Abstract
The fbpA and fbpB genes encoding the 85A and 85B proteins of Mycobacterium tuberculosis H37Rv, respectively, were disrupted, the mutants were examined for their ability to survive, and the strain lacking 85A (ΔfbpA) was tested for its ability to immunize mice. The ΔfbpA mutant was attenuated in mice after intravenous or aerosol infection, while replication of the ΔfbpB mutant was similar to that of the wild type. Complementation of the fbpA gene in ΔfbpA restored its ability to grow in the lungs of mice. The ΔfbpA mutant induced a stronger expression of pulmonary mRNA messages in mice for tumor necrosis factor alpha, interleukin-1 beta (IL-1β), gamma interferon, IL-6, IL-2, and inducible nitric oxide (NO) synthase, which led to its decline, while H37Rv persisted despite strong immune responses. H37Rv and ΔfbpA both induced NO in macrophages and were equally susceptible to NO donors, although ΔfbpA was more susceptible in vitro to peroxynitrite and its growth was enhanced by NO inhibitors in mice and macrophages. Aerosol-infected mice, which cleared a low-dose ΔfbpA infection, resisted a challenge with virulent M. tuberculosis. Mice subcutaneously immunized with ΔfbpA or Mycobacterium bovis BCG and challenged with M. tuberculosis also showed similar levels of protection, marked by a reduction in the growth of challenged M. tuberculosis. The ΔfbpA mutant was thus attenuated, unlike ΔfbpB, but was also vaccinogenic against tuberculosis. Attenuation was incomplete, however, since ΔfbpA revived in normal mice after 370 days, suggesting that revival was due to immunosenescence but not compensation by the fbpB or fbpC gene. Antigen 85A thus affects susceptibility to peroxynitrite in M. tuberculosis and appears to be necessary for its optimal growth in mice.
Tuberculosis is the leading cause of death due to infections in humans today, with at least 2 million deaths reported worldwide in 2002. The causative agent, Mycobacterium tuberculosis, has the unique ability to survive within macrophages by using diverse strategies, to become dormant, and to revive to cause reactivation, which is a leading cause of adult tuberculosis (9, 41). The emergence of multidrug-resistant tuberculosis has posed a threat to effective control of tuberculosis worldwide. Although a vaccine could be the most cost-effective preventive measure, the widely used Mycobacterium bovis BCG vaccine has been found to be ineffective against adult tuberculosis and to have a variable rate of protection against the childhood disease (7, 13). Therefore, the search for a better vaccine continues. Historically, live attenuated vaccines have afforded better protection against many diseases and, not surprisingly, attenuated laboratory strains of M. tuberculosis were among the first to be tested as vaccine candidates. Seminal studies by Trudeau and by Steenken and Gardner demonstrated that the attenuated M. tuberculosis strain R1 did protect animals against virulent organisms (9, 47, 49). Subsequently, however, interest in this direction appears to have waned since M. tuberculosis is a strict pathogen that is capable of becoming latent and later reactivating to cause disease. In recent years, however, multiple mutation strategies have been used to produce attenuated mutants of M. tuberculosis for possible vaccine use or to aid in understanding gene regulation. Enzymes of the biosynthetic pathways have been particularly useful targets. Mutants which cannot synthesize cyclopropane synthetase (pcaA), isocitrate lyase enzyme (icl), or phospholipases had reduced survival in mice (14, 27, 39). Likewise, leucine and pantothenate auxotrophic mutants (ΔleuD, ΔpanD) as well as mutants which had defects in lipid or iron metabolism were all attenuated in SCID or normal mice to various degrees (16, 25, 40, 44). Disruption of genes for nonenzyme proteins has also identified additional functional mutants. Mutants that lack the Erp protein or the C3-binding HbhA protein or those with disruption in the sigma factors were of reduced virulence in mice (11, 21, 30). Finally, attenuated mutants have also been obtained after transposon mutagenesis and after disruption of genes with uncertain functions (26). Despite these successful reports, disruptions of genes have at times been unpredictable. For example, mutants lacking the homologs of Nramp1 protein or the antioxidant genes noxI did not differ from the wild type in growth in mice, while the inactivation of antioxidant gene ahpC did not affect the survival of mutants in mice (8, 12, 46, 48). Paradoxically, disruption of the two-component regulatory system enhanced the virulence in certain strains of M. tuberculosis (37). Together, these efforts indicate that genetic manipulations are a powerful approach to study the pathogenesis of M. tuberculosis although at times they have yielded unexpected results, evidence that the pathogen has multiple complex regulatory pathways that still remain to be explored.
Efforts to interfere with the function of the antigen 85 (Ag85) complex of M. tuberculosis were described previously (4). The Ag85 complex consists of at least three secreted proteins, which have various levels of mycolyl transferase and fibronectin binding activity (6, 43, 53). They have also been implicated in the induction of immune responses since they display antigenic epitopes reactive with T and B cells from humans and animals, besides being vaccinogenic (36, 38). A disruption in their synthesis was thus anticipated to affect the metabolism, in vitro replication, and intracellular growth in macrophages and survival of the wild type in mice. Gene disruption experiments were performed to generate H37Rv mutants defective in the synthesis of Ag85 complex protein A (ΔfbpA) and Ag85 complex protein B (ΔfbpB). Marked phenotypic changes were observed with the ΔfbpA mutant. Unlike the wild-type H37Rv strain and the ΔfbpB mutant, it failed to grow in synthetic medium and required a fatty acid supplement (4). While H37Rv and the ΔfbpB mutant showed progressive growth within human (THP1) and mouse (J774.A1) macrophages, ΔfbpA failed to grow, suggesting that the disruption of fbpA caused a specific attenuation of virulence. In this investigation, we demonstrate that the disruption of the fbpA gene also results in an enhanced susceptibility to peroxynitrite (ONOO) that results in the attenuated growth of ΔfbpA in macrophages and mice. Furthermore, we report that mice previously exposed to or immunized with ΔfbpA resist a subsequent challenge with virulent M. tuberculosis.
MATERIALS AND METHODS
Animals.
Specific pathogen-free male or female C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and used at 4 to 6 weeks of age. They were housed within a biosafety level 3 vivarium, fed pelleted food and water ad libitum, and maintained under Institutional Animal Care and Use Committee-approved conditions.
Bacteria.
M. tuberculosis H37Rv (27294), Erdman (35801), and BCG (Pasteur 35734) reference strains were from the American Type Culture Collection repository. The mutants ΔfbpA and ΔfbpB were produced and characterized as described before (4). CFU from each strain grown on 7H11 agar were subcultured in 7H9 broth, and log-phase organisms were harvested 10 to 12 days later. Washed bacteria were sonicated at 5 W for 15 s to disperse organisms and were stored in aliquots at −70°C in 70% glycerol-phosphate-buffered saline (PBS). One thawed aliquot was diluted 10-fold in saline and plated for CFU counts on 7H11 agar.
Production and evaluation of complemented ΔfbpA mutants.
The integrative strain and the plasmid-complemented strains of ΔfbpA were prepared as described by Armitige et al. (4, 5). The effects of complementation were evaluated by (i) growing the complemented strains in 7H9 broth and performing the Western blot analysis for Ag85 complex proteins with the HYT27 monoclonal antibody as described before (4) and (ii) using the wild-type H37Rv, the ΔfbpA mutant, and the two complemented strains for performing aerosol infections of C57BL/6 mice and analyzing their growth profiles.
Infections of mice and evaluation of growth curves.
C57BL/6 mice of either sex were infected intravenously (i.v.) by using the lateral vein, at a dose of 106 CFU/mouse. Aerosol infections were performed by exposing groups of mice to the wild-type and mutant strains in a Middlebrook aerosol exposure chamber (Glascol, Inc., Terre Haute, Ind.). A sonically dispersed suspension of M. tuberculosis at 106 CFU/ml in saline was nebulized for 30 min and implanted into the lungs at around 2.5 log10 CFU/mouse. Mice were then sacrificed at selected time points, and lungs, livers, and spleens were removed aseptically. Organs were homogenized in PBS with 0.05% Tween 80, and 10-fold dilutions were plated on 7H11 agar for CFU counts.
Histological analysis and evaluation of pulmonary cytokine responses.
Lung tissue collected from mice at selected time points was collected in 10% buffered formalin, and tissue sections were examined after hematoxylin and eosin stains for granuloma formation. To determine pulmonary cytokine responses, C57BL/6 mice were injected i.v with 106 CFU of either ΔfbpA or H37Rv/mouse, and lung tissues were collected into RNAzol at various time points until day 21 after infection. On all days, three mouse lungs were collected per strain and analyzed; due to marked variation in standard deviations, eight mouse lungs were analyzed per strain on day 21, marking the termination of the i.v. experiment. The cytokine message induction was measured by previously standardized methods of bioluminescence-based reverse transcription (RT)-PCR (1-3).
