine that directly stimulates the FLT3-ITD receptor.
FLT3 mutations are detected through polymerase chain reaction (PCR) amplification of WT and mutant alleles.24 Routine application of next-generation sequencing (NGS) often fails to identify FLT3-ITD mutations because the insertions (particularly the long ones) disrupt sequence alignment algorithms used in many NGS platforms. At our own institution, we use conventional multiplex PCR to amplify exons 14 and 20 from genomic DNA to identify FLT3-ITD and D835 mutations and to calculate an allelic ratio, and we use NGS to identify any additional point mutations that may be present.
AML is a polyclonal disease, particularly at initial diagnosis.25 The FLT3 mutant-to-wild type allelic ratio is a reflection of the fraction of leukemia cells that harbor the mutation. Different PCR amplification techniques (eg, different numbers of cycles) will yield different allelic ratios, but regardless, the mutant burden can range from a barely detectable 1% to nearly 100% (which is typically caused by loss of the copy of chromosome 13 harboring the WT allele). High allelic burden at presentation is associated with even worse outcomes.15,26 At relapse, the disease is more oligoclonal, with leukemia cells often containing a very high mutant allelic burden and, at least in vitro, appearing to be more “addicted” to FLT3 signaling.27 One hypothesis that might explain this phenomenon is that, during consolidation chemotherapy, residual leukemia stem/progenitor cells are both protected and selected for by the recurrent exposure to high levels of FL.
Patient 1 (continued)
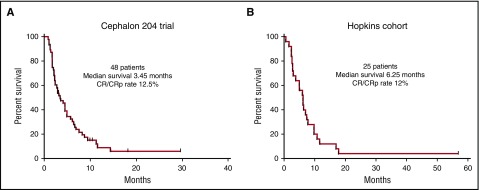
This 48-year-old woman with FLT3-ITD AML relapsing with a first remission duration of <6 months would be predicted to be most likely refractory to salvage chemotherapy.28 Relapsed AML in general has a dismal outcome, but, even worse for this patient, having a FLT3-ITD mutation at relapse is one of the strongest predictors for a very short survival.29,30 Two additional data sets would place her chances of survival at ∼5%. The first is derived from a large randomized study of salvage chemotherapy with or without lestaurtinib.28 Patients in the control arm of this study who had relapsed, like our patient, with a first remission duration of <6 months, received mitoxantrone, etoposide and cytarabine (MEC) as salvage therapy. These patients had an overall response rate of 12.5% (6/48) (Figure 1A) and poor long-term survival. A similar group of relapsed FLT3-ITD patients at our institution treated with a variety of cytarabine-based salvage regimens (without any FLT3 inhibitors) had a similar outcome (Figure 1B). The chemotherapy-resistant nature of this disease is probably related to the pronounced upregulation of prosurvival factors such as MCL-1.31
Figure 1.
Kaplan-Meier survival curves of relapsed and refractory FLT3-ITD AML patients. (A) Patients relapsing after a first remission duration of 1 to 6 months. These 48 patients were enrolled in the control arm of the Cephalon 204 trial28 and were treated with MEC, without any FLT3 inhibitor. (B) 25 patients presenting consecutively over a 4-year period to Johns Hopkins who relapsed after a first remission duration of <6 months, or who were refractory to initial induction therapy. They were all treated with intensive, cytarabine-based chemotherapy, but did not receive any FLT3 inhibitors because none were available at the time. All patients in both data sets harbored FLT3-ITD mutations only (no TKD mutations) and the majority were diagnosed between 2005 and 2009.
There is no Food and Drug Administration–approved therapy for a patient with relapsed AML who has failed a salvage regimen. After the patient was refractory to the salvage regimen with carboplatin and topotecan, she was enrolled in a study of the novel FLT3 TKI quizartinib.32 She achieved a complete remission with incomplete count recovery (CRi) and, on day 56 of therapy underwent an allogeneic transplant using a myeloablative preparative regimen (Busulfan-cyclophosphamide) and an HLA-matched unrelated donor, with post-transplant cyclophosphamide as prophylaxis against graft-versus host disease (GVHD). She developed GVHD of the skin and liver, which was treated with prednisone and tacrolimus. These immunosuppressants were tapered off by day 90 after transplant, and she was then enrolled in a protocol (NCT01578109) in which she received sorafenib as post-transplant maintenance therapy. Because of gastrointestinal toxicity, she was only able to tolerate a dose of 200 mg per day. She remains alive and disease-free, with a normal performance status (and still on sorafenib) as of August 2016, 5 years after her initial diagnosis.
