Abstract
In Helicobacter pylori gastritis, neutrophil activation and migration, which play central roles in the pathogenesis of the disease, are regulated by the neutrophil attractant chemokines interleukin 8 (IL-8) and Groα, whose secretion is induced by H. pylori. However, the modulation of the corresponding chemokine receptors CXCR1 and CXCR2 on human neutrophils under the influence of H. pylori has not been investigated. Incubation of neutrophils with cag+ and cag deletion H. pylori strains resulted in a complete downregulation of the CXCR1 and the CXCR2 receptors after 0.5 h, as tested by fluorescence-activated cell sorter analysis, independent of the cag status. Downregulation of CXCR1 and CXCR2 seems to occur via receptor internalization and rapid degradation, as shown by confocal microscopy and immunoblotting. Neither the proinflammatory cytokines IL-8 and tumor necrosis factor alpha produced by the neutrophils themselves nor H. pylori lipopolysaccharide, which are the known regulators of these two chemokine receptors, was responsible for the downregulation. Reverse transcription-PCR analysis showed that CXCR1 and CXCR2 mRNAs of neutrophils were reduced at a later time than the CXCR1 and CXCR2 proteins. Moreover, cag+ H. pylori strains induced significantly stronger downregulation of CXCR1 and CXCR2 mRNAs than the cag deletion mutant. Therefore, receptor protein and mRNA downregulation seem to be mediated by two independent mechanisms. Data obtained by immunohistochemistry suggested that downmodulation of CXCR1 and CXCR2 on neutrophils may also occur in vivo in the human stomach during H. pylori infection. Downregulation of CXCR1 and CXCR2 expression on neutrophils in H. pylori infection by H. pylori itself may represent a new mechanism of modulating neutrophil migration and activation in the gastric mucosa.
Infection of the gastric mucosa by the gram-negative bacterium Helicobacter pylori is known to cause chronic active gastritis and gastroduodenal ulceration (8, 20, 21, 26). In H. pylori infection, the bacterium colonizes the mucus layer overlying the gastric surface epithelium and causes inflammation of the underlying mucosa, which is called chronic active gastritis. Chronic active gastritis typically is characterized by neutrophils localized in the lamina propria, the epithelial layer, and the foveolar lumen, as well by T and B lymphocytes and macrophages accumulating in the lamina propria.
Neutrophils mediate gastritis activity and are thought to play a central role in the pathogenesis of mucosal ulceration in H. pylori infection, because they not only protect the host from the bacterium but also cause mucosal damage during inflammation (11, 16, 30). In H. pylori gastritis, the neutrophil attractant CXC chemokines interleukin 8 (IL-8) and Groα expressed by lamina propria macrophages and gastric epithelial cells regulate neutrophil migration from the mucosal vessel into the gastric epithelium (10).
Several studies have shown that gastritis activity mediated by neutrophils is associated with increased levels of IL-8 and Groα (7, 24, 30, 37-39). Neutrophils are attracted by these two locally presented chemokines only if they express the appropriate chemokine receptors. IL-8 attracts and activates neutrophils via the receptors CXCR1 and CXCR2. CXCR1 selectively binds IL-8 with high affinity, whereas CXCR2 binds to a range of CXC chemokines, including IL-8 and Groα (15, 19, 29).
In vitro, IL-8- and Groα-directed neutrophil activation and migration have been shown to be regulated by a ligand-dependent internalization and subsequent reexpression of the CXCR1 and CXCR2 receptors (4, 15, 27). In addition, the expression of CXCR1 and CXCR2 can also be regulated by immune modulators, such as the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) and lipopolysaccharides (LPS) of gram-negative bacteria (1).
Therefore, the question arises whether gastritis activity and mucosal damage in H. pylori infection are regulated not only by the expression levels of the neutrophil attractant chemokines IL-8 and Groα, but also by the modulation of the corresponding chemokine receptors CXCR1 and CXCR2 on neutrophils by H. pylori.
H. pylori strains have been subdivided into two major types according to the expression of the virulence factor cytotoxin-associated antigen A (CagA) (36) and the presence of the cag pathogenity island, which encodes a type IV secretion apparatus essential for CagA translocation and IL-8 secretion (14). H. pylori strains possessing the cag island are considered to produce more severe active gastritis than cag deletion strains and are linked to gastroduodenal ulceration (5, 6, 24, 34, 35). cag+ strains induce significantly higher levels of mucosal IL-8 and Groα than cag deletion strains and are thought to be more ulcerogenic (31, 39).
Therefore, in this study, the influence of the H. pylori cag pathogenicity island on CXCR1 and CXCR2 expression on neutrophils was also investigated.
MATERIALS AND METHODS
Isolation of neutrophils.
Peripheral blood neutrophils from three healthy volunteers were isolated by a sedimentation gradient containing sodium diatrizoate and dextran 500 (Axis-Shield, Oslo, Norway) following the instructions of the manufacturer. Cells were harvested and washed twice in RPMI 1640 (Life Technologies, Karlsruhe, Germany). Cell viability was tested using trypan blue; only preparations with a viability of >90% were used.