Susceptibility of M. tuberculosis strains to nitric oxide (NO) in mice and macrophages. (i) In vivo susceptibility to NO.
C57BL/6 mice were infected with ΔfbpA and H37Rv by using a low-dose aerosol as described above. Mice were then fed with a daily oral dose of aminoguanidine (2.5% in water) 5 days per week for 4 weeks, and organ CFU counts were determined at intervals.
(ii) Induction of NO and ROS.
In order to determine whether the decreased growth of ΔfbpA could be due to increased macrophage-mediated killing, the following experiments were done. In an infection experiment repeated thrice, bone marrow (BM)-derived macrophages from C57BL/6 mice, naïve or preactivated with 400 U of gamma interferon (IFN-γ)/ml for 18 h, were used. These macrophages were washed, infected with M. tuberculosis strains at a multiplicity of infection (MOI) of 1:10, and washed to remove nonphagocytosed bacilli, and fresh IFN-γ was added and was replaced every 2 days. In another experiment that was also repeated three times, infected macrophages were incubated in the presence or absence of 10 μg of N-monomethyl arginine (NMMA; Sigma Chemical Co., St. Louis, Mo.)/ml that was replaced on days 3 and 5. CFU counts were determined in triplicate wells of macrophages per strain per time point on days 1, 3, and 7. The macrophage-secreted NO response was measured by titrating culture supernatants of infected, uninfected, and control macrophages 1, 3, and 7 days postinfection by using Griess reagent and colorimetry. Intracellular synthesis of reactive oxygen species (ROS) was measured by infecting macrophages separately at an MOI of 1:10 for 4 h and washing and incubating them. At time points, monolayers were washed thrice with medium and pulsed with 1 μg of dihydrodichlorofluorescein diacetate (DCFDA; Molecular Probes, Inc., Eugene, Oreg.)/ml in medium. DCFDA is cleaved by intracellular esterases, and the resultant DCF is oxidized by ROS to green fluorescent dichlorofluorescein hydrate (DCFH) that gets trapped within cells. Intracellular DCFH was measured by using a Labsystems Ascent Fluoroscan (excitation wavelength, 485 nm; emission wavelength, 530 nm) and was expressed as average fluorescence units per 105 macrophages in quadruplicate wells per strain per time point. For both NO and ROS assays, positive controls included macrophages activated with IFN-γ (400 U/ml).
(iii) In vitro susceptibility of ΔfbpA to NO and peroxynitrite.
Log phase cultures of H37Rv and ΔfbpA grown in 7H9 broth for 12 days were washed, briefly sonicated, matched to McFarland standard 1, and diluted in sterile PBS to CFU concentrations of 106 per ml. 1-Propamine-3-2-hydroxy-2-nitroso-1-propylhydrazine (PAPA-NONOate; Alexis Chemicals, San Diego, Calif.) is a slow-release NO donor that is suitable for the evaluation of bacterial susceptibility to NO (22). Ice-cold solutions of this reagent in 1 M NaOH (or 1 M NaOH as vehicle control) were added to suspension cultures of mycobacteria at 37°C, vortexed, and incubated in a water bath at 37°C for 20 min. Bacteria were then pelleted by centrifugation at 4,000 × g for 15 min, and resuspended in sterile PBS, and cold reagent was added again. This process was repeated for six cycles for each concentration of the NO donor. After the final cycle, M. tuberculosis was resuspended and gently sonicated for dispersal, and aliquots were plated at 10-fold dilutions on 7H1 agar and reduction in viable counts were compared to those of the vehicle-treated cultures. Susceptibility to peroxynitrite (Alexis Chemicals) was tested under identical conditions except that various doses of the compound dissolved in 0.7 M NaOH were prepared with a phosphate buffer immediately before addition to mycobacteria, and the phosphate buffer without peroxynitrite was used as the vehicle control.
Immunizations and evaluation of vaccine efficacy.
Three experiments were performed. In the first experiment, a batch of uninfected C47BL/6 mice were maintained as controls and another batch was aerosol infected with ΔfbpA mutant at 2.5 log10 CFU per mouse. The infected mice were examined for CFU at time points until day 90 to 110, when the CFU counts revealed that lungs had very few culturable CFU. Uninfected control and ΔfbpA-infected mice were then challenged with a suspension of M. tuberculosis strain Erdman via aerosol. Mice were sacrificed 30 days later, and lung CFU were enumerated by plating organ homogenates on 7H11 agar. The next two experiments were performed by using the subcutaneous model of immunization to compare the vaccine effect of the ΔfbpA mutant with that of BCG (36). C57BL/6 mice received two injections of either ΔfbpA or the BCG Pasteur strain given subcutaneously at the base of the tail 2 weeks apart at a dose of 105 CFU/mouse. Age-and-sex-matched unvaccinated mice served as controls. Two weeks (for the second experiment) or 4 weeks (for the third experiment) after the last vaccine dose, unvaccinated and vaccinated mice were challenged with an aerosol dose of the Erdman strain, implanting into the lungs approximately 2.5 log10 CFU/mouse. After the challenge, mice were sacrificed at 30-day intervals to enumerate the CFU for lungs. The vaccine effect was defined as the log10 reduction in growth compared to unvaccinated controls (36, 50). In all three experiments, growth of the challenge Erdman strain in the lungs was confirmed as follows. All CFU platings from BCG-immunized mice were carried out on 7H11 agar with 5 μg of thiophene-2-carboxylic acid/ml. For ΔfbpA-immunized mice, CFU were collected from each plate of growth from multiple dilutions of plating and (i) subcultured into replicate aliquots of 5 ml of 7H9 broth, one of which contained 25 μg of kanamycin/ml and (ii) subcultured into 5 ml of Sauton broth, which does not support the growth of the ΔfbpA mutant. In addition, from the lungs of each mouse sacrificed at every time point, pools of CFU (200 CFU/pool) were subjected to Southern analysis for the fbpA gene following previously described methods (4). Unlike the ΔfbpA mutant, Erdman strain grew in 7H9 broth without kanamycin, grew in Sauton broth, and showed the presence of an intact fbpA gene.
In vitro immunogenicity of the ΔfbpA strain.
C57BL/6 mice were immunized i.v. with a heat-killed suspension of M. tuberculosis (Hk-H37Rv) and boosted 2 weeks later with subcutaneous doses administered 1 week apart. Uninfected mice were controls. Spleens from naïve or immunized mice were obtained 4 weeks after the first dose, depleted of non-T cells by using pan T-cell magnetic beads (Miltenyi, Inc.), and adjusted to 106 cells/ml. C57BL/6 mouse-derived BMs were infected with ΔfbpA, BCG, Hk-H37Rv, or live H37Rv at a MOI of 1:10 or left uninfected for 24 h. Washed monolayers were then overlaid with sensitized or naïve T-cell suspensions, and supernatants were assayed for IFN-γ levels by sandwich enzyme-linked immunosorbent assay (R&D Systems) in three independent experiments. Pools of overlaid T cells collected at various time points from each experiment were stained for intracellular IFN-γ by using an intrakine kit from BD Pharmingen (San Diego, Calif.) and acquired by using a BD-FACSCAN instrument.
Long-term survival of the ΔfbpA strain.
C57BL/6 mice were infected with H37Rv and the ΔfbpA strain by aerosol and were left untreated for 400 days. During the first month, weekly sacrifice was carried out to determine CFU counts. Subsequently, CFU counts were performed on days 90, 180, 270, 300, 370, and 400 postinfection.
RESULTS
Growth of the wild type and mutants in mouse organs after intravenous infection.
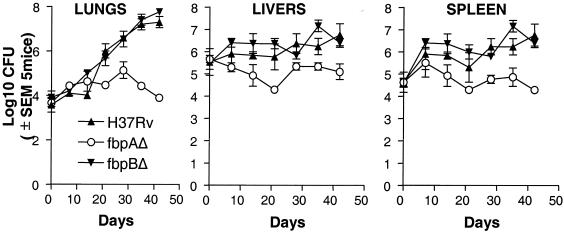
Even though the i.v. route of infection is unnatural, it has been used frequently to evaluate virulence of M. tuberculosis strains (32, 33). An i.v. dose of 106 CFU/mouse was therefore used to first test the growth profiles of ΔfbpA and ΔfbpB mutants in comparison with the wild type. This i.v. dose resulted in a higher implantation in livers and spleens than in lungs, as is the published pattern for mice after i.v. infection (32) (Fig. 1). Nevertheless, both the wild type and the ΔfbpB mutant grew to higher levels in lungs, with modest growth (between 1 and 2 log10) in livers and spleens, followed by a plateau between days 40 to 50 postinfection. In striking contrast, CFU counts of ΔfbpA declined in the lungs, livers, and spleens of mice after an initial modest increase of 1 to 2 log10 over the 30 days. For both the wild type and ΔfbpB, mice became sick between days 40 and 50, with apparent loss of weight and ruffled coats; all mice were sacrificed by day 50. In order to confirm the stability of the phenotypes, ΔfbpA, ΔfbpB, and wild-type H37Rv were recovered from mouse lungs on day 30, passaged in 7H9 broth in vitro three times in 30 days (mutants were grown in presence of antibiotics), and stored frozen as aliquots at −70°C. A year later, thawed mutants and a similarly processed wild-type H37Rv at the same dose were injected i.v. into C57BL/6 mice. Similar growth curves were obtained (data not shown).
