The pH dependence of PMEI, proteins that fine-tune the activity of pectin methylesterases, can be predicted by molecular dynamics simulation and validated in vitro and on pollen tube development.
Abstract
The fine-tuning of the degree of methylesterification of cell wall pectin is a key to regulating cell elongation and ultimately the shape of the plant body. Pectin methylesterification is spatiotemporally controlled by pectin methylesterases (PMEs; 66 members in Arabidopsis [Arabidopsis thaliana]). The comparably large number of proteinaceous pectin methylesterase inhibitors (PMEIs; 76 members in Arabidopsis) questions the specificity of the PME-PMEI interaction and the functional role of such abundance. To understand the difference, or redundancy, between PMEIs, we used molecular dynamics (MD) simulations to predict the behavior of two PMEIs that are coexpressed and have distinct effects on plant development: AtPMEI4 and AtPMEI9. Simulations revealed the structural determinants of the pH dependence for the interaction of these inhibitors with AtPME3, a major PME expressed in roots. Key residues that are likely to play a role in the pH dependence were identified. The predictions obtained from MD simulations were confirmed in vitro, showing that AtPMEI9 is a stronger, less pH-independent inhibitor compared with AtPMEI4. Using pollen tubes as a developmental model, we showed that these biochemical differences have a biological significance. Application of purified proteins at pH ranges in which PMEI inhibition differed between AtPMEI4 and AtPMEI9 had distinct consequences on pollen tube elongation. Therefore, MD simulations have proven to be a powerful tool to predict functional diversity between PMEIs, allowing the discovery of a strategy that may be used by PMEIs to inhibit PMEs in different microenvironmental conditions and paving the way to identify the specific role of PMEI diversity in muro.
The plant cell wall plays a central role in shaping the plant body. The primary plant cell wall is a composite material consisting of cellulose microfibrils embedded in a complex matrix of pectins, xyloglucans, and structural proteins (Carpita and Gibeaut, 1993). Although organized, the plant cell wall is highly dynamic through the synthesis of novel polysaccharides and the action of cell wall-remodeling enzymes during cell elongation and differentiation. Among primary cell walls, pectins represent up to one-third of the cell wall dry mass (Vogel, 2008). They are complex polysaccharides, rich in α-(1-4)-linked d-galacturonic acids, which comprise four main domains: homogalacturonan (HG), rhamnogalacturonan-I, and minor amounts of rhamnogalacturonan-II and xylogalacturonan (Caffall and Mohnen, 2009). In recent years, pectins have been shown to play a central role in modulating plant cell elongation and, ultimately, growth in various developmental processes (Caffall and Mohnen, 2009; Daher and Braybrook, 2015; Levesque-Tremblay et al., 2015b). In particular, fine-tuning the degree of methylesterification (DM) of the HG backbone appears to be of primary importance. HGs are synthetized in a highly methylesterified form (i.e. 80%) and are subsequently demethylesterified in muro by specific enzymes, pectin methylesterases (PMEs), belonging to a large multigenic family of 66 members in Arabidopsis (Arabidopsis thaliana). Among PME proteins, group 1 and group 2 PMEs differ by the presence of a cleavable Pro part in the latter. The chemical modification of pectins leads to changes in their physical properties, conferring to the polysaccharides the ability to rearrange according to the need of remodeling the plant cell wall. Over the past few years, it has been shown that the PME-mediated tuning of the pectin methylesterification status is implicated in primordia emergence at the shoot apical meristem (Peaucelle et al., 2008, 2011a, 2011b), in adventitious root formation (Guénin et al., 2011), in plant-pathogen interactions (Hewezi et al., 2008; Osorio et al., 2008; Raiola et al., 2011), in the control of cell elongation in dark-grown hypocotyls (Derbyshire et al., 2007; Pelletier et al., 2010; Wolf et al., 2012) and pollen tubes (Jiang et al., 2005; Leroux et al., 2015), and in seed development (Levesque-Tremblay et al., 2015a). The regulation of PME activity, therefore, is crucial to control numerous plant developmental processes, and this raises questions concerning the mechanisms by which regulation is mediated.
Specific proteinaceous inhibitors called pectin methylesterase inhibitors (PMEIs) have been identified in various species and are responsible for the fine regulation of in vivo PME activities (Balestrieri et al., 1990; Wolf et al., 2003; Raiola et al., 2004; Hong et al., 2010; Reca et al., 2012). Importantly, similar to the effects observed for PMEs, changes in the level of expression of specific PMEI genes also affect pectin structure in a wide range of developmental processes, including dark-grown hypocotyl elongation (Pelletier et al., 2010; Wolf et al., 2012), seed development and seed mucilage production (Müller et al., 2013; Saez-Aguayo et al., 2013), pollen tube elongation (Woriedh et al., 2013), and plant-pathogen interactions (An et al., 2008; Volpi et al., 2013; Lionetti et al., 2014).
In Arabidopsis, the presence of 76 putative PMEIs obviously questions the specificity of their interactions with PMEs and the existence of specific PME-PMEI pairs. Following the cocrystallization of the tomato (Solanum lycopersicum) PME-kiwi (Actinidia chinensis) PMEI complex, PMEIs were shown to inhibit plant PMEs through the formation of a 1:1 stoichiometric complex in which the PMEI covers the pectin-binding cleft of the PME, thus impeding the interaction between the enzyme and HG (Hothorn et al., 2004, 2010; Di Matteo et al., 2005). In Arabidopsis, only two PMEIs (pollen-expressed AtPMEI1 and AtPMEI2) were characterized at the biochemical level, and their inhibiting capacities were tested on tomato PME (Wolf et al., 2003; Raiola et al., 2004). The recent characterization of the interaction between AtPME3 and AtPMEI7 from Arabidopsis, which overlap in their expression patterns, brought new insights in understanding the specificity of PME-PMEI interactions (Sénéchal et al., 2015a). This study, if extended to other putative PME-PMEI pairs, could help in determining the roles that different PMEI isoforms may have in the physiology of an organism. Considering the numbers of PME and PMEI genes present in the Arabidopsis genome and their overlapping expression patterns (Wang et al., 2013), it is likely that PMEIs are indeed not all equal in their target specificities or inhibiting capacities. In addition, it is plausible that different cell wall microenvironmental conditions (changes in pH and ionic strength) influence the activity of PMEs, either directly or through the modulation of PME-PMEI interactions (Sénéchal et al., 2015a).
One way to address these questions is to have combined approaches involving both biochemical and structural characterization aiming to characterize functional, structural, and dynamic aspects of different PME-PMEI pairs. Recently, computational approaches were proven to be very useful in assessing the functional dynamics of PME-HG complexes (Mercadante et al., 2013, 2014). In the case of PME-PMEI pairs that show high structural similarity and are expressed in exceedingly high numbers, molecular dynamics (MD) simulations are powerful tools that could be used to zoom in on PME-PMEI complexes at the atomic level and sample their dynamics in the different microenvironmental conditions explored by the experiments. This can enhance our understanding of the specificities of PME-PMEI association through the characterization of the dynamics of the complexes, which adds an additional, high-resolution level of detail to the structural information published previously (Di Matteo et al., 2005).
Here, in order to evaluate the potential differences between PMEIs, we elucidated structural and dynamic profiles, as well as the inhibited activity of two PMEIs, which partially overlap in their expression patterns in Arabidopsis: AtPMEI4 (Pelletier et al., 2010) and AtPMEI9 (Sorek et al., 2015). The combination of simulations and experiments has been crucial to elucidate the stability of the complexes and revealed the key residues that could play a role in the specificity and the pH dependence of the inhibition. Therefore, the proposed approach can be transferred to any other PME-PMEI pairs in order to elucidate their characteristics in the framework of pectin remodeling.
RESULTS
AtPMEI4 and AtPMEI9 Are Both Expressed in Roots
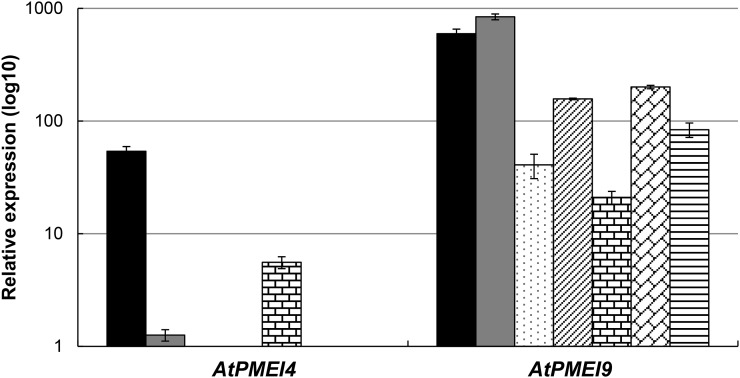
In order to determine the expression pattern of AtPMEI4 and AtPMEI9, their transcript levels were first assessed using reverse transcription-quantitative PCR (RT-qPCR) in distinct organs of Arabidopsis (Fig. 1). AtPMEI4 was expressed mainly in 10-d-old roots and siliques and, to a much lesser extent, in dark-grown hypocotyls. In contrast, AtPMEI9 was highly expressed in all organs considered, particularly in roots and dark-grown hypocotyls. The quantification of AtPMEI genes expressed in 10-d-old roots revealed that AtPMEI9 and AtPMEI4 were the second and 19th most expressed genes, respectively, in this organ (data not shown). Gene expression data suggest that AtPMEI9 is a rather ubiquitous PMEI, which could target a wide range of PMEs, including ubiquitously expressed isoforms, while AtPMEI4 would have more discrete target specificity and/or specific action. Data mining of publicly available databases further revealed that, in roots, AtPMEI4 and AtPMEI9 were both expressed in the elongation zone (Winter et al., 2007). In order to assess whether AtPMEI4 and AtPMEI9 were present at the protein level, cell wall-enriched proteins of 10-d-old roots and 4-d-old dark-grown hypocotyls were extracted and the samples were resolved by SDS-PAGE. Bands were cut out of the gels, and proteins were identified by nano-LC-ESI-MS/MS following trypsin digestion. We were not able to detect peptides corresponding to either AtPMEI4 or AtPMEI9 in these cell wall proteomes, which could be related either to their low abundance at the protein level or to the fact that we considered only weakly bound cell wall proteins in our analysis. However, the expression of a GFP-tagged version of AtPMEI4 under the control of its own promoter revealed that the protein was targeted at the cell wall in 10-d-old roots (Supplemental Fig. S1A). In parallel, data mining of the WallProtDB database (http://www.polebio.lrsv.ups-tlse.fr/WallProtDB/index.php) identified AtPMEI9 as a cell wall protein at later stages of root development, with coverage of 26% of the mature sequence (Supplemental Fig. S1B). Therefore, AtPMEI4 and AtPMEI9 proteins are present in roots.
Figure 1.
AtPMEI4 and AtPMEI9 partially overlap in their expression patterns. Relative expression is shown for AtPMEI4 and AtPMEI9 genes in several organs of Arabidopsis Columbia-0 (Col-0). Roots (black bars) and etiolated hypocotyls (gray bars) were from in vitro-grown Col-0 seedlings, while adult leaves (dotted bars), stems (diagonally striped bars), siliques (bricked bars), floral buds (diagonally bricked bars), and mature seeds (horizontally striped bars) were from Col-0 plants grown on soil. Relative gene expression was measured using stably expressed reference genes (CLA and PEX4) with similar results. Only the results obtained with CLA are shown.