H. pylori strains and coincubation with neutrophils.
The two H. pylori strains NQ315 and NQ1712 were isolated sequentially from a Colombian patient and were considered isogenic, since in a multilocus sequence analysis they had identical sequences in 9 out of 10 gene fragments (12). The second strain (NQ1712) had lost the complete cag pathogenicity island, as demonstrated by an empty-site PCR with primers located in the genes HP0519 and HP0549 flanking the island. Additional confirmation of the presence of the pathogenicity island in strain NQ315 and its complete loss in strain NQ1712 was obtained by specific PCRs for selected individual cag island genes and by DNA microarray hybridization (C. Kraft, A. Stack, C. Josenhans, and S. Suerbaum, unpublished data).
The strains were used for coincubation with neutrophils. H. pylori strains were cultured on blood agar plates (Columbia agar base II; Oxoid, Wesel, Germany) supplemented with 10% horse blood and the following antibiotics: vancomycin (10 mg/liter), polymyxin B (2,500 U/liter), trimethoprim (5 mg/liter), and amphotericin B (4 mg/liter). H. pylori strains were preincubated on plates for 24 h at 37°C under microaerobic conditions for the infection assays to test the bacteria in mid-log phase. For the cell infections, bacteria were harvested, washed once, and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum. The bacteria were coincubated with neutrophils at a multiplicity of infection of 25 bacteria per cell for 0, 0.5, 3, 5, and 24 h at 37°C with 10% CO2.
Purification of H. pylori LPS and culture conditions.
LPS from H. pylori (strain 898-1) was isolated by aqueous phenol extraction (33). The aqueous phases were subsequently pooled and dialyzed for 24 h against demineralized H2O. Further purification of the crude LPS fraction was achieved by Cetavlon precipitation (28). Neutrophils were coincubated with LPS at a concentration of 1 μg/ml for 0, 0.5, and 3 h at 37°C with 10% CO2. Experiments were performed twice with neutrophils from one volunteer.
Measurement of CXCR1 and CXCR2 surface expression on neutrophils by fluorescence-activated cell sorter (FACS) analysis.
Isolated neutrophils were washed with phosphate-buffered saline containing 2% fetal calf serum and incubated for 30 min with monoclonal mouse anti-CXCR1 and anti-CXCR2 antibodies (R&D Systems, Wiesbaden, Germany) in an appropriate dilution. After being washed, the cells were incubated for 15 min with phycoerythrin-conjugated donkey anti-mouse immunoglobulin G (IgG) Fab fragments (Jackson Immunoresearch Laboratories, West Grove, Pa.). After being stained, the cells were analyzed on a FACScan flow cytometer (Becton Dickinson Biosciences, Mountain View, Calif.) with appropriate gatings. Experiments were performed twice with neutrophils from all three volunteers.
Measurement of CXCR1 and CXCR2 mRNAs by semiquantitative reverse transcription (RT)-PCR.
Total RNAs from aliquots of the neutrophils used for FACS analysis were extracted with TRIzol (Sigma, Taufkirchen, Germany) and DNase treated with RNase-free DNase I (MBI Fermentas, St. Leon-Rot, Germany) according to the manufacturers' protocols.
DNase-treated total RNAs from aliquots of 1 μl were taken for reverse transcription using oligo(dT) 15-18 primers (Invitrogen, Karlsruhe, Germany). Subsequent PCR amplifications of the RT reaction were performed using the following primers and conditions: CXCR1 upstream primer (5′-AGG GGC CAC ACC AAC CTT CTG-3′) and CXCR1 downstream primer (5′-AGT GCC TGC CTC AAT GTC TCC A-3′); annealing temperature for CXCR1, 65°C; product length, 364 bp; CXCR2 upstream primer (5′-CAG TTA CAG CTC TAC CCT GCC-3′) and CXCR2 downstream primer (5′-CCA GGA GCA AGG ACA GAC CCC-3′); annealing temperature for CXCR2, 65°C; product length, 451 bp.
All PCRs were performed in the Gene Amp PCR System 2400 (Perkin-Elmer Cetus, Emeryville, Calif.) in a final volume of 25 μl containing 2 μl of cDNA, 2.5 μl of 10× buffer without MgCl2, 1.25 μl of 50 mM MgCl2 (final concentration, 2.5 mM), 5 μl of deoxynucleoside triphosphate mixture (1 mM from each nucleotide; final concentration, 0.2 mM each) (Invitrogen), 10 pmol of each primer (final concentration, 0.4 μM each), and 0.1 μl of Taq DNA polymerase (5 U/μl) (Invitrogen).
The PCR conditions were as follows: initial 94°C for 2 min, 94°C for 30 s, annealing at the primer-specific temperature for 30 s, 30 s at 72°C for 40 cycles, and final 72°C for 5 min.
Ten microliters of each PCR was size fractionated by gel electrophoresis in a 2% agarose gel and analyzed. The semiquantification of the CXCR1 and CXCR2 product bands was performed visually by comparing the bands to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) bands performed in parallel.
Semiquantitative RT PCR was performed with neutrophils from all three volunteers.
Measurement of IL-8 and TNF-α proteins by ELISA.