FIG. 1.
Disruption of fibronectin binding protein A (fbpA) and fbpB genes has different effects on the growth profiles of M. tuberculosis H37Rv in mice. C57BL/6 mice were infected with an intravenous inoculum of 106 CFU of ΔfbpA (○), ΔfbpB (▾), or the wild type (▴) per mouse. Five mice per strain were sacrificed at the time points shown, and CFU counts were determined by plating organ homogenates on 7H11 agar. Both the wild type and the ΔfbpB mutant grow better in the lungs and moderately well in the livers and spleens. ΔfbpA grows initially but declines later in lungs, livers, and spleens. Its growth in lungs is markedly attenuated compared to both the wild type and the ΔfbpB mutant (P values were <0.001 for lung CFU and <0.007 for spleen and liver CFU on days 20 through 50 by the Mann-Whitney U test). SEM, standard error of the mean.
Histopathology of infection in the lungs.
Lungs obtained from mice on day 28 after i.v. infection (Fig. 1) were analyzed after hematoxylin and eosin stains. There were no striking differences between lungs of mice infected i.v. or by aerosol with either ΔfbpA or H37Rv (data not shown).
Expression of cytokine and iNOS messages in the lungs.
Since the growth difference between ΔfbpA and the wild type was most prominent in the lungs, in situ cytokine messages were measured by using RT-PCR. The ΔfbpA mutant induced an increase in message expression for cytokines and inducible NO synthase (iNOS) that were likely expressed by macrophages and for IFN-γ by T cells (Table 1). On day 21, data analyzed statistically on eight mice (t test) showed significant differences (P < 0.05) for tumor necrosis factor alpha (TNF-α), IFN-γ, interleukin-1 beta (IL-1β), IL-6, IL-10, IL-2, and iNOS (Table 2).
TABLE 1.
Expression of lung mRNA messages for cytokines during infection of mice with M. tuberculosis ΔfbpA mutanta
| Day | Results for cytokine indicated (avg ± SD)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α | IFN-γ | IL-1β | iNOS | IL-6 | IL-10 | IL-12 | IL-2 | IL-4 | IL-5 | |
| 0 | 1 ± 0.4 | 1 ± 0.7 | 1 ± 0.3 | 1 ± 1.39 | 1 ± 1.52 | 1 ± 0.75 | 1 ± 0.5 | 1 ± 0.91 | 1 ± 0.88 | 1 ± 1.02 |
| 2 | 2.4 ± 1.1 | 4.4 ± 1.4 | 3.4 ± 1.4 | 2.19 ± 1.89 | 11.04 ± 4.87 | 10.59 ± 1.25 | 2 ± 1.1 | 0.81 ± 0.52 | 0.81 ± 1.18 | 3.29 ± 2.65 |
| 5 | 6.2 ± 5.3 | 4.8 ± 2 | 6.8 ± 2.6 | 2.13 ± 1.39 | 13.15 ± 3.9 | 12.52 ± 4.66 | 3.1 ± 1.7 | 0.85 ± 0.42 | 0.85 ± 1.23 | 3.27 ± 0.64 |
| 7 | 16.9 ± 5.8 | 36.6 ± 22.5 | 28.7 ± 12.1 | 4.34 ± 1.56 | 247.82 ± 92.54 | 23.92 ± 21.27 | 4.6 ± 1.7 | 1.86 ± 0.37 | 1.86 ± 1.32 | 12.25 ± 4.57 |
| 14 | 38.5 ± 15.3 | 34.1 ± 31.5 | 50.6 ± 34.8 | 28.9 ± 3.78 | 140.91 ± 122.23 | 22.12 ± 20.67 | 7.4 ± 7.5 | 0.75 ± 0.68 | 0.85 ± 1.21 | 0.52 ± 0.54 |
| 21 | 155.4 ± 40.1 | 76.8 ± 9.7 | 107.8 ± 26.3 | 157.53 ± 24.83 | 165.19 ± 22.27 | 52.46 ± 0.54 | 33.5 ± 18.4 | 4.89 ± 2.13 | 1.86 ± 5.32 | 4.13 ± 3.96 |
Expression of mRNA in the lungs of C57BL/6 mice following infection with 106 CFU of ΔfbpA was evaluated by quantitative bioluminescence RT-PCR. Samples of cDNA from infected lungs were amplified individually with specific primers, followed by detection using bioluminescence, and were quantitated as β-actin-normalized relative light units. Values obtained for infected and uninfected lungs were compared; data are expressed as mean values ± SDs for three (days 0 to 14) or eight (day 21) mice with duplicate samples for each time point. Avg, average increase (n-fold) in expression of mRNA in mouse lungs.
TABLE 2.
Comparison of lung mRNA expression on day 21 postinfection in mice infected with M. tuberculosis ΔfbpA or H37Rv
| Cytokine or agent | Avg change in lung mRNA expression (n-fold) ± SD for:
|
P value (n = 8)a | |
|---|---|---|---|
| ΔfbpA | Wild-type H37Rv | ||
| TNF-α | 155.4 ± 40.1 | 84.3 ± 84.0 | 0.0486 |
| IFN-γ | 76.8 ± 9.7 | 42.3 ± 40.2 | 0.0333 |
| IL-1β | 107.8 ± 26.3 | 59.7 ± 57.6 | 0.0496 |
| iNOS | 157.5 ± 24.8 | 87.0 ± 82.8 | 0.0369 |
| IL-6 | 165.2 ± 22.3 | 88.0 ± 80.0 | 0.0198 |
| IL-10 | 52.5 ± 0.5 | 27.5 ± 27.0 | 0.0204 |
| IL-12 | 33.5 ± 18.4 | 17.9 ± 17.0 | 0.1000 |
| IL-2 | 4.9 ± 2.1 | 2.6 ± 2.0 | 0.0416 |
| IL-4 | 1.9 ± 1.9 | 2.1 ± 2.0 | 0.8405 |
| IL-5 | 4.1 ± 3.9 | 2.4 ± 2.0 | 0.2911 |
P values of <0.05 were considered significant (t test).
Growth in mouse organs after aerosol infection.
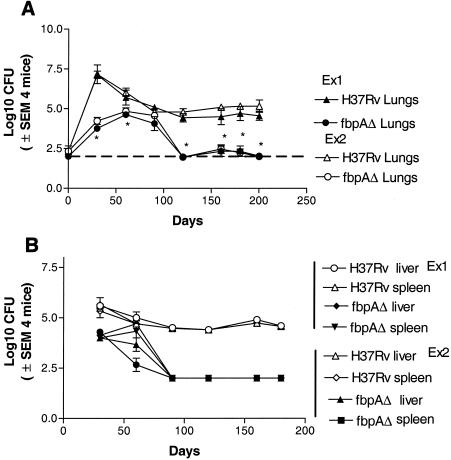
Figure 2A illustrates the results of two independent experiments. The wild type showed an increase over the initial 30 days. Thereafter, the CFU counts declined followed by a stabilization of counts within organs over the next 170 days. The ΔfbpA mutant showed an initial increase in the lungs up to day 30, followed by a gradual decline. By day 90 postinfection, culturable CFU were not demonstrable among lungs. Since the sensitivity of plating is about 100 CFU per organ, the counts between 90 and 180 are shown along a horizontal line representing this level. ΔfbpA disseminated from lungs to livers and spleens by day 30, but in these organs, also, CFU declined to low levels (Fig. 2B).
FIG. 2.
