Arabidopsis CNGC2 is a Ca2+-permeable channel that mediates Ca2+ influx into mesophyll cells near minor veins but has no direct role in the hypersensitive response or leaf senescence.
Abstract
Ca2+ is absorbed by roots and transported upward through the xylem to the apoplastic space of the leaf, after which it is deposited into the leaf cell. In Arabidopsis (Arabidopsis thaliana), the tonoplast-localized Ca2+/H+ transporters CATION EXCHANGER1 (CAX1) and CAX3 sequester Ca2+ from the cytosol into the vacuole, but it is not known what transporter mediates the initial Ca2+ influx from the apoplast to the cytosol. Here, we report that Arabidopsis CYCLIC NUCLEOTIDE-GATED CHANNEL2 (CNGC2) encodes a protein with Ca2+ influx channel activity and is expressed in the leaf areas surrounding the free endings of minor veins, which is the primary site for Ca2+ unloading from the vasculature and influx into leaf cells. Under hydroponic growth conditions, with 0.1 mm Ca2+, both Arabidopsis cngc2 and cax1cax3 loss-of-function mutants grew normally. Increasing the Ca2+ concentration to 10 mm induced H2O2 accumulation, cell death, and leaf senescence and partially suppressed the hypersensitive response to avirulent pathogens in the mutants but not in the wild type. In vivo apoplastic Ca2+ overaccumulation was found in the leaves of cngc2 and cax1cax3 but not the wild type under the 10 mm Ca2+ condition, as monitored by Oregon Green BAPTA 488 5N, a low-affinity and membrane-impermeable Ca2+ probe. Our results indicate that CNGC2 likely has no direct roles in leaf development or the hypersensitive response but, instead, that CNGC2 could mediate Ca2+ influx into leaf cells. Finally, the in vivo extracellular Ca2+ imaging method developed in this study provides a new tool for investigating Ca2+ dynamics in plant cells.
Deciphering the molecular mechanisms underlying plant mineral nutrition will provide a foundation for improving the productivity of modern crops and forests (Karley and White, 2009; Liu et al., 2015; Pilbeam, 2015). Although significant progress has been made in elucidating the cellular and genetic pathways of nitrogen, phosphorus, and potassium uptake and distribution in plants in the past decade (Baker et al., 2015; Krapp, 2015; Wang and Wu, 2015), the corresponding pathways remain largely unknown for Ca (Gilliham et al., 2011; Kumar et al., 2015), an essential mineral that stabilizes cellular structures and acts as an important intracellular messenger (Dodd et al., 2010; Kudla et al., 2010; Reddy et al., 2011; Spalding and Harper, 2011; Yang et al., 2012). Ecological evidence indicates that Ca concentrations have decreased in forest soils and fresh water worldwide over the past century (Johnson et al., 2008; Bedison and Johnson, 2010; Talhelm et al., 2012; Leys et al., 2016). This Ca decrease has increased the susceptibility of forest and crop plants to stressful conditions (Hong-Bo et al., 2008; Genc et al., 2010; Halman et al., 2011) and reduced the diversity of plant species (Närhi et al., 2011). Understanding the genetic mechanisms by which Ca is absorbed by the roots and distributed in the leaves is important for addressing this environmental challenge and for breeding crops with better Ca nutrition (Robertson, 2013).
Ca2+ is absorbed by root cells, uploaded into the xylem, and further transported to the aerial parts of the plant, where it is unloaded from the xylem and redistributed into all types of leaf cells (White, 2001; White and Broadley, 2003; Gilliham et al., 2011). Whereas Ca2+ absorption and distribution have been studied at the physiological level (Kumar et al., 2015), the underlying molecular mechanisms remain largely unknown (Kudla et al., 2010; Spalding and Harper, 2011). Suberin deposits in the roots form a barrier that limits the accumulation of mineral nutrients, including Ca2+, in the shoots (Baxter et al., 2009). Tonoplast-localized Ca2+/H+ transporter CATION EXCHANGER1 (CAX1) and CAX3 transport Ca2+ from the cytosol to the vacuole of Arabidopsis (Arabidopsis thaliana) leaf mesophyll cells (Hirschi et al., 1996; Conn et al., 2011; Manohar et al., 2011; Kumar et al., 2015; Pittman and Hirschi, 2016). A cax1cax3 loss-of-function double mutant exhibits leaf growth defects and early senescence in the leaves when grown in soil and increased sensitivity to high Ca2+ concentrations when grown on agar medium (Cheng et al., 2005). This mutant accumulates higher levels of Ca in the extracellular space than does Columbia-0 (Col-0), as revealed by x-ray analysis and field emission scanning electron microscopy-based mineral element microanalysis of cross sections of frozen-hydrated leaves (Conn et al., 2011).
The identity of the protein responsible for initial extracellular Ca2+ influx into the leaf cells after Ca2+ unloading from the xylem is unknown. In Arabidopsis, CNGC2 is predicted to be a cyclic nucleotide-gated channel and was initially identified as a key component of the hypersensitive response (HR), a rapid, local cell death response evoked by avirulent pathogens (Yu et al., 1998; Clough et al., 2000). The HR is an important self-protection mechanism whereby plants preserve whole leaves by undergoing localized cell death at the infection site. Although the cngc2 mutant, which harbors a disruption in CNGC2, displays no localized cell death when grown in soil, its leaves maintain defense to avirulent pathogen infection (Yu et al., 1998; Clough et al., 2000). This observation has led to the notion that the HR is not necessary for plant cells to withstand infection by avirulent pathogens. Thus, the cngc2 mutant also was named defense no death (dnd1; Yu et al., 1998). However, the soil-grown mutant displays a leaf growth inhibition phenotype, constitutively accumulates high levels of salicylic acid (SA) and reactive oxygen species (Yu et al., 1998; Clough et al., 2000; McDowell et al., 2013), and exhibits conditional early senescence symptoms, reduced Ca concentrations in the leaf (Clough et al., 2000), and altered Ca and magnesium (Mg) contents in the seeds (McDowell et al., 2013). Despite this, and similar to the cax1cax3 double mutant, cngc2 grows as well as the wild-type Col-0 on agar-based medium in sterile conditions, unless the Ca2+ concentration is increased to 10 mm (Chan et al., 2003). To date, no clear mechanism has been proposed to explain all of the phenotypes of the cngc2 mutant. In this study, we show that all of the reported leaf phenotypes of soil-grown cngc2 could be indirect effects of its sensitivity to the Ca2+ supply, suggesting that the primary function of CNGC2 is to mediate the influx of apoplastic Ca2+ into the leaf cells. In addition, we report a new in vivo method to monitor extracellular Ca2+ dynamics in the leaf.
RESULTS
CNGC2 Is Required for Plant Ca Nutrition But Not for Leaf Development and the HR
To test for a role of CNGC2 in Arabidopsis Ca nutrition, we used a hydroponic system to control the amount of Ca2+ available to the root (Fig. 1). Col-0 and cngc2-1 plants were initially grown in medium containing 0.1 mm Ca2+ for 3 weeks and then transferred to medium containing 0.1, 1, 5, or 10 mm Ca2+ for 8 d. When grown in 0.1 mm Ca2+, cngc2-1 grew as well as Col-0 (Fig. 1A); however, when subjected to 1, 5, or 10 mm Ca2+, the cngc2-1 plants but not Col-0 showed [Ca2+]-dependent growth inhibition and developed leaf senescence symptoms (Fig. 1, A and B). The root length (Supplemental Fig. S1A) and fresh weight (Supplemental Fig. S1B) were comparable between the mutant and Col-0 under these conditions. In addition, the Ca2+-sensitive phenotype of cngc2-1 could be complemented by expressing wild-type CNGC2 driven by its native promoter (COM, which denotes complemented with CNGC2; Fig. 1C). Extending the treatment to 30 d strongly inhibited the stem elongation and inflorescence development of cngc2 but not of the Col-0 or COM plants (Fig. 1D). These results demonstrated that CNGC2 has an essential role in Ca2+ nutrition.