IL-8 and TNF-α protein levels were measured in the neutrophil cell culture supernatants by a highly sensitive enzyme-linked immunosorbent assay (ELISA). The supernatants were cleared of bacteria and cellular debris by centrifugation for 1 min at 10,000 × g and stored at −20°C prior to measurement. Cytokine release (IL-8 and TNF-α) into the cell supernatants was quantitated using commercially available kits (IL-8, OPTEIA IL-8 ELISA set from BD Pharmingen, San Diego, Calif., and TNF-α, R&D Systems, Wiesbaden, Germany) according to the instructions provided by the manufacturers.
Cell supernatants were used at appropriate dilutions for measurements. The assay for IL-8 was performed in triplicate, and the TNF-α ELISA was performed in duplicate. Means and standard deviations were calculated.
Confocal microscopy.
Cytospin preparations from the neutrophils incubated with H. pylori were fixed in acetone and stained with monoclonal antibodies directed against CXCR1 and CXCR2 (R&D Systems) at appropriate dilutions, followed by incubation with Cy3-conjugated donkey anti-mouse IgG antibody (Jackson Immunoresearch Laboratories). Nuclei were counterstained with DAPI (Sigma, Hanover, Germany). The stained cells were observed in a confocal microscope (TCS SP2; Leica, Heidelberg, Germany).
Immunoblotting.
Supernatants from neutrophil cultures from two volunteers coincubated with both H. pylori strains for 0.5 and 3 h (see above) were used for the experiments. The supernatants were diluted with Laemmli sampling buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and heated to 95°C for 5 min. Electrophoretic transfer of proteins from polyacrylamide gels to 0.45-μm-pore-size nitrocellulose membranes was performed using a semidry blotting chamber. The blotted membranes were blocked with phosphate-buffered saline containing 0.1% (vol/vol) Tween 20 and 3% (wt/vol) low-fat milk powder, followed by a 1-h incubation with the primary antibodies polyclonal rabbit anti-human CXCR1 and CXCR2 (Santa Cruz, Heidelberg, Germany) at a dilution of 1:200. The secondary antibody peroxidase-coupled goat anti-rabbit (dilution, 1:10,000; Dianova, Hamburg, Germany) was detected with the SuperSignal chemiluminescence kit from Pierce (KMF, St. Augustin, Germany).
Immunohistochemical staining of CXCR1 and CXCR2 in gastric tissue.
Surgical tissue specimens from the antrum and corpus mucosa of 13 patients with chronic active H. pylori gastritis were investigated by immunohistochemistry in this study. Chronic active H. pylori gastritis showed a moderate to severe chronic inflammatory infiltrate of T and B lymphocytes, plasma cells, and monocytes and mild to severe activity with neutrophils in the lamina propria, within the gastric epithelium and the foveolar lumen. In all patients, H. pylori was clearly detectable by a modified Giemsa stain, and the H. pylori density ranged from mild to severe.
For immunohistological analyses, a mouse monoclonal anti-human CXCR1 and CXCR2 IgG antibody (1:200; R&D Systems) was used. Formalin-fixed tissue sections for chemokine receptor staining were deparaffinized and treated with microwaves in 0.01 M citrate buffer three times for 5 min each time. Nonspecific binding sites were blocked with buffered casein solution (Power Block Universal Blocking Reagent; Bio Genex, San Ramon, Calif.) for 10 min at room temperature. Sections were incubated with the first antibody at 4°C overnight. As second-stage reagents, a biotin-streptavidin-peroxidase detection system (Super Sensitive Multilink HRP Detection System; Bio Genex) was used according to the manufacturer's instructions with 3,3′-diaminobenzidine tetra-hydrochloride solution as a substrate. To exclude nonspecific staining, the first antibody was replaced by an appropriate isotype control antibody.
RESULTS
H. pylori downregulates CXCR1 and CXCR2 receptor expression on neutrophils.
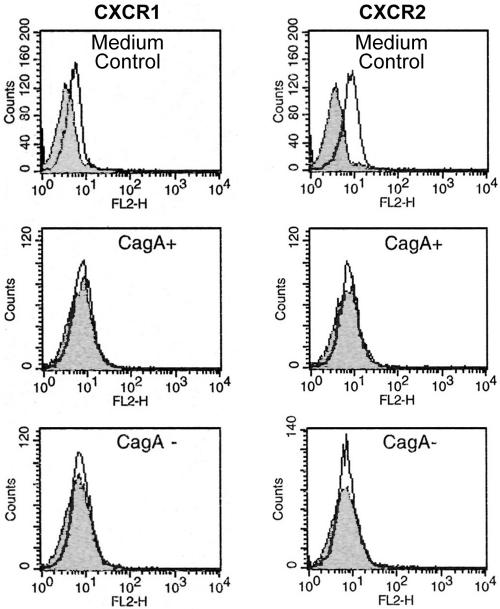
To examine the influence of H. pylori on CXCR1 and CXCR2 receptor expression on neutrophils, purified human neutrophils were incubated with cag+ and cag deletion H. pylori strains (sequential isolates NQ315 and NQ1712) (12). Expression was examined by FACS analysis. Freshly isolated human neutrophils expressed the chemokine receptors CXCR1 and CXCR2. Incubation with a cag+ H. pylori strain and with the corresponding cag deletion mutant resulted in complete downregulation of both chemokine receptors after only 0.5 h in all experiments (n = 3). Downregulation of CXCR1 and CXCR2 receptor proteins was independent of the cag status of H. pylori (Fig. 1). No recovery of the CXCR1 and CXCR2 receptors was found after 3, 5, and 24 h (data not shown).
