Suppression of pectin methyl esterase and jasmonates in synergy with 1-aminocyclopropane-1-carboxylic acid acting via the ethylene signaling pathway induces tyloses in intact, healthy aspen plants.
Abstract
Tyloses are ingrowths of parenchyma cells into the lumen of embolized xylem vessels, thereby protecting the remaining xylem from pathogens. They are found in heartwood, sapwood, and in abscission zones and can be induced by various stresses, but their molecular triggers are unknown. Here, we report that down-regulation of PECTIN METHYLESTERASE1 (PtxtPME1) in aspen (Populus tremula × tremuloides) triggers the formation of tyloses and activation of oxidative stress. We tested whether any of the oxidative stress-related hormones could induce tyloses in intact plantlets grown in sterile culture. Jasmonates, including jasmonic acid (JA) and methyl jasmonate, induced the formation of tyloses, whereas treatments with salicylic acid (SA) and 1-aminocyclopropane-1-carboxylic acid (ACC) were ineffective. SA abolished the induction of tyloses by JA, whereas ACC was synergistic with JA. The ability of ACC to stimulate tyloses formation when combined with JA depended on ethylene (ET) signaling, as shown by a decrease in the response in ET-insensitive plants. Measurements of internal ACC and JA concentrations in wild-type and ET-insensitive plants treated simultaneously with these two compounds indicated that ACC and JA regulate each other’s concentration in an ET-dependent manner. The findings indicate that jasmonates acting synergistically with ethylene are the key molecular triggers of tyloses.
Tyloses, accompanied by gels or gums, are occlusions of xylem conductive tissues in vascular plants (reviewed in De Micco et al., 2016). They are ingrowths of parenchyma cells into the lumens of adjacent tracheary elements, whereas gels and/or gums, depending on plant species, are secreted by the parenchyma cells (Rioux et al., 1998). Tyloses have been reported in many groups of vascular plants, including angiosperms (Chattaway, 1949; Gottwald, 1972; Saitoh et al., 1993), conifers (Chrysler, 1908; Peters, 1974; Dute et al., 1999; Feng et al., 2013), progymnosperms (Scheckler and Galtier, 2003), and ferns (De Micco et al., 2016).
In aspen (Populus tremula × tremuloides), tyloses are formed by ray contact cells (Chafe, 1974). These cells first synthesize secondary wall layers that lignify, together with the walls of adjacent vessel elements (Murakami et al., 1999), then they deposit a tertiary wall layer, called the protective layer, over the secondary wall layer. The protective layer, which has a composition similar to that of the primary cell wall layer and remains unlignified until heartwood forms (Chafe, 1974; Rioux et al., 1998), is instrumental in tylose formation. It is deposited all over the contact ray cell, including the pit membrane that contacts an adjacent vessel element, and this process is usually followed by the deposition of another layer of secondary wall, which lignifies (Chafe, 1974). After the death of the vessel element, the pit membrane arches slightly into the vessel lumen, forming a tylosis initial, which will remain inactive for many years until the contact cell is triggered by a stimulus to form a tylosis in embolized vessels.
Typical triggers of tyloses include infections (Pérez-Donoso et al., 2007; Collins et al., 2009), wounding (Biggs, 1987; Schmitt and Liese, 1993; Sun et al., 2006, 2008), heartwood formation (Taylor et al., 2002), and abscission (Scott et al., 1967; Sexton, 1976; Dute et al., 1999). Within a couple of days following a trigger, the tylosis initial balloons into the embolized vessel lumen. The contact cell protoplast expands into this new space, and the cell nucleus migrates into it (Peters, 1974; Sun et al., 2006). The tylosis nucleus may divide, followed by cytokinesis, or many tyloses may invade the same vessel lumen and fill the space completely. The cell walls of fully grown tyloses typically suberize and/or lignify (Chafe, 1974). In this way, tyloses restrict fungal invasion and growth within heartwood, abscission zones, or necrotic tissues. The ability to form tyloses is an important resistance factor in cotton (Gossypium hirsutum; Shi et al., 1992), and it plays a crucial role in some types of tree decline (McElrone et al., 2010).
The formation of tyloses is usually accompanied by the secretion of gels or gums, which serve the same function, by the parenchyma cells (Rioux et al., 1998). In some species, only one type of occlusion has been found: Tyloses occur in species having vessels with large contact pits and gels or gums in species whose vessels have small contact pits (Chattaway, 1949; Saitoh et al., 1993). In other species, such as grape (Vitis vinifera), tyloses are formed in the summer, whereas gels are produced in the winter (Sun et al., 2008).
Although hormonal triggering of tyloses formation was proposed a long time ago (Bamber, 1976), the nature of the hormone has been elusive. Ethylene (ET) was shown to increase tyloses formation in wounded or infected stems or cuttings (Hillis, 1975; Pérez-Donoso et al., 2007; Mishra et al., 2013), and treatments with ET inhibitors were found to abolish this effect, indicating that ET is involved in tylosis formation (Sun et al., 2007). ET concentration was also observed to increase in the transition zone where the heartwood is being formed (Taylor et al., 2002) and during infections (Pérez-Donoso et al., 2007; McElrone et al., 2010); that is, under circumstances in which tyloses are induced. Recent transcriptomics studies of heartwood formation indicated up-regulation of ET and other stress signaling pathways during this process (Huang et al., 2013; Xu et al., 2013). Moreover, the formation of agarwood, which is the commercially valuable heartwood of Aquilaria sinensis, was intensified by applying methyl jasmonate (MeJA) to the cut stems (Xu et al., 2013), implying the involvement of jasmonates in heartwood induction. However, the induction of tyloses by any of these hormones in intact healthy plants has not previously been demonstrated. Tyloses have been observed in transgenic lignin-deficient poplar lines, but the nature of the substance inducing them was not investigated in these plants (Kitin et al., 2010).
Here we report the formation of tyloses in transgenic aspen with down-regulated expression of the pectin methyl esterase PtxtPME1. These plants and PtxtPME1 overexpressing plants exhibited increased peroxidase activity and levels of hydrogen peroxide as compared to wild-type plants, indicating the activation of stress signaling pathways. We then tested the hormones implicated in stress responses, especially in the jasmonate, ET and salicylic acid (SA) signaling pathways for their ability to induce tyloses in intact plantlets grown in sterile culture. This led to the identification of jasmonates, acting synergistically with ACC in an ET-dependent pathway, as hormonal triggers of tyloses in plants.