Figure 1.
Arabidopsis hydroponics-grown cngc2, cngc4, and cax1cax3 mutants are sensitive to the increasing [Ca2+]. Arabidopsis plants were grown in hydroponic medium supplemented with 0.1 mm Ca2+ for 3 weeks and then transferred to medium containing the indicated concentrations of Ca2+. A, Col-0 and cngc2-1 were treated with 0.1, 1, 5, or 10 mm [Ca2+] for 8 d. B, Leaf fresh weight of plants from A. Asterisks indicate significant differences between Col-0 and cngc2-1: **, P < 0.01. Values represent means ± se; n = 3 biological trials with 40 plants per trial. C, Representative plants of Col-0, cax1cax3, cngc2-1, and cngc2-1 complemented with CNGC2 (COM) treated with 0.1 or 10 mm Ca2+ for 8 d. D, Representative plants of Col-0, cax1cax3 (1/3), cngc2-1 (2-1), and COM treated with 0.1 or 10 mm Ca2+ for 30 d. E, Representative plants of Col-0, cngc2-3, cngc4-5, and cngc2-3cngc4-5 after 8 d of 10 mm Ca2+ treatment.
The Arabidopsis genome contains 20 CNGC genes (Ward, 2001), 15 of which we tested for their involvement in the Ca2+ growth phenotype, using the corresponding T-DNA insertion mutants. Besides cngc2, the only mutant that showed a leaf growth inhibition phenotype when grown on 10 mm Ca2+ was cngc4 (Supplemental Fig. S2). CNGC4 is the closest homolog of CNGC2 in the Arabidopsis genome, and soil-grown cngc4 has the same leaf-growth inhibition phenotype as cngc2 (Jurkowski et al., 2004). To further explore this result, we grew two cngc2 and cngc4 mutant alleles as well as the cngc2cngc4 double mutant and found that all of them showed similar [Ca2+]-sensitive symptoms as the cngc2-1 mutants (Fig. 1E). These results further demonstrated the vital role of CNGC2 in Ca2+ nutrition.
Leaves of soil-grown cngc2-1 plants have spontaneous lesions, occasional cell death spots, and constitutively elevated SA contents and expression of PR1 (Yu et al., 1998; Clough et al., 2000; McDowell et al., 2013). In this study, under 0.1 mm Ca2+ conditions, the leaves of cngc2-1 and Col-0 had similar low levels of staining for cell death (Fig. 2A) and H2O2 (Fig. 2B) as well as basal levels of SA (Fig. 2C) and PR1 gene expression (Fig. 2D). Treatment with 10 mm Ca2+ for 8 d significantly increased cell death (Fig. 2A), H2O2 (Fig. 2B), and SA accumulation (Fig. 2C) as well as PR1 gene expression (Fig. 2D) in the leaves of cngc2-1 but not of Col-0 and the COM plants. These findings demonstrated that the Ca2+ sensitivity of cngc2-1 accounts for the spontaneous high SA, H2O2 production, and cell death spots in cngc2-1 leaves.
Figure 2.
Increasing [Ca2+] promotes cell death and H2O2 and SA production in the leaves of cax1cax3 and cngc2 plants. A, Col-0, cax1cax3, cngc2-1, and cngc2-1 complemented with CNGC2 (COM) were initially grown on 0.1 mm Ca2+ and then treated with 0.1 or 10 mm Ca2+ for 8 d. Leaves with typical symptoms were examined with a dissecting microscope and stained with Trypan Blue for cell death, shown by dark blue spots. Images were taken before and after the staining. B, Plants were treated as in A and stained with 3,3-diaminobenzidine (DAB) for H2O2 accumulation, shown by brown spots. C, Free SA contents in the leaves of Col-0 and cngc2-1 grown in the hydroponics system after 3 d of treatment with 0.1 or 10 mm Ca2+. D, Plants were treated as in C, and PR1 gene expression was examined and normalized to the internal standard and the PR1 expression in Col-0 under 0.1 mm Ca2+ conditions. Values in C and D represent means ± se; n = 3 biological trials. **P < 0.01 between the 0.1 and 10 mm Ca2+ treatments.
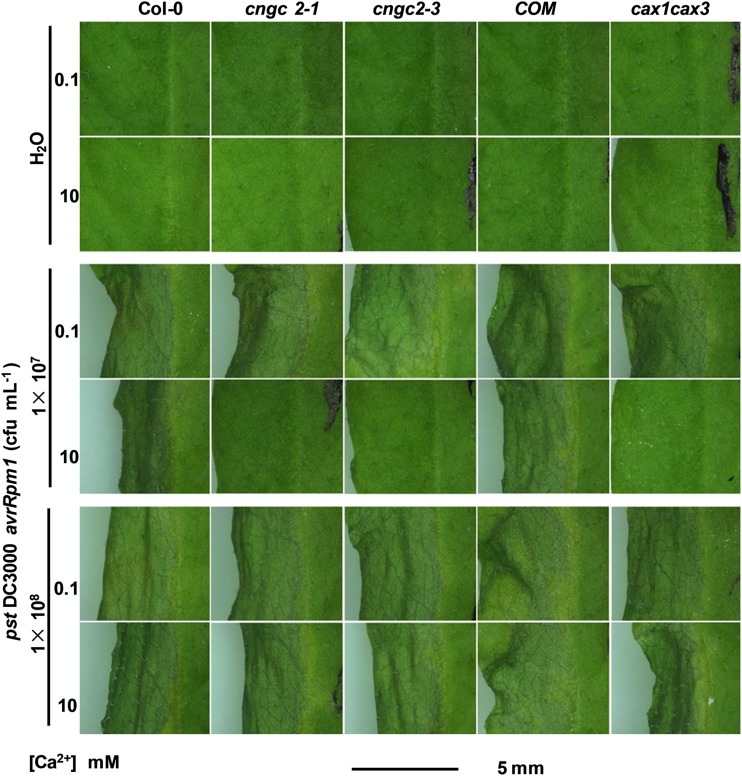
The cngc2 is also named dnd1, since the mutant maintains defense to avirulent pathogens without displaying HR (Yu et al., 1998; Clough et al., 2000). To our knowledge, all of the reported cngc2-1 (dnd1) HR phenotype characterizations were performed with leaves of soil-grown mutant plants showing early senescence or growth inhibition phenotypes (Yu et al., 1998; Clough et al., 2000; Ahn, 2007; Ali et al., 2007; Genger et al., 2008; Chin et al., 2013) similar to those in plants given 10 mm Ca2+ treatment using the hydroponics system in this study (Fig. 1). We reasoned that it was possible that the dnd1 phenotype of cngc2-1 is also Ca2+ supply dependent. To test this hypothesis, we infiltrated low (107 colony-forming units [cfu] mL−1) and high (108 cfu mL−1) densities of the avirulent pathogen Pseudomonas syringae pv tomato DC3000 expressing avrRpm1 into leaves of Col-0, cngc2-1, cngc2-3, and COM plants. Under 0.1 mm Ca2+ growth conditions, all four genotypes displayed a typical HR (Fig. 3). A 3-d treatment with 10 mm Ca2+ did not cause obvious leaf senescence in the mutants and Col-0 but inhibited development of the HR in the leaves of cngc2-1 but not of Col-0 and COM, in response to the low-density pathogen (Fig. 3). However, with the 10 mm Ca2+ treatment, all three genotypes displayed a normal HR in response to the high-density pathogen (108 cfu mL−1; Fig. 3).
Figure 3.