FIG. 1.
H. pylori downregulates the CXCR1 and CXCR2 receptors on purified human neutrophils. Incubation of neutrophils with a cag+ H. pylori strain as well as with the corresponding cag deletion mutant resulted in complete downregulation of both chemokine receptors after only 0.5 h, as determined by FACS analysis. Neutrophils incubated with medium alone (labeled “medium control”) did not show any receptor downregulation. The x axis indicates fluorescence intensity measured on log10 scale, and the y axis indicates event counts per channel on a linear scale.
CXCR1 and CXCR2 receptors on neutrophils are not downregulated by H. pylori LPS and cannot be attributed to increased IL-8 and TNF-α concentrations.
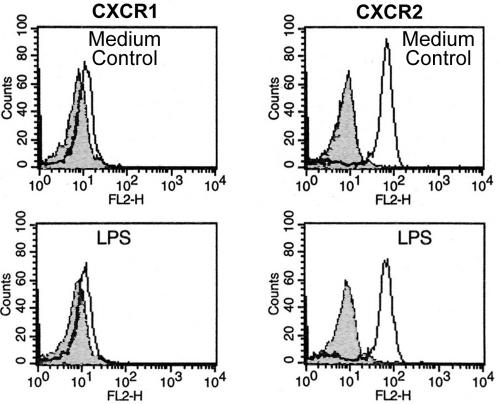
In vitro, LPS from some gram-negative bacteria, as well as the proinflammatory cytokines TNF-α and IL-8, have been shown to downregulate the CXCR1 and CXCR2 receptors on neutrophils (17, 18). Therefore, the effect of purified H. pylori LPS on CXCR1 and CXCR2 expression was investigated. In contrast to the intact live bacteria, purified LPS from H. pylori did not downregulate these two chemokine receptors on neutrophils after 0.5 and 3 h (Fig. 2).
FIG. 2.
Purified LPS from H. pylori does not downregulate the CXCR1 and CXCR2 receptors on human neutrophils. Incubation of neutrophils with LPS from H. pylori did not downregulate the CXCR1 and CXCR2 receptors compared with the medium control, as determined by FACS analysis.
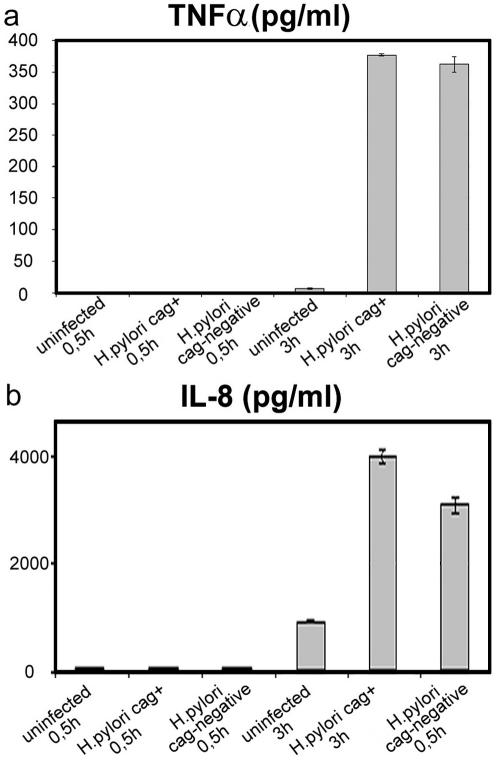
In the next step, we investigated whether downregulation of CXCR1 and CXCR2 by IL-8 was due to an autocrine mechanism. The rationale for these experiments was that H. pylori can induce a variety of cytokines, including IL-8 production by neutrophils (32), which might account for the downmodulation of CXCR1 and CXCR2. Therefore, the kinetics of the H. pylori-stimulated production of IL-8 and TNF-α were examined. No alterations of secreted IL-8 and TNF-α levels could be detected 0.5 h after incubation with cag+ and cag deletion H. pylori strains. After a 3-h incubation with H. pylori, IL-8 was strongly increased and TNF-α was moderately increased in the neutrophil cell culture (Fig. 3). As CXCR1 and CXCR2 receptor protein was already completely downregulated after 0.5 h and IL-8 and TNF-α could not be detected in the neutrophil cell culture at that time, CXCR1 and CXCR2 downregulation is most likely not due to an autocrine mechanism via IL-8 or TNF-α.
FIG. 3.
CXCR1 and CXCR2 receptor downregulation on neutrophils cannot be attributed to increased IL-8 and TNF-α concentrations. Human neutrophils did not express IL-8 (a) and TNF-α (b) 0.5 h after incubation with H. pylori, as determined by ELISA. Three hours after incubation with H. pylori, IL-8 was strongly increased and TNF-α was moderately increased in the neutrophil cell culture. The IL-8 level was higher after incubation with the cag+ than with the cag deletion H. pylori strain. IL-8 measurements were performed in triplicate, and TNF-α measurements were performed in duplicate. Mean values and standard deviations are shown.