Root Growth Is Impaired in Plants with Altered Expression of AtPMEI4 and AtPMEI9
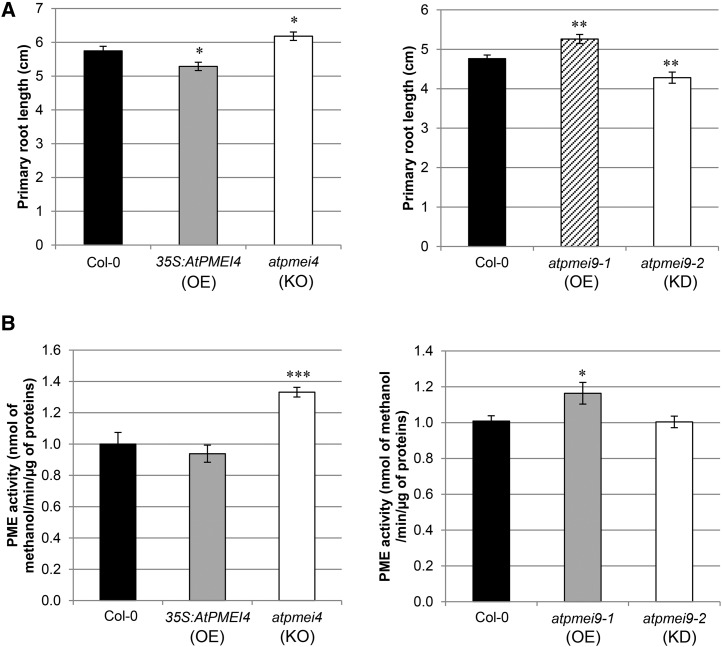
To assess whether AtPMEI4 and AtPMEI9 can have an impact on root development, an organ in which they are both expressed, we characterized transgenic lines with altered gene expression. First, we used 35S::PMEI4-overexpressing lines and the pmei4-1 knockout mutant published previously (Pelletier et al., 2010). We further identified two Arabidopsis lines with altered expression of AtPMEI9 (atpmei9-1 and atpmei9-2). atpmei9-1, presenting a T-DNA inserted in the promoter, is characterized by a 2-fold increase in AtPMEI9 expression in roots, while atpmei9-2, showing an insertion of the T-DNA in an intron, is characterized by a 65-fold decrease in AtPMEI9 expression, as revealed by RT-qPCR (Supplemental Fig. S2). Interestingly, plants altered in the expression of AtPMEI4 showed a distinct root growth phenotype. Indeed, atpmei4-1 root length was increased by ∼10% compared with the wild type, while the PMEI4-overexpressing line showed decreased root length by about 10% (Fig. 2A). In parallel, the phenotypical analysis of atpmei9 lines showed that AtPMEI9 does play a role in root growth, as changes in root length varied in opposite ways when comparing atpmei9-1 and atpmei9-2 alleles (Fig. 2A). Root length of the atpmei9-2 knockdown line was decreased by ∼10% compared with wild-type Col-0, whereas atpmei9-1 roots presented an increase of ∼10% in length. Such opposite phenotypes could be related to the differences in the relative expression of AtPMEI9. This overall shows that a defect in AtPMEI4 or AtPMEI9 expression could lead to opposite phenotypes. A positive correlation vas observed between phenotypes observed for AtPMEI4 and AtPMEI9 lines and the total PME activity measured in roots in the corresponding lines (Fig. 2B). Although it cannot be excluded that changes in the expression of distinct PMEI genes, due to compensation mechanisms in the various lines, could be the source of the phenotypical variability observed, an alternative scenario can be envisaged. Indeed, the differences could be related to the biochemical specificities of AtPMEI4 and AtPMEI9 in inhibiting target PMEs expressed in roots.
Figure 2.
Modification of AtPMEI4 and AtPMEI9 expression has opposite consequences on PME activity and root growth. A, Lengths of 10-d-old roots (left) and 6-d-old roots (right) of wild-type (Col-0), 35S:AtPMEI4, atpmei4, atpmei9-1, and atpmei9-2 lines. Data represent means of length ± se from three independent experiments (n ∼ 50). Significant differences were determined with the parametric Student’s test (*P < 0.05 and **P < 0.01). KD, Knockdown mutant line; KO, knockout mutant line; OE, overexpressor line. B, Total PME activity in 10-d-old (left) and 6-d-old (right) root cell wall-enriched protein extracts from wild-type (Col-0), 35S:AtPMEI4, atpmei4, atpmei9-1, and atpmei9-2 lines. Data represent means of PME activity in nmol methanol min−1 µg−1 protein ± se from three independent extractions and three replicates. Significant differences were determined by the Mann-Whitney statistical test (*P < 0.05 and ***P < 0.001).
AtPME3, among Other PMEs, Is Identified as a Putative Target of AtPMEI4 and AtPMEI9
In order to identify putative PME targets of AtPMEI4 and AtPMEI9 in vivo, proteomic analyses were performed on roots and hypocotyls. Overall, nine PMEs were identified, with sequence coverage ranging from 5% to 31% depending on the isoforms and tissues considered (Table I). Three mature sequences of group 2 PME isoforms were identified in the two organs: AtPME2, AtPME3, and AtPME17, with coverage of 19%, 24%, and 31%, respectively, in roots. In contrast, some PME isoforms were only identified in dark-grown hypocotyls (i.e. AtPME16 and AtPME34) or in roots (AtPME51). These results support the hypothesis that, depending on their expression patterns, PMEs might be the targets of specific PMEI isoforms in planta. Moreover, AtPME3-specific peptides also were recovered in later stages of root development, which suggests that AtPME3 is a major PME isoform expressed in roots. Therefore, considering the above-mentioned results concerning the presence of AtPMEI4 and AtPMEI9 in roots and the identification of AtPME3 in root proteomes (Supplemental Fig. S1), AtPME3/AtPMEI4 and AtPME3/AtPMEI9 interactions are likely to occur in vivo. These two potential pairs were thus used as models to gain more insights into what underlies the specificity, and pH dependence, of PME-PMEI interactions.
Table I. Identification of PMEs in cell wall-enriched extracts from 10-d-old roots and 4-d-old dark-grown hypocotyls of Arabidopsis using nano-LC-ESI-MS/MS.
Values represent the percentage of coverage of the mature protein. –, No peptides identified.
| Protein Name | Locus | Roots | Hypocotyls |
|---|---|---|---|
| AtPME2 | At1g53830 | 19 | 19 |
| AtPME3 | At3g14310 | 24 | 28 |
| AtPME16 | At2g43050 | – | 26 |
| AtPME17 | At2g45220 | 31 | 5 |
| AtPME32 | At3g43270 | – | 15 |
| AtPME34 | At3g49220 | – | 9 |
| AtPME35 | At3g59010 | – | 10 |
| AtPME44 | At4g33220 | – | 19 |
| AtPME51 | At5g09760 | 13 | – |
MD Simulations of the AtPME3/AtPMEI4 and AtPME3/AtPMEI9 Complexes Reveal the Molecular Determinants of the pH-Dependent Inhibitory Capacities of PMEIs
Using MD simulations, the dynamics of AtPMEI4 and AtPMEI9 in complex with AtPME3 were monitored to understand if the conformational dynamics of these PMEIs could predict the specificity in their inhibiting activity toward AtPME3. Therefore, several parameters can be considered, including the strength of the inhibition and the pH dependence of the interaction. Indeed, previous characterization of the AtPMEI7/AtPME3 complex showed that AtPME3 activity was inhibited through the formation of a complex with higher affinity at acidic pH (Sénéchal et al., 2015a). The modeling of PME3 and PMEIs shows a good structural superimposition with the templates. The helices composing the core of the structure of the modeled and template PMEIs overlap naturally. Nevertheless, the PMEIs from Arabidopsis show a longer loop connecting helices αII and αIII when compared with AcPMEI (Supplemental Fig. S3).
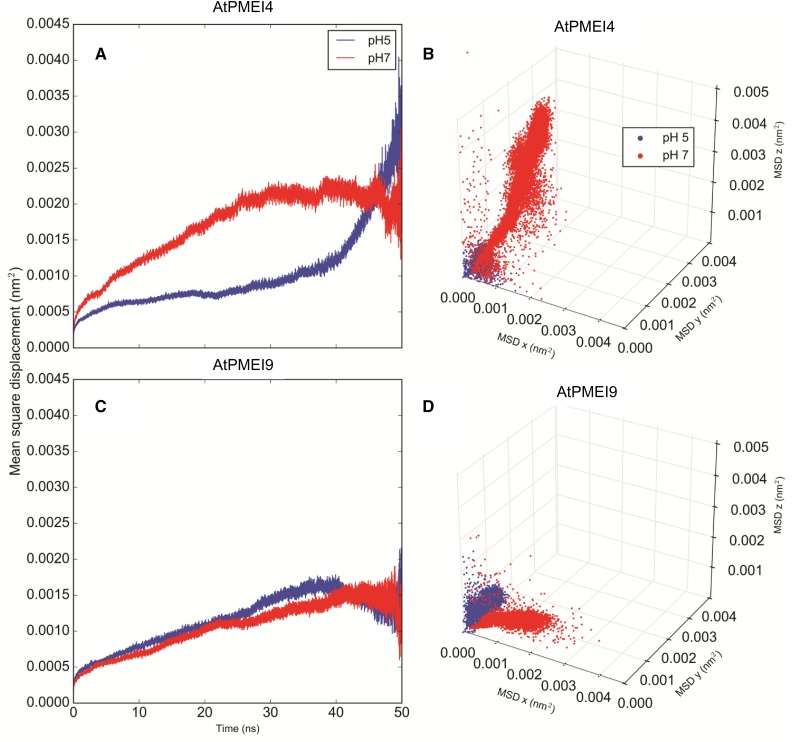
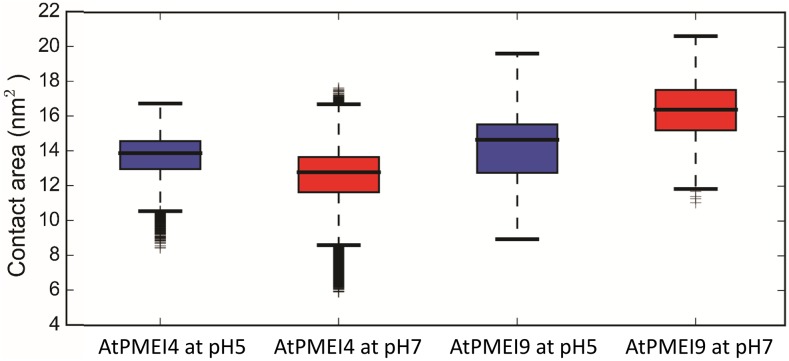
The mean square displacement (MSD) of the center of mass of the two PMEIs with respect to the bound AtPME3 suggests that AtPMEI4 exhibits higher translational motions (Fig. 3, A and B) compared with AtPMEI9 (Fig. 3, C and D) in all three dimensions. In parallel, a lower contact area between AtPME3 and AtPMEI4 can be noted when compared with its association with AtPMEI9 (Fig. 4). On average, along the calculated trajectories, AtPMEI4 establishes a lower number of contacts (676) when bound to AtPME3 compared with the average number of contacts calculated for AtPMEI9 (772; Table II). For the sake of comparison between experiments and computations presented, we consider it legitimate to assume that the number and the nature of the contacts between the partners could qualitatively reflect the inhibiting ability of PMEIs toward a target protein.
Figure 3.
Dynamics of AtPMEI4 and AtPMEI9 as revealed by MD simulations. A and C, MSD of AtPMEI4 and AtPMEI9 inhibitors at pH 5 (blue) and pH 7 (red) averaged along all the collected trajectories after MD of the AtPME3-AtPMEI4/AtPMEI9 complexes. B and D, MSD decomposed in the x, y, and z dimensions. The MSD was calculated for the PMEIs in complex with AtPME3 after removing the translational and conformational motions of AtPME3.
Figure 4.
Contact area between AtPME3 and AtPMEI4/AtPMEI9 at different pH values. The distributions are calculated considering the last 50 ns of the collected trajectories. A higher contact area implies a more stable complex between the partners. As a result of the contacts formed between AtPME3 and the different investigated PMEIs, the contact area can be inversely related with the Kd that has been derived experimentally through means of microscale thermophoresis (MST).
Table II. Average number of contacts between AtPME3 and AtPMEI4/AtPMEI9 as calculated from MD simulations of the two complexes at pH 5 and 7.
| Sample | AtPME3-AtPMEI4 | AtPME3-AtPMEI9 |
|---|---|---|
| pH 5 | 352 | 365 |
| pH 7 | 324 | 407 |
| Sum | 676 | 772 |
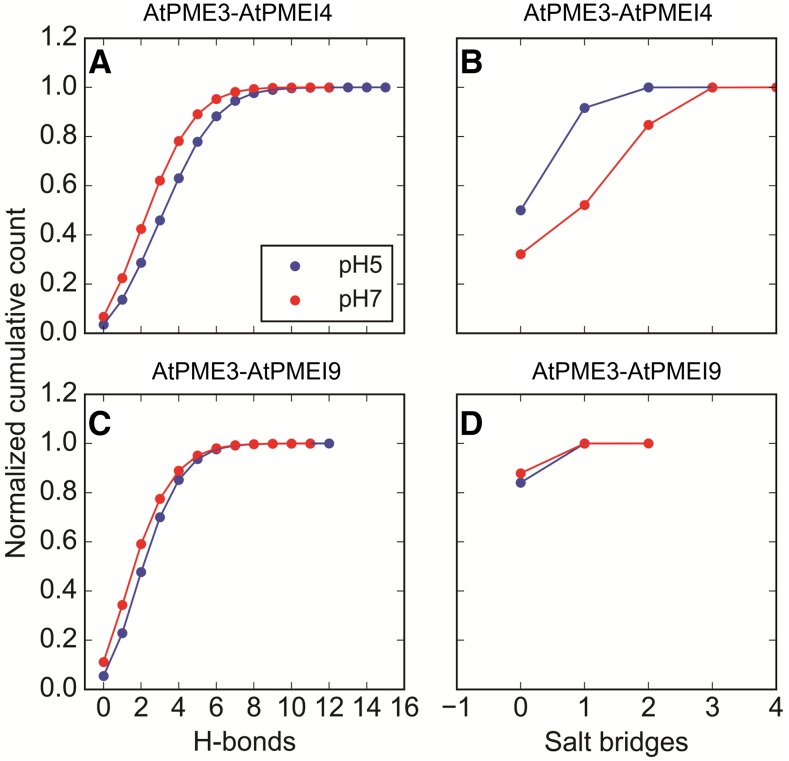
With respect to the pH dependence of the inhibition, a significant difference between acidic and neutral pH can be observed for AtPMEI4: at pH 5, a lower MSD (Fig. 3A) and a higher contact area (Fig. 4) can be observed when compared with the interaction computed at pH 7, reflecting a greater, more stable interaction at acidic pH. On the contrary, in the case of AtPMEI9, the MSD profiles at the two investigated pH values overlap (Fig. 3, C and D), suggesting a much lower pH dependence of the interaction. Hydrogen bonds and salt bridges also were monitored at the dimer interface. The cumulative distributions of the hydrogen bonds computed at the two different pH values show a higher divergence for AtPMEI4 compared with AtPMEI9 (Fig. 5). These observations suggest that the physical interaction between the partners (AtPME3/AtPMEI4 and AtPME3/AtPMEI9) would differ according to the pH.