HR of Arabidopsis Col-0, cngc2, and cax1cax3 mutants. Arabidopsis Col-0, cngc2-1, cngc2-1 complemented with CNGC2 (COM), and cax1cax3 plants were first grown in hydroponic solution supplemented with 0.1 mm Ca2+ for 3 weeks and then subjected to 3-d treatments with 0.1 or 10 mm Ca2+. The leaves were infiltrated with either water or a suspension of P. syringae pv tomato DC3000 avrRpm1 in water at a density of 1 × 107 or 1 × 108 cfu mL−1. Leaves with typical symptoms were photographed 24 h after infection.
These data demonstrated that CNGC2 plays an essential role in plant Ca nutrition and that the previously observed leaf phenotypes of the soil-grown cngc2 mutant could be an indirect result of CNGC2 function in Ca nutrition.
The cngc2 Mutant and cax1cax3 Double Mutants Have Similar Ca-Dependent Phenotypes
To dissect the underlying mechanism of CNGC2-mediated Ca nutrition, we measured total Ca contents in the leaves and roots of Col-0 and cngc2-1 plants that we initially cultured on 0.1 mm Ca2+ and then treated for 3 d with 0.1, 1, or 10 mm Ca2+. The 3-d treatments had no noticeable effects on the leaf growth and development of Col-0 and cngc2-1. Col-0 and cngc2-1 had similar Ca contents in their leaves at 0.1 and 1 mm Ca2+, and cngc2-1 had significantly less Ca than Col-0 at 10 mm Ca2+ (Fig. 4A), which is similar to results with soil-grown plants (Clough et al., 2000; Ma et al., 2010). The total Ca content in cngc2-1 roots was comparable to that of Col-0 (Fig. 4B). The Mg contents showed no difference between Col-0 and the mutant in both leaf and root (Fig. 4, C and D). These data imply that cngc2-1 primarily was defective in Ca2+ distribution in the leaf but not in Ca2+ transport from the root to the leaf. This hypothesis was further supported by the observation that the detached leaves of cngc2-1 developed similar senescence symptoms to those of intact plants under 10 mm [Ca2+] (Fig. 4E). These findings suggested that cngc2-1 was defective primarily in Ca2+ distribution in the leaf but not in Ca2+ transport from the root to the leaf.
Figure 4.
Ca and Mg contents of Col-0 and cngc2-1 plants and their detached leaf responses to [Ca2+]. A to D, Col-0 and cngc2-1 initially were grown under hydroponic conditions with 0.1 mm Ca2+ for 3 weeks, then given 0.1, 1, or 10 mm Ca2+ for 3 d. Ca contents in the leaves (A) and roots (B), and Mg contents in the leaves (C) and roots (D), were measured. Values represent means of three biological replicates ± se. **, Significant difference at P < 0.01 with Student’s t test between Col-0 and cngc2-1 under the same treatment. E, Col-0 and cngc2-1 initially were grown under hydroponic conditions with 0.1 mm Ca2+ for 3 weeks. Fully expanded leaves were detached and inserted into a 200-µL tube with full nutrition medium containing either 0.1 or 10 mm Ca2+ for 8 d. Leaves with typical symptoms are shown.
The two most important genes known to be involved in Arabidopsis leaf Ca2+ nutrition are CAX1 and CAX3, which encode Ca2+/H+ antiporters localized in the tonoplast of leaf mesophyll cells (Hirschi et al., 1996; Manohar et al., 2011; Kumar et al., 2015). The Arabidopsis cax1cax3 double mutant has a very similar phenotype to that of cngc2-1: the soil-grown cax1cax3 plants have growth defects and early senescence phenotypes; moreover, cax1cax3 plants grown on agar-based medium are sensitive to 20 mm Ca2+ (Cheng et al., 2005). Disruption of CAX1 and CAX3 blocks cytosolic Ca2+ storage in the vacuole and causes Ca2+ to overaccumulate in the apoplast (Conn et al., 2011). By analogy, we wondered whether CNGC2 mediates Ca2+ influx into the mesophyll cells and if disrupting CNGC2 causes Ca2+ accumulation in the apoplast, which would account for the mutant’s sensitivity to increasing Ca2+ supply to its roots. If CNGC2, CAX1, and CAX3 indeed function in same pathway mediating extracellular Ca2+ deposit to leaf cells, the cax1cax3 and cngc2 mutants might have similar Ca-dependent phenotypes. To test this hypothesis, we first grew Col-0, cngc2-1, COM, and cax1cax3 plants on the agarose-based medium supplied with different [Ca2+] levels. Similar to cngc2-1, the growth of cax1cax3 plants was no different compared with Col-0 at 0.1 mm Ca2+, but its leaf growth (Supplemental Fig. S3, A and B) and root elongation (Supplemental Fig. S3C) were suppressed significantly by increasing [Ca2+]. In the hydroponic condition with 0.1 mm Ca2+, the cngc2-1 and cax1cax3 mutants grew as well as Col-0 and COM (Fig. 1, C and D); however, the 8-d (Fig. 1C) or 30-d (Fig. 1D) 10 mm Ca2+ treatment inhibited cax1cax3 leaf growth as much as that of cngc2-1, promoted senescence and cell death (Fig. 2A), induced the accumulation of H2O2 (Fig. 2B) and SA (Fig. 2C), and suppressed the HR induced by infection with avirulent pathogen at low density (Fig. 3). These results implied that CNGC2 and CAX1/CAX3 have similar functions in leaf Ca nutrition.
In Vivo Imaging of Extracellular Ca2+ in cngc2 and cax1cax3 Leaves
Disruption of the pathway from the cytosol to the vacuole for Ca2+ storage causes the apoplastic (extracellular) space of cax1cax3 mutants to overaccumulate Ca, which was originally measured using x-ray microanalysis in fixed leaf cross sections (Conn et al., 2011). Although several luminescence- and fluorescence-based methods have been developed to measure intracellular Ca2+ distribution and dynamics in living plant cells (Knight et al., 1991; Behera et al., 2013), there has not been an in vivo method reported to date to visualize extracellular Ca2+ distribution in plant cells. In animal and human cells, Oregon Green BAPTA 488 5N (OGB-5N), a well-established membrane-impermeable low-affinity Ca2+ fluorescent dye (White and McGeown, 2002; Jiménez-Moreno et al., 2008), has been used to monitor extracellular Ca2+ dynamics (Rusakov and Fine, 2003), endocytosis-mediated extracellular Ca2+ uptake (Gerasimenko et al., 1998; Sherwood et al., 2007), and intracellular Ca2+ dynamics in giant cells like oocytes (Callamaras and Parker, 2000) and muscle cells (Hollingworth et al., 2009) through microinjection or the pipette during patch-clamp whole-cell recordings. Here, we developed a method to use OGB-5N in plants to track extracellular Ca2+ transport and distribution in the leaf.
We first grew Col-0 and cngc2-1 in the hydroponic system with 0.1 mm Ca2+ and then applied a 3-d 0.1 or 10 mm Ca2+ treatment. Afterward, we placed the petioles of detached mature leaves from the hydroponics-cultured plants into the corresponding growth solutions containing OGB-5N. At time 0, no fluorescence was observed in all tested leaves (Fig. 5A). After 20 min, clear green fluorescence with similar patterns and intensity appeared in the vascular systems of Col-0 and cngc2-1 in the 0.1 and 10 mm Ca2+ conditions (Fig. 5A). After 4 h of staining, leaves of both Col-0 and cngc2-1 treated with 0.1 mm Ca2+ showed extracellular [Ca2+] ([Ca2+]ext)-dependent green fluorescence primarily in the third and minor veins, with no obvious difference between genotypes in terms of pattern and intensity (Fig. 5A). No detectable fluorescence was present in the nonvascular tissues (e.g. veinlet, the leaf areas divided by the veins; Fig. 5A). Thus, it appears that Ca2+ unloaded from the vascular system was actively redistributed into other leaf cells and that very little free Ca2+ was extracellular. In the leaves treated with 10 mm Ca2+, the [Ca2+]ext-dependent green fluorescence was still restricted to the vascular tissues in Col-0 but it spread to the nonvascular tissues (veinlet) in cngc2-1 leaves (Fig. 5A). This difference implied that disrupting CNGC2 causes extracellular Ca2+ accumulation around the leaf cells. Furthermore, in a separate set of experiments, extracellular Ca2+ accumulation around the leaf cells was observed with two alleles of cngc2 and cax1cax3 and was rescued back to the Col-0 phenotype in the complementation line COM (Fig. 5B).
