Abstract
Using pharmacologic and biochemical criteria, we evaluated whether uptake of four different Chlamydia trachomatis serovars, D, E, K, and L2, was dependent upon lipid rafts. Our data suggest that lipid raft-mediated entry is not required for C. trachomatis infection of cultured epithelial cells.
Microbes often enter into nonphagocytic cells by usurping normal host cell endocytic pathways. In addition to clathrin-mediated endocytosis, various nonclathrin endocytic mechanisms have been identified, including uptake through caveolae, macropinosomes, and a separate constitutive pathway (reviewed in reference 24). These non-clathrin-dependent pathways do not use known coat complexes for cargo recruitment and budding of transport intermediates but are heterogeneous in nature. A subset has been identified that utilizes lipid rafts, specialized dynamic, detergent-resistant regions of the plasma membrane enriched in cholesterol, glycosphingolipids, glycosylphosphatidylinositol-anchored proteins, and some membrane proteins, including caveolin (2, 33). A subtype of lipid rafts, caveolae, has a distinct cave-like morphology in the plasma membrane. Whereas caveolin is widely distributed, caveolae are found in only a few cell types and tissues (16).
An increasing number of pathogens, including some bacteria, viruses, and even parasites, have been suggested to enter cells through lipid rafts (reviewed in reference 8). These conclusions are based on pharmacologic, biochemical, and cell biological assays. Inhibition of pathogen entry by drugs that are known to extract or bind to membrane cholesterol, such as methyl-β-cyclodextrin (MβCD), filipin, and nystatin, has been used as evidence for lipid raft involvement (35). However, MβCD has pleiotropic effects, including disruption of clathrin-mediated endocytosis, and should not be used as the sole criterion for defining lipid raft-dependent processes. Lipid rafts have also been defined by their resistance to extraction with nonionic detergents at 4°C (hence, they are often referred to as detergent-resistant membranes [DRMs]) and their sedimentation in low-density membrane fractions upon sucrose gradient centrifugation. The colocalization of caveolin or GM1 with pathogens is additional evidence that raft-mediated entry may be involved (7). However, the criterion used to define lipid rafts is at best imprecise and still evolving.
Chlamydia spp. are obligate intracellular parasites that are associated with many important human diseases and undergo a dimorphic developmental cycle (13). They alternate between an extracellular, spore-like form, the elementary body (EB), and an intracellular form, the metabolically active but noninfectious reticulate body. The specific bacterial ligand and host receptor(s) that mediate entry are still unclear. Heparan sulfate acts as a bridging molecule for a relatively weak and reversible interaction (12, 38, 41, 42) that is followed by a stronger, more specific binding to an unidentified secondary receptor (5, 11). Internalization is accompanied by induction of a microvillus-like structure over a large portion of the host cell in a process that is dependent upon actin polymerization (3) and is mediated through Rac (4). Upon uptake, the organism is sequestered in a membrane-bound compartment, termed the chlamydial inclusion, which rapidly separates from the endocytic pathway and avoids phagolysosomal fusion (30, 31). After replicating by binary fission within the ever-enlarging inclusion over a 48- to 72-h time frame, the host cell is lysed and the bacteria are released.
Despite recent advances, the entry mechanism of Chlamydia trachomatis is still unclear. Expression of a dominant negative allele of dynamin did not diminish entry, suggesting that neither clathrin nor caveola-mediated uptake is involved. However, these findings do not rule out the possibility that lipid rafts are involved. To further investigate this hypothesis, we carefully tested whether C. trachomatis serovars L2, D, E, and K are associated with lipid rafts, caveolin, or GM1 during entry. In contrast to some published studies (18, 25, 36), we were unable to find evidence that C. trachomatis entry is associated with or requires lipid rafts.