H. pylori downregulates CXCR1 and CXCR2 on neutrophils via internalization and rapid degradation.
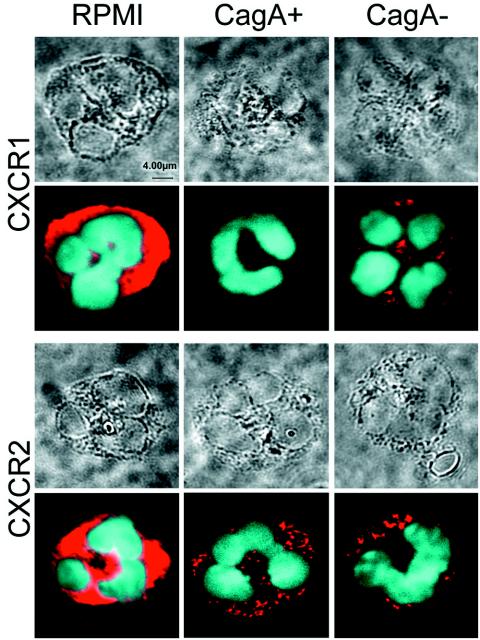
To further clarify the mechanism of CXCR1 and CXCR2 receptor downregulation on neutrophils by H. pylori, confocal microscopy and immunoblotting of the culture supernatant were performed. Freshly isolated human neutrophils showed strong CXCR1 and CXCR2 fluorescence signals in confocal microscopy at the membranes and in the cytoplasm of the cells (Fig. 4). The same staining intensity and distribution pattern of CXCR1 and CXCR2 was observed after 0.5, 3, and 5 h of incubation in medium without H. pylori. Incubation with cag+ and cag deletion H. pylori strains resulted in complete loss of the fluorescence signals at the cell membrane after only 0.5 h. Some CXCR1 and CXCR2 signals remained in the cytoplasm. Immunoblotting of the neutrophil culture supernatant with polyclonal antibodies against CXCR1 and CXCR2 was also used to identify proteolytic fragments of the receptors (18). Specific receptor products were not detectable in the supernatant (data not shown). In conclusion, our data suggest that H. pylori causes downregulation of the CXCR1 and CXCR2 chemokine receptors on neutrophils via internalization and rapid cytoplasmic degradation.
FIG. 4.
H. pylori downregulates the CXCR1 and CXCR2 receptors on neutrophils, as shown by confocal microscopy. Purified human neutrophils showed strong CXCR1 and CXCR2 expression at the cell membrane and in the cytoplasm after 0.5 h of incubation in RPMI medium. Incubation with a cag+ and a cag deletion H. pylori strain for 0.5 h resulted in a strong reduction of the fluorescence signals, with complete loss of the signals at the cell membrane and only some CXCR1 and CXCR2 signal in the cytoplasm. Fluorescence microscopy is shown in the lower part of the figure, and transmission light microscopy of the same cells is shown in the upper part of the figure.
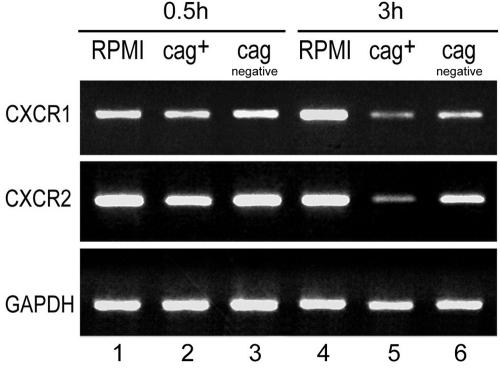
cag+ H. pylori causes more severe downregulation of CXCR1 and CXCR2 mRNAs in purified human neutrophils than cag deletion H. pylori.
The influence of H. pylori on the chemokine receptor CXCR1 and CXCR2 mRNA transcription of neutrophils was tested. Freshly isolated neutrophils expressed the mRNAs for the chemokine receptors CXCR1 and CXCR2. H. pylori induced a reduction of the CXCR1 and CXCR2 mRNAs in neutrophils, but not before 3 h after incubation. The H. pylori cag+ strain induced significantly stronger downregulation of CXCR1 and CXCR2 mRNAs in neutrophils than the cag deletion mutant (Fig. 5). This is in contrast to the CXCR1 and CXCR2 receptor protein, which was already downregulated 0.5 h after coincubation with H. pylori, independent of the cag status.
FIG. 5.
cag+ H. pylori induced more severe downregulation of CXCR1 and CXCR2 mRNAs in purified human neutrophils than cag deletion H. pylori. Neutrophils incubated in RPMI for 0.5 and 3 h strongly expressed CXCR1 and CXCR2 mRNAs (lanes 1 and 4). H. pylori downregulated CXCR1 and CXCR2 mRNAs in neutrophils 3 h after incubation (lanes 5 and 6). CXCR1 and CXCR2 mRNAs were not downregulated 0.5 h after coincubation with H. pylori (lanes 2 and 3). The H. pylori cag+ strain (lane 5) downregulated CXCR1 and CXCR2 mRNAs significantly more strongly than the cag deletion mutant (lane 6).
In vivo downregulation of CXCR1 and CXCR2 on neutrophils during H. pylori infection.
To demonstrate that downmodulation of chemokine receptors on neutrophils may also play a role in H. pylori infection in vivo in the human gastric mucosa, patients with chronic active H. pylori gastritis were investigated by immunohistochemistry for the expression of these receptors.