The ΔfbpA mutant is attenuated in mice after low-dose aerosol-induced infection. C57BL/6 mice were aerosol implanted with ΔfbpA or H37Rv, and CFU counts in lungs (A) and spleens and livers (B) were determined over time. Results of two independent experiments performed a year apart are shown, with four mice per strain per time point being sacrificed for CFU counts in each experiment. After an initial increase, CFU of ΔfbpA mutant in the lungs declined to low levels by day 90 postimplantation (A). H37Rv grows progressively until day 30 and then stabilizes over the next 150 days. ΔfbpA disseminates to spleens and livers, where it declines (B), while the wild type persists in spleens and livers in significant numbers. The broken horizontal line indicates the sensitivity of plating. SEM, standard error of the mean. (A) *, P value was <0.005 for ΔfbpA compared to H37Rv. (B) *, P value was <0.002 compared to H37Rv by the Mann-Whitney U test.
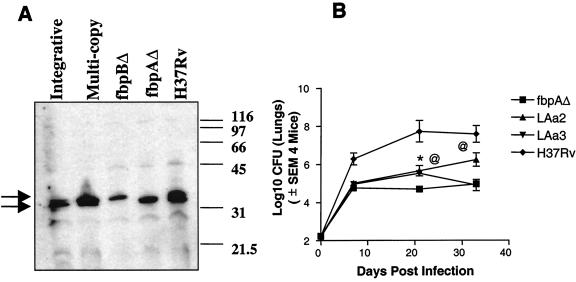
Effect of complementation of the fbpA gene in the ΔfbpA mutant.
The complemented mutants were cultured in vitro, and culture supernatants were tested by using the HYT27 monoclonal antibody (kind gift of Kris Huygen, Pasteur Institute, Brussels, Belgium) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting as previously described (4). Figure 3A demonstrates that the ΔfbpA mutant lacks the antigen 85A (Ag85A) protein band and the ΔfbpB mutant lacks the Ag85B protein band. Use of multicopy plasmid transfectants shows restoration of the Ag85A protein, as does the integrative vector for the Ag85A (fbpA) gene. The wild type, the ΔfbpA mutant, and the complemented strains were then used for aerosol infection of mice. Figure 3B shows the enhanced growth of the wild type and the attenuated growth of the ΔfbpA mutant in the lungs. Interestingly, the integrative strain (LAa2) grew to higher levels than the ΔfbpA strain, thus indicating that complementation restored the function of fbpA gene (P value of <0.008 compared to ΔfbpA on day 33). The growth of the multicopy plasmid-bearing strain (LAa3) was significant initially (P value of <0.01 on day 21) but later declined. The ΔfbpA and the complemented strains also disseminated to livers and spleens by day 21 after aerosol infection, although they showed little growth in either organ until day 33, when the experiment was terminated (data not shown).
FIG. 3.
Effect of complementation of the fbpA gene in the ΔfbpA mutant. (A) The ΔfbpA mutant was complemented by using an integrative vector and multicopy plasmids. Western blot analysis of sodium dodecyl sulfate-polyacrylamide gel electrophoresis run on proteins isolated from H37Rv, ΔfbpA, and ΔfbpB mutants reveals that the ΔfbpA mutant lacks the antigen 85A protein band and the ΔfbpB mutant lacks the antigen 85B protein band. Use of multicopy plasmid transfectants shows restoration of the Ag85A protein, as does integrative vector for the Ag85A (fbpA) gene. The monoclonal antibody HYT27, which recognizes all three proteins of Ag85 complex, was used in the blots. Molecular weight standards (in thousands) are indicated on the left. Arrows indicate overlapping protein recognition. (B) C57BL/6 mice were aerosol implanted with wild-type H37Rv, ΔfbpA, and the two complemented strains (LAa2 integrative and LAa3 multicopy plasmid). At the indicated time points, four mice per strain were sacrificed and lung CFU was determined by plating on 7H11 agar. The growth of the ΔfbpA mutant is attenuated and similar to the profiles described above. Both of the complemented mutants grow better than the ΔfbpA mutant, although the integrative strain grows better than the strain with the multicopy plasmid. *, P value was <0.01 for LAa3 compared to ΔfbpA; @, P value was <0.01 day 21 and <0.008 on day 33 for LAa2 compared to ΔfbpA by the Mann-Whitney U test. Standard errors of the means (SEMs) for ΔfbpA are too low to be plotted.
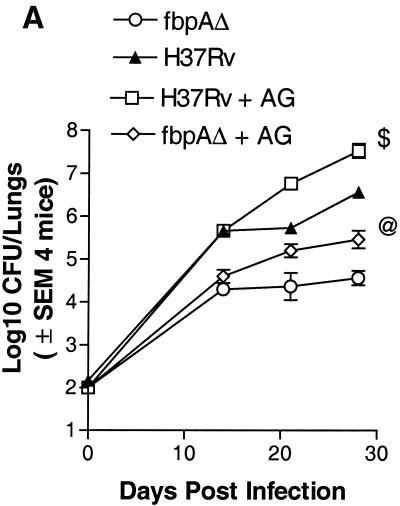
In vivo and in vitro susceptibility to NO in ΔfbpA and H37Rv. (i) In vivo susceptibility.
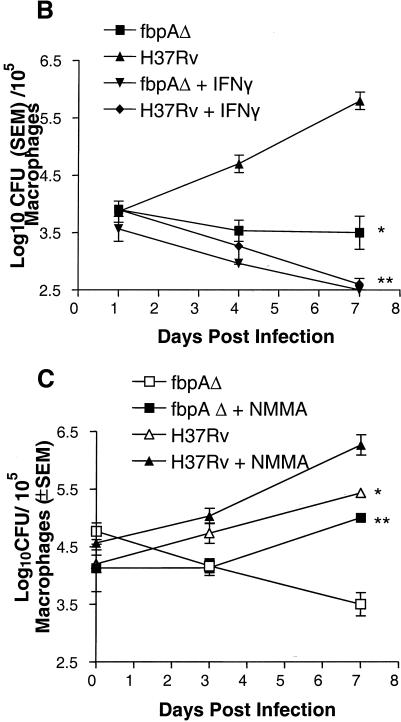
When mice were treated with a NO inhibitor, there was an enhancement of the growth of ΔfbpA compared to that of the untreated control (Fig. 4A).
FIG. 4.
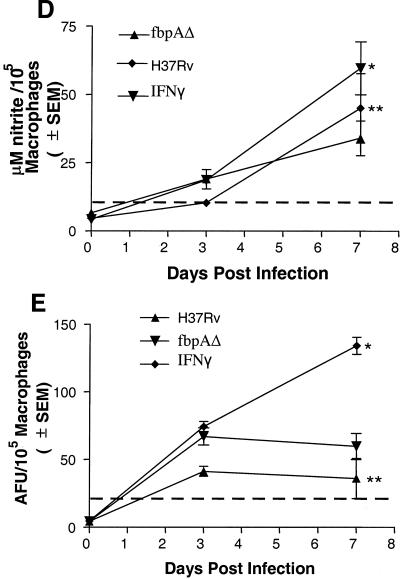
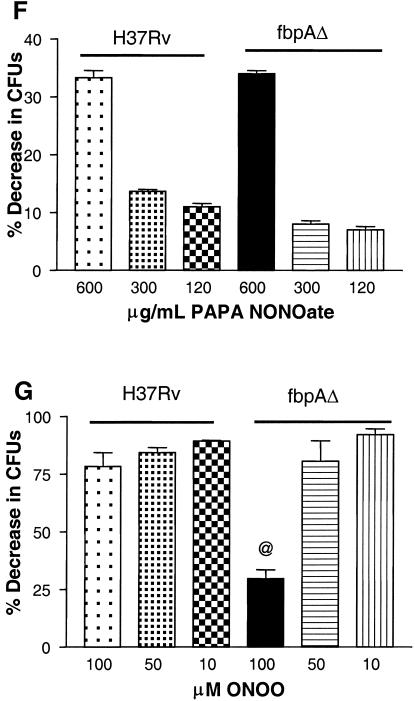
Effect of nitric oxide (NO) on ΔfbpA growth in mice and macrophages. (A) Mice infected and treated with aminoguanidine (AG) show enhanced growth of ΔfbpA and of the wild type. @, P < 0.009; $, P < 0.008 (Mann-Whitney U test). (B and C) Naïve, IFN-γ-treated (400 U/ml) or NMMA-treated mouse macrophages were infected with ΔfbpA or H37Rv, and growth curves were plotted. (B) IFN-γ reduces baseline CFU. *, P value was <0.007 for ΔfbpA compared to H37Rv; **, P value was <0.02 for ΔfbpA compared to IFN-γ treatment and <0.003 for H37Rv compared to IFN-γ treatment (t test). (C) NMMA enhances growth of both strains. *, P value was <0.02 for ΔfbpA compared to NMMA treatment and <0.03 for H37Rv compared to NMMA treatment (t test). (D and E) Macrophages infected with ΔfbpA and H37Rv were measured for NO by using Griess reagent and ROS for 7 days postinfection with a DCFDA fluorescent probe. ΔfbpA and H37Rv induce similar levels of NO and ROS. (D) *, P value was <0.01 for IFN-γ compared to both strains; **, no significant difference found for ΔfbpA compared to H37Rv. (E) *, P value was <0.03 for IFN-γ compared to both strains; no significant difference was found for ΔfbpA compared to H37Rv. (F and G) Strains were treated with the NO donor, PAPA-NONOate, or peroxynitrite and measured for CFU counts, and results are expressed as the percent decrease from the vehicle control. ΔfbpA and H37Rv show nearly identical susceptibility to NO (F), but ΔfbpA is more susceptible to the 100 μM dose of peroxynitrite (G). @, P value was <0.01 (t test) for ΔfbpA compared to H37Rv. SEM, standard error of the mean.