We first confirmed that acute depletion of plasma membrane cholesterol by the cholesterol-chelating agent MβCD inhibits C. trachomatis entry. C. trachomatis was propagated as previously described (40). HeLa 229 cells (obtained from the American Type Culture Collection) were seeded onto acid-treated 12-mm coverslips at a density of 104 cells/coverslip and grown overnight at 37°C in minimal essential medium (MEM; Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL). The cells were washed extensively with serum-free MEM and pretreated for 30 min with MβCD (Sigma) diluted into MEM. The cells were then infected for 1 h with C. trachomatis serovar LGV L2, E, D, or K in the presence of MβCD diluted into either MEM (L2) or sucrose-phosphate-glutamic acid medium (SPG; D, E, and K) at a multiplicity of infection (MOI) of 1 to 5 (9). HeLa cells were then washed with phosphate-buffered saline (PBS) and grown overnight in the presence of MEM supplemented with 10% FBS and 1 mg of heparin (Sigma)/ml to inhibit further uptake of C. trachomatis. Twenty hours postinfection, HeLa cells were fixed with ice-cold methanol and stained with a fluorescein isothiocyanate (FITC)-conjugated mouse anti-Chlamydia antibody and Evan's blue (Meridian Diagnostics). The extent of C. trachomatis infection was determined by scoring the percentage of HeLa cells containing inclusions as revealed by immunofluorescence (IF) microscopy (a minimum of 300 cells/coverslip were counted, and experiments were done in triplicate). The infection efficiency was set to 100% for control conditions, and all other treatments were normalized to this value.
Similar to the published results of others (18), we found that MβCD inhibited infectivity of serovars L2, E, D, and K (data not shown). Maximal inhibition was achieved at a concentration of 3 mM, resulting in a decrease in infectivity of approximately 60% for serovars L2 and E and 50% for serovars D and K (P < 0.002 by Student's unpaired two-tailed t test, drug versus no drug for each serovar). Treatment of infected cells with MβCD for 2 h postinfection did not decrease the efficiency of infection relative to that in untreated controls (data not shown).
To evaluate whether MβCD inhibited binding or entry, HeLa cells were pretreated for 30 min with MβCD (3 mM) or heparin (1 mg/ml), an agent known to decrease serovar L2 and E binding and entry (38, 42), and subsequently infected for 1 h (MOI of 1 to 5) at 4°C in the presence of these reagents. The percentage of HeLa cells with associated C. trachomatis was determined by IF microscopy. Due to clumping and the small size of EBs, this method was more accurate than determining the average number of EBs per cell. A minimum of 200 cells per slip was counted, and all experiments were performed in triplicate. Extraction of cholesterol with MβCD had no effect on the binding of either serovar L2 or E with HeLa cells, while treatment with heparin led to dramatic reductions in binding, approximately 80% of control treatments (P < 0.0001, Student's two-tailed unpaired t test [data not shown]). We also determined whether MβCD itself was toxic to C. trachomatis. Pretreatment of serovar L2 or E for 1 h at 37°C with MβCD (3 mM) or medium alone was followed by extensive washing to remove any residual MβCD. The resulting EBs were used to infect HeLa cells, and the percentage of infected cells was determined 20 h postinfection. No reduction in infectivity was observed following pretreatment with MβCD of serovar L2 or E EBs, demonstrating that MβCD was acting upon host cells and not bacteria (data not shown). Finally, HeLa cells that had been pretreated and infected in the presence of a 3 mM MβCD-83 μM cholesterol complex exhibited infection efficiencies similar to those of untreated cells (data not shown). Together, these data support the idea that the reduction in infectivity was occurring at the level of chlamydial entry rather than development.
Given the potential pleiotropic effects of extensive cholesterol extraction on membrane structure and function, we utilized the more specific lipid raft-disrupting reagents filipin and nystatin. These drugs bind to plasma membrane cholesterol without extracting it, and they are used widely to demonstrate the involvement of lipid rafts and caveolae in biological processes (35). Pretreatment and infection of HeLa cells in the presence of either filipin (2.5 or 5 μg/ml; Sigma) or nystatin (12.5 or 25 μg/ml; Sigma) did not significantly alter the infectivity of C. trachomatis serovars L2, E, D, and K relative to that of untreated control infections (data not shown). These reagents also had no effect on the morphology of the chlamydial inclusion (data not shown). It was not possible to do progeny assays due to the toxicity of the drugs during extended tissue culture times.
Despite using concentrations of nystatin and filipin that have been reported in the literature to disrupt caveolae and lipid rafts (35), our results contrasted with those of Norkin et al. and Stuart et al. (25, 36). These investigators reported that nystatin and filipin inhibited the entry of serovars E, F, and K, but not L2, into HeLa cells. At the concentrations of filipin and nystatin used in our study, a dramatic shift in the association of caveolin from DRM to non-DRM fractions was observed, suggesting that the drugs were effective (Fig. 1; also, see below).