Patient 2
A 46-year-old man developed fatigue and was found to have a WBC count of 400 000, mostly blasts. His past medical history was remarkable only for tobacco use. He was diagnosed with AML and underwent urgent leukapheresis followed by induction therapy with a 7+3 regimen. His AML was characterized by normal cytogenetics, an NPM1 mutation, and a FLT3-ITD mutation migrating at 363 bp (the allelic ratio was not reported by the commercial laboratory performing the test). He recovered normal peripheral blood counts, but his bone marrow showed 1% residual AML by flow cytometric determination of a leukemia-associated phenotype. His left ventricular ejection fraction, which had been normal prior to induction, was reduced to 40%. He received a cycle of HiDAc and achieved a complete remission (CR). An allogeneic transplant was planned, but given his reduced ejection fraction, this was delayed until his cardiac status could improve. He was given 2 more cycles of HiDAc, but on recovery from the third cycle he again had circulating blasts and recurrent disease. He enrolled in a clinical trial of decitabine combined with an inhibitor of exportin 1, but his disease was refractory to this. He was referred urgently to our institution with a WBC count of 85 000, 95% of which were blasts. The AML again had normal cytogenetics, an NPM1 mutation, and the FLT3-ITD mutation migrating at 363 bp. The allelic ratio was 25:1 (mutant:wild type). He was enrolled on a trial of ASP2215 (gilteritinib), a novel FLT3 inhibitor. He experienced tumor lysis syndrome, but was stabilized, and 8 weeks after treatment his bone marrow was morphologically clear of AML. He underwent an allogeneic transplant using a matched unrelated donor and reduced-intensity conditioning because of his reduced ejection fraction. Post-transplant, he was started on sorafenib (off-label, nonprotocol) 200 mg twice daily but developed GVHD of the skin and gut shortly thereafter, so this drug was stopped. His GVHD responded to steroids, and he was resumed on sorafenib. He remains in remission, with a normal functional status, 2.5 years after his diagnosis.
FLT3 inhibitors
Both patients 1 and 2 failed standard therapy, and treatment with a FLT3 TKI stabilized their disease sufficiently to allow them to undergo an allogeneic transplant. Because mutations conferring resistance to FLT3 TKIs have been reported to emerge fairly rapidly,33,34 these patients were moved briskly to transplant before such an event could occur. Although the majority of such patients (with relapsed/refractory disease) die, with or without FLT3 TKIs, these 2 cases are by no means unusual to us, or to numerous other investigators around the world. In vitro data indicate that in the relapsed or refractory setting, FLT3-ITD AML cells are addicted to the aberrant FLT3 signaling,27 a property that, in these 2 patients, may well have been selected for during the repeated courses of ineffective chemotherapy. Presently, there are no data from randomized trials demonstrating that FLT3 inhibition confers a survival benefit in the relapsed/refractory setting, and thus none has received regulatory approval. We arrive, therefore, at a central conundrum in this field: the only treatment that may offer a consistent level of efficacy is available exclusively in clinical trials or as off-label use (in the case of sorafenib).