RESULTS
Transgenic Aspen Plants with Expression of PtxtPME1 Down-Regulated Form Tyloses
Since tylosis formation necessitates very prominent and rapid extension of the pit membrane-protective layer sandwich, which is known to contain a large amount of acidic and methylesterified homogalacturonan (HG; Plavcová and Hacke, 2011), we tested whether modifying the level of HG esterification would affect tylosis formation. We previously observed that transgenic aspen with altered PtxtPME1 expression exhibited alterations in PME activity and HG methylesterification pattern in developing wood (Siedlecka et al., 2008). We therefore grew two PtxtPME1 overexpressing and two PtxtPME1 down-regulated lines along with wild-type plants in the greenhouse for 3 months and examined their wood for the presence of tyloses. PtxtPME1 expression levels in the overexpressing and down-regulated lines, compared to the wild type, are shown in Supplemental Figure S1. Tyloses (Fig. 1) were never observed in the wild-type or in overexpressing lines, but they were found in all trees of the two most suppressed lines that were analyzed. These tyloses were formed close to the cambium in greenhouse-grown trees; thus, they could not be associated with heartwood formation, which does not differentiate in such tissues or in trees as young as these. Moreover, there were no clear signs of infection in the trees that formed tyloses.
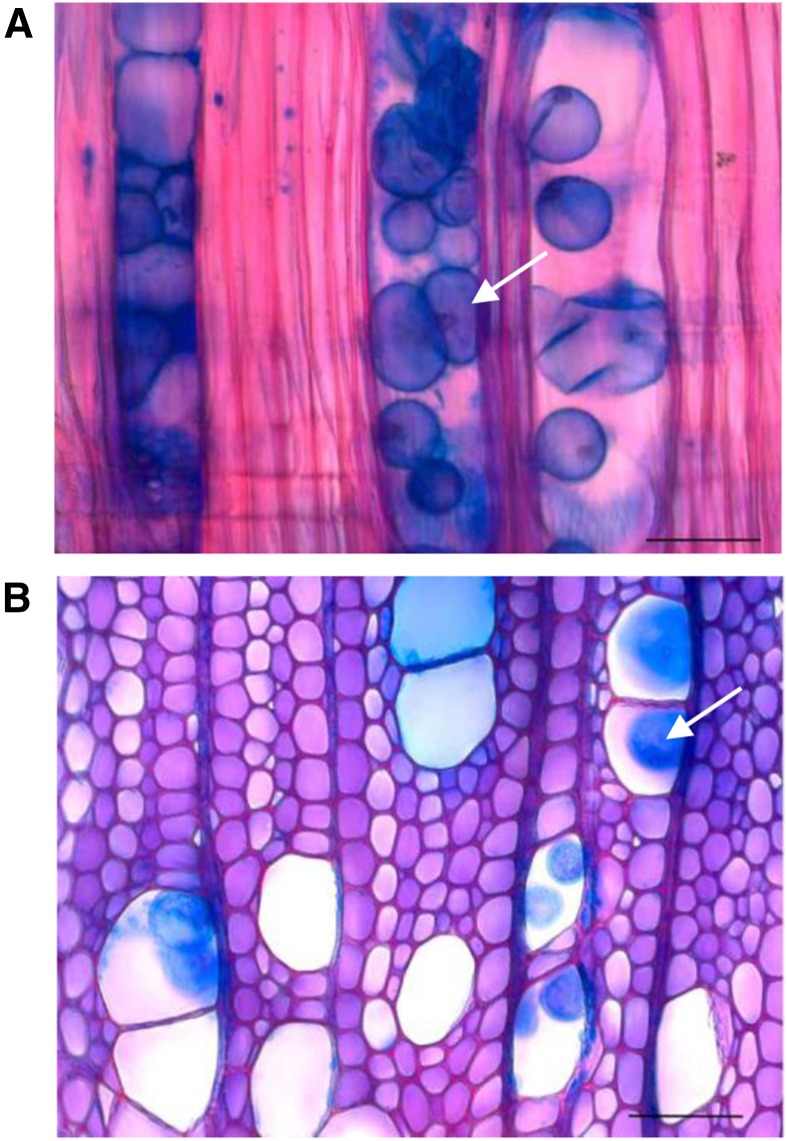
Figure 1.
Tyloses (arrows) found in developing wood of aspen trees with suppressed expression of PtxtPME1. Shown are a longitudinal (A) and a transverse (B) section of the developing wood of line 5 (Siedlecka et al., 2008) approximately 0.5 mm from the cambium, stained with safranin and alcian blue. Bar = 50 μm.
PME Deregulated Lines Exhibit Increased Levels of Peroxidase and H2O2
The formation of tyloses in PtxtPME1 suppressed lines could result from alterations in HG methylesterification, which is expected to affect cell wall extensibility (Derbyshire et al., 2007; Siedlecka et al., 2008; Müller et al., 2013) or from activation of the stress responses that could have been triggered in these lines by cell wall integrity sensing mechanisms (Hamann, 2012). Biotic stresses and wounding are known inducers of tyloses (Schmitt and Liese, 1993; Pérez-Donoso et al., 2007; Sun et al., 2008; Collins et al., 2009), and plants with modified cell wall constituents frequently exhibit symptoms of the activation of biotic stress response pathways (Hamann, 2012). We therefore investigated whether the PME deregulated lines exhibited any sign of the oxidative stress that accompanies biotic stresses (Bolwell et al., 2002; Demidchik, 2015). Peroxidase activity was detected in stems and leaves by means of the 3,3′-diaminobenzidine (DAB) reaction with exogenous H2O2 (Thordal-Christensen et al., 1997; Ganesan and Thomas, 2001). In the leaves, the peroxidase signals were prominent in the vicinity of stomata, whereas in the wood they were most distinct near vessel elements (Fig. 2). The peroxidase signals in the leaves and stems were increased in both suppressed and overexpressing PtxtPME1 lines compared to the wild type (Fig. 2, A and B). Moreover, the H2O2 signals visualized by oxidation of DAB (Thordal-Christensen et al., 1997) were found to be very prominent in the vascular bundles of the leaves, and they were increased in both types of PME deregulated line (Fig. 2C), but more markedly so in the PME-suppressed lines.
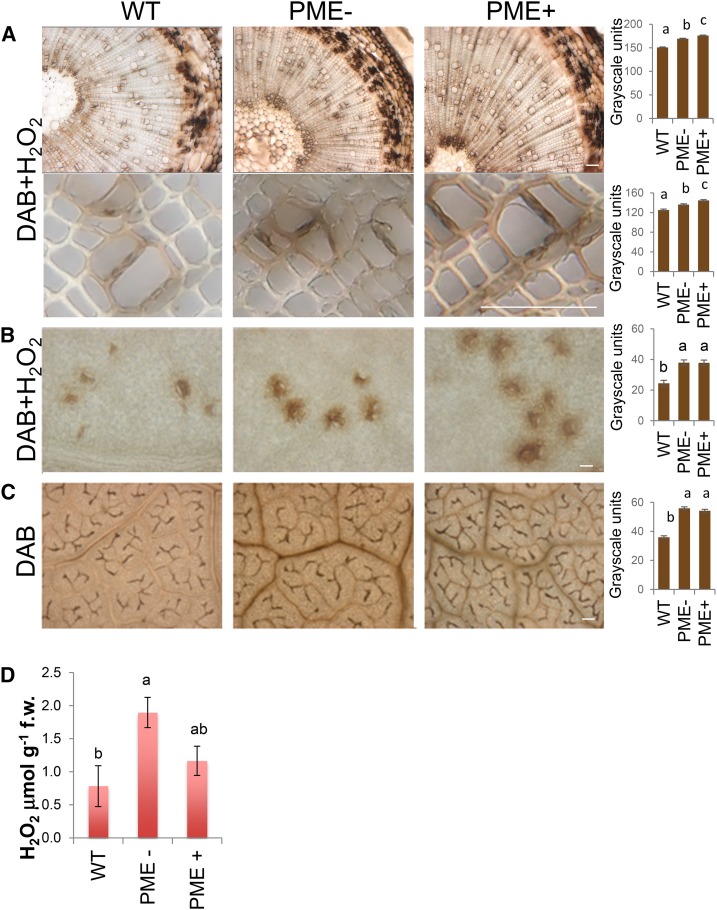
Figure 2.