Figure 5.
Fluorescence microscopy of extracellular Ca2+ in leaves of Col-0, cngc2, and cax1cax3. Col-0, cngc2-1, cax1cax3, and cngc2-1 complemented with CNGC2 (COM) were hydroponically grown at 0.1 mm Ca2+ for 3 weeks and then given 3-d treatments of 0.1 or 10 mm Ca2+. Fully expanded leaves were detached and inserted into 200-μL tubes with their corresponding growth medium in the presence of 20 μm Ca2+-dependent fluorescent dye OGB-5N. A, Bright-field and fluorescence images of these leaves were taken at different time points of the OGB-5N treatment with either 2× or 10× objective lenses. The areas defined by the yellow squares in the 4-h (2×) images were magnified and are shown in the 4-h (10×) images. B, Treatment was the same as in A, and images were taken at 4 h.
To visualize the extracellular Ca2+ distribution in the leaf with higher spatial resolution, we used confocal laser scanning microscopy. The cell wall boundaries of the leaf cells were defined using the red fluorescent dye propidium iodide. At 0.1 mm Ca2+, extracellular Ca2+ was detected exclusively in the vascular system (veins), with very little if any green fluorescence detected in the surrounded leaf areas (veinlet) of Col-0, cax1cax3, cngc2-1, and COM plants (Fig. 6). At 10 mm Ca2+, the [Ca2+]ext-dependent green fluorescence was still almost exclusively present in the vascular tissues (veins) of Col-0 and COM, especially the minor veins, with very limited signal in the nonvascular leaf areas (veinlet; Fig. 6). However, a significant amount of extracellular Ca2+-dependent green fluorescence was detected around veinlet areas in cngc2 and cax1cax3 leaves (Fig. 6).
Figure 6.
Confocal microscopy of extracellular Ca2+ in leaves of Col-0, cngc2, and cax1cax3 plants. A, The plant growth and treatment procedures were the same as in Figure 7. The extracellular Ca2+ (Ext Ca2+) and the cell wall boundary of the epidermal cells were visualized using the Ca2+-dependent green fluorescent dye OGB-5N and red fluorescent dye propidium iodide, respectively, and observed by confocal laser scanning microscopy. Ext Ca2+ + Cell Wall indicates the merged images of Ext Ca2+ and Cell Wall. COM denotes the cngc2-1 line complemented with CNGC2. B, Relative fluorescence intensity between the propidium iodide-dependent red and OGB-5N-dependent green signals was compared between Col-0 and mutants under 0.1 or 10 mm Ca2+ conditions in the vein and nonvein (veinlet) areas. Values represent means of three biological trials ± se, with five leaves per trial and five areas per leaf. **P < 0.01 between the 0.1 and 10 mm Ca2+ treatments.
Ca in plants can be in a water-soluble ionic form or chelated by cell wall components and other acidic compounds like oxalate (Gilliham et al., 2011). It has been suggested that Ca2+ overaccumulation in the extracellular space of the cax1cax3 leaf can increase Ca2+ deposition into the cell wall and decrease Ca2+ in the cytoplasm (Conn et al., 2011). To investigate this hypothesis, Ca in the leaves of Col-0, cngc2-1, and cax1cax3 with 3 d of exposure to either 0.1 or 10 mm Ca2+ was sequentially extracted with water, acetic acid, and HCl to isolate water-soluble Ca2+, the Ca2+ bound to the cell wall, and Ca2+ in complexes with acid compounds, respectively. After water and acetic acid extractions, we did not detect Ca in the HCl extract. With the 0.1 mm Ca2+ treatment, there was no significant difference between Col-0 and cngc2-1 or cax1cax3 in either water-soluble (Fig. 7, A and C) or acetic acid-soluble (Fig. 7, B and D) Ca2+ contents. However, at 10 mm Ca2+, cngc2-1 and cax1cax3 plants had significantly less water-soluble Ca2+ (Fig. 7, A and C) but more acetic acid-soluble Ca2+ than Col-0 (Fig. 7, B and D). This result was consistent with the hypothesis that increasing the accumulation of Ca2+ in the extracellular space promotes Ca2+ deposition into the cell wall of cngc2 and cax1cax3 plants under high-Ca2+ growth conditions.
Figure 7.
Water- and acetic acid-soluble Ca in the leaves of Col-0, cngc2-1, and cax1cax3. Col-0, cngc2-1, and cax1cax3 plants were grown hydroponically with 0.1 mm Ca2+ for 3 weeks and then given a 3-d treatment with 0.1 or 10 mm Ca2+. Fully expanded leaves were detached and extracted with water (A and C) for Ca content measurement. After the water extraction, the concentrated tissues were sequentially extracted with acetic acid (AA) for Ca content measurement (B and D). Values represent means ± se; n = 3 biological trials, five replicates per trial, and leaves from four plants per replicate. **, P < 0.01.
CNGC2 Is Expressed around Minor Veins and Encodes a Ca2+ Influx Channel
The Ca2+ overaccumulation in the extracellular space of cngc2-1 leaf cells appeared preferentially in the areas surrounding the minor veins (Figs. 5 and 6), which might reflect cell-specific expression of CNGC2. For the 12-d-old seedlings grown on the agarose-based medium, CNGC2 promoter activity was detected primarily in the leaves (Supplemental Fig. S4A) and weakly in the vascular tissue of the roots (Supplemental Fig. S4, B and C). For the mature plants grown on the hydroponics system, the CNGC2 promoter activity was weakly detected in the stem (Supplemental Fig. S4D), flowers (Supplemental Fig. S4E), developing siliques (Supplemental Fig. S4F), and not in the roots (Supplemental Fig. S4G). In the leaf, besides the activity found in the cells surrounding trichomes (Supplemental Fig. S4H), CNGC2 was expressed specifically in the vascular tissues and the leaf cells proximate to the vascular system, especially in the leaf areas around the free endings of minor veins, under both 0.1 and 10 mm Ca2+ conditions (Fig. 8A).
Figure 8.
CNGC2 minor vein area-specific expression and inward Ca2+ channel activity in HEK293T cells. A, GUS reporter gene-based CNGC2 gene expression pattern (blue areas) in the leaf at 0.1 mm Ca2+. The red circles indicate the positions of the minor vein free-ending areas. The pattern in the leaf at 10 mm Ca2+ was similar to that at 0.1 mm. B, CNGC2 was transiently expressed in HEK293T cells. Patch-clamp whole-cell recording was used to monitor current across the plasma membrane under continuous voltage change from +20 to –180 mV. Representative recordings are shown under the indicated conditions. C, Average current-voltage curves are shown for the cell transformed with empty vector and recorded with 200 μm 8-bromoadenosine cAMP (8-Bromo-cAMP) and 10 mm Ca2+ in the bath solution (n = 7) and the cells transformed with CNGC2 in bath solution containing 200 μm 8-Bromo-cAMP and 10 mm Ca2+ (n = 6), 10 mm Ca2+ + 1 mm GdCl3 (n = 5), or 30 mm Ca2+ (n = 9). Values represent means ± se.