Figure 5.
Hydrogen bonds and salt bridges between AtPME3 and AtPMEI4/AtPMEI9. A and C, Cumulative distributions of the hydrogen bonds (H-bonds) formed at pH 5 (blue) and pH 7 (red). B and D, Cumulative distributions of the salt bridges formed at pH 5 (blue) and pH 7 (red).
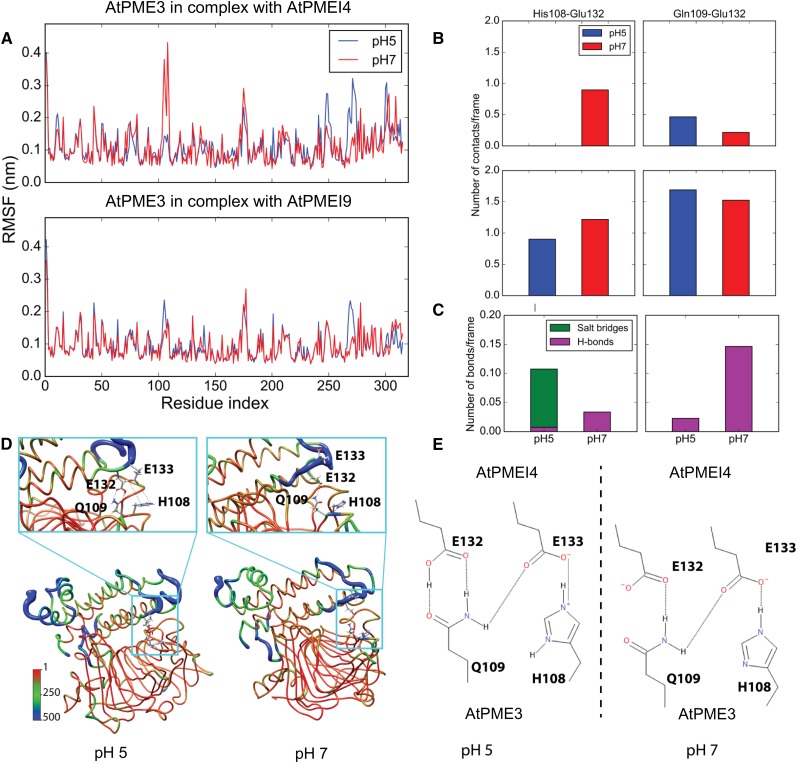
The root mean square fluctuation (RMSF) of AtPME3 when bound to the two investigated inhibitors also was monitored in order to track, along the AtPME3 structure, the main regions affected by changes in dynamics at different pH values and to identify key intermolecular interactions that contribute to the different profiles observed at different pH values. Differences in the RMSF trace of AtPME3 at different pH values can only be observed when the protein is bound to AtPMEI4. At pH 7, the region comprising residues 108 to 110 of AtPME3 shows a considerably higher RMSF than at pH 5 and corresponds to part of the structure that lies at the interface with the inhibitor (Fig. 6A). This region of AtPME3 interacts with the loops connecting helices αI and αII and helices αIII and αIV of the AtPMEI4 and AtPMEI9 inhibitors (Fig. 6). On the other hand, the longer loop characterizing the AtPMEIs does not show differences at the two different pH values sampled, as they do not interact stably with PME3 (Fig. 6). At pH 7, the increased dynamics of AtPME3 corresponds to an increased mobility of the AtPMEI4 loops at the complex’s interface when compared with AtPMEI9 (Supplemental Fig. S4). This favors the disruption of specific interactions occurring at the interface between the partners. Interestingly, the estimated pKa of His-108 is 6.09 (Table II), allowing the residue to swap its protonation state and to become positively charged upon lowering the pH from 7 to 5. Glu-132 and Glu-133 of AtPMEI4, present at the dimer interface with AtPME3, have calculated pKa values of 5.13 and 4.58, respectively. At pH 5, Glu-132 is protonated, while it bears a negative charge at pH 7. Glu-133, on the contrary, is deprotonated and negatively charged at both pH values. Therefore, at pH 5, a stable salt bridge can be formed between His-108 on AtPME3 and Glu-133 on AtPMEI4, stabilizing the interaction between Gln-109 and Glu-132, which form two hydrogen bonds (Fig. 6, D and E). At pH 7, on the other hand, the negative charges of the deprotonated Glu-132 and Glu-133 and the lack of positive charge on His-108 destabilize the complex interface. The effect of protonation and deprotonation on the stability of the above-mentioned interactions can be seen in the number of contacts established by His-108/Gln-109 on AtPME3 and Glu-132/Glu-133 on AtPMEI4 (Fig. 6, B and C). Overall, this set of contacts is responsible for the observed differences in the RMSF profiles of AtPME3 bound to AtPMEI4 and AtPMEI9 (Fig. 6A). Importantly, Glu-132 in AtPMEI4 is replaced by a Ser in AtPMEI9, suggesting that the residue may be crucially important for tuning the interaction at different pH values.
Figure 6.
Structural determinants of the pH-dependent activity of AtPMEI4. A, RMSF profile of AtPME3 in complex with AtPMEI4 (top) and AtPMEI9 (bottom). B, Average number of contacts per frame for specific amino acids between AtPME3 and AtPMEI4 at pH 5 (blue) and pH 7 (red). C, Average number of hydrogen bonds (H-bonds; magenta) and salt bridges (green) between AtPME3 and AtPMEI4 (left)/AtPMEI9 (right) at pH 5 and pH 7. D, Representative conformations of AtPME3-AtPMEI4 complexes at pH 5 (left) and pH 7 (right). The structures are shown in ribbons colored accordingly and with a diameter proportional to the B-factors back calculated from the RMSF obtained from MD simulations and shown in A. The residues identified as important for tuning the pH-dependent activity of AtPMEI4 (His-108 and Gln-109 on AtPME3 and Glu-132 and Glu-133 on AtPMEI4) are shown in ball and sticks and colored by atom types (carbon, gray; hydrogen, white; oxygen, red; nitrogen, blue). E, Two-dimensional representation of the interactions between the residues participating in the pH-dependent activity of AtPMEI4.
Altogether, MD simulations predict a difference in behavior of the two complexes, regarding (1) the affinity of AtPME3 toward the two inhibitors and (2) the existence of a stronger pH dependence of the AtPME3/AtPMEI4 complex stability compared with that of the AtPME3/AtPMEI9 complex.
Recombinant AtPMEI4 and AtPMEI9 Are Produced as Active Forms in Escherichia coli
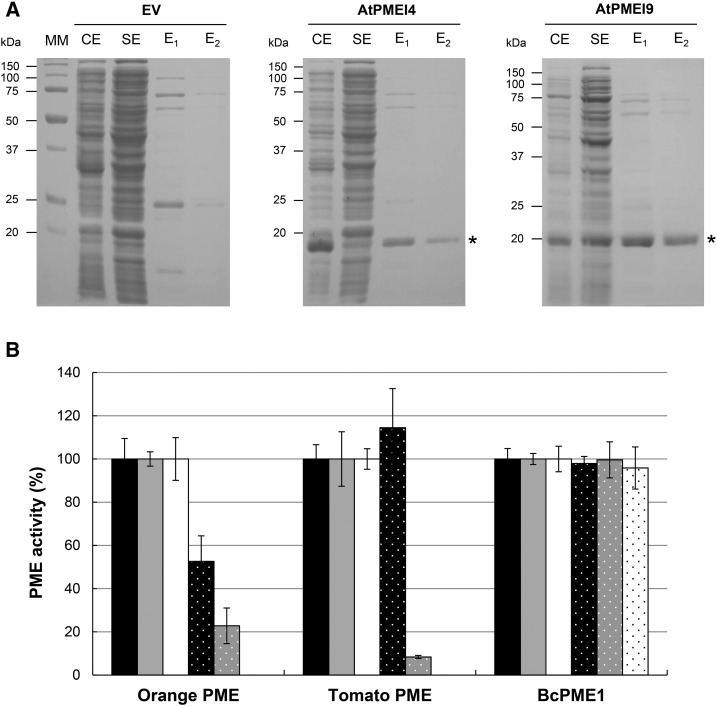
In order to biochemically characterize the PME-inhibiting capacities of AtPMEI4 and AtPMEI9, and potentially confirm the predictions, the proteins were expressed in E. coli. The coding sequences minus the putative signal peptide were cloned downstream and in frame with the 6xHis-coding sequence present in an inducible expression vector. When compared with E. coli containing the empty vector, proteins extracted from induced cultures harboring the 6xHis-AtPMEI4 or the 6xHis-AtPMEI9 construct showed the presence of an extra band of ∼20 kD on SDS-PAGE gels (Fig. 7A). The bands were purified and further analyzed by nano-LC-ESI-MS/MS following trypsin digestion, showing 17 and 20 unique peptides corresponding to 77% and 80% of the AtPMEI4 and AtPMEI9 primary sequences, respectively (Supplemental Fig. S5). The purified fractions were subsequently used for PME activity inhibition tests on purified PME proteins and cell wall-enriched protein extract.
Figure 7.
Recombinant AtPMEI4 and AtPMEI9 inhibit plant PMEs. A, Expression of 6xHis-AtPMEI4 and 6xHis-AtPMEI9 in E. coli and purification with Ni-NTA agarose. SDS-PAGE analysis is shown for proteins in crude extract (CE), soluble extracts (SE), and fractions eluted from Ni-NTA with 250 mm imidazole (E1 and E2). From left to right, SDS-PAGE with proteins from IPTG-induced cultures containing empty vector (EV) or recombinant vector with AtPMEI4 and AtPMEI9. MM, Molecular mass markers. B, Inhibitory capacity of PMEIs on orange PME, tomato PME, and BcPME1 at pH 5. Experiments were carried out using 1 milliunit of commercial orange PME, 1 milliunit of commercial tomato PME, 0.5 milliunit of purified BcPME1, and 0.125 µg of purified PMEIs. Black bars, Boiled AtPMEI4; gray bars, boiled AtPMEI9; white bars, boiled AcPMEI as controls; black bars with spots, AtPMEI4; gray bars with spots, AtPMEI9; white bars with spots, AcPMEI. Results are means ± sd of three replicates.
We first determined if recombinant AtPMEI4 and AtPMEI9 were able to inhibit PME activity. Therefore, we used commercial orange (Citrus spp.) PME, commercial tomato PME, and purified BcPME1, a fungal PME from Botrytis cinerea, and resulting PME activities were compared with those obtained with PMEI purified from kiwi (AcPMEI) as a control. Using a gel diffusion assay at pH 5 and appropriate standard curves, the relation between the diameter of the halo and PME activity was established. One milliunit of orange/tomato PME and 0.5 milliunit of BcPME1 were incubated for 30 min in the presence of 0.125 µg of purified 6xHis-AtPMEI4, 6xHis-AtPMEI9, and AcPMEI (Fig. 7B). The results showed striking differences in the inhibiting capacity of recombinant AtPMEI9 and of AcPMEI compared with the recombinant AtPMEI4. The inhibition of orange PME activity was 50%, 75%, and 100% with 6xHis-AtPMEI4, 6xHis-AtPMEI9, and AcPMEI, respectively. The inhibiting capacity of PMEIs also appeared to differ depending on the considered PME target. The commercial tomato PME activity was not inhibited by recombinant AtPMEI4, while it was inhibited at 95% and 100% when recombinant AtPMEI9 and purified AcPMEI, respectively were used. Despite these strong differences in the inhibiting capacity toward plant PMEs, 6xHis-AtPMEI4, 6xHis-AtPMEI9, and AcPMEI were not able to impair PME activity from BcPME1, suggesting that these three inhibitors are only able to inhibit plant PMEs.