Hydrogen peroxide levels and peroxidase activity are increased in the leaves and the wood of PME up- and down-regulated lines (PME+ and PME−, respectively). A and B, Visualization of endogenous peroxidase activity (brown), after reaction with H2O2 and DAB, in the wood (A) and the leaves (B). C, H2O2 (brown) distribution in the leaves after staining with DAB. A to C, Representative reaction among two transgenic lines per construct, each represented by six trees. Bar = 100 μm. Densitometry of images of all trees is shown on the right. D, H2O2 levels in the leaves of PME+ and PME− lines as compared to the wild type. In A to D, Means ± se; two independent lines were analyzed per genotype with two trees each. Means accompanied by the same letters are not statistically different (Tukey test, P ≤ 5%).
Levels of spectrophotometrically measured H2O2 were higher in the leaves of PtxtPME1 down-regulated plants than in the wild type, and the same tendency was also observed in PtxtPME1 up-regulated plants, but the difference in the latter case was smaller and not statistically significant (Fig. 2D). Taken together, the data on peroxidase activity and hydrogen peroxide levels point to elevated oxidative stress in the leaves and wood of PME-deregulated lines, especially when PtxtPME1 is suppressed.
Hormonal Induction of Tyloses in Intact Plantlets Grown in Vitro
To test whether the biotic stresses and wounding-related hormonal signaling pathways can trigger tyloses induction, we set up a sterile culture system that allowed treatment of the intact plants with different hormones applied to the medium. When SA, jasmonate (JA), and a precursor of ET, 1-aminocyclopropane-1-carboxylic acid (ACC), were applied to the medium at a concentration of 100 µm, only JA was effective in tylosis induction, and only in 25% of the treated plants (Fig. 3; Table I). The tyloses were accompanied by gels that stained blue with alcian blue (Fig. 3, A–E). We also tested MeJA at concentrations between 10 and 250 µm and found that it induced tyloses and gels in a similar manner to JA at the highest concentration applied (Fig. 3, C and F; Supplemental Table S1). Treatments with jasmonates at the concentrations that induced tyloses inhibited height increase and wood production (Fig. 3, G and H).
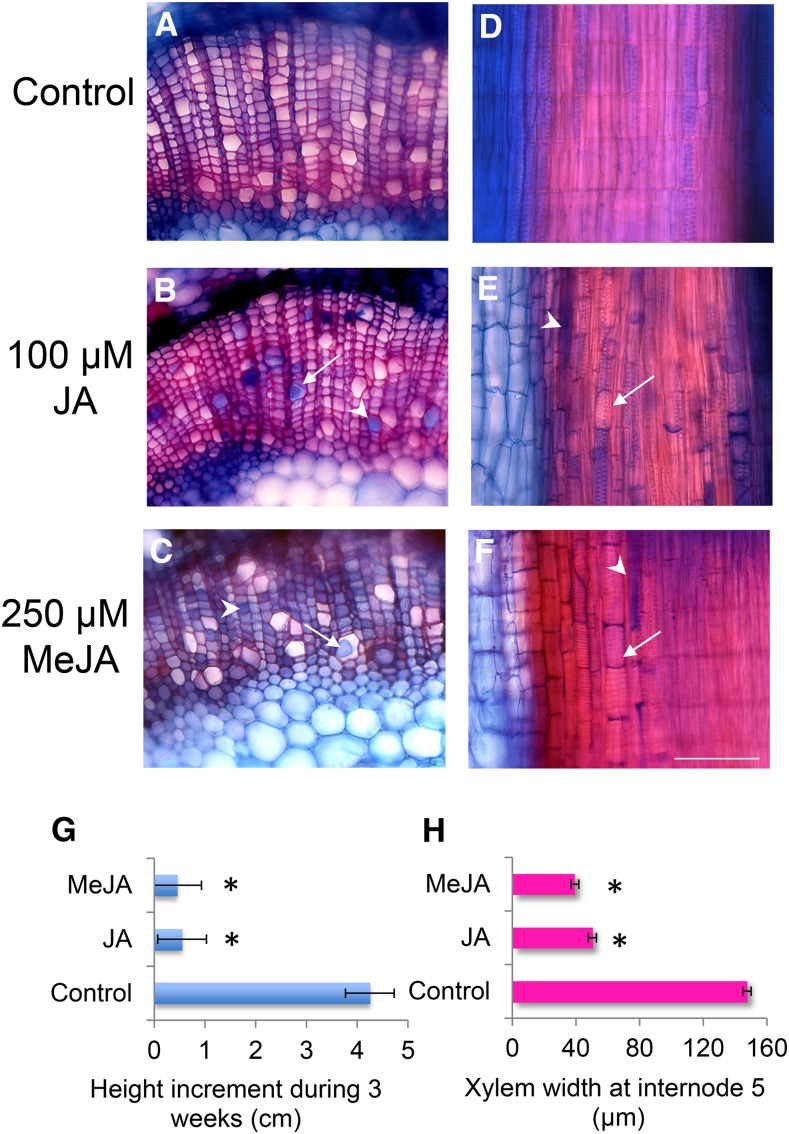
Figure 3.
Treatment of intact aspen plants with jasmonates induces formation of tyloses (arrows) and gels (arrowheads) and inhibits plant growth. The panels show transverse (A–C) and radial longitudinal (D–F) sections through the secondary xylem of aspen plants treated with either JA in DMSO, MeJA in DMSO, or DMSO (control) for 2 weeks. Bar (same for all micrographs) = 100 μm. G and H, Inhibition of plant (G) and xylem (H) growth by jasmonate treatment. Data are means for two plants per treatment; asterisk indicates significant difference from the control according to a Tukey post ANOVA test at P ≤ 5%.
Table I. JA induces tyloses in intact aspen plants grown in sterile culture.