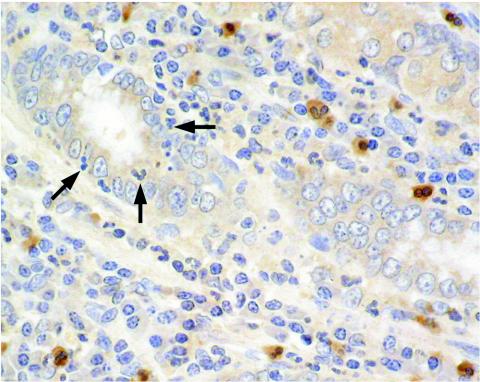
CXCR1 expression on neutrophils in patients with H. pylori gastritis seems to be dependent on the localization of the neutrophils in the mucosa. While some CXCR1-expressing neutrophils were detectable in all patients, many neutrophils clearly did not express the CXCR1 receptor. Neutrophils in the lamina propria frequently expressed the CXCR1 receptor, whereas neutrophils infiltrating the gastric epithelium or localized in the foveolar lumen, where they are in closer contact with H. pylori, did not express CXCR1 (Fig. 6).
FIG. 6.
Downmodulation of the CXCR1 receptor on neutrophils may also occur during H. pylori infection in the human stomach. Neutrophils in the lamina propria expressed the CXCR1 receptor, whereas neutrophils infiltrating gastric epithelium or localized in the foveolar lumen (arrows), where they are in closer contact with H. pylori, lacked CXCR1 expression.
The CXCR2 receptor was only sporadically expressed by neutrophils. Most neutrophils did not show CXCR2 receptor expression (data not shown).
These data suggest that CXCR1 and CXCR2 receptor downregulation on neutrophils may also occur in vivo in H. pylori gastritis, thus modulating the responsiveness of neutrophils to the neutrophil attractant chemokines IL-8 and Groα. Staining with an appropriate isotype-matched antibody was negative.
DISCUSSION
Our data suggest that neutrophil activation and migration in H. pylori infection is regulated not only by the expression of the neutrophil attractant chemokines IL-8 and Groα, but also by modulation of the corresponding chemokine receptors CXCR1 and CXCR2 on neutrophils by H. pylori.
We found that H. pylori downregulates CXCR1 and CXCR2 expression on neutrophils. In vitro neutrophil activation and migration are known to be regulated by two separate pathways of CXCR1 and CXCR2 modulation: a tyrosine kinase-independent pathway induced by IL-8 and a tyrosine kinase-dependent pathway induced by the proinflammatory cytokine TNF-α, as well as by bacterial LPS (17, 18).
Because H. pylori is known to induce IL-8 and TNF-α production in neutrophils, we investigated the expression kinetics of these two cytokines in neutrophil cell culture experiments. As IL-8 and TNF-α could not be detected in the neutrophil cell culture after 0.5 h of incubation with H. pylori and CXCR1 and CXCR2 receptor protein was already completely downregulated at that time, it seems most likely that downregulation of these chemokine receptors is not mediated by IL-8 and TNF-α. However, the possibility that IL-8 released from neutrophils is consumed by binding to the CXCR1 and CXCR2 receptors and is therefore not measurable cannot be completely excluded.
In contrast to LPS from enterobacteria, which is also described as downregulating CXCR1 and CXCR2 expression on neutrophils, purified LPS from H. pylori appeared to have no effect on the regulation of the chemokine receptors CXCR1 and CXCR2 on neutrophils. This may reflect the low biological activity of H. pylori LPS (2, 22, 23).
The mechanisms for CXCR1 and CXCR2 downregulation by H. pylori are not clear. CXCR1 and CXCR2 receptor protein regulation by H. pylori does not seem to be under the influence of known receptor regulators, such as the inflammatory cytokines IL-8 and TNF-α and bacterial LPS. Therefore, other so far unknown mechanisms induced by H. pylori seem to be responsible for the downregulation of these two chemokine receptors. As receptor regulation occurred very rapidly in our experiments and no production of the cytokines IL-8 and TNF-α was detectable at this early time point, it seems likely that CXCR1 and CXCR2 receptor regulation on neutrophils is a direct effect of H. pylori.
Although H. pylori is a noninvasive bacterium, bacterial products can enter the extracellular space by specific secretion pathways, autolysis, and membrane vesicle formation and come into direct contact with neutrophils infiltrating the lamina propria (3, 13, 25, 28). Furthermore, H. pylori may be in contact with neutrophils after transepithelial migration in severe active gastritis and after epithelium destruction or tight-junction opening.
Surprisingly, CXCR1 and CXCR2 mRNAs of neutrophils were downregulated at a later time than the receptor protein. Therefore, the rapid receptor protein downregulation seems not to be mediated by reduced mRNA transcription.
Receptor shedding or receptor internalization could explain this rapid CXCR1 and CXCR2 receptor downregulation independent of mRNA. By confocal microscopy and immunoblotting, we could in fact show that CXCR1 and CXCR2 downregulation on neutrophils by H. pylori is most probably due to receptor internalization and rapid degradation of these chemokine receptors.