Recombinant AtPMEI4 and AtPMEI9 Have Distinct Capacity to Inhibit PME from Root Cell Wall-Enriched Protein Extracts
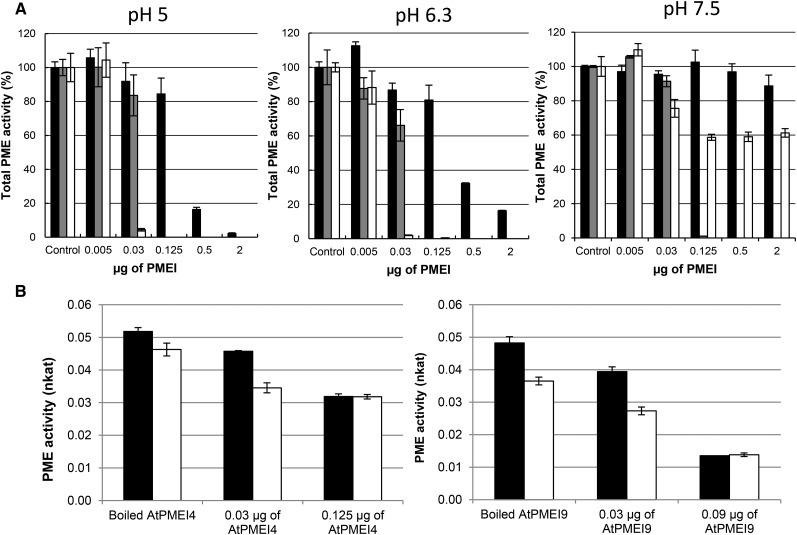
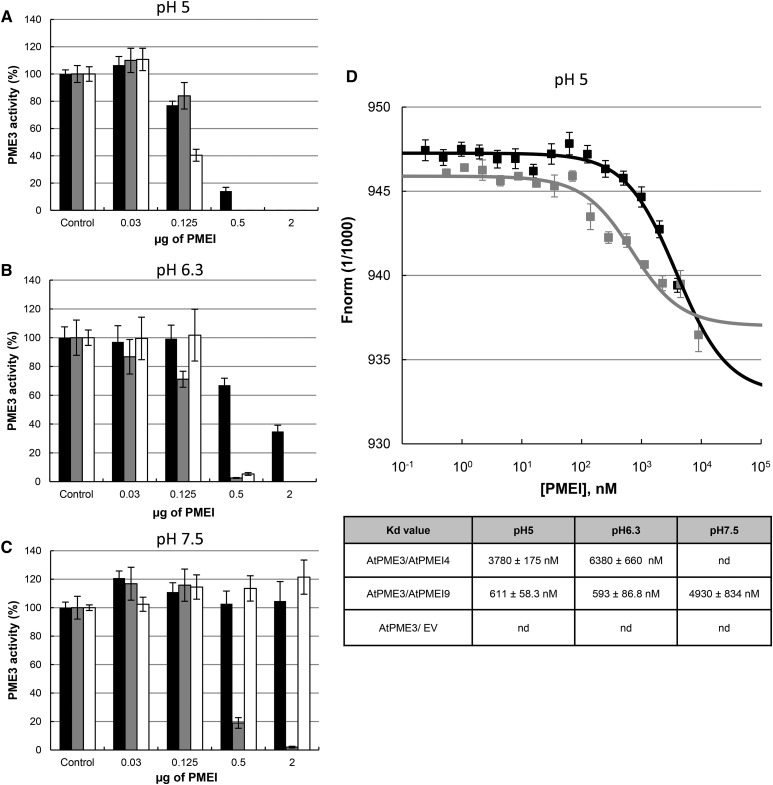
The respective inhibiting capacity of recombinant AtPMEI4 and AtPMEI9 on Arabidopsis PMEs was tested subsequently on cell wall-enriched protein extracts from roots, where they should interact with their target PMEs. MD simulations suggested that interaction between PME and PMEI could be influenced by pH. One milliunit of total PME activity from cell wall-enriched root protein extracts, measured by the Klavons and Bennett method (pH 7.5), was incubated with increasing quantities of purified inhibitors and assayed at three pH values (5, 6.3, and 7.5) in gel diffusion assays. As expected, PME activity was higher at neutral to basic pH compared with acidic pH (Supplemental Fig. S6A). Despite the differences in PME activity observed at each pH, for a more comprehensive representation of the results, we chose to present the effects of recombinant AtPMEI4 and AtPMEI9 on root PME activity as a percentage of the control activity. Our results showed that the strength of the inhibition of root PME activity by recombinant AtPMEI4 was related to both pH and the quantity of inhibitor used (Fig. 8A). For example, when using 0.5 µg of 6xHis-AtPMEI4, PME activity was reduced to 20% and 30% at pH 5 and 6.3, respectively. At pH 7.5, no inhibition of PME activity was observed. When the quantity of 6xHis-AtPMEI4 was increased up to 2 µg, only a residual PME activity was measured at pH 5, while PME activity was 15% and 90% of the control at pH 6.3 and 7.5, respectively. This shows that AtPMEI4’s inhibiting capacity is highly pH dependent and that the protein does not target all PME isoforms present in cell wall-enriched protein extracts from roots. Similar to what was shown previously using orange and tomato PMEs, 6xHis-AtPMEI9 had a stronger inhibiting capacity toward PME activity in cell wall-enriched protein extracts from roots compared with that of recombinant AtPMEI4 (Fig. 8A). At pH 5, 6.3, and 7.5, when using 0.125 µg or more of 6xHis-AtPMEI9, PME activity was inhibited by 100%. Therefore, the inhibition of total PME activity by recombinant AtPMEI9 appears less pH dependent, which may be related to the role of pH in the tuning of the PME-PMEI interaction. AcPMEI, used as a control, showed similar features to 6xHis-AtPMEI9 when considering the inhibition capacity toward PME activity from cell wall-enriched protein extracts at pH 5 and 6.3. However, at pH 7.5, AcPMEI behaved similarly to 6xHis-AtPMEI4, as its efficiency in inhibiting PME activity from cell wall-enriched protein extracts was largely decreased. This overall suggests, depending on the PMEI isoforms, distinct effects of pH in the inhibition, which could play a role in the control of PME activity in specific cell wall microenvironments. Similar results were obtained with cell wall-enriched protein extracts from hypocotyls (Supplemental Fig. S6B). It is also worth noticing that the inhibiting capacity of PMEI, notably AtPMEI4, differs when considering distinct organs, which might be related to the presence of specific targets in given organs (Fig. 8A; Supplemental Fig. S6B).
Figure 8.
Effects of recombinant AtPMEI4 and AtPMEI9 on PMEs from root cell wall-enriched protein extracts. A, Recombinant AtPMEI4 and AtPMEI9 show distinct inhibitory capacities and pH dependence on PMEs from 10-d-old root cell wall-enriched protein extracts. Experiments were carried out using 1 milliunit of PME activity of this extract at pH 5 (left), pH 6.3 (middle), and pH 7.5 (right) and various quantities of purified PMEIs: AtPMEI4 (black bars), AtPMEI9 (gray bars), and AcPMEI (white bars). Controls represent extract with boiled PMEI. Results are means ± sd of three replicates. B, Effects of AtPMEI4 (left) and AtPMEI9 (right) on PME activity from wild-type (black bars) and atpme3 (white bars) root total protein extracts at pH 5. Experiments were carried out using 1 milliunit of PME activity of these extracts at pH 5, and the addition of AtPMEI4 and AtPMEI9 at different concentrations was performed. Experiments were performed in triplicate.
We showed in vitro that recombinant AtPMEI4 and AtPMEI9 were able to target PMEs in cell wall-enriched protein extracts from roots. One of the main PME isoforms present in root is AtPME3 (Table I). In order to demonstrate that AtPME3 activity can be modulated by recombinant AtPMEI4 and AtPMEI9 in complex extracts, gel diffusion assays were performed on cell wall-enriched protein extracts from roots of the wild type and the atpme3 mutant at pH 5 (Fig. 8B). Our results confirmed that PME activity was lower in atpme3 mutants compared with the wild type due to the absence of the AtPME3 protein (Guénin et al., 2011). Following the addition of 0.03 µg of recombinant AtPMEI4 and AtPMEI9, PME activities in the wild type and atpme3 mutants decreased due to the inhibition of PME isoforms from roots (Fig. 8A). After the addition of 0.125 µg of 6xHis-AtPMEI4, the differences in PME activity between the two genotypes disappeared, showing that recombinant AtPMEI4 was able to inhibit supernumerary isoforms in the wild-type genotype, among them AtPME3. Similar results were obtained when adding 0.09 µg of 6xHis-AtPMEI9, as its inhibiting capacity was higher than AtPMEI4 (Fig. 8B). Results obtained at pH 7.5 showed that the difference in PME activity between wild-type and atpme3 extracts was maintained upon the incubation of AtPMEI4, confirming the inability of AtPMEI4 to inhibit PME at this pH. These results are in favor of an inhibition of AtPME3 activity by either of the PMEIs in complex extracts. Therefore, we assessed these interactions biochemically.
Recombinant AtPMEI4 and AtPMEI9 Differed in the pH Dependence of the Inhibition of AtPME3 Activity
Following the expression and characterization of AtPME3 (Sénéchal et al., 2015a), we used the purified proteins to assess whether 6xHis-AtPMEI4 and 6xHis-AtPMEI9 differed in their inhibiting capacities and pH dependence toward this specific PME target. This should help confirm the accuracy of MD simulation as a predictive approach.
At pH 5, when using 0.125 μg (625 nm) of purified inhibitor, the inhibition of AtPME3 activity was ∼20% with recombinant AtPMEI4 and AtPMEI9 and ∼60% with AcPMEI (Fig. 9A). When increasing the PMEI quantity to 0.5 μg (2,500 nm), the inhibition of AtPME3 activity was 100% with 6xHis-AtPMEI9 and AcPMEI, while a residual AtPME3 activity (∼15%) was measured for 6xHis-AtPMEI4. At pH 6.3, the inhibiting capacity of 6xHis-AtPMEI4 was reduced compared with what was measured at pH 5: for 0.125 and 0.5 μg of PMEI, 6xHis-AtPMEI4-mediated inhibition of AtPME3 was 0% and 40%, respectively (compared with 20% and 85% at pH 5). For 6xHis-AtPMEI9 and AcPMEI, the inhibition of AtPME3 activity was similar to what was observed at pH 5 (Fig. 9B). When using a neutral to basic pH of 7.5, a clear difference in the inhibiting capacity of the three PMEIs was revealed. Similar to what was shown on cell wall-enriched protein extracts, up to 2 µg (10,000 nm) of 6xHis-AtPMEI4 and AcPMEI appeared inefficient in inhibiting AtPME3 activity. In contrast, when using 0.5 μg of 6xHis-AtPMEI9, AtPME3 activity was totally inhibited (Fig. 9C). Overall, this suggests a distinct pH dependence of the AtPME3/PMEI interaction depending on the PMEIs considered.
Figure 9.
Recombinant AtPMEI4 and AtPMEI9 show distinct pH-dependent inhibition capacities toward recombinant AtPME3 activity. A to C, Inhibition of AtPME3-6xHis activity by 6xHis-AtPMEI4, 6xHis-AtPMEI9, and AcPMEI using 1 milliunit of PME3 at pH 5 (A), pH 6.3 (B), and pH 7.5 (C) and various quantities of purified PMEIs: 6xHis-AtPMEI4 (black bars), 6xHis-AtPMEI9 (gray bars), and AcPMEI (white bars). Results are means ± sd of three replicates. D, Molecular interaction studies between recombinant AtPME3, 6xHis-AtPMEI4, and 6xHis-AtPMEI9 by MST. The graph at top corresponds to the interaction of recombinant PME3 measured at pH 5 with 6xHis-AtPMEI4 (black bars) and 6xHis-AtPMEI9 (gray bars). Concentrations on the x axis are plotted in nm. The table at bottom corresponds to the Kd values determined for the same experiment performed at pH 5, 6.3, and 7.5. Kd values are calculated in nm. nd, Not detected.