(ii) Susceptibility in macrophages.
The role of NO against ΔfbpA was further evaluated with macrophage cultures. Naïve and IFN-γ activated macrophages were infected for survival studies, and NO synthesis was inhibited. H37Rv replicated and increased over 7 days by 1 log10 in naïve macrophages, and ΔfbpA declined (Fig. 4B). In activated macrophages, both groups showed a >1 log10 decrease from baseline CFU levels. Inhibition of NO in macrophages with NMMA increased the growth of ΔfbpA and that of H37Rv (Fig. 4C). Macrophages were then measured for the magnitudes of NO and ROS. The positive control, IFN-γ, induced higher levels of nitrite or ROS in macrophages than either organism, while ΔfbpA and H37Rv induced similar levels of nitrite (Fig. 4D) or ROS in macrophages (Fig. 4E).
(iii) Broth susceptibility studies.
H37Rv and ΔfbpA were then exposed to various doses of the slow-release NO donor PAPA-NONOate. The two strains showed a similar susceptibility to extraneous NO mediated killing as shown in Fig. 5F, which is representative of three independent experiments with similar results. However, when the strains were exposed to three cycles of peroxynitrite, a difference emerged. While H37Rv was completely resistant for three cycles of exposure, ΔfbpA showed a significant susceptibility to peroxynitrite at 100 μM (P value of <0.01 [t test] compared to H37Rv) (Fig. 5G).
FIG. 5.
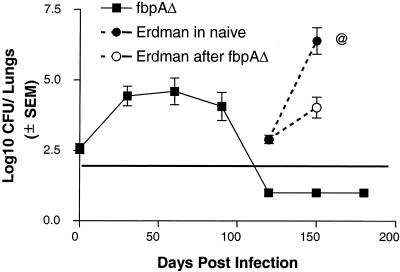
Effect of prior infection with the ΔfbpA mutant on subsequent challenge with the Erdman strain. C57BL/6 mice were left uninfected or were aerosol infected with ΔfbpA. ΔfbpA reaches baseline levels by day 90. Mice (four per time point) were then challenged with the Erdman strain via aerosol. Mice previously infected with the ΔfbpA mutant reduce the growth of the challenge Erdman strain by more than 2 log10 (○) compared to untreated and challenged mice (•). SEM, standard error of the mean.
Effect of prior infection of mice with ΔfbpA on subsequent challenge with Erdman strain.
The gradual decline of ΔfbpA to low levels in lungs, livers, and spleens by day 100 postinfection suggested that the mice could offer resistance to a challenge infection, thereby indicating a vaccinogenic effect for ΔfbpA. To test this hypothesis, mice were infected by aerosol and rested until day 90. CFU counts for lungs showed that there were very few culturable CFU. Mice were then challenged by the aerosol route with the Erdman strain, and an identical number of age-and-sex-matched uninfected mice were used as unprotected controls. The Erdman strain grew to very high levels among the unprotected mice (Fig. 5). In contrast, mice previously exposed to ΔfbpA showed marked reductions of growth of the challenge strain (>2 log10 difference). In order to confirm that the growth of organisms represented the Erdman strain and not that of reactivated ΔfbpA, three strategies were used. First, CFU randomly picked up from 7H11 agar showed the absence of the kanamycin marker present in the ΔfbpA mutant. Second, colonies from 7H11 agar were subcultured in Sauton broth, where they grew; in contrast, ΔfbpA is unable to grow in Sauton broth. Third, the presence of the fbpA gene in selected pools of CFU was confirmed by Southern analysis.
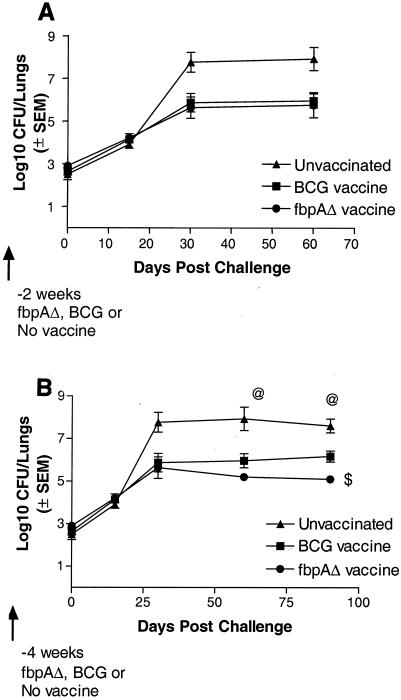
Vaccinogenic effects of ΔfbpA.
The National Institutes of Health model of vaccine testing (36) was used, with the modifications that both the ΔfbpA strain and BCG were given twice before the challenge and that the challenge Erdman strain was implanted in the mouse lungs at an aerosol dose of about 2.5 log10 CFU per mouse. In the first experiment, mice were immunized twice through the subcutaneous route and challenged with the Erdman strain by the aerosol route 2 weeks after the second vaccine dose. Figure 6A demonstrates that both BCG and ΔfbpA were equally effective in reducing the growth of the challenge strain (by about 2 log10). The second experiment was performed 1 year later with the ΔfbpA strain that had been passaged occasionally in 7H9 broth containing kanamycin and subsequently stored frozen for 6 months at −70°C. In this experiment, mice were immunized as described above but challenged after a rest period of 4 weeks after the second vaccine dose. Mice were monitored for a longer period of time. Again, both BCG and ΔfbpA vaccines showed protective effects (Fig. 6B). However, on days 60 and 90 postchallenge, the ΔfbpA strain showed a better protective effect than BCG (P value of <0.01 on day 90 compared to BCG-induced protection). In both of these vaccine experiments, the growth of the challenge strain was confirmed by using the methods described above.
FIG. 6.
The ΔfbpA mutant is vaccinogenic against a challenge with the virulent Erdman strain in mice. C57BL/6 mice were left unimmunized (▴) or were immunized subcutaneously with two doses of live ΔfbpA (•) or BCG (Pasteur; ▪) 2 weeks apart, each at 106 CFU/mouse. Mice were challenged at either 2 (A) or 4 (B) weeks after the second dose of vaccine with an aerosol dose of the Erdman strain implanting into the lungs at ∼500 CFU/mouse. Four mice were sacrificed for each experiment at the time points shown, and lung CFU were determined. (A) Both BCG and ΔfbpA strains reduce the growth of the Erdman challenge strain at 2 weeks postvaccination. (B) ΔfbpA is more effective when the challenge is done at 4 weeks postvaccination. The Erdman strain grows better in naïve mice. $, P value was <0.01 for ΔfbpA compared to BCG (Mann-Whitney U test); @, P value was <0.009 for the Erdman strain compared to the ΔfbpA or BCG vaccine group (Mann-Whitney U test). SEM, standard error of the mean.
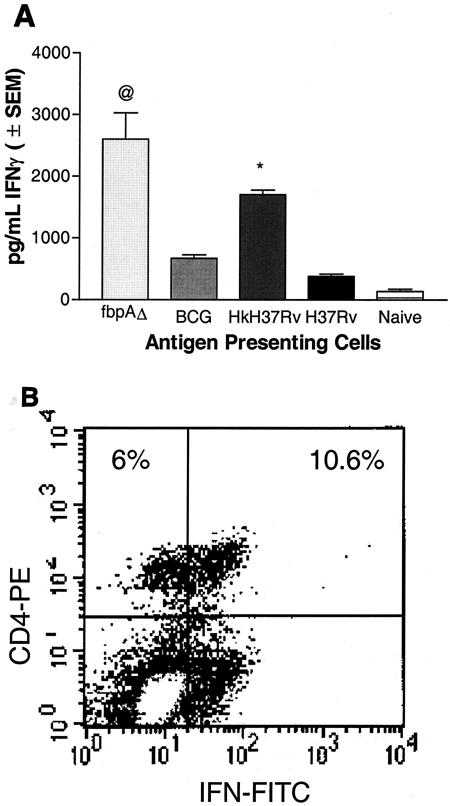
In vitro immunogenicity of ΔfbpA.