Interestingly, cag+ H. pylori strains induced more severe downregulation of CXCR1 and CXCR2 than cag deletion H. pylori strains on the mRNA level, whereas no differences between cag+ and cag deletion H. pylori strains could be seen on the receptor protein level. These findings suggest that CXCR1 and CXCR2 mRNA downregulation is a cag-dependent process, whereas protein downregulation is a cag-independent process.
In contrast to the receptor protein regulation, mRNA regulation, which occurs at a later time, may be induced by the influence of cytokines during H. pylori infection. This may explain differences in mRNA regulation between cag+ and cag deletion H. pylori strains, because cag+ strains of H. pylori are known to induce a stronger cytokine response than cag deletion strains. In our experiments, neutrophils incubated with cag+ and cag deletion strains did not differ significantly in their TNF-α expression, whereas IL-8 secretion by neutrophils tended to be higher in cag+ than in cag deletion strains 3 h after incubation.
Therefore, it may be suggested that the rapid downregulation of the CXCR1 and CXCR2 receptors on neutrophils may be a direct effect of H. pylori via receptor internalization and cytoplasmic degradation. In contrast, in the process of mRNA downregulation, which occurs at a later time, H. pylori-induced cytokines may play a role and so prevent receptor reexpression.
In another very recent study, phagocytosing neutrophils were found to downregulate the CXCR1 and CXCR2 receptors very rapidly via receptor shedding. In that study, however, CXCR1 and CXCR2 mRNAs showed a constant level during phagocytic stimulation, which is in contrast to our findings. Further studies are needed to show whether this mechanism plays a role in H. pylori infection (9).
Based on our immunohistochemical data, downregulation of the CXCR1 and CXCR2 receptors on neutrophils may also occur in vivo during H. pylori infection in the human stomach and modulate the responsiveness of neutrophils to neutrophil attractant chemokines in this manner.
Our findings suggest that H. pylori regulates neutrophil activation and migration in the gastric mucosa by the modulation of the neutrophil-activating and -attracting chemokines IL-8 and Groα, as well as by the modulation of the corresponding chemokine receptors CXCR1 and CXCR2 on neutrophils. Gastritis activity and mucosal damage seem to be complex processes, in which ligand expression, as well as corresponding receptor expression, is regulated under the influence of the bacterium itself. Once neutrophils in the gastric mucosa are in the presence of the pathogen H. pylori, there is less need for those neutrophils to continue to migrate in response to inflammatory chemokines and more need for antimicrobial functions, such as superoxide generation and production of cytokines and chemokines. Because these defense mechanisms not only protect the host from the bacterium but also cause mucosal damage during inflammation, downregulation of CXCR1 and CXCR2 receptor expression may represent a new pathomechanism of H. pylori.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft grants EC 203/1-3 and EC 203/1-2. S.S. and C.J. were supported by grant Su 13313-3 from the Deutsche Forschungsgemeinschaft.
We thank E. Bachmann, E. Schmitt, and B. Meyer for their excellent technical assistance. We also thank E. Geisinger for reading the manuscript.
Editor: J. D. Clements
REFERENCES
- 1.Bhattacharya, C., S. Samanta, S. Gupta, and A. K. Samanta. 1997. A Ca2+-dependent autoregulation of lipopolysaccharide-induced IL-8 receptor expression in human polymorphonuclear neutrophils. J. Immunol. 158:1293-1301. [PubMed] [Google Scholar]
- 2.Birkholz, S., U. Knipp, C. Nietzki, R. J. Adamek, and W. Opferkuch. 1993. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol. Med. Microbiol. 6:317-324. [DOI] [PubMed] [Google Scholar]
- 3.Cao, P., M. S. McClain, M. H. Forsyth, and T. L. Cover. 1998. Extracellular release of antigenic proteins by Helicobacter pylori. Infect. Immun. 66:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuntharapai, A., and K. J. Kim. 1995. Regulation of the expression of IL8 receptor A/B by IL8: possible functions of each receptor. J. Immunol. 155:2587-2594. [PubMed] [Google Scholar]
- 5.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, and R. Rappuoli. 1993. Molecular characterisation of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabtree, J. E., J. D. Taylor, J. I. Wyatt, R. V. Heatley, T. M. Shallcross, D. S. Tompkins, and B. J. Rathbone. 1991. Mucosal recognition of Helicobacter pylori 120 kda protein, peptic ulceration, and gastric pathology. Lancet 338:332-335. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree, J. E., J. I. Wyatt, L. K. Trejdosiewicz, P. Peichl, P. H. Nichols, N. Ramsay, P. N. Primrose, and I. J. Lindley. 1994. Interleukin-8 expression in Helicobacter pylori, normal and neoplastic gastroduodenal mucosa. J. Clin. Pathol. 47:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon, M. F. 1991. Helicobacter pylori and peptic ulceration: histopathological aspects. J. Gastroenterol. Hepatol. 6:125-130. [DOI] [PubMed] [Google Scholar]
- 9.Doroshenko, T., Y. Chaly, V. Savitskiy, O. Maslakova, A. Portyanko, I. Gorudko, and N. N. Voitenok. 2002. Phagocytosing neutrophils downregulate the expression of chemokine receptors CXCR1 and CXCR2. Blood 100:2668-2671. [DOI] [PubMed] [Google Scholar]