To investigate whether recombinant AtPME3/AtPMEI4 and AtPME3/AtPMEI9 interact physically in vitro, the binding affinities of purified AtPME3 with different concentrations of recombinant AtPMEI4 and AtPMEI9 were determined by MST analyses at three different pH values (5, 6.3, and 7.5). This sensitive method assesses the protein-protein interaction in vitro by measuring the motion of molecules along microscopic temperature gradients and detects changes in their hydration shell, charge, or size (Wienken et al., 2010; Jerabek-Willemsen et al., 2014). Recombinant AtPME3 was labeled with the blue fluorescent dye. A constant concentration of AtPME3 was added in capillaries with serial dilutions of recombinant unlabeled 6xHis-AtPMEI4 or 6xHis-AtPMEI9. The resulting fluorescence of AtPME3, which is dependent on the interaction of the protein with inhibitors, was measured in each capillary and the dissociation constant (Kd) was calculated. MST measurements for AtPME3/AtPMEI4 and AtPME3/AtPMEI9 interactions revealed distinct interactions between AtPME3 and the two PMEIs (Fig. 9D; Supplemental Fig. S7). MST curves for recombinant AtPME3/AtPMEI4 and AtPME3/AtPMEI9 interactions at pH 5 (Fig. 9D) showed that each pair of proteins can interact and Kd values could be determined for each interaction. The higher Kd calculated for AtPME3/AtPMEI4 (3,780 versus 611 nm for AtPME3/AtPMEI9) corresponded to a weaker interaction, which could explain the lower inhibition of AtPME3 activity measured when using AtPMEI4 (Fig. 9A). At pH 6.3, MST curves and Kd values confirmed that AtPME3/AtPMEI4 and AtPME3/AtPMEI9 interactions occurred (Supplemental Fig. S7A), which fitted with the inhibition of AtPME3 activity. As for pH 5, AtPME3 was more tightly associated to AtPMEI9 than to AtPMEI4 (Fig. 9D). Finally, at pH 7.5, only an interaction between recombinant AtPME3 and AtPMEI9 (Supplemental Fig. S7B) was measured, with an associated Kd value at 4,930 nm. These results showed that (1) AtPME3 and AtPMEI4 do not interact at neutral pH, which is consistent with the lack of inhibition of AtPME3 activity measured by gel diffusion assay (Fig. 9C), and (2) the association between the two partners in the AtPME3/AtPMEI9 complex is weaker at pH 7.5 compared with acidic pH. Overall, this showed that, in contrast to 6xHis-PMEI4, 6xHis-PMEI9 interacts with AtPME3 whatever the pH. This leads to an inhibition of AtPME3 activity at any of the pH values considered, even if the force of interaction changes according to the pH. Overall, biochemical analyses show that AtPMEI4 and AtPMEI9 are not equal in their pH dependence, which might play a role in tuning plant development.
Recombinant AtPMEI4 and AtPMEI9 Have Distinct Effects on Pollen Tube Elongation
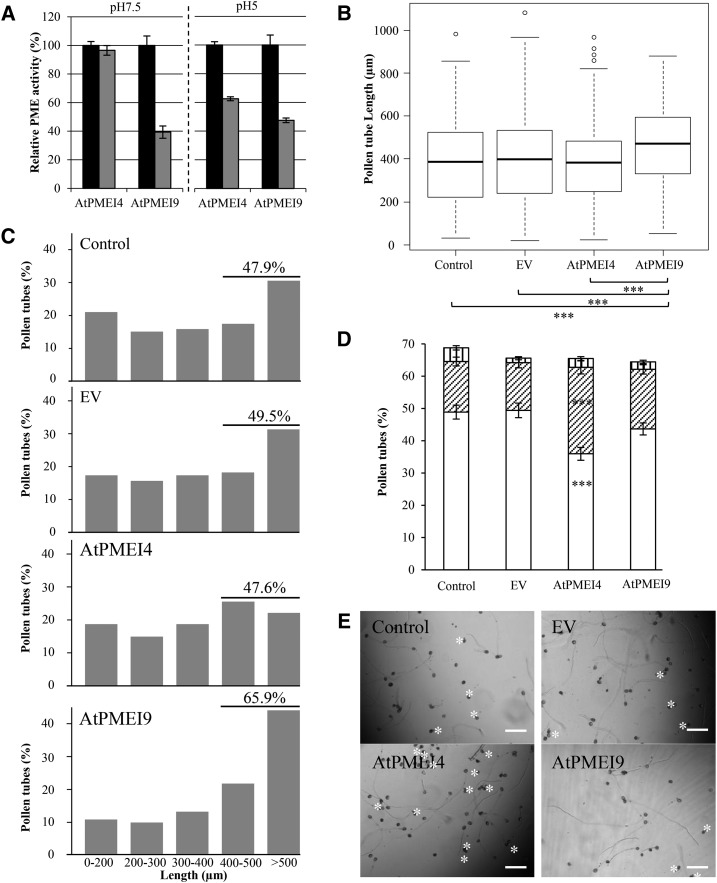
Pollen tube elongation involves apical growth and drastic changes in cell wall chemistry and structure, particularly of pectins. This is notably sustained by the fact that pollen grain germination and pollen tube growth were reported previously to be impaired when PME expression was altered or following the application of purified PMEIs. Thus, pollen appears as a suitable model to assess the developmental consequences of the application of PMEI differing in their biochemistry. We first tested whether recombinant AtPMEI4 and AtPMEI9, which, to our knowledge, are not expressed in pollen, could inhibit pollen-specific PME activity. For this purpose, we chose to perform gel diffusion assays at pH 5 and 7.5, for which the inhibition ability of AtPMEI4 and AtPMEI9 differs (Fig. 10A). As measured for cell wall-enriched protein extracts from roots, PME activity from pollen tubes cultured for 6 h was inhibited at both pH values with recombinant AtPMEI9. In contrast, PME activity was inhibited only at pH 5 with AtPMEI4. In consequence, we assessed whether exogenous application of PMEIs could have distinct consequences on pollen tube elongation at restrictive pH. Arabidopsis pollen being described to germinate at rather neutral pH (Boavida and McCormick, 2007), this is a suitable system in which to highlight potential different consequences upon the application of AtPMEI4 and AtPMEI9. After the application of recombinant AtPMEI4 or AtPMEI9 (tested at 0.5 µm, corresponding to 1.25 µg of recombinant inhibitors in 125 µL), germination of pollen grains and elongation of pollen tubes were measured and compared with three different controls: (1) liquid germination medium; (2) in the presence of 1.25 µg of purified proteins extracted from induced cultures of E. coli containing the empty vector; or (3) in the presence of boiled AtPMEI4 and AtPMEI9 (1.25 µg; Supplemental Fig. S9). Pollen grain germination of the control reached 30% and 70% after 2 and 6 h of culture, respectively, and no difference in germination rate was observed upon the application of AtPMEI (data not shown). However, the data showed a significant increase in pollen tube length after 2 and 6 h of growth in the presence of recombinant AtPMEI9 compared with controls (Fig. 10B; Supplemental Fig. S8A). No difference in the overall pollen tube length distribution was observed when comparing the application of exogenous AtPMEI4 to the two controls after 2 and 6 h of culture (Fig. 10C; Supplemental Fig. S8, B–E). In contrast, pollen tube length distribution was affected in AtPMEI9-treated pollen grains by shifting the tube length toward longer categories as early as 2 h after starting the experiment (Supplemental Fig. S8). A total of 65.9% of the tubes reached at least 400 µm in the presence of 6xHis-AtPMEI9 after 6 h of growth, while only ∼48% of pollen tubes reached 400 µm in the control condition or with 6xHis-PMEI4 or empty vector (Fig. 10C). Finally, germinated pollen tubes were classified after 6 h of culture in three categories based on their shapes (normal, burst, and misshaped). Observations revealed a statistically significant increase in tubes bursting when treated with the recombinant AtPMEI4 than in the other treatments, including recombinant AtPMEI9 (Fig. 10, D and E). Taken together, the data revealed distinct effects of the application of the two PMEIs at a pH for which they show distinct PME activity-inhibiting capacities.
Figure 10.
Effects of AtPMEI4 and AtPMEI9 on Arabidopsis pollen germination, 6-h-old pollen tube length, and PME activity from 6-h-old pollen tube protein extracts. A, PME activity from total 6-h-old pollen tube protein extract was measured at pH 7.5 and 5 after adding 1.25 µg of AtPMEI4 or AtPMEI9 (gray bars); 1.25 µg of boiled AtPMEI (black bars) was added for controls. This experiment was performed in three biological replicates. B, Pollen tube length dispersions after 6 h of growth. Statistical comparisons between samples were performed using the unilateral pairwise Wilcoxon rank-sum test. ***, P < 0.001. C, Pollen tube length distribution after 6 h of growth. D, Percentage of normal (white bars), burst (diagonally striped bars), and misshaped (vertically striped bars) pollen tubes. E, Representative images of pollen tubes. Stars show burst tubes. Data are means ± sd of three independent replicates. EV, Empty vector. Bars = 120 µm.
DISCUSSION
In recent years, the fine-tuning of the DM of HGs has appeared as a key element in the control of plant growth and development. A number of studies have indeed shown that changes in the expression of PME isoforms have dramatic consequences on cell elongation and, ultimately, in the shaping of the plant body (for review, see Sénéchal et al., 2014b; Levesque-Tremblay et al., 2015b). One key feature of the control of PME activity is its regulation via a 1:1 interaction of the protein with proteinaceous inhibitors, PMEIs (Di Matteo et al., 2005). As an alternative mean of assessing the role of the DM of HG in controlling plant development, one can analyze the consequences of changes in the level of expression of PMEIs. This has proven to be particularly informative, as the constitutive overexpression of distinct PMEI isoforms has distinct consequences on plant development. For instance, the overexpression of the pollen-expressed AtPMEI5 induced a strong root-waving phenotype, while the overexpression of the root-expressed AtPMEI4 did not (Pelletier et al., 2010; Wolf et al., 2012). Surprisingly, the constitutive overexpression of pollen-expressed AtPMEI1 and AtPMEI2 did not have similar effects to that of AtPMEI5 in roots (Lionetti et al., 2007). Thus, it could be hypothesized that the overexpression of distinct PMEI genes triggers specific changes in signaling pathways. Alternatively, as a more direct effect, different PMEI isoforms could interact with specific PMEs with consequent effects on the tuning of HG and development. We believe that our study brings new insights to understanding the diversity of the biochemical specificities of PMEI isoforms and the determinants of PME-PMEI interaction. In view of the size of the PME and PMEI gene families in plants, in particular in Arabidopsis (Wang et al., 2013), it is tempting to consider that, depending on the organs and plant developmental stages considered, specific PME-PMEI pairs could occur. Such a large number of genes prevents scientists from expressing and testing every single PME-PMEI pair. Therefore, computational approaches based on molecular modeling and MD simulations appear necessary to push forward our understanding of the regulation of PME activity and, hence, the fine control of the DM of HG in vivo. In particular, MD simulations are powerful tools with which to rationalize and predict experimental findings; hence, their utilization in investigating carbohydrate-binding enzymes is increasing steadily (Massa et al., 2010; Mercadante et al., 2013, 2014; Bernardi et al., 2014). Although in molecular mechanics, the shift of protonation is not yet easily possible during the simulations, comparing simulations in which residues are differently protonated accordingly to their pKa can be effective to study the effects of pH on macromolecular interactions, especially considering the full-atom resolution at which MD normally operates. In the case presented here, MD simulations predicted the structural basis of the differences between two PMEIs in their ability to bind AtPME3 in distinct pH microenvironments. MD predictions were confirmed by the collected experimental observations.
When using AtPME3 as a target PME, two main conclusions could be inferred from the simulations. First, the strength of the interaction between the two partners of the complex was predicted to be higher when using AtPMEI9 compared with AtPMEI4, as reflected by the higher number of contacts (Table II). The number of interfacial contacts in protein-protein complex formation and stability was recently linked directly to the stability of the complexes (Vangone and Bonvin, 2015). The biochemical approach, consisting of complementary PME inhibition assays and Kd calculations, supported the MD simulations: AtPMEI9 was a stronger inhibitor of AtPME3 activity than AtPMEI4 (Fig. 9, A–C). This could be related to a greater interaction between the two proteins as at pH 5 and 6.3; the Kd values describing the interaction of AtPME3 with AtPMEI9 are 5- to 10-fold lower than those of the AtPME3/AtPMEI4 complex (Fig. 9D; Supplemental Fig. S7). As a more general feature, AtPMEI9 is a stronger inhibitor of PME activity from roots and dark-grown hypocotyls, showing that it is likely to target a wide range of PMEs in planta (Figs. 7B and 8A; Supplemental Fig. S6B). Second, the pH dependence of the PME-PMEI interaction was assessed, and MD simulations predicted different behavior of the two proteins tested. The AtPME3/AtPMEI4 interaction was predicted to be more pH dependent than the AtPME3/AtPMEI9 interaction. The lack of differences in the conformational dynamics of AtPMEI9 in complex with AtPME3 at the two different pH values (pH 5 and 7) was corroborated by the experimental observation that, in the investigated pH range (from slightly acidic to around neutrality), the binding affinity for AtPME3 was still measurable (Fig. 9D; Supplemental Fig. S7). In contrast, no Kd value could be determined at pH 7.5 for the AtPME3/AtPMEI4 interaction (Fig. 9D; Supplemental Fig. S7B). Along the same line, measured inhibition of PME activity confirmed the pH-independent mode of action of AtPMEI9 versus the pH-dependent one of AtPMEI4 (Figs. 7B and 8A; Supplemental Fig. S7); thus, the inhibition of PME activity by AtPMEI9 can be considered as pH independent (total inhibition of PME activity is reached) compared with that observed for AtPMEI4 of kiwi PMEI.