Macrophages infected with the live ΔfbpA strain induced a stronger IFN-γ response (P value of <0.007 compared to BCG or H37Rv) in overlaid T cells than in macrophages infected with either BCG or live H37Rv (Fig. 7A). Hk-H37Rv also induced significant levels of IFN-γ, consistent with its ability to undergo phagosome-lysosome (PL) fusion (P value of <0.009 compared to BCG or H37Rv). Neither naïve macrophages nor naïve T cells showed significant levels of IFN-γ (data not shown). Immunophenotyping of overlaid T cells confirmed that the IFN-γ response was correlated with an expansion of T cells, as shown in a representative panel of ΔfbpA-induced CD4+ IFN-γ+ T cells (Fig. 7B).
FIG. 7.
Immunogenicity of macrophages infected with H37Rv and ΔfbpA. (A) BM-derived macrophages from C57BL/6 mice were infected with ΔfbpA, BCG, live or heat-killed (Hk) H37Rv, or left uninfected. Splenic T cells from naïve mice or mice immunized with Hk-MTB were layered on macrophages, and the supernatants were tested for IFN-γ. ΔfbpA induces a stronger early IFN-γ response than BCG or live wild type. @, P value was <0.007 compared to BCG or H37Rv (t test). The positive control, Hk-H37Rv, also induced a higher response (P value was <0.009 compared to BCG or H37Rv). (B) Overlaid T cells were stained for phenotype and IFN-γ intrakine and analyzed by flow cytometry. ΔfbpA induces an early activation of IFN-γ-producing CD4+ T cells consistent with enhanced IFN-γ secretion (results shown are representative of three experiments). SEM, standard error of the mean.
Revival of the ΔfbpA strain.
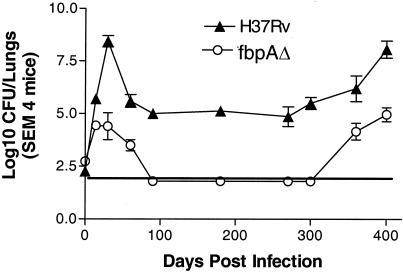
The wild type and the ΔfbpA strain showed growth curves essentially similar to those shown in Fig. 2, until day 180. Organs of ΔfbpA mice remained devoid of culturable CFU, while those infected with H37Rv generally yielded 4 to 5 log10 organisms per set of lungs and a lesser number in the liver and spleen (data not shown). By day 370 postinfection, however, CFU counts for H37Rv-infected lungs increased and mice progressively became sick, consistent with the increased growth of organisms, and several mice died of infection (Fig. 8). Interestingly, by day 370, ΔfbpA organisms also revived and increasing numbers of organisms were recovered from lungs but not from spleens and livers (data not shown). These mice became sick, with loss of weight, sluggish movement, and ruffled coat, but they did not die. All surviving mice were sacrificed on day 400. Figure 8 thus shows that the wild type revived to yield elevated CFU counts, while ΔfbpA showed an increase from undetectable levels.
FIG. 8.
Revival of wild-type H37Rv and the ΔfbpA mutant in mice. C57BL/6 mouse lungs were aerosol infected with ΔfbpA (○) and H37Rv (▴) (∼200 CFU/mouse). After an initial growth period, H37Rv stabilizes in lungs until day 300, after which organisms resume growth. Growth of the ΔfbpA mutant declines but resumes after day 350. SEM, standard error of the mean.
DISCUSSION
In a previous study undertaken to understand the effects of disrupting Ag85 complex, ΔfbpA was found to be unable to grow in macrophages, while both ΔfbpB and the wild type were growth competent (4). In the present study, disruption of fbpA and fbpB genes was also found to cause markedly different effects on the in vivo growth of H37Rv. Tuberculosis in mice is dependent upon the dose and route of infection. Large-dose intravenous infection resulted in a markedly reduced growth of ΔfbpA in the lungs and a limited growth in the spleen. Since progressive growth in the lungs of mice is generally considered to reflect the virulence of M. tuberculosis strains, this result suggested that ΔfbpA was attenuated while ΔfbpB was not. In mice, aerosol infections are more lethal than those by the intravenous route (32, 33). Even after aerosol infection, however, ΔfbpA was unable to grow progressively in the lungs of mice and, after an initial spurt of growth, showed a gradual decline to baseline levels by day 100 (Fig. 2). This decline in growth was likely due to the loss of Ag85A, since the ΔfbpA strain lacks Ag85A and the integrative and plasmid-complemented strains for fbpA gene grew better than ΔfbpA after aerosol infection of mice (Fig. 3). The complementation experiments used both the plasmid-complemented strain and the integrated strain since our previous unpublished studies showed a possible loss of plasmids from mutants during in vivo growth. Indeed, the plasmid strain LaA3 grew as well as the integrative LaA2 strain up to day 21 and then declined, suggesting possible loss of plasmid. While there is no clear explanation for why the integrative strain did not grow as well as the wild type, its growth was significantly higher than that of the ΔfbpA strain on both day 21 and day 28 postinfection (Fig. 3). The restoration of increased growth in complemented strains of ΔfbpA strain thus suggests that antigen 85A is essential for the optimal in vivo growth of M. tuberculosis.
The gradual decline of the ΔfbpA mutant after intravenous infection was in striking contrast to the continued presence of the wild type in the lungs. Initial histological observations demonstrated no significant differences in lungs, livers, or spleens. Thus, lung immune responses were measured by using RT-PCR (1-3). While pulmonary messages for various cytokines increased in both ΔfbpA- and H37Rv-infected mice, on day 21 postinfection, ΔfbpA induced a significantly larger magnitude of response for the cytokines TNF-α, IL-1β, IFN-γ, IL-6, and IL-2 (Table 2). Increased iNOS levels correlated well with an increase in IL-1β, a feedback regulator of iNOS in mice (34). The protective role of many of these cytokines in tuberculosis has already been established, and so the decline in the growth of ΔfbpA appeared to be due to their increased synthesis and iNOS.
Nevertheless, we sought to define more precisely the role of NO as a defense mechanism against ΔfbpA, because despite many studies demonstrating the protective role of NO against tuberculosis in mice (31), at least one study suggested that the wild type is able to overcome the lethal effects of NO (20). Figure 4A to C demonstrates that the inhibition of NO in mice or macrophages enhances the growth of ΔfbpA, while IFN-γ activation of macrophages leads to a better killing of the mutant. While a role for NO as a bactericidal mechanism was apparent from these studies, it was paradoxical to observe that both ΔfbpA and H37Rv induced similar levels of NO and intracellular H2O2 (Fig. 4D to E). Our studies were therefore extended to test the hypothesis that ΔfbpA was more susceptible than the wild type to NO or ONOO. Figure 4G and H illustrate that ΔfbpA was more susceptible than the wild type to ONOO. It is interesting to recall here that attenuated strains of mycobacteria, such as Mycobacterium smegmatis and BCG, were found to be more susceptible to ONOO while the wild-type M. tuberculosis was resistant (55). Thus, ΔfbpA responds to ONOO more like the attenuated mycobacterial strains.
It is pertinent to note that the ΔfbpA mutant did not grow, even within naïve macrophages. While a free radical (NO and superoxide)-independent mechanism of killing mycobacteria may exist in murine macrophages, we sought alternative explanations. Preliminary studies found that the phagosomes of the ΔfbpA mutant rapidly colocalize with acidotropic LysoTrackers within naïve mouse macrophages, unlike the wild type (10). Curiously, the cell wall composition of ΔfbpA may in part be responsible for the enhanced maturation and loss of viability in naïve macrophages. That Ag85 complex proteins have mycolyl transferase function was reported before (6). In a recent study, it was found that Ag85A was responsible for the synthesis of trehalose-dimycolate (TDM) and ΔfbpA was found to be markedly deficient in cell wall TDM (5). Inactivation of Ag85C has also been reported to alter the cell wall lipid composition in M. tuberculosis (19). Interestingly, TDM, in turn, has been suggested to play a role in the prevention of PL fusion in macrophages (45). Although the underlying molecular mechanisms remain to be elucidated, we tentatively propose that because of reduced TDM, ΔfbpA phagosomes may become more amenable to phagosome maturation and thus become more susceptible to degradation and antimycobacterial action within macrophages, unlike the wild type, which is well-known to avoid PL fusion in macrophages (41).