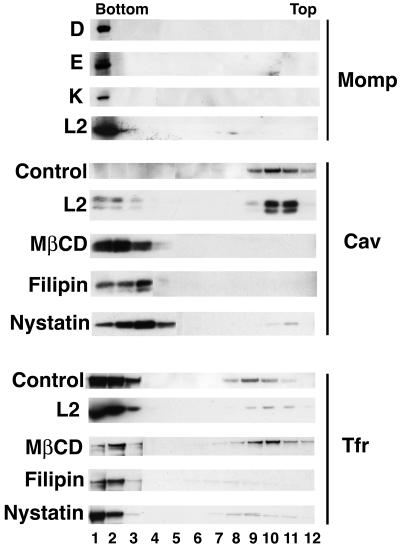
FIG. 1.
C. trachomatis does not cofractionate with DRMs. HeLa cells were infected with the indicated C. trachomatis serovars for 1 h at 37°C, and DRMs were prepared as described in the text. The fractionation profiles for each of the C. trachomatis serovars, as visualized by staining with an antibody to MOMP, are shown in the upper panel. The middle and lower panels show the fractionation profiles for untreated HeLa cells (control), L2-infected HeLa cells, and HeLa cells treated with the lipid-disrupting agents MβCD, filipin, and nystatin and immunoblotted with an antibody to caveolin (middle panel) or TfR (lower panel) as described in the text. The caveolin and TfR sedimentation in serovar D-, E-, and K-infected cells was similar to that seen in the L2-infected cells (data not shown). In some samples, caveolin migrated as a doublet, possibly due to phosphorylation (20).
We further addressed whether C. trachomatis was associated with lipid rafts by subcellular fractionation of infected cells. Due to their buoyant nature, DRMs can be isolated from cells by sucrose density gradient fractionation following cell lysis with Triton X-100 at 4°C (34). HeLa cells were infected with C. trachomatis serovar L2, D, E, or K for 1 h, and the infected cells were extracted with Triton X-100 at 4°C followed by centrifugation through a sucrose gradient as previously described (34), with the following modifications. To both synchronize and improve the efficiency of infection, the cultures were centrifuged upon addition of EBs for 5 min at 1,000 rpm in a Sorvall RT6000B centrifuge. As controls, we also prepared lysates from HeLa cells treated with filipin (5 μg/ml), nystatin (25 μg/ml), or MβCD (3 mg/ml). Following treatment, cells were washed three times with PBS, harvested by scraping, and lysed in ice-cold TEN buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 1 mM EDTA) containing 1% Triton X-100 and protease inhibitors (Complete cocktail; Roche) for 20 min on ice. To ensure complete lysis, cells suspensions were passed five times through a 27-gauge needle. Lysates were centrifuged at 800 × g for 10 min, and the postnuclear supernatant was diluted to 40% sucrose in TEN buffer by the addition of an equal volume of 80% sucrose dissolved in TEN buffer. The 40% sucrose solution (600 μl) was overlaid with 2,400 μl of 30% sucrose and 600 μl of 5% sucrose. The resulting gradient was centrifuged in an SW55Ti rotor (Beckmann Coulter) for 20 h at 200,000 × g at 15°C. Twelve equivalent 300-μl fractions were collected beginning at the top of the gradient and diluted twofold with 2× sodium dodecyl sulfate sample buffer containing 10 mM dithiothreitol and boiled for 5 min. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on either 12% gels (for the detection of major outer membrane protein [MOMP] and caveolin) or 8% gels (for the detection of transferrin receptor [TfR]). Proteins were transferred to Immobilon membranes (Millipore) by semidry blotting, blocked with a 5% solution of skim milk powder, and detected by enhanced chemiluminescence (Amersham Biosciences) according to the manufacturer's protocol. All antibodies (goat anti-C. trachomatis MOMP antibody [Cortex Biochem], rabbit anticaveolin antibody [BD Transduction Laboratories], mouse anti-TfR [I. Trowbridge, Salk Institute, La Jolla, Calif.], goat anti-rabbit immunoglobulin G [IgG]-Alexa 488 [Molecular Probes], goat anti-mouse IgG-Alexa 568 [Molecular Probes], rabbit anti-goat IgG-horseradish peroxidase [HRP] [Calbiochem], and goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP [Amersham Biosciences]) were used at a 1:5,000 dilution.