*P ≤ 0.05 and **P ≤ 0.01; χ2 test.
| Age of Treated Plants | Treatment | % of Plants with Tyloses (No. of Plants Treated) |
|---|---|---|
| 3 Weeks |
100 µm JA |
25% (16)* |
| Control |
0% (16) |
|
| 6 Weeks |
100 µm JA |
25% (28)** |
| Control | 0% (30) |
To test the responses of PME-deregulated plants in vitro, two lines of each PME-up-regulated and PME-down-regulated genotype, and wild-type plants, were treated with MeJA at two different concentrations, 100 and 250 μm, and with 100 μm SA alone and combined with MeJA. Although the plants with down-regulated PME expression produced tyloses spontaneously in the greenhouse (Fig. 1), we did not observe such a reaction in the plants cultivated in vitro (Supplemental Table S1). However, when treated with 100 μm MeJA, a concentration that was not inductive in the wild type, 25% of the PME deregulated plants formed tyloses (Supplemental Table S1). Both PME-up-regulated and PME-down-regulated plants showed a similar response to 100 μm MeJA. SA, as seen before with the wild type, was ineffective, and when applied together with MeJA, it abolished the inductive effect of MeJA in PME deregulated plants.
ET has been implicated in tyloses formation in stem cuttings (Sun et al., 2006; Mishra et al., 2013), and endogenous levels of ET were found to be elevated in situations when tyloses were initiated (Hillis, 1975; Taylor et al., 2002). However, no induction of tyloses was observed in ACC-treated intact aspen plants in this study. One possibility is that ACC is effective only in wounded plants in which wound-generated signals such as jasmonates are present. The jasmonates are well known to be involved in wound responses in plants (Wasternack and Hause, 2013) including poplar (Babst et al., 2009). Intact aspen plants were therefore treated with a combination of 100 μm JA and 100 μm ACC or with each of these hormones alone. As seen before, ACC alone was not effective, and JA alone induced the formation of tyloses in 20% of the plants treated (Fig. 4; Table II). When the two hormones were combined, tyloses were observed in 90% of plants. This synergistic effect was manifested as a substantially increased frequency of plants forming tyloses, whereas the number of tyloses in individual plants was not visibly affected. This indicates that the combination of ACC and JA is a trigger that acts like an on-off switch at the organism level.
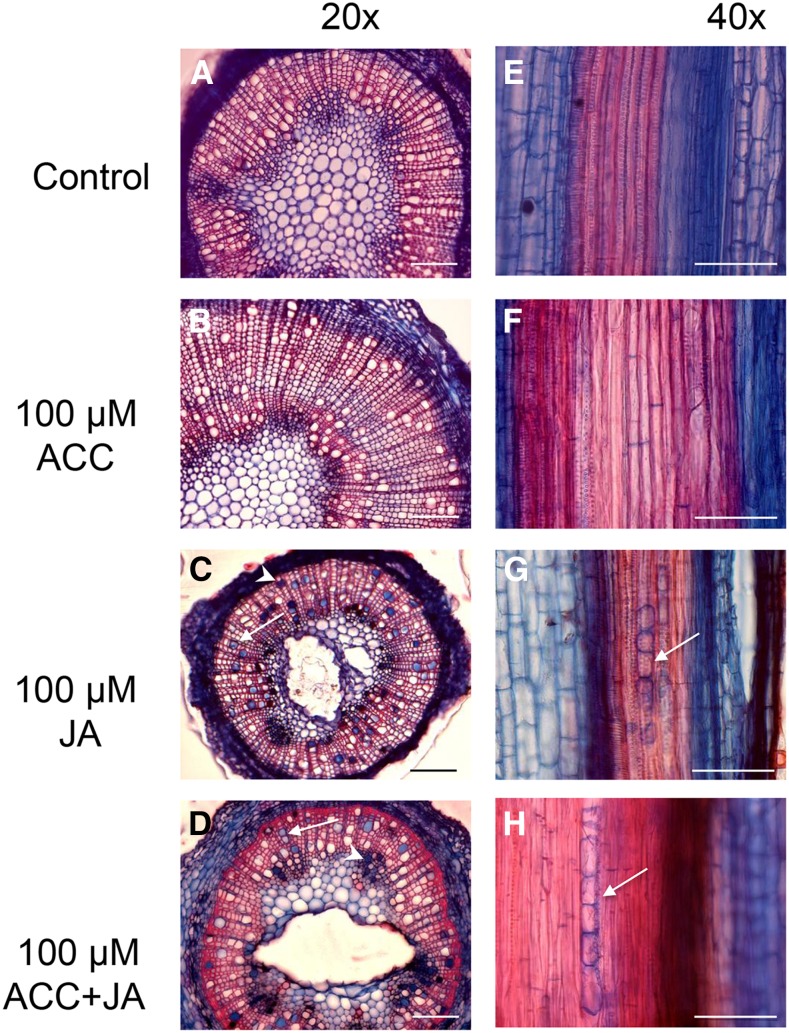
Figure 4.
ACC combined with JA, or JA alone, can induce the formation of tyloses (arrows) and gels (arrowheads) in aspen cuttings. Transverse (A–D) and radial longitudinal (E–H) sections of aspen stems 3 weeks after treatment with hormones or DMSO (control). Bar = 100 µm.
Table II. ACC stimulates the inductive effects of JA, synergistically inducing tyloses.
*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. ns, not significant.
| Treatment | % of Plants with Tyloses (No. of Plants Treated) | χ2 vs. Noninductive Treatments | χ2 vs. Control |
|---|---|---|---|
| 100 µm ACC |
0% (10) |
||
| 100 µm JA |
20% (10) |
* |
ns |
| 100 µm JA + 100 µm ACC |
90% (10) |
*** |
** |
| Control | 0% (10) |
ACC is the immediate precursor of ET in the biosynthetic reaction catalyzed by ACC oxidase. However, there are also observations suggesting that ACC has a role independently of that of ET (Tsang et al., 2011). We therefore investigated whether the effect of ACC on JA-induced tyloses formation depends on ET signaling by treating aspen lines having reduced ET sensitivity (Love et al., 2009) with ACC and JA and comparing their reaction to that of wild-type plants (Table III). Reduced ET sensitivity in transgenic lines resulted in a significantly lower frequency of tyloses induction. Line 1E, in which ET perception was more strongly affected, was also more affected with respect to tyloses induction. The synergistic effect of ACC on JA-induced tyloses formation is therefore dependent on the ET signaling pathway.
Table III. The effects of ACC are mediated by ethylene.