Well over a dozen small-molecule FLT3 TKIs have been investigated in early-phase trials,35-42 and 5 (midostaurin, sorafenib, quizartinib, gilteritinib, and crenolanib) are currently being investigated or have recently been studied in randomized phase 3 studies. Of these, quizartinib and gilteritinib seem to have the most activity as single agents, at least in the relapsed/refractory setting. Most recently, the results of a randomized, placebo-controlled trial of induction chemotherapy with or without midostaurin for newly diagnosed patients ages 18 to 59 with FLT3-mutated AML (C10603/RATIFY) indicated a survival benefit for patients receiving midostaurin.43 Midostaurin, a pan-kinase inhibitor with some in vivo activity against FLT3, is actually the least effective of these agents clinically when administered as monotherapy, likely because of its multitargeted nature (and corresponding lack of potency and selectivity for FLT3). And yet, that very characteristic of midostaurin may have made it uniquely suited, among all the other FLT3 TKIs, for efficacy in the polyclonal disease that is newly-diagnosed FLT3-mutant AML.44 To this point, the use of midostaurin was demonstrated to have clinical benefit even in the FLT3-TKD patients. Given that FLT3-TKD mutations are probably only rarely drivers of the disease, this suggests that partial inhibition of multiple kinases, rather than direct inhibition of FLT3, was probably the mechanism of benefit. Likewise, the use of sorafenib, another multitargeted TKI, in combination with induction chemotherapy, was associated with improved event-free survival, in unselected AML patients.45 A simple hypothesis based on the clinical and laboratory evidence would be that pan-kinase inhibition would be more effective in the up-front setting and that the more potent, selective inhibitors would be relatively ineffective as monotherapy in newly diagnosed patients. The utility of more selective inhibitors in the newly diagnosed patients might be to use them to prevent outgrowth of the FLT3-addicted clones that invariably seems to happen during or immediately after chemotherapy. With regard to post-transplant maintenance therapy, there are 2 formal studies of post-transplant sorafenib that have been conducted, both nonrandomized.46,47 In addition, there are several published patient series describing the activity of single-agent sorafenib in the postallogeneic transplant setting.48-50 Although all of this is encouraging, the benefit of FLT3 inhibition needs to be established with a well-designed randomized study. Until that time, sporadic off-label usage of agents such as sorafenib is likely to continue based on limited data published to date.
Patient 3
A 60-year-old man with an unremarkable past medical history developed dyspnea, weakness, and night sweats. He was found to have a WBC count of 82 000, with 62% blasts, and was diagnosed with AML. The AML had normal cytogenetics, with an NPM1 mutation and 2 distinct FLT3-ITD mutations migrating at 342 bp and 366 bp. The mutant-to-wild type allelic ratios were 0.1:1 and 1.24:1, respectively. He was treated on a cooperative group protocol (NCT01802333) and received a combination of idarubicin (12 mg/m2 daily for 3 days) and cytarabine (6 g/m2 by continuous infusion over 96 hours), achieving a CR. He then enrolled on an institutional protocol (NCT01578109) of sorafenib as peritransplant maintenance, and subsequently underwent an allogeneic transplant using reduced-intensity conditioning and his haplo-identical son as the bone marrow donor. He resumed sorafenib post-transplant. He is alive, in remission, although he suffers from mild but persistent palmar-plantar erythrodysesthesia (hand-foot syndrome) from the sorafenib, 15 months after his original diagnosis.
Patient 4
A 68-year-old woman with an unremarkable past medical history developed dyspnea and fever and was noted to have a WBC count of 178 000, 85% of which were monoblasts. The cytogenetics were normal, there was an NPM1 mutation. A FLT3-ITD mutation, migrating at 351 bp, was present with a mutant-to-wild type allelic ratio of 0.59:1. Her creatinine was 3.0 mg/dL and her uric acid was 18.4 mg/dL. She received hydration, allopurinol, raspuricase, and was cyto-reduced over 3 days with hydroxyurea. She was induced with 7+3 and achieved a CR. Because of delays in identifying a suitable allogeneic donor, she was treated with a single cycle of HiDAc (using 1.5 g/m2 twice daily on days 1, 3, and 5). On recovery from this cycle, she underwent an allogeneic transplant on a cooperative group protocol (NCT01597778) using reduced-intensity conditioning and 2 partially matched cord blood units. She was then initiated on the same protocol of sorafenib as peritransplant maintenance as were Patients 1 and 3. She remains in remission, still on sorafenib, with a normal functional status 2 years after her initial diagnosis.