As shown in Fig. 1, for untreated cells (control, middle panel), the lipid raft marker caveolin was found predominantly in fractions obtained from the top of the gradient, while most of the TfR was found in fractions obtained from the bottom of the gradient (control, lower panel). The distribution of these markers did not change in cells infected with serovar L2 (middle and lower panels) or D, E, and K (data not shown). Importantly, for each of the C. trachomatis serovars tested (upper panel), the vast majority of MOMP was found in fractions obtained from the bottom of the gradient, indicating that the bacteria were not associating with lipid rafts under these conditions. In contrast, exposure of the HeLa cells to MβCD, filipin, or nystatin resulted in the near-complete loss of caveolin association with the DRM fractions (middle panel); instead, caveolin partitioned with fractions associated with TfR (bottom panel). We note that the caveolin-containing fractions in nystatin-treated cells were shifted slightly less than the corresponding fractions in MβCD- and filipin-treated cells.
During the course of our studies, Dautry-Varsat and colleagues reported that C. trachomatis serovar L2 associated with DRM fractions as judged by Triton X-100 extraction at 4°C followed by sucrose gradient fractionation (18). In their experiments, equivalent amounts of protein from each fraction were compared, whereas we analyzed equal volumes of each fraction. Since lipid rafts comprise only a small percentage of the total cellular protein (19), it is more biologically accurate to compare equal volumes from each fraction rather than to normalize for protein concentration. The latter approach would overestimate the proportion of caveolin in fractions that contain lower levels of total protein.
As an alternative method to assess the role of lipid rafts in C. trachomatis entry, we determined the effect of antibody-mediated patching of the β-subunit of cholera toxin (CTxβ) bound to ganglioside GM1-containing lipid rafts on C. trachomatis infection. It was previously shown that patching of lipid rafts leads to lipid raft coalescence and alterations in protein localization, signaling events, and endocytosis (15, 21, 23, 27). Exogenous addition of CTxβ also blocks bladder epithelial cell invasion by uropathogenic Escherichia coli, which is known to utilize lipid rafts for entry (7).
Lipid raft patching was induced as described previously (10) with the following modifications. HeLa cells were seeded at a density of 2× 104 cells/12-mm coverslip and grown overnight at 37°C. Twenty hours later HeLa cells were washed three times with PBS and incubated for 30 min at 4°C in MEM containing a 10-μg/ml solution of CTxβ labeled with Alexa Fluor 594 (Molecular Probes). Cells were then washed with PBS and incubated in MEM containing either a 1:200 dilution of a goat anti-CTxβ polyclonal antibody (Molecular Probes) or a control polyclonal antibody (rabbit anti-Pseudomonas aeruginosa ExoT polyclonal antibody [17]) for a further 30 min at 4°C. At the conclusion of this incubation, the cells were shifted to 37°C for 30 min. They were washed with PBS and infected with the various C. trachomatis serovars at an MOI of 1 to 5 for 1 h in SPG. Following washing with PBS, the infected cells were grown for 20 h at 37°C in the presence of MEM supplemented with 10% FBS and 1 mg of heparin/ml. In parallel experiments, uninfected cells treated as described above were fixed with 4% paraformaldehyde (Sigma) and mounted onto slides, and the efficiency of patching was determined by IF microscopy. Twenty hours postinfection HeLa cells were fixed with ice-cold methanol and processed for IF microscopy using a FITC-conjugated mouse anti-Chlamydia antibody (Meridian Diagnostics), and the percentage of infected cells was determined by IF microscopy as described above.