The near complete clearance of organisms from lungs, livers, and spleens of ΔfbpA-infected mice and the strong immune responses present before the decline of organisms suggested that these mice might have become immune and may be able to resist subsequent infections. The reduced efficacy of the BCG vaccine to protect against adult tuberculosis has led to a search for more effective vaccines, which has included investigations of subunit antigens of M. tuberculosis, bacterial vectors expressing M. tuberculosis proteins, BCG or M. tuberculosis hyperexpressing M. tuberculosis proteins, and naked DNA encoding M. tuberculosis proteins (36, 54). Many of these candidates have failed to surpass BCG in the mouse protection model, and it was of interest to determine whether ΔfbpA could work as a vaccine.
Thus, mice which had cleared a previous aerosol-induced infection were first challenged with the virulent Erdman strain (Fig. 5) in a key experiment that brought out a difference between the ΔfbpA mutant and the BCG vaccine. First, North et al. immunized mice i.v. with 105 BCG organisms, allowed the infection to stabilize over 50 days, cleared organisms by a 10-day course of chemotherapy, and then challenged the mice with the Erdman strain by aerosol (33). Despite the induction of pulmonary immunity, the challenged organisms in vaccinated mice grew nearly identically to those in unvaccinated controls during the first 20 days. Subsequently, the challenge Erdman strain grew less than the organisms in the unvaccinated group, which died by day 100. In contrast, in our study, mice which naturally cleared the ΔfbpA mutant in the lungs over the first 100 days limited the growth of the challenge Erdman strain for the first 30 days, showing about a 2 log10 difference from the control. This result suggested that ΔfbpA may be an effective vaccine. Two additional experiments were conducted to confirm its ability to immunize mice (Fig. 6). In these experiments, mice immunized with the ΔfbpA mutant and challenged 4 weeks later showed better protection than those immunized with BCG and better protection than those challenged 2 weeks later. We hypothesize that the ability of ΔfbpA to induce stronger pulmonary immune responses (Table 2) may translate into a better ability to protect mice after vaccination. Additional evidence was obtained in this direction. M. tuberculosis is well-known to inhibit PL fusion and antigen presentation mechanisms (38, 41). Consistent with this notion, macrophages infected with live M. tuberculosis or BCG induced far less IFN-γ than macrophages infected with the live ΔfbpA mutant (Fig. 7). That Hk-H37Rv was able to induce IFN-γ is indirect evidence that ΔfbpA also underwent phagosome maturation and antigen processing that led to a rapid expansion of CD4+ IFN-γ T cells and perhaps has a better ability to immunize mice. We recognize here that vaccine evaluations using animal models are subject to multiple variations and that efficacy of vaccines needs careful interpretation (28, 36, 50, 52). Thus, there will be a need to lengthen the duration between the vaccine dose and the challenge, to alter the dose and route of the vaccine, and to test guinea pigs and rabbits in order to establish that ΔfbpA is better than BCG in terms of offering protection. However, recent reports have indicated that attenuated mutants of M. tuberculosis may either equal or exceed the level of protection offered by BCG, and our studies suggest that the ΔfbpA strain is a novel addition to the group (44).
Ag85 complex proteins and their encoding DNA are markedly immunogenic and vaccinogenic in animal models (15, 17, 18, 23, 24, 29, 42, 51). However, our finding that ΔfbpA is immunogenic in mice is not inconsistent with these observations. First, attenuated ΔfbpA still has potentially numerous antigens that can successfully immunize mice against challenge. In a recent study, M. tuberculosis DNA encoding a single novel antigen by itself offered protection comparable to BCG in mice (36). Second, there may be subtle differences in the immunogenicity of the individual components of the Ag85 complex. For example, Ag85B has been found to be more frequently able to induce T-cell stimulation and proliferation than Ag85A (38, 42). Ag85B could therefore be compensating for the lack of Ag85A in the ΔfbpA mutant in terms of immunogenicity. Finally, there is an intriguing possibility that the lack of Ag85A may have reduced the ability of the mutant to prevent PL fusion in macrophages, thereby enhancing its ability to present antigens to sensitized T cells (10).
That Ag85A, Ag85B, and Ag85C shared many biochemical properties hinted initially that Ag85B and Ag85C proteins could compensate for the absence of Ag85A and allow the mutant to resume growth over time in mice. Mice were therefore infected with a low aerosol dose and left untreated for prolonged periods. However, the ΔfbpA strain revived in mice only after a quiescent period of nearly 300 days (Fig. 8). Since even the wild-type H37Rv began to increase in counts by this time, we suggest that the intact immune system of mice kept the growth of H37Rv in check while suppressing that of ΔfbpA and that the advent of immunosenescence in mice allowed the revival of both strains. Incidentally, the revival of M. tuberculosis in aged mice is consistent with a similar previous report (35). On the other hand, the marked decline and later revival of the ΔfbpA strain suggest the novel observation that Ag85A is probably essential for optimal growth in mouse organs, since neither Ag85B nor Ag85C compensated and facilitated the revival of ΔfbpA during the prolonged period of quiescence (days 100 to 300). Ag85A therefore could be more directly related to the virulence of M. tuberculosis than either Ag85B or Ag85C through hitherto unexplored mechanisms. Thus, disruption of fbpA and fbpB genes thought to be similar in function has yielded two different phenotypes; one of these is attenuated and vaccinogenic, while the other resembles the wild type, suggesting that M. tuberculosis genes may have unanticipated functions that become apparent in vivo.
Acknowledgments
We thank Kris Huygen, Pasteur Institute, Brussels, Belgium, for HYT27 monoclonal antibody.
This investigation was supported by NIH grant AI49534-01.
Editor: J. D. Clements
REFERENCES
- 1.Actor, J. K., T. Kuffner, C. S. Dezzutti, R. L. Hunter, and J. McNicholl. 1998. A flash-type bioluminescent immunoassay that is more sensitive than radioimaging: quantitative detection of cytokine cDNA in activated and resting human cells. J. Immunol. Methods 211:65-77. [DOI] [PubMed] [Google Scholar]
- 2.Actor, J. K., M. Olsen, C. Jagannath, and R. L. Hunter. 1999. Relationship between survival, organism containment and granuloma formation in acute murine tuberculosis. J. Interferon Cytokine Res. 19:1183-1193. [DOI] [PubMed] [Google Scholar]
- 3.Actor, J. K., E, Breij, H. Hoffmann, R. A. Wetsel, R. L. Hunter, and C. Jagannath. 2001. A role for complement C5 in organism containment and granulomatous response during murine tuberculosis in mice. Scand. J. Immunol. 53:464-474. [DOI] [PubMed] [Google Scholar]
- 4.Armitige, L. Y., C. Jagannath, A. Wanger, and S. J. Norris. 2000. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages. Infect. Immun. 68:767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armitige, L. Y., S. J. Norris, A. Wanger, T. R. Sievert, and K. Takayama. Submitted for publication.
- 6.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 7.Bloom, B. R., and P. M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis, p. 531-537. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 8.Boechat, N., B. Lagier-Roger, S. Petit, Y. Bordat, J. Rauzier, A. J. Hance, B. Gicquel, and J.-M. Reyrat. 2002. Disruption of the gene homologous to mammalian Nramp1 in Mycobacterium tuberculosis does not affect virulence in mice. Infect. Immun. 70:4124-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, F. M. 1998. Mycobacterial pathogenesis: a historical perspective. Front. Biosci. 3:123-132. [DOI] [PubMed] [Google Scholar]
- 10.Copenhaver, R. H., R. L. Hunter, and C. Jagannath. 2001. An attenuated mutant of M. tuberculosis H37Rv that lacks the expression of antigen 85 complex A rapidly acquires late endosomal markers and undergoes phagosome-lysosome fusion in macrophages, p. 708, U-48. Abstr. 101st Gen. Meet. Am. Soc. Microbiol. 2001. American Society for Microbiology, Washington, D.C.