Although highly variable depending on species and organs considered, typical apoplastic pH values were reported to be in a range of 4.5 to 7 (Yu et al., 2000). It could thus be assumed that the differences in the pH-mediated inhibition of PME activity determined for AtPMEI4 (pH dependent) and AtPMEI9 (pH independent) in our conditions could be of importance in vivo. Our findings suggest that, overall, the strength and pH dependence of the PME inhibition would be governed mainly by structural determinants from the inhibitor rather than the target enzymes. The inhibiting capacity of the recombinant AtPMEI4 appeared very similar to that reported previously for AtPMEI7 (Sénéchal et al., 2015a). In contrast, 6xHis-AtPMEI9 showed distinct behavior: higher inhibiting capacity and less pH dependence. Several PMEIs were reported previously to differ in their pH dependence, and several potential mechanisms were proposed (Bonavita et al., 2016), but our study brings new insights on how this could be mediated dynamically at an atomistic scale and on the complexity of the interaction between PMEs and PMEIs. Until now, the characterization of specific PME-PMEI pairs from the same species has remained scarce. Early studies, using purified pollen-expressed AtPMEI1 and AtPMEI2 from Arabidopsis and tomato PME, have shown that the inhibition of PME activity was rather pH independent within a 5.5 to 8.5 range (Raiola et al., 2004). AtPME3 was shown subsequently to be a putative interacting protein of AtPMEI1 and AtPMEI2 (Lionetti et al., 2007), but the existence of such an interaction in vivo is unlikely, considering the localization of expression of AtPME3 (Guénin et al., 2011). Depending on the PMEI isoforms and PME targets considered, the pH dependence of the PMEI-mediated inhibition of PME activity appears variable. Using plants impaired for AtPME3 expression (Guénin et al., 2011), we provide evidence that, in complex extracts, AtPMEI4 and AtPMEI9 can indeed target AtPME3 in a pH-dependent manner. Although the demonstration of the pH dependence of the interaction in vivo will be required, we believe that it could be an additional way of regulating PME activity in specific cell wall microenvironmental conditions (i.e. pH and ion concentrations; Hocq et al., 2017).
To assess whether the differences in the biochemical characteristics of AtPMEI4 and AtPMEI9 can have significance in development, we first applied purified recombinant AtPMEI4 and AtPMEI9 on pollen grains and followed the pollen tube elongation at pH 7.5. The pollen tube is a powerful model in which to relate cell elongation to cell wall characteristics, as reported previously (Lehner et al., 2010; Mollet et al., 2013). Although apparently not expressed in pollen, both AtPMEI4 and AtPMEI9 did show an inhibitory effect on the PME activity of pollen tubes (Fig. 10A). The pH dependence of this inhibition was similar to what was observed also in roots and hypocotyls. A number of PMEs were identified in the pollen proteome, which could be targeted by AtPMEI4 and AtPMEI9 in a similar way to that observed for AtPME3 (Holmes-Davis et al., 2005).
Based on our results, AtPMEI9 had an impact on pollen tube elongation when applied at pH 7.5 after only 2 h, which may be related to the inhibitory activity of AtPMEI9 at this pH. Therefore, in those conditions, exogenous application of active AtPMEI9 would favor pollen tube elongation. In contrast, an increase in pollen burst was observed upon exogenous application of AtPMEI4, which was not observed for AtPMEI9. This could be surprising, given the fact that AtPMEI4 did not show any PME-inhibiting effect at pH 7.5. However, the data are in agreement with a previous report using exogenous application of kiwi PMEI in the same conditions (Paynel et al., 2014). Other results, using distinct pH conditions and PMEIs, also showed that exogenous application induced a burst of maize (Zea mays) pollen tubes (Woriedh et al., 2013). The results obtained for AtPMEI9 compared with AtPMEI4 confirm that the two PMEIs differ, not only in their abilities to inhibit PME activity at distinct pH values in vitro but also in the modulation of pectin structure and ultimately of cell wall mechanics allowing elongation. Transgenic lines impaired for the expression of AtPMEI4 and AtPMEI9 showed that root growth was altered in opposite ways (Fig. 2A). In particular, the overexpression of AtPMEI9 leads to increased root growth, which correlated with the increased pollen tube length upon application of this protein. Root growth was shown previously to be impaired in AtPMEI5-overexpressing lines (Wolf et al., 2012), which suggests that the biochemical characteristics of the PMEIs could trigger distinct phenotypes.
Based on our findings regarding the biochemical characterization of AtPMEI4 and AtPMEI9, it appears that PMEIs from Arabidopsis differ in their ability to regulate PME activity. AtPMEI4 shows that the dynamics of such an inhibitor is highly pH dependent for the presence of key protonatable amino acids interacting with AtPME3. These residues may include a Glu (Glu-132), which is part of the loop connecting helices αI and αII of AtPMEI4, and a His residue (His-108) of AtPME3. The presence of acidic residues to regulate a pH-dependent interaction represents a parallelism with a previously reported case in which the interaction between an invertase from tobacco (Nicotiana tabacum) and its inhibitor can be finely tuned as a function of the pH through a short motif that embeds acidic residues at the binding interface. The same motif also is inserted into a loop connecting two helices of the invertase inhibitor, which is structurally highly similar to PMEIs (Hothorn et al., 2010). The analysis of AtPMEI4 and AtPMEI9 sequences also revealed that most of the Glu residues at the PME-PMEI interface are not conserved in AtPMEI9, which could make it less pH dependent. Overall, the computational structural biology approach employed in this study was proven to be also helpful in identifying some of the many protonatable residues that may have a crucial role in determining the pH-dependent activity of PMEIs and ultimately in regulating plant cell wall remodeling at different stages of the plant life.
With such a high number of different PME and PMEI isoforms, the identification of research approaches with a high predictive potential, such as molecular modeling and MD, is of key importance to provide knowledge regarding the general strategies employed by plants to finely regulate their cell wall metabolism. In addition to the ability to rationalize experimental evidence, such approaches also are able to help formulate new hypotheses in which residues mutate in order to modify the PMEI mode of action, with immediate advantages in understanding their function in vivo.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type Col-0 plants or the atpme3 mutant line were grown in a phytotronic chamber on plates (16-h photoperiod at 120 µmol m−2 s−1 and 22°C) or on soil (16-h photoperiod at 100 µmol m−2 s−1 and 23°C/19°C day/night) as described (Pelletier et al., 2010; Sénéchal et al., 2014a) with transfer to light referred as time 0 for all experiments. Various organs (10-d-old roots, 4-d-old dark-grown hypocotyls, and 4-week-old leaves, stems, floral buds, siliques, and mature seeds) were harvested and immediately frozen in liquid nitrogen. They were subsequently ground to a fine powder in a ball mill and kept frozen (−80°C) until processing.
RNA Extraction and Gene Expression Analysis by RT-qPCR
RNA was extracted from 100 mg of powder from the various organs as described previously (Verwoerd et al., 1989). DNA was removed using the Turbo DNA-free kit (Ambion; catalog no. AM1907) according to the manufacturer’s protocol. cDNA synthesis was performed using 4 µg of DNA-free RNA, 50 µm oligo(dT)20, and the SuperScript III First-Strand Synthesis SuperMix (Invitrogen; catalog no. 18080-400) following the manufacturer’s guidelines. RT-qPCR was performed on 1:20 diluted cDNA using the SYBR Green I Master Mix (Roche; catalog no. 04887352001) and the LightCycler 480 Real-Time PCR System (Roche). Oligonucleotide primers used to amplify AtPMEI4 and AtPMEI9 transcripts were PMEI4-F (5′-AAACGGCATGCAACTCAACAAC-3′)/PMEI4-R (5′-CGGACTTGATGGTGGAGGAATAGG-3′) and PMEI9-F (5′-CGTCGCCAAACTAACCAAAGAGAC-3′)/PMEI9-R (5′-AGCTAACCGGTCCACGCTATTG-3′). The reference genes CLATHRINE (CLA; CLA-F, 5′-GTTTGGGAGAAGAGCGGTTA-3′, and CLA-R, 5′-CTGATGTCACTGAACCTGAACTG-3′), PEROXIN4 (PEX4; PEX4-F, 5′-CTTGGACGCTTCAGTCTGTG-3′, and PEX4-R, 5′-TGAACCCTCTCACATCACCA-3′), ELONGATION FACTOR1a (EF1a; EF1a-F, 5′-TGGTGACGCTGGTATGGTTA-3′, and EF1a-R, 5′-TCCTTCTTGTCCACGCTCTT-3′), and ADENINE PHOSPHORIBOSYL TRANSFERASE1 (APT1; APT1-F, 5′-GAGACATTTTGCGTGGGATT-3′, and APT1-R, 5′-CGGGGATTTTAAGTGGAACA-3′) were used as internal controls to calculate the relative expression of genes of interest (Gutierrez et al., 2009).
Proteomic Analyses by Nano-LC-ESI-MS/MS
Cell wall-enriched proteins from 10-d-old roots or 4-d-old hypocotyls were extracted from 50 mg of frozen material using 50 mm sodium acetate, 1 m LiCl buffer at pH 5 (Baldwin et al., 2014) or 50 mm Na2HPO4, 20 mm citric acid, 1 m NaCl, 0.01% Tween 20 buffer at pH 7 (Pilling et al., 2000), respectively, for 1 h at 4°C under shaking. The extracts were clarified by centrifugation at 20,000g for 30 min at 4°C, and the supernatants were filtered using an Amicon ultracentrifugal filter 0.5 mL/10 kD (Millipore; catalog no. UFC5010BK) in order to remove salts. Protein concentration was determined by the Bradford method (Bradford, 1976) using a protein assay kit (Bio-Rad; catalog no. 500-0006). Equal amounts of proteins for roots and hypocotyls or maximum quantities of purified recombinant PMEIs were resolved by SDS-PAGE using Mini-protean TGX gels (Bio-Rad; gradient, 4%–20%; catalog no. 456-1094) at a constant voltage of 200 V for 45 min. Proteins were stained with Coomassie Blue G-250 (Bio-Rad; catalog no. 161-0787) and destained with distilled water. Following the excision of bands and trypsin hydrolysis, all digested peptide mixtures were separated online using nano-liquid chromatography and analyzed by nano-electrospray tandem mass spectrometry according to the method described previously (Sénéchal et al., 2014a). The experiments were performed on an Ultimate 3000 RSLC system coupled with an LTQ-Orbitrap XL mass spectrometer (ThermoFisher Scientific).
Molecular Modeling and MD Simulations of AtPME3-AtPMEI4/AtPMEI9 Complexes
The structures of AtPME3, AtPMEI4, and AtPMEI9 were modeled using the software MODELER version 9.12 (Sali and Blundell, 1993) and adopting as templates a PME from tomato (Solanum lycopersicum; SlPME) and a PMEI from kiwi fruit (Actinidia chinensis; AcPMEI), which were cocrystallized previously in a specific 1:1 stoichiometric complex (Di Matteo et al., 2005). Therefore, the coordinates of the partners in complex were used to obtain complexes of AtPME3 and AtPMEI4/AtPMEI9 as observed for the complex of tomato PME and kiwi fruit PMEI. MD simulations were performed on the modeled AtPME3/AtPMEI4 and AtPME3/AtPMEI9 complexes at both pH 5 and 7 using the GROMACS package version 4.6.7 (Van Der Spoel et al., 2005). Hydrogen atoms were added considering the pKa values empirically calculated for each protonatable residue using the program PROPKA version 3.0 (Olsson et al., 2011). The protonation states calculated for each residue of AtPME3 and AtPMEI4 or AtPMEI9 involved in the complex are reported in Table III. Molecular topologies were then built adopting the parameters provided in the amber99sb*-ILDN force field (Lindorff-Larsen et al., 2010). The complexes were placed in a cubic box and solvated using TIP3P water molecules (Jorgensen et al., 1983). In order to neutralize the net charge of the system, Na+ or Cl− ions were added, replacing water molecules in excess. Additionally, for each system, Na+ and Cl− ions also were added to reach an ionic strength of 100 mm. The complexes were then minimized using a steep descent algorithm. The minimized complexes were equilibrated in two steps. An initial equilibration of 500 ps was carried out in the NVT ensemble, in which the temperature was coupled to a value of 300 K every 0.5 ps using a V-rescale thermostat (Bussi et al., 2007). In this step, random velocities following a Maxwell distribution calculated at 300 K were assigned to each particle. Different sets of initial velocities differentiated the replicates that were run in this study. Equilibration was continued for an additional 500 ps in the NPT ensemble, in which temperature and pressure were kept constant at 300 K and 1 atm every 0.5 ps, using a V-rescale thermostat and Parrinello-Rahman barostat (Parrinello and Rahman, 1981), respectively. During the NVT and NPT equilibration steps, water was allowed to relax around the solute, and the coordinates of the protein atoms were isotropically restrained using a potential of 1,000 kJ mol−1 nm2. Production runs were then performed for six replicates of 100 ns each (total simulated time = 600 ns) employing a time step of 2 fs. Bonds between atoms were constrained using a LINCS algorithm (Hess et al., 1997; Hess, 2008). The interactions between the particles composing the system were calculated by computing a neighbor list between atoms at every picosecond along the simulated trajectory. The cutoff used to compute van des Waals and short-term Coulomb interactions was set at 1 nm. Long-range electrostatic calculations were computed using the particle mesh Ewald summation method (Darden et al., 1993). Temperature and pressure were coupled every 2 ps at the values of 300 K and 1 atm as described previously for the NPT equilibration step. Trajectories were collected every 1 ps, and the analysis was carried out only on the last 50 ns, using both in-house and GROMACS tools. The analysis of the contacts between the partners included the identification of hydrogen bonds, salt bridges, and any other nonspecific contact between atoms. Distance criteria for the identification of hydrogen bonds, salt bridges, and other nonspecific contacts were 0.35, 0.4, and 0.5 nm, respectively. In order to consistently describe the structure of the modeled proteins and the interaction between PMEIs and PME3, we adopt the same nomenclature used by Di Matteo et al. (2005), in which the three short helices appearing at the N-terminal end of the inhibitor are defined as αa, αb, and αc, whereas the helices composing the four-helix bundle partially interacting with PME3 are defined as αI to αIV (Supplemental Fig. S3).