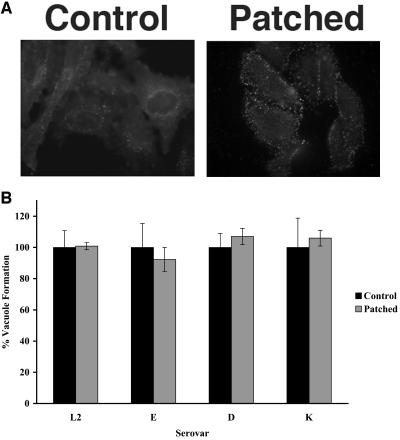
Figure 2 (upper panel) shows the distribution of CTxβ 1 h postinfection following treatment with either an anti-CTxβ antibody (patched) or a control antibody. In the absence of an antibody specific for CTxβ, diffuse staining of GM1 was observed, while incubation with an antibody directed against CTxβ led to dramatic punctate staining, demonstrating efficient patching of GM1 containing lipid rafts. Although patching of GM1 was striking, no significant effect was observed on C. trachomatis infection of patched HeLa cells relative to control infections (Fig. 2, lower panel). These results are consistent with our observation that the raft-disrupting agents filipin and nystatin failed to inhibit C. trachomatis infection.
FIG. 2.
CTxβ-mediated patching of GM1 does not impair C. trachomatis infection. HeLa cells were subjected to CTxβ-mediated patching of GM1 and infected with the indicated C. trachomatis serovar. (Upper) The effect of patching 1 h postinfection was revealed by IF microscopy using fluorescent-conjugated CTxβ. (Lower) At 20 h postinfection, the total number of inclusions was determined by IF microscopy for 10 fields. The fraction of control cells infected with C. trachomatis was set to 100%, and the fraction of infected cells following patching was normalized to this value. Error bars represent the standard deviations for an experiment done in triplicate and did not reach statistical significance compared to the control sample by Student's two-tailed unpaired t test.
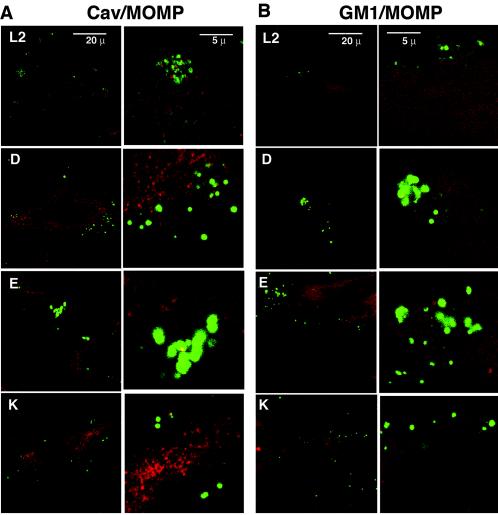
Finally, we used conventional and confocal IF microscopy to determine whether the EBs colocalized with known lipid raft markers. HeLa cells were infected with serovar L2, D, E, or K for 1 h prior to fixation for IF microscopy and stained with antibodies to MOMP and either the lipid raft protein caveolin or a fluorescent conjugate of CTxβ, which binds the lipid raft marker GM1. Caveolin staining of uninfected cells revealed the expected punctate staining pattern, and fluorescent CTxβ staining revealed primarily a plasma membrane distribution of GM1 (data not shown); interestingly, these two lipid raft components did not colocalize with each other under the conditions of our experiments, suggesting that they identify nonoverlapping lipid raft populations (data not shown). By both laser scanning confocal IF microscopy (Fig. 3) and conventional IF microscopy (data not shown), negligible colocalization of caveolin or GM1 with attached and/or entering EBs could be detected after 1 h of infection. There was also no obvious change in the subcellular distribution of either of these two raft-associated markers (data not shown).
FIG. 3.
C. trachomatis EBs do not colocalize with caveolin or GM1. HeLa cells were infected with the indicated serovars for 1 h and then fixed and stained with antibodies to MOMP (green) and to caveolin (red [A]) or with fluorescent-conjugated CTxβ (red [B]). The samples were examined by laser scanning confocal microscopy using a 60× high numerical aperture oil immersion objective on a Zeiss LSM2 microscope. A single representative 0.35-μm x-y slice is shown. The pictures in the right-hand column of each panel represent a 4× digital enlargement of a section from the corresponding picture in the left column.