- 11.de Mendonca-Lima, L., Y. Bordat, E. Pivert, C. Recchi, O. Neyrolles, A. Maitournam, B. Gicquel, and J.-M. Reyrat. 2003. The allele encoding the mycobacterial Erp protein affects lung disease in mice. Cell. Microbiol. 5:65-73. [DOI] [PubMed] [Google Scholar]
- 12.Domenech, P., A. S. Pym, M. Cellier, C. E. Barry III, and S. T. Cole. Inactivation of the Mycobacterium tuberculosis Nramp orthologue (mntH) does not affect virulence in a mouse model of tuberculosis. FEMS Microbiol. Lett. 207:81-86. [DOI] [PubMed]
- 13.Fine, P. M. 2001. BCG: the challenge continues. Scand. J. Infect. Dis. 33:243-245. [DOI] [PubMed] [Google Scholar]
- 14.Glickman, M. S., J. S. Cox, and W. R. Jacobs. 2000. A novel mycolic acid cyclopropane synthetase is required for coding, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 15.Hess, J., L. Grode, J. Hellwig, P. Conradt, I. Gentschev, W. Goebel, C. Ladel, and S. H. Kaufmann. 2000. Protection against murine tuberculosis by an attenuated recombinant Salmonella typhimurium vaccine strain that secretes the 30-kDa antigen of Mycobacterium bovis BCG. FEMS. Immunol. Med. Microbiol. 27:283-289. [DOI] [PubMed] [Google Scholar]
- 16.Hondalus, M. K., S. Bardarov, R. Russell, J. Chan, W. R. Jacobs, Jr., and B. R. Bloom. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 68:2888-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Masleša-Galić. 2000. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, M., C. Raynaud, M.-A. Lanéelle, C. Guilhot, C. Laurent-Winter, D. Ensergueix, B. Gicquel, and M. Daffé. 1999. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol. Microbiol. 31:1573-1587. [DOI] [PubMed] [Google Scholar]
- 20.Jung, Y. J., R. LaCourse, L. Ryan, and R. J. North. 2002. Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide synthase 2-independent defense in mice. J. Exp. Med. 196:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushal, D., B. G. Schroeder, S. Tyagi, T. Yoshimatsu, C. Scott, C. Ko, L. Carpenter, J. Mehrotra, Y. C. Manabe, R. D. Fleischmann, and W. R. Bishai. 2002. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proc. Natl. Acad. Sci. USA 99:8330-8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keefer, L. K. 1996. NONOates (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 268:281-293. [DOI] [PubMed] [Google Scholar]
- 23.Lowrie, D. B., and C. L. Silva. 2000. Enhancement of immunocompetence in tuberculosis by DNA vaccination. Vaccine 18:1712-1716. [DOI] [PubMed] [Google Scholar]
- 24.Lozes, E., K. Huygen, J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, P. Vandenbussche, J. P. Van Vooren, A. Drowart, J. B. Ulmer, and M. A. Liu. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 15:830-833. [DOI] [PubMed] [Google Scholar]
- 25.Manabe, Y. C., B. J. Saviola, L. Sun, J. R. Murphy, and W. R. Bishai. 1999. Attenuation of virulence in Mycobacterium tuberculosis expressing a constitutively active iron repressor. Proc. Natl. Acad. Sci. USA 96:12844-12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdam, R, A., S. Quan, D. A. Smith, S. Bardarov, J. C. Betts, F. C. Cook, E. U. Hooker, A. P. Lewis, P. Woollard, M. J. Everett, P. T. Lukey, G. J. Bancroft, W. R. Jacobs, Jr., and K. Duncan. 2002. Characterization of a Mycobacterium tuberculosis H37Rv transposon library reveals insertions in 351 ORFs and mutants with altered virulence. Microbiology 148:2975-2986. [DOI] [PubMed] [Google Scholar]
- 27.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 28.McMurray, D. N. 2001. Determinants of vaccine induced resistance in animal models of pulmonary tuberculosis. Scand. J. Infect. Dis. 33:175-178. [DOI] [PubMed] [Google Scholar]
- 29.Morris, S., C. Kelley, A. Howard, Z. Li, and F. M. Collins. 2000. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine 18:2155-2163. [DOI] [PubMed] [Google Scholar]
- 30.Mueller-Ortiz, S. L., E. Sepulveda, M. R. Olsen, C. Jagannath, A. R. Wanger, and S. J. Norris. 2002. Decreased infectivity despite unaltered C3 binding by a ΔhbhA mutant of Mycobacterium tuberculosis. Infect. Immun. 70:6751-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North, R. J. 1995. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than via the intravenous route. J. Infect. Dis. 172:1550-1553. [DOI] [PubMed] [Google Scholar]
- 33.North, R. J., R. Lacourse, and L. Ryan. 1999. Vaccinated mice remain more susceptible to Mycobacterium tuberculosis infection initiated via the respiratory route than the intravenous route. Infect. Immun. 67:2010-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obermeier, F., V. Gross, J. Scholmerich, and W. Falk. 1999. Interleukin-1 production by mouse macrophages is regulated in a feedback fashion by nitric oxide. J. Leukoc. Biol. 66:829-836. [DOI] [PubMed] [Google Scholar]
- 35.Orme, I. M. 1988. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am. Rev. Respir. Dis. 137:716-718. [DOI] [PubMed] [Google Scholar]
- 36.Orme, I. M., D. M. McMurray, and J. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 115-118. [DOI] [PubMed]
- 37.Parish, T., D. A. Smith, S. Kendall, N. Casali, G. J. Bancroft, and N. G. Stoker. 2003. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect. Immun. 71:1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandra, L., E, Noss, W. H. Boom, and C. V. Harding. 2001. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomsal formation of peptide-major histocompatibility complex II complex and is inhibited by live bacilli that decrease phagosome maturation. J. Exp. Med. 194:1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raynaud, C., C. Guilhot, J. Rauzier, Y. Bordat, V. Pelicic, R. Manganelli, I. Smith, B. Gicquel, and M. Jackson. 2002. Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 45:203-217. [DOI] [PubMed] [Google Scholar]
- 40.Rindi, L., L. Fattorini, D. Bonanni, E. Iona, G. Freer, D. Tan, G. Deho, G. Orefici, and C. Garzelli. 2002. Involvement of the fadD33 gene in the growth of Mycobacterium tuberculosis in the liver of BALB/c mice. Microbiology 148:3873-3880. [DOI] [PubMed] [Google Scholar]
- 41.Russell, D. G. 2001. Mycobacterium tuberculosis: here today and here tomorrow. Nat. Rev. Mol. Cell Biol. 2:569-577. [DOI] [PubMed] [Google Scholar]
- 42.Russo, D. M., N. Kozlova, D. L. Lakey, and D. Kernodle. 2000. Naive human T cells develop into Th1 effectors after stimulation with Mycobacterium tuberculosis-infected macrophages or recombinant Ag85 proteins. Infect. Immun. 68:6826-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacchettini, J. C., and D. R. Ronning. 2000. The mycobacterial antigens 85 complex—from structure to function: response. Trends Microbiol. 10:441-444. [DOI] [PubMed] [Google Scholar]
- 44.Sambandamurthy, V. K., X. Wang, B. Chen, R. G. Russell, S. Derrick, F. M. Collins, S. L. Morris, and W. R. Jacobs, Jr. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 8:1171-1174. [DOI] [PubMed] [Google Scholar]
- 45.Spargo, B. J., L. M. Crowe, T. Ioneda, B. L. Beaman, and J. H. Crowe. 1991. Cord factor (α,α-trehalose 6,6′-dimycolate) inhibits fusion between phospholipid vesicles. Proc. Natl. Acad. Sci. USA 88:737-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Springer, B., S. Master, P. Sander, T. Zahrt, M. McFalone, J. Song, K. G. Papavinasasundaram, M. J. Colston, E. Boettger, and V. Deretic. 2001. Silencing of oxidative stress response in Mycobacterium tuberculosis: expression patterns of ahpC in virulent and avirulent strains and effect of ahpC inactivation. Infect. Immun. 69:5967-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steenken, W., and L. U. Gardner. 1943. Vaccinating properties of avirulent dissociates of five different strains of tubercle bacilli. Yale J. Biol. Med. 15:393-403. [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart, G. R., S. Ehrt, L. W. Riley, J. W. Dale, and J. McFadden. 2000. Deletion of the putative antioxidant noxR1 does not alter the virulence of Mycobacterium tuberculosis H37Rv. Tuber. Lung Dis. 80:237-242. [DOI] [PubMed] [Google Scholar]
- 49.Trudeau, E. L. 1893. Eye tuberculosis and antitubercular tuberculosis. N. Y. Med. J. 58:97-101. [Google Scholar]
- 50.Turner, J., E. R. Rhoades, M. Keen, J. T. Belisle, A. A. Fran, and I. M. Orme. 2000. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect. Immun. 68:1706-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulmer, J. B. 2001. Tuberculosis DNA vaccines. Scand. J. Infect. Dis. 33:246-248. [DOI] [PubMed] [Google Scholar]
- 52.Weigeshaus, E. H., and D. W. Smith. 1989. Evaluation of the protective potency of new tuberculosis vaccines. Rev. Infect. Dis. 11(Suppl. 2):S484-S490. [DOI] [PubMed] [Google Scholar]
- 53.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing, Z. 2001. The hunt for new tuberculosis vaccines: anti-TB immunity and rational design of vaccines. Curr. Pharm. Des. 7:1015-1037. [DOI] [PubMed] [Google Scholar]
- 55.Yu, K., C. Mitchell, Y. Xing, R. S. Magliozzo, B. R. Bloom, and J. Chan. 1999. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79:191-198. [DOI] [PubMed] [Google Scholar]