Patients 3 and 4 represent our most common approach to newly-diagnosed FLT3-ITD AML, namely, conventional induction therapy followed as rapidly as possible by allogeneic transplant, avoiding repeated rounds of consolidation chemotherapy. Patients with FLT3-ITD mutations tend to be somewhat younger than FLT3/WT patients (probably because the mutations are less common in AML arising out of myelodysplasia), and thus Patients 3 and 4 are in a typical age range. This is consistent with the reports from other groups.51 At our institution, 66% of patients with FLT3-ITD AML are between the ages of 30 and 64, and only 8.8% are above age 70.
It is our firm view that allogeneic transplant is the best consolidation therapy for patients with FLT3-ITD AML.52 Although there have been no prospective randomized trials investigating this issue, retrospective reviews of clinical databases (with one controversial exception53,54) conclude that transplant results in the best outcomes for these patients. Although there is ample evidence that a co-occurring NPM1 mutation mitigates the effect of a FLT3-ITD mutation on survival, the data indicate that only in cases in which there is an NPM1 mutation and the FLT3 mutant-to-wild type allelic ratio is low does conventional chemotherapy even equal the outcomes from allogeneic transplant.55-57 Laboratory techniques used to estimate allelic ratio are far from standardized and are dependent on the blast percentage of the specimen analyzed (which can be influenced by the quality of the aspirate etc.). Therefore, because allelic ratio determinations can be unreliable, we favor offering transplant to all patients in remission. It should be noted that we do not favor one donor source over another, although we prefer using younger donors whenever possible. Most patients receive reduced-intensity conditioning, although for fit younger patients we routinely use myeloablative conditioning.
Although the use of allogeneic transplant as consolidation therapy for FLT3-ITD AML is no longer controversial, the use of post-transplant maintenance with a FLT3 inhibitor certainly is. FLT3 TKIs clearly have single-agent activity (eg, see Patients 1 and 2),58,59 and there are numerous reports of the benefit of these agents in combination with an allogeneic effect.47,48,50,60 These agents are associated with some toxicities such as nausea, hypertension, palmar-plantar-erthrodysesthesia, QTc interval prolongation, and diarrhea. Although they appear to be generally safe in the post-transplant setting,46 there have simply been no randomized trials establishing their benefit. Furthermore, patients enrolled in the C10603/RATIFY trial did not receive midostaurin post-transplant, and there are certainly long-term survivors from that group.43 To address this crucial question, the Blood and Marrow Transplantation-Clinical Trials Network (BMT-CTN) is launching BMT-CTN 1506, a multicenter, randomized, double-blind, placebo-controlled trial of a FLT3 TKI (gilteritinib) as post-transplant maintenance. Given that there will be numerous participating centers in the United States, Europe, Canada, Japan, Korea, and Australia, we would urge that any patient for whom an allogeneic transplant is planned be referred for participation in this trial.
Recommendations
Approximately half of the patients with FLT3-mutated disease are referred to our institution with relapsed or refractory disease. The other half are newly diagnosed. The patient cases described here represent who we see—although our success rate at achieving long-term survival in the relapsed and refractory patients remains poor overall.
Our current approach to the newly diagnosed FLT3-ITD AML patient can be summarized as follows. First, we induce with conventional cytarabine-based chemotherapy. During the initial hospitalization for induction, and as soon as the results of the FLT3 mutation test are known, we perform HLA typing of the patient and initiate a search for a donor. Our preference is to use whichever source of stem cells is most rapidly available—matched siblings, matched or partially-matched unrelated donors, haplo-identical related donors, or umbilical cord blood. During aplasia, because most of these patients have pronounced leukocytosis at presentation, we examine the cerebrospinal fluid for the presence of leukemic meningitis and instill one dose of prophylactic intrathecal cytarabine and hydrocortisone. Once the patient recovers blood counts from induction, we confirm remission with a bone marrow biopsy and aspirate. We then proceed to an allogeneic transplant, with the preparative regimen typically starting within a few weeks of the biopsy confirming remission. We do not use the allelic ratio or NPM1 mutation status at the time of diagnosis to decide whether a patient with FLT3-ITD AML should undergo transplant—all of them are transplanted (and we do not have an age limit at our institution for allogeneic transplant). The only FLT3-ITD AML patients that might not derive benefit from transplant are those with a low mutant allelic burden, and these data are derived from retrospective trials using nonstandardized PCR assays.55-57 Furthermore, these ratios are typically only available at academic institutions. Finally, we do not believe we are doing harm by transplanting all FLT3-ITD AML patients, because there are data from meta-analyses indicating that patients with either high- or intermediate-risk AML benefit from allogeneic transplant.61 If a donor is not immediately available, we will administer a single cycle of HiDAc as consolidation, with the transplant planned to begin 45 to 60 days after day 1 of the cytarabine. Once the patient has undergone stem cell infusion and achieved engraftment, we currently enroll them on an institutional protocol to receive sorafenib as post-transplant maintenance. However, we anticipate that shortly after the time of this printing, BMT-CTN 1506 (randomized, gilteritinib vs placebo) will be open for accrual, and all such patients will be referred for enrollment in this trial.