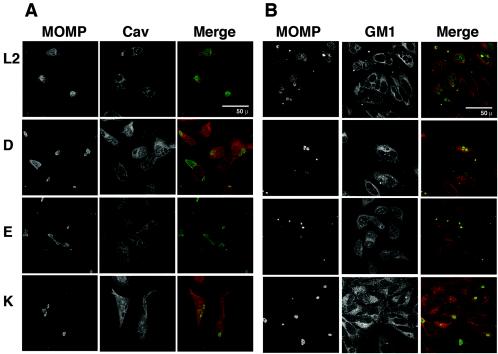
C. trachomatis inclusions have been shown to have properties that suggest that they are extensions of the trans-Golgi network. In particular, they have been shown to receive cholesterol and sphingolipids, components of lipid rafts, from the trans-Golgi network (6, 14). We therefore determined whether C. trachomatis inclusions colocalized with caveolin or GM1 late in the infection process. HeLa cells were infected with serovars L2, D, E, and K for 20 h and examined by conventional (data not shown) and confocal IF (Fig. 4) microscopy. Although the size and number of inclusions per cell varied with the different serovars, neither caveolin nor GM1 (as revealed by staining with fluorescent CTxβ) was observed to colocalize with inclusions as revealed in these selected single 0.35-μm x-y sections. In the FITC channel, the MOMP-stained inclusions appeared as holes in the cell stained with an antibody against caveolin (Fig. 4A) or with CTxβ (Fig. 4B). Together, these findings suggest that although the inclusion is known to intercept host cell sphingolipids, it does not contain or receive GM1 or caveolin.
FIG. 4.
C. trachomatis inclusions do not colocalize with caveolin or GM1. HeLa cells were infected with the indicated serovar for 1 h and washed, and infection was allowed to proceed for 20 h. The samples were fixed, stained, and examined by laser scanning confocal microscopy. The FITC, tetramethyl rhodamine isocyanate, and merged layers of a single representative 0.35-μm x-y slice for each serovar are shown. (A) In the merged micrographs (third column), MOMP staining is shown in green and the caveolin staining is shown in red. Note that the caveolin staining and MOMP staining are mutually exclusive. (B) In the merged micrographs (third column), MOMP staining is shown in green and the GM1 staining, as revealed by fluorescent-conjugated CTxβ, is shown in red. Note that the GM1 staining and MOMP staining are mutually exclusive.
It has been reported that caveolin colocalizes with serovar E, F, and K inclusions at both early and late times after infection (25, 36). It is possible that this discrepancy relates to differences in the antibody; we used a rabbit polyclonal antibody, whereas a mouse monoclonal antibody was used in other published studies (36). However, it seems extremely unlikely that our polyclonal antibody would have a more restricted reactivity than the monoclonal antibody that they used. Furthermore, in the published studies (25, 36), the IF micrographs show staining throughout the inclusion, a finding that implies that the interior of the inclusion, including the reticulate bodies, is stained with the caveolin antibody. Although it is possible that EBs acquired caveolin during entry, particularly since this protein binds cholesterol (22), we could not visualize an association of caveolin with EBs during entry. We suggest that there may have been spurious cross-reactivity between the mouse monoclonal antibody and a chlamydial protein. In support of our findings that caveolin is not required for C. trachomatis entry, it has been reported that C. trachomatis infection of caveolin-deficient FRT cells is no less efficient than that of FRT cells that express caveolin (36).
In summary, by multiple approaches we have performed an exhaustive study to examine whether lipid raft-dependent entry pathways are utilized by C. trachomatis. Our studies confirm that MβCD inhibits C. trachomatis entry (18). However, interpretation of experiments using this drug is complicated by the fact that cholesterol depletion is likely to have pleiotropic effects on membrane processes (28, 37). Our further in-depth experiments indicated that pathways that involve lipid rafts are not required for the uptake and development of four different serovars of C. trachomatis under the conditions of our assays. First, neither nystatin nor filipin inhibited entry of serovars L2, D, E, and K, as judged by inclusion formation. Second, CTxβ-induced aggregation of GM1, a component of some lipid rafts, failed to diminish chlamydial entry and intracellular development. Third, the vast majority of bacteria did not cosediment with caveolin in DRMs, a marker for lipid rafts, after Triton X-100 extraction at 4°C; rather, most of the bacteria cosedimented with the TfR-containing fractions. Fourth, by neither conventional nor confocal IF microscopy could we detect colocalization between caveolin and GM1 and either bound or internalized EBs or with mature inclusions. Fifth, overexpression of an N-terminal deletion mutant of caveolin, CavDGV, which has been reported to inhibit trafficking of cholesterol to the plasma membrane (29), did not affect L2 entry or development (unpublished results). Sixth, previously published experiments that failed to show inhibition of entry of C. trachomatis by genistein (9), which blocks caveolar endocytosis (26, 32, 39), support the notion that caveola-mediated endocytosis is not necessary. Seventh, we and others have failed to find an association of glycosylphosphatidylinositol-linked proteins with C. trachomatis during either entry or inclusion development and failed to find colocalization (unpublished data and reference 18). While it is possible that C. trachomatis has multiple modes of entry, including through pathways that involve lipid rafts, our results strongly argue against an exclusive role for this mode of entry under the conditions of our experiments. Finally, the observation that dominant negative alleles of dynamin failed to inhibit internalization (1) argues against caveolin- or clathrin-mediated entry as the only pathway of uptake.