ET-insensitive lines 1E and 3A show a decrease in the frequency of plants with tyloses after inductive treatment with JA and ACC compared to the wild type. Asterisks indicate significant difference from the wild type at P ≤ 0.05 (*) or P ≤ 0.001 (***); χ2 test.
| Treatment | % of Plants with Tyloses (No. of Plants Treated) | ||
|---|---|---|---|
| Wild Type |
1E |
3A |
|
| 100 µm JA + 100 µm ACC | 70% (10) | 10%*** (10) | 50%* (10) |
Levels of JA and ACC in Intact Plantlets Treated with These Hormones
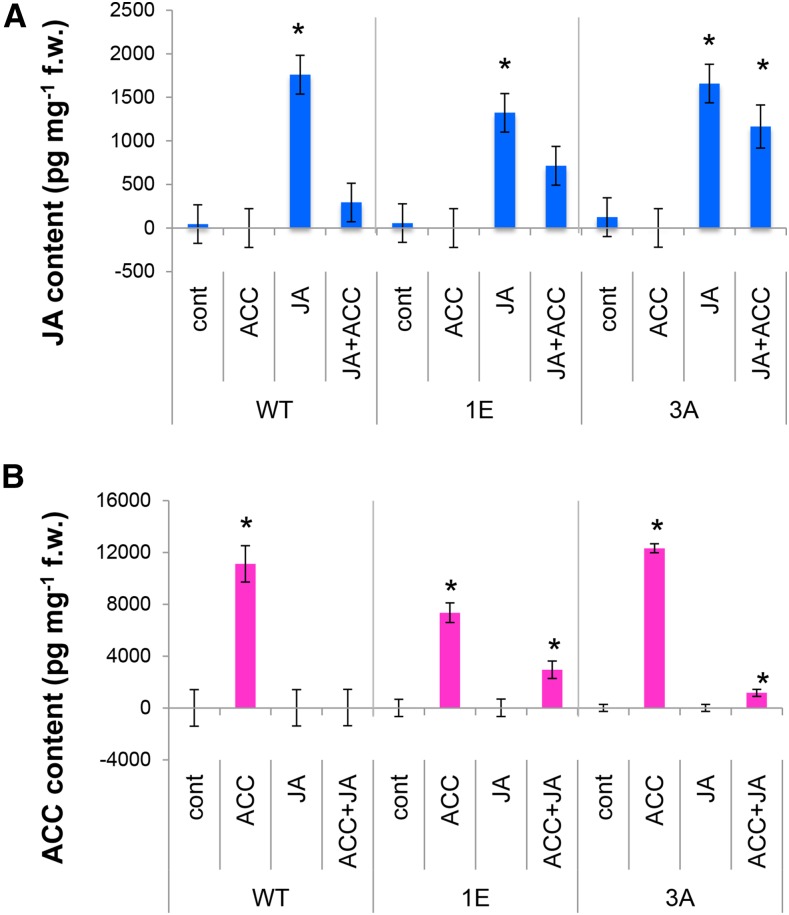
To learn more about the synergism of JA and ACC in tyloses induction, the internal levels of these hormones were analyzed 2 weeks after they had been applied individually or in combination to wild-type plants and to plants with reduced ET sensitivity (Fig. 5).
Figure 5.
Internal levels of JA (A) and ACC (B) after ACC and JA treatments in wild-type aspen and in transgenic aspen lines 1E and 3E, which have reduced sensitivity to ethylene. Means ± se; n = 5 biological replicates. Asterisk indicates a significant difference between control and hormonal treatment for each genotype (post ANOVA Tukey test, P ≤ 5%).
JA application increased internal JA levels in the stems in both wild-type and ET-insensitive plants compared to mock-treated plants (control; Fig. 5A). This indicates that maintenance of high JA levels after JA application does not depend on ET signaling. Interestingly, simultaneous application of JA and ACC to wild-type plants resulted in greatly decreased JA levels, down to a level similar to that observed in control plants. Thus, the synergism between JA and ACC does not involve an increase in JA level mediated by ACC; rather, ACC application almost completely abolishes the high internal JA levels caused by exogenous JA application. The reduction of high JA levels in plants treated simultaneously with JA and ACC was less pronounced in the ET-insensitive lines 1E and 3A, suggesting that it may depend on ET signaling.
Plants treated with ACC also exhibited very much increased levels of this compound compared to control (Fig. 5B). This was observed in both wild-type and ET-insensitive plants, suggesting that maintenance of high ACC levels following exogenous ACC application does not depend on ET signaling. Combined JA and ACC treatments completely abolished the increase in internal ACC level caused by exogenous ACC in the wild type, while it decreased the internal ACC level in ET-insensitive plants, suggesting that the decrease in internal ACC caused by JA treatment was at least in part dependent on ET signaling.
DISCUSSION
Deregulation of PME Triggers Defense Responses
This work provides several lines of evidence indicating that the deregulation of PME activates a broad spectrum of defense responses in aspen wood. First, intact PME down-regulated plants were seen to form tyloses in developing wood (Fig. 1). Tyloses in this location are typically found in wounded stems. This suggests that wound response signaling is activated in PME down-regulated plants. Secondly, the PME up- and down-regulated lines showed up-regulation of oxidative stress, as indicated by increased peroxidase activity in the wood and leaves and increased hydrogen peroxide levels in the leaves (Fig. 2). This was more prominent in the PME down-regulated lines that formed tyloses, suggesting that the oxidative status is altered by the activation of wound stress signaling in these lines. Finally, the PME-deregulated lines were more sensitive to treatment with 100 µm MeJA, which induced tyloses formation in these lines but was ineffective in wild-type plants (Supplemental Table S1). In findings similar to these observations, the activation of constitutive defense responses has been observed in strawberry (Fragaria spp.) lines overexpressing a PME encoded by FaPE1 (Osorio et al., 2008) and in the Arabidopsis (Arabidopsis thaliana) qua2-1 mutant, which is affected in HG methyl transferase (Raggi et al., 2015), whereas analysis of selected stress-related transcripts in Arabidopsis pme3 mutants and in PMEI-1- or PMEI-2-overexpressing plants failed to detect any constitutive alteration in their expression (Raiola et al., 2011).
Although the molecular mechanisms involved in the activation of stress signaling in the wood of PME deregulated aspen plants are not presently known, recent studies in Arabidopsis have shown that HG methylesterification plays a crucial role in cell wall integrity sensing via at least two pathways: the BR signaling pathway, which involves receptor-like protein kinase RLP44 interacting with BRI1 (Wolf et al., 2012, 2014), and the wall-associated kinase (WAK) pathway comprising WAK1 and WAK2, which bind to pectins and oligogalacturonides in a methylesterification-dependent way and further signal the presence of stress using the MPK3 cascade (Kohorn, 2016). Deesterification of HG in PtxtPME1-up-regulated lines (Siedlecka et al., 2008) would make this polymer more susceptible to endogenous polygalacturonases and pectate lyases, leading to an increase in the formation of deesterified oligogalacturonides known as DAMP signals, which trigger downstream hormonal pathways (Simpson et al., 1998; Denoux et al., 2008; Galletti et al., 2008; Wolf et al., 2014; Benedetti et al., 2015). A high level of HG methylesterification in the PtxtPME1-down-regulated lines, on the other hand, could decrease cellular adhesion and cell wall rigidity (Siedlecka et al., 2008), and this might trigger mechanosensing responses. The mechanosensing pathway and biotic stress responses converge on the ET, JA, and reactive oxygen species (ROS) signaling pathways or via ROS pathways that are independent of JA or ET (Hernández-Blanco et al., 2007; Tsang et al., 2011; Benikhlef et al., 2013). This might explain the fact that both types of PME deregulation induce similar activation of ROS (Fig. 2) and make plants more susceptible to the induction of tyloses by jasmonates (Supplemental Table S1).