Table III. Calculated pKa values for the residues that switch protonation state between pH 5 and 7 for AtPME3, AtPMEI4, and AtPMEI9.
| Residue | AtPME3 | AtPMEI4 | AtPMEI9 |
|---|---|---|---|
| Asp-118 | 6.59 | – | – |
| Asp-153 | 5.00 | – | – |
| Asp-188 | 5.49 | – | – |
| Glu-44 | 5.28 | – | – |
| Glu-225 | 5.08 | – | – |
| Glu-243 | 5.55 | – | – |
| His-108 | 6.09 | – | – |
| His-169 | 5.43 | – | – |
| Asp-84 | – | 5.72 | – |
| Asp-129 | – | 5.01 | – |
| Glu-132 | – | 5.13 | – |
| His-3 | – | 5.94 | – |
| His-70 | – | 6.17 | – |
| His-164 | – | 5.42 | – |
| Asp-35 | – | – | 5.40 |
| Asp-131 | – | – | 5.10 |
| Glu-126 | – | – | 6.71 |
| Glu-135 | – | – | 5.10 |
| His-21 | – | – | 6.35 |
| His-32 | – | – | 6.47 |
| His-101 | – | – | 6.44 |
| His-152 | – | – | 6.20 |
Cloning of AtPMEI4 and AtPMEI9 Coding Sequences into Expression Plasmids
The sequence encoding from Thr-26 to the end of AtPMEI4 was amplified from RIKEN clone pda18258 using 5′-GAGGGATCCACCCCACCAGGCACGTAC-3′ and 5′-GAGAAGCTTCAAACGTACCCATGCACATGC-3′ oligonucleotides and Taq polymerase.
The sequence encoding from Thr-24 to the end of AtPMEI9 was amplified from cDNAs synthesized from Arabidopsis Col-0 dark-grown hypocotyl RNA using 5′-GAGGGATCCACCCCAAACCGGTCGGAATC-3′ and 5′-GAGAAGCTTAAGGAAACTTCACACTTCGCTTC-3′ oligonucleotides. A first amplification was done using Taq polymerase. After migration on an agarose gel, the eluted product was amplified again using Phusion Hot Start II DNA polymerase (ThermoFisher). Both PCR fragments were digested by BamHI and HindIII and inserted into the same sites of pQE30 (Qiagen) downstream of a 6xHis tag to give fusion proteins named 6xHis-AtPMEI4 and 6xHis-AtPMEI9, respectively. The nucleotidic sequence of the PCR fragment was verified by nucleotide sequencing (MWG-Eurofins). A silent mutation was found in the Thr-24 codon (ACC→ACA) of 6xHis-AtPMEI9. Escherichia coli strain Rosetta-gami (Novagen) was transformed subsequently with each construct.
Purification of Recombinant AtPMEI4 and AtPMEI9, AcPMEI, AtPME3, and BcPME1
Purification of recombinant 6xHis-AtPMEI4 and 6xHis-AtPMEI9 was performed according to methods described previously (Sénéchal et al., 2015a) using Ni-NTA agarose resin (Qiagen; catalog no. 30210). After incubation of soluble protein extracts with affinity resin and washes, the bound 6xHis-AtPMEI4 or 6xHis-AtPMEI9 was eluted with 50 mm Na2HPO4, 300 mm NaCl, and 250 mm imidazole buffer at pH 8. Regarding AcPMEI (from kiwi fruit), extraction from mature fruit and purification were performed according to Balestrieri et al. (1990) and Dedeurwaerder et al. (2009). Purification of AtPME3-6xHis (from Arabidopsis, expressed in tobacco [Nicotiana tabacum]) and recombinant BcPME1 (PME from Botrytis cinerea strain B05.10, produced in Pichia pastoris strain GS115) was performed according to methods described previously (Kars et al., 2005; L’Enfant et al., 2015; Sénéchal et al., 2015a). Protein concentration was determined as described previously. Purified proteins were analyzed by SDS-PAGE using a 12% acrylamide/bis-acrylamide gel and stained with Coomassie Brilliant Blue G-250 (Bio-Rad; catalog no.161-0406). In the text, purified AtPMEI4 and AtPMEI9 are either referred to as recombinant AtPMEI4 and recombinant AtPMEI9 or 6xHis-AtPMEI4 and 6xHis-AtPMEI9.
Inhibition Test of PME Activity with Recombinant AtPMEI4/AtPMEI9 and AcPMEI by Gel Diffusion Assay
Inhibition of PME activity by PMEIs was quantified by gel diffusion assay (Downie et al., 1998) with the following modifications (Ren and Kermode, 2000). Protein extractions from Arabidopsis wild-type (Col-0) organs (roots and etiolated hypocotyls) and 6-h-old pollen tubes from 400 flowers, protein concentration determination, and PME activity quantification (mU or nmol min−1) were performed as described by Sénéchal et al. (2015a). For each experiment, 1 mU of PME3-6xHis, orange (Citrus spp.) PME (Sigma; catalog no. P5400), tomato PME (Sigma; catalog no. P6763), and total PME activity from Arabidopsis cell wall-enriched protein extracts were measured using a colorimetric assay described by Baldwin et al. (2014) in a volume of 5 µL and preincubated for 30 min at room temperature with 5 µL containing various amounts of 6xHis-AtPMEI4, 6xHis-AtPMEI9, or AcPMEI. For BcPME1, 0.5 mU activity was used. Then, the reaction mixtures were loaded onto each well on the gels containing pectins at DM > 85% (Sigma; catalog no. P9561) and prepared at various pH values (5, 6.3, and 7.5). After incubation at 37°C for 16 h, gels were stained with a 0.02% (w/v) Ruthenium Red solution for 1 h and washed with distilled water. Diameters of the red halos around each well were measured using the ImageJ software (http://rsbweb.nih.gov/ij/), and PME activity based on red-stain diameter was quantified using a standard curve, derived from commercial orange PME, as described previously (Bourgault and Bewley, 2002).
MST Binding Assay
The molecular interaction between purified AtPME3-6xHis and 6xHis-AtPMEI4 or 6xHis-AtPMEI9 was measured using MST as described by Wienken et al. (2010) and Jerabek-Willemsen et al. (2014). In all experiments, AtPME3-6xHis was labeled with the dye NT-495 NHS from Monolith NT.115 Protein Labeling (NanoTemper; catalog no. L003) and resuspended in a 50 mm sodium phosphate buffer, pH 7.5. 6xHis-AtPMEI4 or 6xHis-AtPMEI9 was transferred in the appropriate buffer: 50 mm sodium acetate buffer, pH 5, and 50 mm sodium phosphate buffer (50 mm sodium phosphate, 300 mm NaCl), pH 6.3 and pH 7.5, for binding experiments at pH 5, 6.3, and 7.5 respectively. When testing the binding with AtPMEI9 at pH 7.5, the buffer was supplemented with 250 mm imidazole. Buffer exchange was performed using a PD SpinTrap G-25 column (GE Healthcare; catalog no. 28-9180-04) following the manufacturer’s protocol. For different assays, a constant concentration of labeled AtPME3-6xHis (83 or 166 nm depending on experiments) was titrated with increasing concentrations of nonlabeled recombinant AtPMEI4 and AtPMEI9 at the different pH values. Thermophoresis analyses and Kd determination were performed according to the method described previously by Sénéchal et al. (2015a).
Pollen Tube Growth
In vitro pollen tube growth was assessed as described by Leroux et al. (2015). Pollen grains were grown in a liquid germination medium (GM) according to the method described by Boavida and McCormick (2007). To harvest pollen grains, flowers (40 per 1.5-mL tube) were submerged in 1 mL of GM (pH 7.5) containing 5 mm CaCl2∣2H2O, 0.01% (w/v) H3BO3, 5 mm KCl, 1 mm MgSO4∣7H2O, and 10% (w/v) Suc. Tubes were shaken with a vortex to release the pollen grains in the GM. Flowers were removed using tweezers, and the pollen suspension was then centrifuged at 4,000g for 7 min. A total of 125 μL of fresh GM was added to the pellet supplemented with 0.5 µm (corresponding to 1.25 µg) of recombinant AtPMEI4 or AtPMEI9, an equivalent quantity of purified proteins from the empty expression vector, and an equivalent volume of protein buffer or boiled AtPMEI4 or AtPMEI9 (controls). Pollen grains were transferred onto 96-well plates and grown in a growth chamber in the dark at 22°C. Twenty images per sample were acquired after 2 and 6 h of culture, and ImageJ software (Abramoff et al., 2004) was used to determine the percentage of germination, to measure pollen tube length, and to classify the pollen tubes regarding their phenotypes (normal, burst, or misshaped). Measurements of the pollen tube length were repeated for two independent biological replicates with three technical replicates. The data were analyzed statistically by Student’s t test (GraphPad Software; www.graphpad.com). Differences were considered statistically significant at P ≤ 0.05 (*), highly significant at P < 0.01 (**), and extremely significant at P < 0.001 (***).
Accession Numbers
Nucleotide sequence data from this article can be found in The Arabidopsis Information Resource database under the following accession numbers: AtPME3, AT3G14310; AtPMEI4, AT4G25250; AtPMEI9, AT1G62770; CLA, AT5G46630; PEX4, AT5G25760; EF1a, AT5G60390; and APT1, AT1G27450. Protein data from this article can be found in UniprotKB database under the following accession numbers: AtPME3, O49006; AtPMEI4, Q9SB38; AtPMEI9, Q9SI72; BcPME1, Q9C2Y1; tomato PME, P14280; and AcPMEI, P83326.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. AtPMEI4, AtPMEI9, and AtPME3 proteins are found in Arabidopsis roots.
Supplemental Figure S2. Expression of AtPMEI9 in 6-d-old roots of atpmei9-1 and atmpei9-2 alleles.
Supplemental Figure S3. Structural alignment of the template and modeled PMEIs and PME-PMEI complexes.
Supplemental Figure S4. Dynamics of AtPME3-AtPMEI4/AtPMEI9 complexes at different pH values.
Supplemental Figure S5. Identification by nano-LC-ESI-MS/MS of purified 6xHis-AtPMEI4 and 6xHis-AtPMEI9.
Supplemental Figure S6. PME activity on Arabidopsis hypocotyls and roots.
Supplemental Figure S7. Molecular interaction studies between recombinant AtPME3 and 6xHis-AtPMEI4 and 6xHis-AtPMEI9 by MST.
Supplemental Figure S8. Pollen tube growth is enhanced upon 2 h of AtPMEI9 exogenous application.
Supplemental Figure S9. Pollen tube growth after exogenous application of boiled AtPMEI4 and AtPMEI9.
Acknowledgments
We thank Bill Williams (Massey University) for initial discussions concerning MD simulations and Herman Höfte (Institut Jean Pierre Bourgin, Institut National de la Recherche Agronomique) for the kind gift of the pmei4-1 mutant and the 35S:PMEI4::GFP Arabidopsis transgenic line.
Glossary
- HG
homogalacturonan
- DM
degree of methylesterification
- MD
molecular dynamics
- RT-qPCR
reverse transcription-quantitative PCR
- Col-0
Columbia-0
- MSD
mean square displacement
- RMSF
root mean square fluctuation
- MST
microscale thermophoresis
- GM
germination medium
Footnotes
This work was supported by the Trans Channel Wallnet project, which was selected by the INTERREG IVA program France (Channel)-England European cross-border cooperation program (to L.H.), by the Agence Nationale de la Recherche (grant nos. ANR-09-BLANC-0007-01 GROWPEC and ANR-12-BSV5-0001 GALAPAGOS), by the Conseil Régional de Picardie (to F.S.), and by the Institut Universitaire de France (to J.P.).