If remission is not achieved, or if the patient is referred to us already having failed induction, our preference is to enroll the patient on a FLT3 TKI–based salvage trial. If no such trial is available, we use the combination of sorafenib (off-label) and azacitidine as published.62 This regimen induces synergistic differentiation of FLT3-ITD AML cells, and as an added bonus, is not associated with an increase in plasma FL levels.62,63 Clinically, we have found that sorafenib/azacitadine therapy often requires 3 to 4 cycles to achieve maximal antileukemic effect (eg, loss of detectable FLT3-ITD mutation). If sorafenib simply cannot be obtained, we recommend enrollment in any available clinical trial. We are obviously not enthusiastic about conventional salvage chemotherapy regimens under these circumstances (Figure 1). We regard patients who present with an isolated FLT3-TKD mutation as intermediate risk. They may or may not be consolidated with allogeneic transplant depending on other circumstances.
Treatment of FLT3-mutant AML in the (near?) future
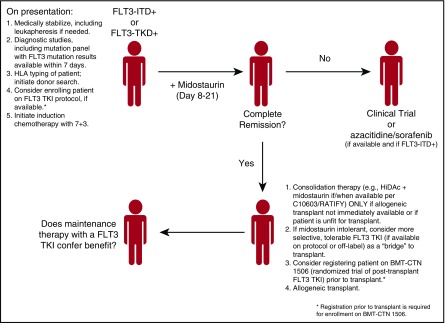
The recent results of the C10603/RATIFY trial have generated considerable enthusiasm in the field, in that we may actually have a new treatment option, midostaurin, for FLT3-mutant AML patients. Given that registration trials for 2 more selective TKIs (quizartinib and gilteritinib) are currently accruing, and that there are numerous other FLT3 inhibitors in development,47 we will indulge ourselves in a brief bout of wishful thinking as to how we might treat the patient with FLT3-mutant AML in the future (Figure 2). With the polyclonal nature of newly diagnosed AML, induction chemotherapy in combination with midostaurin—for patients with either FLT3-ITD or FLT3-TKD mutations—using the C10603/RATIFY approach in which midostaurin is administered immediately after chemotherapy, seems logical. Admittedly, this approach may only be of benefit in patients under age 60—the C10603/RATIFY only enrolled younger patients, and a trial of sorafenib combined with induction chemotherapy for older, otherwise nonselected patients was not beneficial because of increased toxicity.64 We plan to use the chemotherapy regimen of C10603/RATIFY, namely cytarabine 200 mg/m2 per day for 7 days and daunorubicin 60 mg/m2 per day for 3 days with midostaurin. In those patients with an ITD mutation, it may make more sense to transition them to a more selective, potent (and tolerable) FLT3 inhibitor, proceeding to an allogeneic transplant, and then maintaining the patient post-transplant with one of these selective agents. These latter concepts, however, must be tested in clinical trials before any firm conclusions are made. Such trials are in active development at this time.
Figure 2.
Proposed flowchart for the treatment of patients with FLT-3-mutated AML. The final point, maintenance therapy in the post-transplant setting, remains an open question, one it is hoped will be addressed by randomized trials.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health, National Cancer Institute (NCI Leukemia SPORE P50 CA100632) (M.L.).