Taken together, our results strongly suggest that lipid raft-mediated entry is not required for C. trachomatis entry into cultured epithelial cells. Further studies will be required to elucidate the uptake pathway of Chlamydia and may shed new insights into fundamental mechanisms of eukaryotic endocytic pathways.
Acknowledgments
We thank Craig Nelson for excellent technical support, Rob Parton for the caveolin constructs, and Keith Mostov and members of the Engel lab for advice and encouragement.
This work was supported by a grant from the National Institutes of Health to J.N.E. (R01 AI42806). S. van Ijendoorn was supported by a long-term fellowship from the Human Frontier Science Program. During a portion of this work, C.E. was supported by a fellowship from the American Lung Association.
Editor: F. C. Fang
REFERENCES
- 1.Boleti, H., A. Benmerah, D. M. Ojcius, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J. Cell Sci. 112:1487-1496. [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 3.Carabeo, R. A., S. S. Grieshaber, E. Fischer, and T. Hackstadt. 2002. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect. Immun. 70:3793-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carabeo, R. A., S. S. Grieshaber, A. Hasenkrug, C. Dooley, and T. Hackstadt. 2004. Requirement for the Rac GTPase in Chlamydia trachomatis invasion of non-phagocytic cells. Traffic 5:418-425. [DOI] [PubMed] [Google Scholar]
- 5.Carabeo, R. A., and T. Hackstadt. 2001. Isolation and characterization of a mutant Chinese hamster ovary cell line that is resistant to Chlamydia trachomatis infection at a novel step in the attachment process. Infect. Immun. 69:5899-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carabeo, R. A., D. J. Mead, and T. Hackstadt. 2003. Golgi-dependent transport of cholesterol to the Chlamydial inclusion. Proc. Natl. Acad. Sci. USA 100:6771-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan, M. J., G. Li, J. S. Shin, J. L. Carson, and S. N. Abraham. 2004. Bacterial penetration of bladder epithelium through lipid rafts. J. Biol. Chem. 279:18944-18951. [DOI] [PubMed] [Google Scholar]
- 8.Duncan, M. J., J. S. Shin, and S. N. Abraham. 2002. Microbial entry through caveolae: variations on a theme. Cell Microbiol. 4:783-791. [DOI] [PubMed] [Google Scholar]
- 9.Fawaz, F., C. van Ooij, S. Mutka, and J. Engel. 1997. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host proteins including cortactin. Infect. Immun. 65:5301-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fra, A. M., E. Williamson, K. Simons, and R. G. Parton. 1994. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 269:30745-30748. [PubMed] [Google Scholar]
- 11.Fudyk, T., L. Olinger, and R. S. Stephens. 2002. Selection of mutant cell lines resistant to infection by Chlamydia trachomatis and Chlamydia pneumoniae. Infect. Immun. 70:6444-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez-Martin, C. B., D. M. Ojcius, R. Hsia, R. Hellio, P. M. Bavoil, and A. Dautry-Varsat. 1997. Heparin-mediated inhibition of Chlamydia psittaci adherence to HeLa cells. Microb. Pathog. 22:47-57. [DOI] [PubMed] [Google Scholar]
- 13.Hackstadt, T. 1999. Cell biology, p. 101-138. In R. Stephens (ed.), Chlamydia. ASM Press, Washington, D.C.