Deregulation of many different cell wall biosynthetic processes has been shown to constitutively activate JA- and ET-dependent and -independent stress signaling pathways (Ellis et al., 2002; Hernández-Blanco et al., 2007) and to increase resistance to abiotic stresses (Keppler and Showalter, 2010) or pathogens (Vogel et al., 2002, 2004; Hernández-Blanco et al., 2007; Manabe et al., 2011; Pogorelko et al., 2013; Pawar et al., 2016). The induction of tyloses as a result of cell wall defects has been studied only in transgenic poplars with reduced lignin biosynthesis (Kitin et al., 2010), but the current study on PME deregulated plants suggest that increased susceptibility to tyloses formation is likely to be a concern in other cell wall-related mutants and transgenic plants. This is an important consideration for the deployment of plants with genetically engineered cell walls, since such tyloses restrict water conductivity in these plants and result in low productivity.
Roles of Jasmonates and Ethylene in Induction of Tyloses
Tyloses are typically induced to form in the embolized vessels by wounding, pathogen attack, and preceding the heartwood formation (Biggs, 1987; Schmitt and Liese, 1993; Taylor et al., 2002; Sun et al., 2006, 2008; Pérez-Donoso et al., 2007; Collins et al., 2009), which are the physiological processes in which jasmonates have been implicated (Wasternack and Hause, 2013; Huang et al., 2013; Xu et al., 2013), but no direct involvement of jasmonates in tyloses formation has yet been demonstrated. This study provides the evidence that jasmonates are capable of inducing tyloses and their accompanying gels in plants. The key finding is that these hormones, when externally applied, can induce tyloses in intact healthy sterile plants (Figs. 3 and 4; Tables I and II; Supplemental Table S1). Applying hormones externally leads to increases in their internal concentration in the stem tissues where the tyloses are formed (Fig. 5). Although several observations have implicated ET in tylose induction (Hillis, 1975; Pérez-Donoso et al., 2007; Sun et al., 2007; Mishra et al., 2013), we found that treatments with ACC alone were not capable of inducing tyloses in intact aspen stems. However, when applied simultaneously with jasmonates, ACC acted synergistically, increasing the proportion of plants that formed tyloses (Fig. 4; Table II). Moreover, we demonstrate that this synergistic action of ACC is related to ET signaling, since it is diminished in plants with suppressed ET perception (Table II).
Synergism of the action of jasmonates and ET is known in many different responses, such as the reactions to herbivory and necrotrophic pathogen attack (Wasternack and Hause, 2013), but it has not previously been shown for tylose induction. On the other hand, JA acting synergistically with the ET-releasing compound ethephon has been found to induce gummosis in tulips (Skrzypek et al., 2005), suggesting a similar induction cascade. The synergistic effect of ACC and JA applied simultaneously to plants cannot be attributed to increased concentrations of these compounds in the stem tissues, as we were expecting based on reports of positive interactions between jasmonates and ET biosynthesis (Yu et al., 2009). Rather, and unexpectedly, simultaneous applications of JA and ACC drastically decreased the internal ACC or JA concentrations compared to the levels observed in plants given either of these compounds individually (Fig. 5). Although the mechanism by which these compounds disappear is presently unknown, one likely possibility is that in aspen stems JA and ACC are conjugated to form JA-ACC. Such a conjugate was found at levels comparable to those of other JA conjugates in Arabidopsis, and the JAR1 protein was reported to catalyze its biosynthesis in vitro (Staswick and Tiryaki, 2004). This explanation is compatible with the simultaneous decrease in both ACC and JA observed in aspen supplied simultaneously with these compounds. Another, nonexclusive, possibility is that when ACC and JA are provided simultaneously, they stimulate each other’s metabolic conversion to the active compounds ET and JA-Ile, thus augmenting the physiological response. In aspen, both tyloses induction and the disappearance of ACC and JA from the system are apparently dependent on ET signaling, since both these responses were weaker in plants with suppressed ET signaling than in wild-type plants. It is possible that tylose induction and the metabolism of ACC and JA are parts of the same signaling response.
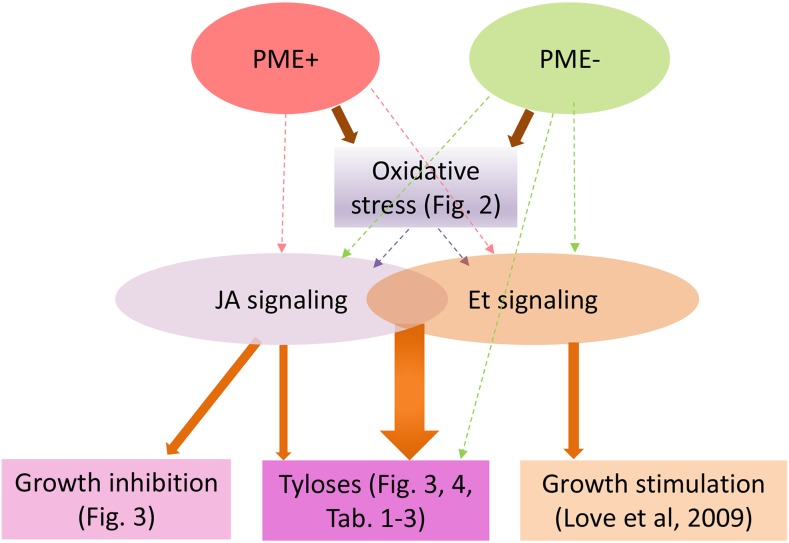
The proposed pathways of tyloses induction emerging from this study are schematically presented in Figure 6. Although the intermediate steps in the pathway leading from PME suppression to tylose induction and from PME alteration to JA or ET signaling are not yet understood, as discussed above, we demonstrated that PME alteration induces oxidative stress and that simultaneous JA and ET signaling is the key component of the tyloses induction.
Figure 6.
Proposed pathways leading to tyloses induction based on experiments presented in this study. Solid arrows refer to interactions shown by specific figures in this article or by Love et al. (2009). Stippled arrows indicate different possible interactions suggested by our data or by the literature.
MATERIALS AND METHODS
Plant Lines Used in This Study
All transgenic lines were produced in a hybrid aspen (Populus tremula × tremuloides) background. Lines with modified expression of PtxtPME1 (GenBank accession number AJ277547), carrying the PtxtPME1 sense cDNA controlled by the 35S CaMV promoter, were described by Siedlecka et al. (2008), and lines carrying the antisense fragment controlled by the 35S promoter were described by Berthold et al. (2006). Three suppressed lines (sense 5, sense 10B, and antisense 6N) and two overexpressing sense lines (2B and 7) were used in this study (Siedlecka et al., 2008; Supplemental Fig. S1). Transgenic lines with reduced sensitivity to ET were described by Love et al. (2009).