Authorship
Contribution: K.W.P. and M.L. wrote the manuscript.
Conflict-of-interest disclosure: M.L. receives research funding from Novartis and Astellas, and serves as a consultant for Novartis, Daiichi-Sankyo, Astellas, and Arog.
Correspondence: Mark Levis, Kimmel Cancer Center at Johns Hopkins, 1650 Orleans St, Room 2M44, Baltimore, MD 21287; e-mail: levisma@jhmi.edu.
References
- 1.Cheson BD, Bennett JM, Kopecky KJ, et al. ; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642-4649. [DOI] [PubMed] [Google Scholar]
- 2.Dosil M, Wang S, Lemischka IR. Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol Cell Biol. 1993;13(10):6572-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosnet O, Bühring HJ, deLapeyrière O, et al. Expression and signal transduction of the FLT3 tyrosine kinase receptor. Acta Haematol. 1996;95(3-4):218-223. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S, Fukuda S, Lee Y, et al. Essential role of signal transducer and activator of transcription (Stat)5a but not Stat5b for Flt3-dependent signaling. J Exp Med. 2000;192(5):719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adolfsson J, Månsson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295-306. [DOI] [PubMed] [Google Scholar]
- 6.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8(11):1207-1216. [DOI] [PubMed] [Google Scholar]
- 7.Kenins L, Gill JW, Holländer GA, Wodnar-Filipowicz A. Flt3 ligand-receptor interaction is important for maintenance of early thymic progenitor numbers in steady-state thymopoiesis. Eur J Immunol. 2010;40(1):81-90. [DOI] [PubMed] [Google Scholar]
- 8.Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 1998;91(4):1101-1134. [PubMed] [Google Scholar]
- 9.Wodnar-Filipowicz A, Lyman SD, Gratwohl A, Tichelli A, Speck B, Nissen C. Flt3 ligand level reflects hematopoietic progenitor cell function in aplastic anemia and chemotherapy-induced bone marrow aplasia. Blood. 1996;88(12):4493-4499. [PubMed] [Google Scholar]
- 10.Carow CE, Levenstein M, Kaufmann SH, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87(3):1089-1096. [PubMed] [Google Scholar]
- 11.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911-1918. [PubMed] [Google Scholar]
- 12.Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93(9):3074-3080. [PubMed] [Google Scholar]
- 13.Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21(16):2555-2563. [DOI] [PubMed] [Google Scholar]
- 14.Kottaridis PD, Gale RE, Langabeer SE, Frew ME, Bowen DT, Linch DC. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100(7):2393-2398. [DOI] [PubMed] [Google Scholar]
- 15.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326-4335. [DOI] [PubMed] [Google Scholar]
- 16.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59-66. [DOI] [PubMed] [Google Scholar]
- 17.Fröhling S, Schlenk RF, Breitruck J, et al. ; AML Study Group Ulm. Acute myeloid leukemia. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372-4380. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113(4):983-988. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434-2439. [DOI] [PubMed] [Google Scholar]
- 20.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110(4):1262-1270. [DOI] [PubMed] [Google Scholar]
- 21.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738-1752. [DOI] [PubMed] [Google Scholar]
- 22.Sato T, Yang X, Knapper S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng R, Bailey E, Nguyen B, et al. Further activation of FLT3 mutants by FLT3 ligand. Oncogene. 2011;30(38):4004-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy KM, Levis M, Hafez MJ, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003;5(2):96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61(19):7233-7239. [PubMed] [Google Scholar]
- 27.Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravandi F, Kantarjian H, Faderl S, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res. 2010;34(6):752-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevallier P, Labopin M, Turlure P, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25(6):939-944. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114(24):5034-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114(14):2984-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Man CH, Fung TK, Ho C, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119(22):5133-5143. [DOI] [PubMed] [Google Scholar]
- 35.O’Farrell AM, Foran JM, Fiedler W, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9(15):5465-5476. [PubMed] [Google Scholar]
- 36.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669-3676. [DOI] [PubMed] [Google Scholar]