- 14.Hackstadt, T., M. A. Scidmore, and D. D. Rockey. 1995. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc. Natl. Acad. Sci. USA 92:4877-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harder, T., and K. Simons. 1997. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 9:534-542. [DOI] [PubMed] [Google Scholar]
- 17.Hauser, A. R., S. Fleiszig, P. J. Kang, K. Mostov, and J. N. Engel. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66:1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jutras, I., L. Abrami, and A. Dautry-Varsat. 2003. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect. Immun. 71:260-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisanti, M. P., Z. Tang, P. E. Scherer, and M. Sargiacomo. 1995. Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol. 250:655-668. [DOI] [PubMed] [Google Scholar]
- 20.Mastick, C. C., M. J. Brady, and A. R. Saltiel. 1995. Insulin stimulates the tyrosine phosphorylation of caveolin. J. Cell Biol. 129:1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayor, S., K. G. Rothberg, and F. R. Maxfield. 1994. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science 264:1948-1951. [DOI] [PubMed] [Google Scholar]
- 22.Murata, M., J. Peranen, R. Schreiner, F. Wieland, T. V. Kurzchalia, and K. Simons. 1995. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. USA 92:10339-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy, P., G. Vereb, Z. Sebestyen, G. Horvath, S. J. Lockett, S. Damjanovich, J. W. Park, T. M. Jovin, and J. Szollosi. 2002. Lipid rafts and the local density of ErbB proteins influence the biological role of homo- and heteroassociations of ErbB2. J. Cell Sci. 115:4251-4262. [DOI] [PubMed] [Google Scholar]
- 24.Nichols, B. J., and J. Lippincott-Schwartz. 2001. Endocytosis without clathrin coats. Trends Cell Biol. 11:406-412. [DOI] [PubMed] [Google Scholar]
- 25.Norkin, L. C., S. A. Wolfrom, and E. S. Stuart. 2001. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res. 266:229-238. [DOI] [PubMed] [Google Scholar]
- 26.Parton, R. G., B. Joggerst, and K. Simons. 1994. Regulated internalization of caveolae. J. Cell Biol. 127:1199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelkmans, L., and A. Helenius. 2002. Endocytosis via caveolae. Traffic 3:311-320. [DOI] [PubMed] [Google Scholar]
- 28.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J. F. Hancock, and R. G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98-105. [DOI] [PubMed] [Google Scholar]
- 30.Scidmore, M. A., E. R. Fischer, and T. Hackstadt. 2003. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect. Immun. 71:973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scidmore-Carlson, M., and T. Hackstadt. 2000. Chlamydia internalization and intracellular fate. Subcell. Biochem. 33:459-478. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, D. K., A. Choudhury, R. D. Singh, C. L. Wheatley, D. L. Marks, and R. E. Pagano. 2003. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278:7564-7572. [DOI] [PubMed] [Google Scholar]
- 33.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 34.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 35.Smart, E. J., and R. G. Anderson. 2002. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 353:131-139. [DOI] [PubMed] [Google Scholar]
- 36.Stuart, E. S., W. C. Webley, and L. C. Norkin. 2003. Lipid rafts, caveolae, caveolin-1, and entry by chlamydiae into host cells. Exp. Cell Res. 287:67-78. [DOI] [PubMed] [Google Scholar]
- 37.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taraktchoglou, M., A. A. Pacey, J. E. Turnbull, and A. Eley. 2001. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infect. Immun. 69:968-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomsen, P., K. Roepstorff, M. Stahlhut, and B. van Deurs. 2002. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell 13:238-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ooij, C., S. van Ijzendoorn, M. Nishijima, K. Hantada, K. Mostov, and J. Engel. 2000. Host derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell Microbiol. 2:627-638. [DOI] [PubMed] [Google Scholar]
- 41.Wuppermann, F. N., J. H. Hegemann, and C. A. Jantos. 2001. Heparan sulfate-like glycosaminoglycan is a cellular receptor for Chlamydia pneumoniae. J. Infect. Dis. 184:181-187. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, J. P., and R. S. Stephens. 1992. Mechanism of Chlamydia trachomatis attachment to eukaryotic host cells. Cell 69:861-869. [DOI] [PubMed] [Google Scholar]