Growth Conditions in the Greenhouse
Transgenic and wild-type plants were grown in a greenhouse under natural light conditions, supplemented with metal halogen lamps, with an 18-h-light/6-h-dark photoperiod at a temperature of 22°C/15°C (day/night). They were watered daily and fertilized once a week with a SuperbraS nutrient solution (Supra Hydro). When the plants reached a height of about 2 m, stem segments from internodes 20 to 39 were harvested, frozen in liquid nitrogen, and stored at −80°C until required for use.
Growth Conditions in Vitro
Aspen lines were propagated from cuttings in vitro on Murashige and Skoog medium (2.2 g L−1, pH 5.6; Sigma-Aldrich), solidified with Phytagel (Sigma-Aldrich) at 2.7 g L−1, in polypropylene containers with OS140-ODS140 gas-exchange spore filters (Combiness), under a 16-h-light/8-h-dark photoperiod, temperature 22°C/18°C (day/night), illuminated with Phillips Master TLD 58W/830 lights at approximately 90 μmol m−2 s−1.
Analysis of Peroxidase and H2O2 in the Leaves and Stems
Peroxidase activity and the presence of H2O2 were determined histochemically in the leaves of transgenic and wild-type plants grown in the greenhouse and stems of such plants grown in vitro. Stem transverse sections (60 μm) were cut using a vibratome (Leica) and fixed in 4% glutaraldehyde in PBS (pH 7.2) for 2 h at 4°C, followed by rinsing in PBS (pH 7.6). Leaf pieces approximately 10 × 10 mm were used fresh without fixation. For peroxidase activity, stem sections were incubated in 50 mm Tris-HCl buffer (pH 7.6) for 10 min and then in 0.002% DAB, 0.01% H2O2, and 50 mm Tris-HCl buffer (pH 7.6) for 50 min (Graham and Karnovsky 1966), whereas leaf pieces were placed in 0.001% DAB at pH 5.2, prepared according to Thordal-Christensen et al. (1997), to which H2O2 was added to a final concentration of 5 mm, incubated for 25 min, and cleared by boiling in 95% ethanol for 30 min to remove chlorophyll. H2O2 was detected histochemically in the leaves by the DAB method (Thordal-Christensen et al., 1997), by incubating leaf pieces in 0.001% DAB at pH 5.2 for 24 h, followed by clearing as above. Control sections/leaf pieces were boiled for 30 min before incubation with substrates. The stem sections and cleared leaves were mounted in glycerol and observed using a Zeiss universal microscope. Densitometry was carried out on sections and whole mounts using Image J, by grayscale intensity within a 4 × 4 pixel window placed over stained anatomical features randomly selected in 10 to 20 locations per specimen, using two to three trees per line, and two lines per PME condition, and three to six wild-type trees.
H2O2 content in the leaves was measured according to Velikova et al. (2000). A sample of 500 mg of leaves (without main veins) was frozen in liquid nitrogen, ground to a fine powder, then mixed with 5 mL of 0.1% (w/v) TCA on ice, and centrifuged at 12,000g for 15 min. The top 1 mL of the supernatant was then collected and its H2O2 content was analyzed after reaction with iodine. The 2 mL reaction volume contained 0.5 mL of the leaf extract, 0.5 mL 10 mm potassium phosphate buffer, pH 7.0, and 0.5 mL 1 m KI. The absorbance was measured at 390 nm, against a blank sample prepared with 0.1% TCA instead of the leaf extract, after 15 min incubation. A standard curve was prepared with H2O2 in the reaction buffer.
Hormonal Induction of Tyloses in Vitro
Plantlets grown in vitro with well-developed roots, typically 3 weeks old unless otherwise stated, were used for the hormone application experiments. The hormone stocks (0.2%, w/v or v/ v, in 2% DMSO [Duchefa Biochemie]), pH 5.6 adjusted with KOH, were filter sterilized. The filter-sterilized hormones were applied in volumes of approximately 2 mL to the sterile medium to give a final concentration of 100 μm unless otherwise stated. Control treatments consisted of 2% DMSO without hormones. JA and (−)-jasmonic acid methyl ester (MeJA) were from OlChemIm, and ACC was from Sigma-Aldrich.
Microscopy Analysis of Tyloses
Plantlets were analyzed for the presence of tyloses after 2 weeks of hormone treatment. Stem segments 2 cm in length, collected directly above the medium, which contained the vascular cambium at the time of treatment, were fixed in 4% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2) for 24 h at 4°C, washed in buffer and deionized water, dehydrated in an ethanol series, and stored in 70% ethanol at room temperature. Before sectioning, the segments were rehydrated. Stem transverse (1 cm above medium) and longitudinal (radial and tangential) sections 35-μm thick were made using a vibratome (Leica). Sections were stained with 0.33% (w/v) safranin, 16.67% (v/v) ethanol, and 0.67% (w/v) alcian blue for 15 min, washed, mounted in 50% glycerol, and observed using a Zeiss Axioplan 2 microscope with Axiovision software.
Analysis of ACC and JA Levels
Hormones were analyzed in the stem segments directly above the sites analyzed for the presence of tyloses. These segments were flash frozen in liquid nitrogen and stored at −80°C. Approximately 20 mg of stem segments were ground and cooled in liquid nitrogen for 30 min in a mixer mill with two tungsten beads per Eppendorf tube. Subsequently, 1 mL of extraction mix, MeOH:H2O (8:2 v/v), containing 1 ng of 2H4-ACC (Sigma-Aldrich) internal standard and either 10 or 0.5 ng of 2H6-±-JA (OlChemIm) internal standard for JA-treated and untreated samples, respectively, was added per sample, and the mill was run again for 3 min at 30 Hz with tungsten beads. After removing the beads with a magnet, the samples were centrifuged for 5 min at 100g. The supernatants (approximately 200 µL), along with blank samples containing extraction mix only, were transferred into kimble vials and the methanol was evaporated off leaving the aqueous phase in a speed-vac for approximately 1 h at 37°C. The pH of the samples obtained was adjusted to 3.0 by adding 300 µL of 1% HOAc per sample. For purification by solid phase extraction, each 150 mg MCX SPE column (Waters) was activated with 3 mL of methanol and then equilibrated with 3 mL of HOAc. The samples were applied to the columns, which were then washed with 2 mL of 20% methanol in 1% HOAc. JA was eluted with 2 mL of methanol, and ACC was eluted with 2 mL of 4 m NH3 in 60% methanol. The eluates were dried down in a speed-vac and transferred to LC-MS vials. The samples were stored at −80°C until required for derivatization and final liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Derivatization of ACC with AccQ-Tag
ACQ derivatization of the ACC fraction was performed using an AccQ⋅Tag kit from Waters according to the manufacturer’s protocol. In brief the dried extracts were resuspended in 20 µL of 20 mm HCl, and 60 µL of AccQ⋅Tag Ultra borate buffer was added to each vial. Then, 20 µL of freshly prepared AccQ⋅Tag derivatization solution was added and the sample was immediately vortexed for 10 s. After mixing, the samples were allowed to stand for 30 min at room temperature followed by 10 min at 55°C.