Free-ending areas of these minor veins are the primary places at which mineral ions, including Ca2+, are unloaded from the vascular system and redistributed into the surrounding leaf cells (Ahn, 2007). Thus, it seemed that CNGC2 might function as a Ca2+ influx transporter mediating Ca2+ influx into the leaf cells immediately after Ca2+ unloading from the vascular tissues. Indirect evidence has suggested that CNGC2 is a Ca2+ influx channel: the plasma membrane of cngc2 guard cells lacks the cAMP-activated Ca2+ influx current (Ali et al., 2007), and CNGC2 mediates a cAMP-induced increase in cytosolic Ca2+ when it is expressed in mammalian HEK293 cells (Leng et al., 1999). Recently, CNGC18, one of 20 CNGCs in Arabidopsis, was demonstrated to have Ca2+ influx channel activity when expressed in HEK293T cells, as measured by a patch-clamp whole-cell configuration (Gao et al., 2014, 2016) with an experimental setup similar to that used for previous recordings of the CRACM1 Ca2+ channel (Vig et al., 2006). To test directly for CNGC2 channel activity, we used the same protocol to express and study CNGC2 in HEK293T cells.
With 10 mm Ca2+ in the bath, an inward current was observed in HEK293T cells expressing CNGC2 but not in the cells with empty vector (Supplemental Fig. S5). The CNGC2-dependent current showed a moderate response to the membrane-permeable lipophilic cAMP analog 8-bromoadenosine cAMP (Supplemental Fig. S5). There are five significant ions in the recording solutions: Ca2+, Na+, Cs+, Mg2+, and Cl− (Vig et al., 2006; Gao et al., 2014, 2016). At room temperature (23°C), with the assumption that only a single ion has the ability to cross the membrane, the theoretical equilibrium potentials for the individual ions are 140 mV (Ca2+), 69 mV (Na+), −63 mV (Cs+), −13 mV (Mg2+), and −48 mV (Cl−). The measured reverse membrane potential for the CNGC2-dependent current was close to 0 mV (Supplemental Fig. S5), which was not close enough to any of the theoretical equilibrium potentials that the ionic composition of the current could be determined. However, additional observations suggested that the CNGC2-dependent current had a Ca2+ influx component. First, this was strongly inhibited by adding Gd3+, an inward Ca2+ channel inhibitor, into the bath solution (Fig. 8, B and C). Second, the theoretical equilibrium potential for Ca2+, with 175 nm free Ca2+ inside the cell, is predicted to increase from 140 to 154 mV when the bath [Ca2+] is increased from 10 to 30 mm; experimentally, the reverse potential of the CNGC2-dependent current showed a similarly positive shift from 0 to around 17 mV (Fig. 8, B and C). In addition, the measured reverse membrane potential for the CNGC2-dependent current (Fig. 8, B and C) was similar to those reported for CNGC18 (Gao et al., 2014, 2016) and CRACM1 (Vig et al., 2006) Ca channels under the same ionic recording conditions. These data support the conclusion that CNGC2 has Ca2+ influx channel activity.
CNGC2 shows inward K+ channel activity when expressed in oocytes (Leng et al., 1999). By contrast, CNGC18 does not have inward K+ channel activity in HEK293T cells (Gao et al., 2014). Here, we did not detect inward K+ channel activity in the CNGC2-expressing HEK293T cells (Supplemental Fig. S5), although the KAT1 positive control showed typical inward K+ channel activity (Supplemental Fig. S6). In summary, we conclude that CNGC2, similar to CNGC18 (Gao et al., 2014), can mediate Ca2+ but not K+ influx across the plasma membrane in HEK293T cells.
DISCUSSION
Plant growth and development depend on mineral nutrients absorbed by the roots and redistributed into leaves through the apoplast (extracellular space) and symplast (intracellular compartments) pathways, facilitated by transpiration and membrane-located mineral ion transporters, respectively (Kumar et al., 2015). Ca is an essential plant macronutrient with key roles in cellular signaling and structural integrity (Dodd et al., 2010; Kudla et al., 2010; Reddy et al., 2011; Yang et al., 2012). The physiological pathways mediating Ca absorption and distribution in plants are well studied (White and Broadley, 2003). However, the corresponding genetic mechanisms are largely unknown (Kudla et al., 2010; Spalding and Harper, 2011). In this study, we have provided several lines of evidence to support a key role of CNGC2 in mediating Ca2+ influx into Arabidopsis leaf cells after unloading from the vascular tissues (Fig. 9). First, CNGC2 shows Ca2+ influx channel activity when expressed in HEK293T cells (Fig. 8, B and C). Second, CNGC2 is expressed predominantly in the leaf cells surrounding the free endings of the minor veins, which are the primary sites for Ca2+ unloading from the vascular tissues into the leaf cells (Fig. 8A). Third, disrupting CNGC2 caused a significant amount of Ca2+ to accumulate in the extracellular space of leaf epidermal cells, similar to cax1cax3 double mutants, which are known to be defective in Ca2+ transport from the extracellular space to the intracellular compartments (Figs. 5 and 6). Finally, at 0.1 mm Ca2+, the cngc2 mutant grew as well as the wild type; however, increasing the [Ca2+] induced cell death, H2O2 and SA accumulation (Fig. 2), as well as growth inhibition in the leaves of cngc2-1 but not of the wild type (Fig. 1), which indicates the importance for plant cells to maintain low extracellular Ca2+ through CNGC2-mediated deposition of Ca2+ into leaf cells.
Figure 9.
Model of CNGC2, CAX1, and CAX3 function in the transport of Ca2+ from the apoplast to the vacuole in Arabidopsis leaves. Ca2+ is absorbed by roots and transported upward to the apoplastic space of the leaf through the xylem driven by the leaf transpiration stream. After the Ca2+ in the xylem unloads into the apoplastic space of the leaf cells, it subsequently is transported into the cytosol through the plasma membrane-localized CNGC2 channel. The Ca2+ in the cytosol is sequestered into the vacuole via the tonoplast-localized Ca2+/H+ antiporters CAX1 and CAX3.
Since the discovery of the first CNGC2 loss-of-function mutant dnd1 (cngc2-1) in 1998, it has been noted that the mutant leaves on soil-grown plants are smaller and senesce earlier than those of wild-type plants (Yu et al., 1998). In addition, the mutant leaves have constitutively higher accumulation of H2O2 and SA than the wild-type leaves when grown in soil (Yu et al., 1998; Clough et al., 2000). However, the mutant grows normally, like the wild type, when grown on agar medium in petri dishes unless the [Ca2+] increases to 10 mm (Chan et al., 2003). In this study, by controlling the Ca2+ supply to the roots through a hydroponic growth system (Fig. 1), we demonstrated that it was the increasing [Ca2+]ext that was responsible for the observed leaf phenotypes of the mutant. It is possible that disrupting CNGC2 decreases the Ca2+ uptake capacity of the leaf cells near the minor veins. With low Ca2+ supply, the remaining Ca2+ uptake capacity of the cngc2 leaf cells still would efficiently absorb the Ca2+ in the extracellular space that was unloaded from the vascular tissues, to maintain a low [Ca2+]ext. However, when the Ca2+ supply increased, the amount of Ca2+ absorbed by the mutant leaf cells would not be able to keep pace with the amount of Ca2+ unloading from the vascular tissue, which in turn would lead to Ca2+ overaccumulation in the extracellular space of the mutant cells (Fig. 9).
The data presented in this study demonstrate that maintaining a low [Ca2+]ext is essential for the physiological functions of leaf cells and that CNGC2 plays a key role in this process. The increase of the [Ca2+]ext due to the inactivation of CNGC2 interrupts multiple aspects of leaf function through largely unknown cellular and genetic pathways. Increasing [Ca2+]ext activates class III peroxidase in the apoplast, which further promotes the degradation of H2O2 (Hepler and Winship, 2010). The plasma membrane-localized superoxide-generating NADPH oxidase RBOHD can be activated by the cytosolic [Ca2+] either directly or indirectly, which is responsible for the H2O2 burst in the apoplast (Hepler and Winship, 2010). In addition, H2O2 can activate a Ca2+-permeable channel in the plasma membrane of the root and guard cells (Hepler and Winship, 2010). An increase in cytosolic [Ca2+] can activate SA biosynthesis through Ca2+-binding proteins (Seyfferth and Tsuda, 2014). However, it is unknown how the increase of [Ca2+]ext is linked to H2O2 and SA production. Moreover, increases in [Ca2+]ext can suppress water flow in leaf, which might interfere with photosynthesis (Gilliham et al., 2011).