- 37.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54-60. [DOI] [PubMed] [Google Scholar]
- 38.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113(17):3938-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratz KW, Cho E, Levis MJ, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortes JE, Kantarjian H, Foran JM, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. 2013;31(29):3681-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galanis A, Ma H, Rajkhowa T, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. 2014;123(1):94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borthakur G, Kantarjian H, Ravandi F, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone R, Mandrekar S, Sanford BL, et al. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose c consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): an international prospective randomized (rand) p-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]) [abstract]. Blood. 2015;126(23):6.26138538 [Google Scholar]
- 44.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Röllig C, Serve H, Hüttmann A, et al. ; Study Alliance Leukaemia. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691-1699. [DOI] [PubMed] [Google Scholar]
- 46.Chen YB, Li S, Lane AA, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(12):2042-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pratz K, Gojo I, Karp J, et al. Prospective study of peri-transplant use of sorafenib as remission maintenance for FLT3-ITD patients undergoing allogeneic transplantation [abstract]. Blood. 2015;126(23):3164. [Google Scholar]
- 48.Metzelder SK, Schroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353-2359. [DOI] [PubMed] [Google Scholar]
- 49.Safaian NN, Czibere A, Bruns I, et al. Sorafenib (Nexavar) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leuk Res. 2009;33(2):348-350. [DOI] [PubMed] [Google Scholar]
- 50.Sora F, Chiusolo P, Metafuni E, et al. Sorafenib for refractory FMS-like tyrosine kinase receptor-3 (FLT3/ITD+) acute myeloid leukemia after allogenic stem cell transplantation. Leuk Res. 2011;35(3):422-423. [DOI] [PubMed] [Google Scholar]
- 51.Lazenby M, Gilkes AF, Marrin C, Evans A, Hills RK, Burnett AK. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia. 2014;28(10):1953-1959. [DOI] [PubMed] [Google Scholar]
- 52.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology Am Soc Hematol Educ Program. 2013;2013:220-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658-3665. [DOI] [PubMed] [Google Scholar]
- 54.Meshinchi S, Arceci RJ, Sanders JE, et al. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108(1):400, author reply 400-401. [DOI] [PubMed] [Google Scholar]
- 55.Pratcorona M, Brunet S, Nomdedéu J, et al. ; Grupo Cooperativo Para el Estudio y Tratamiento de las Leucemias Agudas Mieloblásticas. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734-2738. [DOI] [PubMed] [Google Scholar]
- 56.Schlenk RF, Kayser S, Bullinger L, et al. ; German-Austrian AML Study Group. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441-3449. [DOI] [PubMed] [Google Scholar]
- 57.Ho AD, Schetelig J, Bochtler T, et al. ; Study Alliance Leukemia. Allogeneic Stem Cell Transplantation Improves Survival in Patients with Acute Myeloid Leukemia Characterized by a High Allelic Ratio of Mutant FLT3-ITD. Biol Blood Marrow Transplant. 2016;22(3):462-469. [DOI] [PubMed] [Google Scholar]
- 58.Levis M, Perl A, Dombret H, et al. Final results of a Phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation [abstract]. Blood. 2012;120(21):673a. [Google Scholar]
- 59.Levis M, Perl A, Altman JK, et al. Results of a first-in-human, phase I/II trial of ASP2215, a selective, potent inhibitor of FLT3/Axl in patients with relapsed or refractory (R/R) acute myeloid leukemia (AML) [abstract]. J Clin Oncol. 2015;33(suppl). Abstract 7003. [Google Scholar]
- 60.Sammons SL, Pratz KW, Smith BD, Karp JE, Emadi A. Sorafenib is tolerable and improves clinical outcomes in patients with FLT3-ITD acute myeloid leukemia prior to stem cell transplant and after relapse post-transplant. Am J Hematol. 2014;89(9):936-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravandi F, Alattar ML, Grunwald MR, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang E, Ganguly S, Rajkhowa T, Gocke CD, Levis M, Konig H. The combination of FLT3 and DNA methyltransferase inhibition is synergistically cytotoxic to FLT3/ITD acute myeloid leukemia cells. Leukemia. 2016;30(5):1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110-3118. [DOI] [PubMed] [Google Scholar]