- 10.Eck, M., B. Schmauβer, K. Scheller, A. Toksoy, M. Kraus, T. Menzel, H. K. Müller-Hermelink, and R. Gillitzer. 2000. CXC chemokines Groα/IL8 and IP-10/MIG in Helicobacter pylori gastritis. Clin. Exp. Immunol. 22:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst, P. B., S. E. Crowe, and V. E. Reyes. 1997. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology 113:35-42. [DOI] [PubMed] [Google Scholar]
- 12.Falush, D., C. Kraft, N. S. Taylor, P. Correa, J. G. Fox, M. Achtman, and S. Suerbaum. 2001. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. USA 98:15056-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiocca, R., V. Necchi, P. Sommi, V. Ricci, J. Telford, T. L. Cover, and E. Solcia. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188:220-226. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, W. E., J. Lee, W. J. Kuang, G. C. Rice, and W. I. Wood. 1991. Structure and functional expression of a human interleukin-8 receptor. Science 253:1278-1280.1840701 [Google Scholar]
- 16.Israel, D. A., and R. M. Peek. 2001. Pathogenesis of Helicobacter pylori-induced gastric inflammation. Aliment. Pharmacol. Ther. 15:1271-1298. [DOI] [PubMed] [Google Scholar]
- 17.Khandaker, M. H., L. Xu, R. Rahimpour, G. Mitchell, M. E. DeVries, J. G. Pickering, S. K. Singhal, R. D. Feldman, and D. J. Kelvin. 1998. CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J. Immunol. 161:1930-1938. [PubMed] [Google Scholar]
- 18.Khandaker, M. H., G. Mitchell, L. Xu, J. D. Andrews, R. Singh, H. Leung, J. Madrenas, S. S. Ferguson, R. D. Feldman, and D. J. Kelvin. 1999. Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood 93:2173-2185. [PubMed] [Google Scholar]
- 19.Lee, J., R. Horuk, G. C. Rice, G. L. Bennett, T. Camerato, and W. I. Wood. 1992. Characterization of two high affinity human interleukin-8 receptors. J. Biol. Chem. 267:16283-16287. [PubMed] [Google Scholar]
- 20.Marshall, B. J. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273-1275. [PubMed] [Google Scholar]
- 21.Marshall B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1314. [DOI] [PubMed] [Google Scholar]
- 22.Moran, A. P. 1995. Cell surface characteristics of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 10:271-280. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, H., S. Birkholz, L. P. Andersen, and A. P. Moran. 1994. Neutrophil activation by Helicobacter pylori lipopolysaccharides. J. Infect. Dis. 170:135-139. [DOI] [PubMed] [Google Scholar]
- 24.Peek, R. M., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to CagA+ Helicobacter pylori strains. Lab. Investig. 73:760-770. [PubMed] [Google Scholar]
- 25.Phadnis, S. H., M. H. Parlow, M. Levy, D. Ilver, C. M. Caulkins, J. B. Connors, and B. E. Dunn. 1996. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauws, E. A., W. Langenberg, H. J. Houthoff, H. C. Zanen, and G. N. Tytgat. 1988. Campylobacter pyloridis-associated chronic active antral gastritis. Gastroenterology 94:33-40. [PubMed] [Google Scholar]
- 27.Samanta, A. K., J. J. Oppenheim, and K. Matsushima. 1990. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J. Biol. Chem. 265:183-187. [PubMed] [Google Scholar]
- 28.Schraw, W., M. S. McClain, and T. L. Cover. 1999. Kinetics and mechanisms of extracellular protein release by Helicobacter pylori. Infect. Immun. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher, C., I. Clark-Lewis, M. Baggiolini, and B. Moser. 1992. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc. Natl. Acad. Sci. USA 89:10542-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimoyama, T., and J. E. Crabtree. 1998. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut 43(Suppl. 1):2-5. [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoyama, T., S. M. Everett, M. F. Dixon, A. T. Axon, and J. E. Crabtree. 1998. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J. Clin. Pathol. 51:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweet, M. J., and D. A. Hume. 1996. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 60:8-26. [DOI] [PubMed] [Google Scholar]
- 33.Westphal, M., and L. Jann. 1965. Purification of bacterial flagellin. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 34.Xiang, Z., M. Bugnoli, R. Rappuoli, A. Covacci, A. Ponzetto, and J. E. Crabtree. 1993. Helicobacter pylori: host response in peptic ulceration. Lancet 341:900-901. [DOI] [PubMed] [Google Scholar]
- 35.Xiang, Z., M. Bugnoli, A. Ponzetto, A. Morgando, N. Figura, A. Covacci, R. Petracca, C. Pennatini, S. Censini, D. Armellini, and R. Rappuoli. 1993. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128 kDa protein (CagA) of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 12:739-745. [DOI] [PubMed] [Google Scholar]
- 36.Xiang, Z., S. Censini, P. F. Bayeli, J. L. Telford, N. Figura, R. Rappuoli, and A. Covacci. 1995. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 63:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, and J. Imanishi. 1996. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 110:1744-1752. [DOI] [PubMed] [Google Scholar]
- 38.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, K. Kashima, and J. Imanishi. 1997. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut 41:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, T. Tanahashi, K. Kashima, and J. Imanishi. 1998. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 42:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]