It is well documented that the leaves of soil-grown dnd1 (cngc2-1) mutants lack an HR but maintain their defenses against avirulent pathogen infection (Yu et al., 1998; Ali et al., 2007). In this study, the mutant leaf had a perfect HR when its root was supplied with 0.1 mm Ca2+ (Fig. 3). Three-day treatment with high [Ca2+] (10 mm) could suppress the HR induced by infection with the pathogen at low density but not at high density (Fig. 3). These data demonstrated that CNGC2 is not essential for the HR. The mutant’s defective HR in response to the low-density avirulent pathogen in this study could be an indirect consequence of the overaccumulation of Ca2+ in the apoplast. We hypothesize that the increase of [Ca2+]ext enhances the interaction of the Ca2+ with pectin and further strengthens the cell wall (Hepler and Winship, 2010), which could protect the leaf cell from death as a response to the avirulent pathogen (Choi et al., 2013; Mortimer et al., 2013; Johansson et al., 2014) but also could limit cell extensibility and decrease its growth rate. This possibility is supported by the findings that cax1cax3 mutant leaf cell wall rigidity is increased under high [Ca2+] conditions (Conn et al., 2011).
In conclusion, this study not only identifies CNGC2 as the key factor mediating Ca2+ influx into the leaf cells after Ca2+ is unloaded into the apoplast from the vascular tissues but also provides an explanation for the growth and HR-defective phenotypes of the soil-grown cngc2-1 (dnd1) mutant known since 1998 (Yu et al., 1998; Ali et al., 2007). Finally, the in vivo extracellular Ca2+ imaging method developed in this study provides a new tool for investigating Ca2+ dynamics in plant cells.
MATERIALS AND METHODS
Arabidopsis Growth Conditions and Pathogen Inoculation
To grow Arabidopsis (Arabidopsis thaliana) hydroponically, the seeds were sterilized with 75% and 100% ethanol for 15 and 5 min, respectively. The ethanol-treated seeds were dried on sterilized filter paper inside a hood. Twelve seeds were evenly distributed on an agarose-based control medium inside a petri dish, which contained the control solution with 0.1 mm CaCl2 (Wang et al., 2015). After a 3-d treatment at 4°C, the petri dishes with seeds were put horizontally inside a growth chamber with 12/12-h light/dark period and 100 to 120 μmol m−2 s−1 light intensity. Three-week-old seedlings were transferred to a 5-mL tube hydroponics box containing the same nutrient solution as a control but without sugar and agar. The hydroponic solution was changed weekly. After another 3 weeks, the solution was changed to that with different concentrations of Ca2+ and other nutrients for the times indicated in the text. The cngc2-1 (dnd1), cngc2-3 (salk_066908), cngc4-1 (dnd2), cngc4-5 (salk_081369), and cax1cax3 mutants were reported previously (Clough et al., 2000; Cheng et al., 2005; Genger et al., 2008; Liu et al., 2011; Chin et al., 2013). The cngc2-3cngc4-5 double mutant was identified using PCR in the F2 population from F1 seeds created by cross-pollination of the two single mutants. Other cngc SALK mutants (Alonso et al., 2003) and primers used for homozygous identification are listed in Supplemental Table S1.
For the hypersensitive response assay, after 3-d treatments with either 0.1 or 10 mm Ca2+, we infiltrated the avirulent pathogen Pseudomonas syringae pv tomato DC3000 avrRpm into the abaxial side of the leaves with a 1-mL syringe as described previously (Li et al., 2013).
Ca and Mg Content Measurement by Atomic Absorption Spectrometry
The leaf or root tissues for Ca and Mg content measurements in Figure 4 were harvested and dried in an oven at 105°C for 5 to 6 d. The dried tissues were weighed and ground to powder, which was further extracted with 1.5 m HCl for 2 d. The samples were centrifuged at 12,000g for 30 min, and the supernatants were used for Ca and Mg measurement with an atomic absorption spectrometer (TAS-990; Beijing Purkinje General).
For the sequential Ca extraction in Figure 7, the leaves were immediately frozen in liquid nitrogen after harvest and ground into fine powder for weighing (Borer et al., 2004, 2012). The powder was transferred into a 50-mL tube, and 20 mL of ultrapure water from the ELGA Option-Q ultrapure water system was added. The basal Ca content in the water was below 10 μm as measured with the Amplite Rhod Red fluorescence-based Ca quantification kit (Biolite). The powder-containing tube was shaken at 180 rpm, at room temperature, overnight and subsequently centrifuged at 8,000g for 30 min. The supernatant was saved as the water-soluble Ca for Ca measurement. The pellet was washed once with the ultrapure water and put into a 50°C oven overnight. The dried pellet was dissolved in 20 mL of acetic acid and shaken at 180 rpm, at room temperature, overnight. After centrifugation at 8,000g for 30 min, the supernatant was saved as the acetic acid-soluble Ca for Ca measurement. The pellet was subjected to further extraction with HCl.
Reactive Oxygen Species and Cell Death Assays in Arabidopsis Leaf
Arabidopsis leaf cell death was monitored with a Trypan Blue (Sigma-Aldrich) staining assay as described at http://commonweb.unifr.ch/biol/pub/mauchgroup/staining.html. The method for the 3,3-diaminobenzidine (Sigma-Aldrich)-based reactive oxygen species staining assay was as described previously (Zou et al., 2015).
SA Measurement and Real-Time Reverse Transcription-PCR
Arabidopsis plants were cultured in hydroponic conditions with 0.1 mm Ca2+ for 3 weeks and then given either 0.1 (as a control) or 10 mm Ca2+ treatment. Afterward, the well-expanded leaves were collected and ground into fine powder in liquid nitrogen, 50 to 100 mg of which was added into 1 mL of extraction buffer containing propanol:water:HCl (2:1:0.002, v/v/v) and 50 ng of 2H-labeled SA as an internal standard. The mixture was rotated at 100 rpm at 4°C for 1 h, after which 1 mL of dichloromethane was added and the sample was rotated at 100 rpm and 4°C for another 1 h. The mixture was then centrifuged at 12,000g at 4°C for 30 min. The tissue debris was found between the two layers of liquid phases. Solution (200 μL) from the bottom phase was taken for total SA quantification as described previously (Pan et al., 2010).
For RNA extraction, the leaf tissue fine powder was extracted with the TransZol Plant kit (Transgen Biotech). cDNA was synthesized with TransScript All-in-One First Strand cDNA Synthesis Supermix for qPCR with One-Step gDNA Removal (Transgen Biotech) following the manufacturer’s instructions. Primers for the internal standards TUBULIN β-SUBUNIT (At5g44340) and PR1 (At2g14610) were reported previously (Ma et al., 2010). Quantitative real-time PCR was performed using a SYBR Premix Ex Taq II (TaKaRa) in a qTOWER 2.2 real-time PCR system (Analytik). The level of gene transcript accumulation was first normalized to the internal standard TUBULIN β-SUBUNIT (At5g44340) and expressed relative to the PR1 gene value in Col-0 under the 0.1 mm Ca2+ condition.