References
- Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11: 36–43 [Google Scholar]
- An SH, Sohn KH, Choi HW, Hwang IS, Lee SC, Hwang BK (2008) Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228: 61–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin L, Domon JM, Klimek JF, Fournet F, Sellier H, Gillet F, Pelloux J, Lejeune-Hénaut I, Carpita NC, Rayon C (2014) Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 104: 37–47 [DOI] [PubMed] [Google Scholar]
- Balestrieri C, Castaldo D, Giovane A, Quagliuolo L, Servillo L (1990) A glycoprotein inhibitor of pectin methylesterase in kiwi fruit (Actinidia chinensis). Eur J Biochem 193: 183–187 [DOI] [PubMed] [Google Scholar]
- Bernardi RC, Cann I, Schulten K (2014) Molecular dynamics study of enhanced Man5B enzymatic activity. Biotechnol Biofuels 7: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boavida LC, McCormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Bonavita A, Carratore V, Ciardiello MA, Giovane A, Servillo L, D’Avino R (2016) Influence of pH on the structure and function of kiwi pectin methylesterase inhibitor. J Agric Food Chem 64: 5866–5876 [DOI] [PubMed] [Google Scholar]
- Bourgault R, Bewley JD (2002) Gel diffusion assays for endo-β-mannanase and pectin methylesterase can underestimate enzyme activity due to proteolytic degradation: a remedy. Anal Biochem 300: 87–93 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126: 014101. [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Daher FB, Braybrook SA (2015) How to let go: pectin and plant cell adhesion. Front Plant Sci 6: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J Chem Phys 98: 10089–10092 [Google Scholar]
- Dedeurwaerder S, Menu-Bouaouiche L, Mareck A, Lerouge P, Guerineau F (2009) Activity of an atypical Arabidopsis thaliana pectin methylesterase. Planta 229: 311–321 [DOI] [PubMed] [Google Scholar]
- Derbyshire P, McCann MC, Roberts K (2007) Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo A, Giovane A, Raiola A, Camardella L, Bonivento D, De Lorenzo G, Cervone F, Bellincampi D, Tsernoglou D (2005) Structural basis for the interaction between pectin methylesterase and a specific inhibitor protein. Plant Cell 17: 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie B, Dirk LM, Hadfield KA, Wilkins TA, Bennett AB, Bradford KJ (1998) A gel diffusion assay for quantification of pectin methylesterase activity. Anal Biochem 264: 149–157 [DOI] [PubMed] [Google Scholar]
- Guénin S, Mareck A, Rayon C, Lamour R, Assoumou Ndong Y, Domon JM, Sénéchal F, Fournet F, Jamet E, Canut H, et al. (2011) Identification of pectin methylesterase 3 as a basic pectin methylesterase isoform involved in adventitious rooting in Arabidopsis thaliana. New Phytol 192: 114–126 [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Bussell JD, Pacurar DI, Schwambach J, Pacurar M, Bellini C (2009) Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21: 3119–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B. (2008) P-LINCS: a parallel linear constraint solver for molecular simulation. J Chem Theory Comput 4: 116–122 [DOI] [PubMed] [Google Scholar]
- Hess B, Bekker H, Berendsen H, Fraaije J (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18: 1463–1472 [Google Scholar]
- Hewezi T, Howe P, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ (2008) Cellulose binding protein from the parasitic nematode Heterodera schachtii interacts with Arabidopsis pectin methylesterase: cooperative cell wall modification during parasitism. Plant Cell 20: 3080–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocq L, Pelloux J, Lefebvre V (2017) Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci 22: 20–29 [DOI] [PubMed] [Google Scholar]
- Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S (2005) Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 5: 4864–4884 [DOI] [PubMed] [Google Scholar]
- Hong MJ, Kim DY, Lee TG, Jeon WB, Seo YW (2010) Functional characterization of pectin methylesterase inhibitor (PMEI) in wheat. Genes Genet Syst 85: 97–106 [DOI] [PubMed] [Google Scholar]
- Hothorn M, Van den Ende W, Lammens W, Rybin V, Scheffzek K (2010) Structural insights into the pH-controlled targeting of plant cell-wall invertase by a specific inhibitor protein. Proc Natl Acad Sci USA 107: 17427–17432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn M, Wolf S, Aloy P, Greiner S, Scheffzek K (2004) Structural insights into the target specificity of plant invertase and pectin methylesterase inhibitory proteins. Plant Cell 16: 3437–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerabek-Willemsen M, André T, Wanner R, Roth HM, Duhr S, Baaske P, Breitsprecher D (2014) Microscale thermophoresis: interaction analysis and beyond. J Mol Struct 1077: 101–113 [Google Scholar]
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79: 926 [Google Scholar]
- Kars I, Krooshof GH, Wagemakers L, Joosten R, Benen JAE, van Kan JAL (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J 43: 213–225 [DOI] [PubMed] [Google Scholar]
- Lehner A, Dardelle F, Soret-Morvan O, Lerouge P, Driouich A, Mollet JC (2010) Pectins in the cell wall of Arabidopsis thaliana pollen tube and pistil. Plant Signal Behav 5: 1282–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Enfant M, Domon JM, Rayon C, Desnos T, Ralet MC, Bonnin E, Pelloux J, Pau-Roblot C (2015) Substrate specificity of plant and fungi pectin methylesterases: identification of novel inhibitors of PMEs. Int J Biol Macromol 81: 681–691 [DOI] [PubMed] [Google Scholar]
- Leroux C, Bouton S, Kiefer-Meyer MC, Fabrice TN, Mareck A, Guénin S, Fournet F, Ringli C, Pelloux J, Driouich A, et al. (2015) PECTIN METHYLESTERASE48 is involved in Arabidopsis pollen grain germination. Plant Physiol 167: 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque-Tremblay G, Müller K, Mansfield SD, Haughn GW (2015a) HIGHLY METHYL ESTERIFIED SEEDS is a pectin methyl esterase involved in embryo development. Plant Physiol 167: 725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque-Tremblay G, Pelloux J, Braybrook SA, Müller K (2015b) Tuning of pectin methylesterification: consequences for cell wall biomechanics and development. Planta 242: 791–811 [DOI] [PubMed] [Google Scholar]
- Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, Shaw DE (2010) Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78: 1950–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D (2007) Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol 143: 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Raiola A, Cervone F, Bellincampi D (2014) Transgenic expression of pectin methylesterase inhibitors limits tobamovirus spread in tobacco and Arabidopsis. Mol Plant Pathol 15: 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa C, Guarnaccia C, Lamba D, Anselmi C (2010) Insight into the structure of an endopolygalacturonase from the phytopathogen Burkholderia cepacia: a biochemical and computational study. Biochimie 92: 1445–1453 [DOI] [PubMed] [Google Scholar]
- Mercadante D, Melton LD, Jameson GB, Williams MAK (2014) Processive pectin methylesterases: the role of electrostatic potential, breathing motions and bond cleavage in the rectification of Brownian motions. PLoS ONE 9: e87581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadante D, Melton LD, Jameson GB, Williams MAK, De Simone A (2013) Substrate dynamics in enzyme action: rotations of monosaccharide subunits in the binding groove are essential for pectin methylesterase processivity. Biophys J 104: 1731–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Leroux C, Dardelle F, Lehner A (2013) Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants (Basel) 2: 107–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B, Haughn G, Kermode AR (2013) Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol 161: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson MHM, Søndergaard CR, Rostkowski M, Jensen JH (2011) PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J Chem Theory Comput 7: 525–537 [DOI] [PubMed] [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V (2008) Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J 54: 43–55 [DOI] [PubMed] [Google Scholar]
- Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52: 7182–7190 [Google Scholar]
- Paynel F, Leroux C, Surcouf O, Schaumann A, Pelloux J, Driouich A, Mollet JC, Lerouge P, Lehner A, Mareck A (2014) Kiwi fruit PMEI inhibits PME activity, modulates root elongation and induces pollen tube burst in Arabidopsis thaliana. Plant Growth Regul 74: 286–297 [Google Scholar]
- Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H (2011a) Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Louvet R, Johansen JN, Höfte H, Laufs P, Pelloux J, Mouille G (2008) Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol 18: 1943–1948 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Louvet R, Johansen JN, Salsac F, Morin H, Fournet F, Belcram K, Gillet F, Höfte H, Laufs P, et al. (2011b) The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Development 138: 4733–4741 [DOI] [PubMed] [Google Scholar]
- Pelletier S, Van Orden J, Wolf S, Vissenberg K, Delacourt J, Ndong YA, Pelloux J, Bischoff V, Urbain A, Mouille G, et al. (2010) A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol 188: 726–739 [DOI] [PubMed] [Google Scholar]
- Pilling J, Willmitzer L, Fisahn J (2000) Expression of a Petunia inflata pectin methyl esterase in Solanum tuberosum L. enhances stem elongation and modifies cation distribution. Planta 210: 391–399 [DOI] [PubMed] [Google Scholar]
- Raiola A, Camardella L, Giovane A, Mattei B, De Lorenzo G, Cervone F, Bellincampi D (2004) Two Arabidopsis thaliana genes encode functional pectin methylesterase inhibitors. FEBS Lett 557: 199–203 [DOI] [PubMed] [Google Scholar]
- Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, Cervone F, Bellincampi D (2011) Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant Microbe Interact 24: 432–440 [DOI] [PubMed] [Google Scholar]
- Reca IB, Lionetti V, Camardella L, D’Avino R, Giardina T, Cervone F, Bellincampi D (2012) A functional pectin methylesterase inhibitor protein (SolyPMEI) is expressed during tomato fruit ripening and interacts with PME-1. Plant Mol Biol 79: 429–442 [DOI] [PubMed] [Google Scholar]
- Ren C, Kermode AR (2000) An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol 124: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Aguayo S, Ralet MC, Berger A, Botran L, Ropartz D, Marion-Poll A, North HM (2013) PECTIN METHYLESTERASE INHIBITOR6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell 25: 308–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234: 779–815 [DOI] [PubMed] [Google Scholar]
- Sénéchal F, Graff L, Surcouf O, Marcelo P, Rayon C, Bouton S, Mareck A, Mouille G, Stintzi A, Höfte H, et al. (2014a) Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Ann Bot (Lond) 114: 1161–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, L’Enfant M, Domon JM, Rosiau E, Crépeau MJ, Surcouf O, Esquivel-Rodriguez J, Marcelo P, Mareck A, Guérineau F, et al. (2015a) Tuning of pectin methylesterification: pectin methylesterase inhibitor 7 modulates the processive activity of co-expressed pectin methylesterase 3 in a pH-dependent manner. J Biol Chem 290: 23320–23335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, Wattier C, Rustérucci C, Pelloux J (2014b) Homogalacturonan-modifying enzymes: structure, expression, and roles in plants. J Exp Bot 65: 5125–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N, Szemenyei H, Sorek H, Landers A, Knight H, Bauer S, Wemmer DE, Somerville CR (2015) Identification of MEDIATOR16 as the Arabidopsis COBRA suppressor MONGOOSE1. Proc Natl Acad Sci USA 112: 16048–16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) GROMACS: fast, flexible, and free. J Comput Chem 26: 1701–1718 [DOI] [PubMed] [Google Scholar]
- Vangone A, Bonvin AM (2015) Contacts-based prediction of binding affinity in protein-protein complexes. eLife 4: e07454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17: 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. (2008) Unique aspects of the grass cell wall. Curr Opin Plant Biol 11: 301–307 [DOI] [PubMed] [Google Scholar]
- Volpi C, Raiola A, Janni M, Gordon A, O’Sullivan DM, Favaron F, D’Ovidio R (2013) Claviceps purpurea expressing polygalacturonases escaping PGIP inhibition fully infects PvPGIP2 wheat transgenic plants but its infection is delayed in wheat transgenic plants with increased level of pectin methyl esterification. Plant Physiol Biochem 73: 294–301 [DOI] [PubMed] [Google Scholar]
- Wang M, Yuan D, Gao W, Li Y, Tan J, Zhang X (2013) A comparative genome analysis of PME and PMEI families reveals the evolution of pectin metabolism in plant cell walls. PLoS ONE 8: e72082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienken CJ, Baaske P, Rothbauer U, Braun D, Duhr S (2010) Protein-binding assays in biological liquids using microscale thermophoresis. Nat Commun 1: 100. [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Grsic-Rausch S, Rausch T, Greiner S (2003) Identification of pollen-expressed pectin methylesterase inhibitors in Arabidopsis. FEBS Lett 555: 551–555 [DOI] [PubMed] [Google Scholar]
- Wolf S, Mravec J, Greiner S, Mouille G, Höfte H (2012) Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol 22: 1732–1737 [DOI] [PubMed] [Google Scholar]
- Woriedh M, Wolf S, Márton ML, Hinze A, Gahrtz M, Becker D, Dresselhaus T (2013) External application of gametophyte-specific ZmPMEI1 induces pollen tube burst in maize. Plant Reprod 26: 255–266 [DOI] [PubMed] [Google Scholar]
- Yu Q, Tang C, Kuo J (2000) A critical review on methods to measure apoplastic pH in plants. Plant Soil 219: 29–40 [Google Scholar]