GUS Reporter Gene-Based Promoter Activity Assay
A 2,186-bp DNA fragment was amplified from the genome region upstream of the CNGC2 start codon with Col-0 genomic DNA as a template. The primers used were 5′-CTAGAAGCTTCTCCAAGCCAAGCCTGGTTAAAATCGG-3′ and 5′-ATTGGATCCGATTGAAATAGAGGAACCACCATGGGAG-3′, the underlined portions of which are restriction enzyme sites for HindIII and BamHI, respectively. The amplified DNA fragment was integrated into the HindIII and BamHI cloning sites upstream of the GUS reporter gene inside the binary vector pORE R1. Agrobacterium tumefaciens strain GV3101 harboring the 2,186-bp DNA fragment-containing pORE R1 vector (Coutu et al., 2007) was used to transform Arabidopsis (Clough and Bent, 1998). Transgenic Arabidopsis screening and GUS assays were as described previously (Zou et al., 2015).
HEK293T Cell Culture, Transfection, and Patch-Clamp Whole-Cell Recording
The CNGC2 coding sequence was amplified with primers 5′-CGGAATTCATGCCCTCTCACCCCAACTTCAT-3′ and 5′-CCCCGGGTTATTCGAGATGATCATGCGGTCG-3′, the underlined portions of which are restriction enzyme sites for EcoRI and SmaI, respectively. The fragment was integrated into the EcoRI and SmaI sites of the pCI-neo vector. HEK293T cells were purchased from the Institute of Biotechnology and Cell Biology, Chinese Academy of Science. The procedures for HEK293T cell culture, transfection, and patch-clamp whole-cell recording were as described previously (Vig et al., 2006; Gao et al., 2014, 2016). For cAMP treatment, 200 μm 8-bromoadenosine cAMP (Sigma-Aldrich) was used. The theoretical equilibrium potentials were calculated with an online Nernst Potential Calculator at http://www.physiologyweb.com/calculators/nernst_potential_calculator.html.
Observation of Extracellular Ca2+ Distribution with Fluorescence and Confocal Microscopy
The low-affinity cell-impermeable Ca2+ fluorescent dye OGB-5N with excitation and emission at 494 and 521 nm, respectively, was purchased from Thermo Fisher Scientific (O-6812) and dissolved in DMSO to make a 5 mm stock stored at −20°C without exposure to light. For visualizing extracellular Ca2+ distribution, a fully expanded mature leaf was detached from a hydroponically grown Arabidopsis plant, and its petiole was put into a PCR tube containing 100 μL of growth solution including 20 μm OGB-5N and either 0.1 or 10 mm CaCl2 for the indicated periods of time. Afterward, the leaf was stained with 20 μm propidium iodide for 10 min. Images presented in Figure 5 were acquired with a fluorescence microscope (Nikon Eclipse Ti). Images presented in Figure 6 were acquired with a confocal laser scanning microscope (Zeiss 710).
Accession Numbers
Sequence data for this article can be found in The Arabidopsis Information Resource at www.arabidopsis.org under the following accession numbers: AtCNGC2 (At5g15410), AtCNGC4 (At5g54250), AtCAX1 (At2g38170), and AtCAX3 (At3g51860).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Root responses of cngc2-1 to different Ca2+ concentrations in a hydroponic system.
Supplemental Figure S2. Responses of hydroponically grown Arabidopsis cngc mutants to Ca2+.
Supplemental Figure S3. Ca-dependent growth of cngc2-1 and cax1cax3 seedlings.
Supplemental Figure S4. CNGC2 promoter-driven GUS reporter gene expression in Arabidopsis young seedlings and mature plants.
Supplemental Figure S5. CNGC2 shows inward Ca2+ channel activity in HEK293T cells.
Supplemental Figure S6. Inward K+ channel activity of CNGC2 and KAT1 in HEK293T cells.
Supplemental Table S1. Primer list for the identification of Arabidopsis cngc homozygous mutants.
Supplementary Material
Acknowledgments
We thank Dr. Yong-Fei Wang at the Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, for instruction on HEK293T cell-based patch-clamp experiments as well as Dr. Kendal D. Hirschi at Texas A&M University and Dr. Ying-Shin Chen at the Agricultural Biotechnology Research Center, Taiwan, for cax1cax3 seeds.
Glossary
- Col-0
Columbia-0
- HR
hypersensitive response
- SA
salicylic acid
- cfu
colony-forming units
- OGB-5N
Oregon Green BAPTA 488 5N
Footnotes
This work was supported by the National Natural Science Foundation of China (grant nos. 31571448 and 31171364 to Z.Q.), by the Collaborative Innovation Center of Grassland, Ecology, and Husbandry, Department of Education of Inner Mongolia Autonomous Region, People’s Republic of China (to Z.Q.), and by the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (grant no. CAAS-ASTIP-2016-IGRCAAS to Z.W. and X.H.).
References
- Ahn IP. (2007) Disturbance of the Ca2+/calmodulin-dependent signalling pathway is responsible for the resistance of Arabidopsis dnd1 against Pectobacterium carotovorum infection. Mol Plant Pathol 8: 747–759 [DOI] [PubMed] [Google Scholar]
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA (2007) Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19: 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Baker A, Ceasar SA, Palmer AJ, Paterson JB, Qi W, Muench SP, Baldwin SA (2015) Replace, reuse, recycle: improving the sustainable use of phosphorus by plants. J Exp Bot 66: 3523–3540 [DOI] [PubMed] [Google Scholar]
- Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009) Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5: e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedison JE, Johnson AH (2010) Seventy-four years of calcium loss from forest soils of the Adirondack Mountains, New York. Soil Sci Soc Am J 74: 2187–2195 [Google Scholar]
- Behera S, Krebs M, Loro G, Schumacher K, Costa A, Kudla J (2013) Ca2+ imaging in plants using genetically encoded Yellow Cameleon Ca2+ indicators. Cold Spring Harb Protoc 2013: 700–703 [DOI] [PubMed] [Google Scholar]
- Borer CH, Hamby MN, Hutchinson LH (2012) Plant tolerance of a high calcium environment via foliar partitioning and sequestration. J Arid Environ 85: 128–131 [Google Scholar]
- Borer CH, Schaberg PG, DeHayes DH, Hawley GJ (2004) Accretion, partitioning and sequestration of calcium and aluminum in red spruce foliage: implications for tree health. Tree Physiol 24: 929–939 [DOI] [PubMed] [Google Scholar]
- Callamaras N, Parker I (2000) Ca2+-dependent activation of Cl− currents in Xenopus oocytes is modulated by voltage. Am J Physiol Cell Physiol 278: C667–C675 [DOI] [PubMed] [Google Scholar]
- Chan CW, Schorrak LM, Smith RK Jr, Bent AF, Sussman MR (2003) A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol 132: 728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, DeFalco TA, Moeder W, Yoshioka K (2013) The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol 163: 611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Kim NH, Lee YK, Hwang BK (2013) The pepper extracellular xyloglucan-specific endo-β-1,4-glucanase inhibitor protein gene, CaXEGIP1, is required for plant cell death and defense responses. Plant Physiol 161: 384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK Jr, Bent AF (2000) The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 97: 9323–9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, Schreiber AW, Baumann U, Moller I, Cheng NH, Stancombe MA, Hirschi KD, Webb AA, et al. (2011) Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23: 240–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu C, Brandle J, Brown D, Brown K, Miki B, Simmonds J, Hegedus DD (2007) pORE: a modular binary vector series suited for both monocot and dicot plant transformation. Transgenic Res 16: 771–781 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Gao QF, Fei CF, Dong JY, Gu LL, Wang YF (2014) Arabidopsis CNGC18 is a Ca2+-permeable channel. Mol Plant 7: 739–743 [DOI] [PubMed] [Google Scholar]
- Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, Sun SJ, Dong JY, Liu H, Wang YF (2016) Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc Natl Acad Sci USA 113: 3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc Y, Tester M, McDonald GK (2010) Calcium requirement of wheat in saline and non-saline conditions. Plant Soil 327: 331–345 [Google Scholar]
- Genger RK, Jurkowski GI, McDowell JM, Lu H, Jung HW, Greenberg JT, Bent AF (2008) Signaling pathways that regulate the enhanced disease resistance of Arabidopsis “defense, no death” mutants. Mol Plant Microbe Interact 21: 1285–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV (1998) Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol 8: 1335–1338 [DOI] [PubMed] [Google Scholar]
- Gilliham M, Dayod M, Hocking BJ, Xu B, Conn SJ, Kaiser BN, Leigh RA, Tyerman SD (2011) Calcium delivery and storage in plant leaves: exploring the link with water flow. J Exp Bot 62: 2233–2250 [DOI] [PubMed] [Google Scholar]
- Halman J, Schaberg P, Hawley G, Hansen C (2011) Potential role of soil calcium in recovery of paper birch following ice storm injury in Vermont, USA. For Ecol Manage 261: 1539–1545 [Google Scholar]
- Hepler PK, Winship LJ (2010) Calcium at the cell wall-cytoplast interface. J Integr Plant Biol 52: 147–160 [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93: 8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S, Gee KR, Baylor SM (2009) Low-affinity Ca2+ indicators compared in measurements of skeletal muscle Ca2+ transients. Biophys J 97: 1864–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Bo S, Li-Ye C, Ming-An S (2008) Calcium as a versatile plant signal transducer under soil water stress. BioEssays 30: 634–641 [DOI] [PubMed] [Google Scholar]
- Jiménez-Moreno R, Wang ZM, Gerring RC, Delbono O (2008) Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J 94: 3178–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ON, Fantozzi E, Fahlberg P, Nilsson AK, Buhot N, Tör M, Andersson MX (2014) Role of the penetration-resistance genes PEN1, PEN2 and PEN3 in the hypersensitive response and race-specific resistance in Arabidopsis thaliana. Plant J 79: 466–476 [DOI] [PubMed] [Google Scholar]
- Johnson AH, Moyer A, Bedison JE, Richter SL, Willig SA (2008) Seven decades of calcium depletion in organic horizons of Adirondack forest soils. Soil Sci Soc Am J 72: 1824–1830 [Google Scholar]
- Jurkowski GI, Smith RK Jr, Yu IC, Ham JH, Sharma SB, Klessig DF, Fengler KA, Bent AF (2004) Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “defense, no death” phenotype. Mol Plant Microbe Interact 17: 511–520 [DOI] [PubMed] [Google Scholar]
- Karley AJ, White PJ (2009) Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Curr Opin Plant Biol 12: 291–298 [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352: 524–526 [DOI] [PubMed] [Google Scholar]
- Krapp A. (2015) Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Curr Opin Plant Biol 25: 115–122 [DOI] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh UM, Manohar M, Gaur VS (2015) Calcium transport from source to sink: understanding the mechanism(s) of acquisition, translocation, and accumulation for crop biofortification. Acta Physiol Plant 37: 1722 [Google Scholar]
- Leng Q, Mercier RW, Yao W, Berkowitz GA (1999) Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol 121: 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys BA, Likens GE, Johnson CE, Craine JM, Lacroix B, McLauchlan KK (2016) Natural and anthropogenic drivers of calcium depletion in a northern forest during the last millennium. Proc Natl Acad Sci USA 113: 6934–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang J, Ma C, Zhao Y, Wang Y, Hasi A, Qi Z (2013) Glutamate receptor-like channel3.3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis. Plant Physiol 162: 1497–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Aung K, Tseng CY, Chang TY, Chen YS, Chiou TJ (2011) Vacuolar Ca2+/H+ transport activity is required for systemic phosphate homeostasis involving shoot-to-root signaling in Arabidopsis. Plant Physiol 156: 1176–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YB, Pan XB, Li JS (2015) A 1961-2010 record of fertilizer use, pesticide application and cereal yields: a review. Agron Sustain Dev 35: 83–93 [Google Scholar]
- Ma W, Smigel A, Walker RK, Moeder W, Yoshioka K, Berkowitz GA (2010) Leaf senescence signaling: the Ca2+-conducting Arabidopsis cyclic nucleotide gated channel2 acts through nitric oxide to repress senescence programming. Plant Physiol 154: 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar M, Shigaki T, Hirschi KD (2011) Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol (Stuttg) 13: 561–569 [DOI] [PubMed] [Google Scholar]
- McDowell SC, Akmakjian G, Sladek C, Mendoza-Cozatl D, Morrissey JB, Saini N, Mittler R, Baxter I, Salt DE, Ward JM, et al. (2013) Elemental concentrations in the seed of mutants and natural variants of Arabidopsis thaliana grown under varying soil conditions. PLoS ONE 8: e63014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Yu X, Albrecht S, Sicilia F, Huichalaf M, Ampuero D, Michaelson LV, Murphy AM, Matsunaga T, Kurz S, et al. (2013) Abnormal glycosphingolipid mannosylation triggers salicylic acid-mediated responses in Arabidopsis. Plant Cell 25: 1881–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Närhi P, Middleton M, Gustavsson N, Hyvönen E, Sutinen ML, Sutinen R (2011) Importance of soil calcium for composition of understory vegetation in boreal forests of Finnish Lapland. Biogeochemistry 102: 239–249 [Google Scholar]
- Pan X, Welti R, Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5: 986–992 [DOI] [PubMed] [Google Scholar]
- Pilbeam DJ. (2015) Breeding crops for improved mineral nutrition under climate change conditions. J Exp Bot 66: 3511–3521 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Hirschi KD (2016) CAX-ing a wide net: cation/H+ transporters in metal remediation and abiotic stress signalling. Plant Biol (Stuttg) 18: 741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, Ali GS, Celesnik H, Day IS (2011) Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23: 2010–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. (2013) Modulating plant calcium for better nutrition and stress tolerance. ISRN Botany 2013: 952043 [Google Scholar]
- Rusakov DA, Fine A (2003) Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron 37: 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfferth C, Tsuda K (2014) Salicylic acid signal transduction: the initiation of biosynthesis, perception and transcriptional reprogramming. Front Plant Sci 5: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood MW, Prior IA, Voronina SG, Barrow SL, Woodsmith JD, Gerasimenko OV, Petersen OH, Tepikin AV (2007) Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA 104: 5674–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Harper JF (2011) The ins and outs of cellular Ca2+ transport. Curr Opin Plant Biol 14: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhelm AF, Pregitzer KS, Burton AJ, Zak DR (2012) Air pollution and the changing biogeochemistry of northern forests. Front Ecol Environ 10: 181–185 [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Y, Yang J, Ma C, Zhang Y, Ge T, Qi Z, Kang Y (2015) Arabidopsis ROOT HAIR DEFECTIVE3 is involved in nitrogen starvation-induced anthocyanin accumulation. J Integr Plant Biol 57: 708–721 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu WH (2015) Genetic approaches for improvement of the crop potassium acquisition and utilization efficiency. Curr Opin Plant Biol 25: 46–52 [DOI] [PubMed] [Google Scholar]
- Ward JM. (2001) Identification of novel families of membrane proteins from the model plant Arabidopsis thaliana. Bioinformatics 17: 560–563 [DOI] [PubMed] [Google Scholar]
- White C, McGeown G (2002) Imaging of changes in sarcoplasmic reticulum [Ca2+] using Oregon Green BAPTA 5N and confocal laser scanning microscopy. Cell Calcium 31: 151–159 [DOI] [PubMed] [Google Scholar]
- White PJ. (2001) The pathways of calcium movement to the xylem. J Exp Bot 52: 891–899 [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR (2003) Calcium in plants. Ann Bot (Lond) 92: 487–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Punshon T, Guerinot ML, Hirschi KD (2012) Plant calcium content: ready to remodel. Nutrients 4: 1120–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IC, Parker J, Bent AF (1998) Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA 95: 7819–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JJ, Li XD, Ratnasekera D, Wang C, Liu WX, Song LF, Zhang WZ, Wu WH (2015) Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 27: 1445–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.