Derivatization of JA with Bromocholine
The JA fraction was derivatized with bromocholine essentially according to Kojima et al. (2009). To each sample, 20 μL of water, 4 μL of 500 mm bromocholine (Sigma-Aldrich) in 70% acetonitrile and 0.8 μL of trimethylamine (Sigma-Aldrich) were added. The solution was mixed and incubated at 80°C for 130 min, evaporated to dryness in a speed-vac concentrator, and subsequently reconstituted with 50 μL of 0.1% formic acid and subjected to LC-MS/MS.
LC-MS/MS Analysis
Analytes were separated using an HP 1200 LC system from Agilent Technologies, consisting of a G1379B online vacuum degasser, a G1312B binary pump, a G1316B thermostated column compartment, and a G1367D autosampler with a G1330B autosampler thermostat. For ACC analysis, a 1-µL aliquot of the sample was injected onto a 2.1 × 50-mm, 1.7-µm Kinetex HPLC column (Phenomenex) held at 55°C in a column oven. The gradient elution buffers were A (water and 0.1% formic acid) and B (acetonitrile and 0.1% formic acid), and the flow rate was 500 µL min−1. The initial condition was 0.1% B; from 0.54 to 7 min the proportion of B was increased linearly from 0.1 to 9.1%. From 7 to 10 min, B was increased linearly to reach 21.2%. To elute the more nonpolar compounds, the proportion of B was rapidly increased to 80% at 11 min and kept there for 1 min. From 12 to 12.5 min, the column was returned to its initial conditions (0.1% B), and it was equilibrated for 5 min before injection of the subsequent sample. For JA analysis the flow rate was 300 µL min−1. The initial condition was 1% B, from 1 to 10 min, the proportion of B was increased linearly to 100% then kept there for 1 min, and from 11 to 11.5 min, the column was returned to its initial conditions (1% B). It was equilibrated for 3 min before injection of the subsequent sample.
Derivatized ACC and JA were detected with an Agilent 6460 triple quadrupole mass spectrometer equipped with a jet stream electrospray source operating in positive ion mode. The jet stream gas temperature was 325°C with a gas flow of 6 L h−1, and a sheath gas temperature equal to 400°C and flow rate of 12 L h−1; nebulizer pressure was set to 25 p.s.i. The ion spray voltage was set at 4 kV, and nozzle voltage was set to 0 V. For the multiple-reaction monitoring (MRM) experiment, fragmentation conditions for each ACC and JA derivative were optimized using MassHunter MS Optimizer software (Agilent Technologies). The optimized fragmentation voltages were 135 V for ACC-Acq and 2H4-ACC-Acq, and 90 V for JA-choline and 2H6-JA-choline. The collision energies were 20 V for ACC derivatives and 10 V for JA derivatives. The following MRM transitions were recorded: ACC-Acq and 2H4-ACC-Acq 272→171 and 276→171, respectively; JA-choline and 2H6-JA-choline 209→59 and 215→59, respectively. Samples were analyzed in MRM Mode with a dwell time of 200 ms. Nitrogen was used as collision gas. The LC-MS/MS analysis was controlled by the MassHunter software package, v 4.00, and data processing was carried out using an in-house script.
Accession Numbers
Sequence data for PtxtPME1 can be found in GenBank under accession number AJ2775.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Quantification of relative levels of PtxtPME1 transcripts.
Supplemental Table S1. Methyl-jasmonic acid induces tyloses in intact wild-type aspen plants grown in sterile culture.
Supplemental Figure S1. Quantification of relative levels of PtxtPME1 transcripts, in relation to the internal calibrator 18S rRNA and to the wild type, in two independent up-regulated lines (PME+) and two independent down-regulated lines (PME−).
Supplemental Table S1. Methyl-jasmonic acid induces tyloses in intact wild-type aspen plants grown in sterile culture.
Supplementary Material
Acknowledgments
We thank Alexander Makoveychuk for pilot experiments optimizing hormonal dosages and for preparation of all cuttings for hormonal treatments.
Glossary
- ET
ethylene
- MeJA
methyl jasmonate
- HG
homogalacturonan
- DAB
3,3′-diaminobenzidine
- JA
jasmonate
- ACC
1-aminocyclopropane-1-carboxylic acid
- ROS
reactive oxygen species
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MRM
multiple-reaction monitoring
Footnotes
Articles can be viewed without a subscription.
This work was supported by VR and Formas grants to E.J.M., by a FORE travel grant to J.L., by the Berzelius Center for Tree Biotechnology, and by the Knut and Alice Wallenberg Foundation who funded the purchase of mass spectrophotometer instrumentation.
References
- Babst BA, Sjödin A, Jansson S, Orians CM (2009) Local and systemic transcriptome responses to herbivory and jasmonic acid in Populus. Tree Genet Genomes 5: 459–474 [Google Scholar]
- Bamber RK. (1976) Heartwood, its function and formation. Wood Sci Technol 10: 1–8 [Google Scholar]
- Benedetti M, Pontiggia D, Raggi S, Cheng Z, Scaloni F, Ferrari S, Ausubel FM, Cervone F, De Lorenzo G (2015) Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc Natl Acad Sci USA 112: 5533–5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benikhlef L, L’Haridon F, Abou-Mansour E, Serrano M, Binda M, Costa A, Lehmann S, Métraux JP (2013) Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol 13: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold F, Mellerowicz E, Sundberg BW (2006) New transgenic plants and method of their production. Patent No. WO2006/068603-A1
- Biggs AR. (1987) Occurrence and location of suberin in wound reaction zones in xylem of 17 tree species. Phytopathology 77: 718–725 [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53: 1367–1376 [PubMed] [Google Scholar]
- Chafe SC. (1974) Cell wall formation and “protective layer” development in the xylem parenchyma of trembling aspen. Protoplasma 80: 335–354 [DOI] [PubMed] [Google Scholar]
- Chattaway M. (1949) The development of tyloses and secretion of gum in heartwood formation. Aust J Sci Res Ser B 2: 227–240 [Google Scholar]
- Chrysler MA. (1908) Tyloses in tracheids of conifers. New Phytol 7: 198–204 [Google Scholar]
- Collins BR, Parke JL, Lachenbruch B, Hansen EM (2009) The effects of Phytophthora ramorum infection on hydraulic conductivity and tylosis formation in tanoak sapwood. Can J Res 39: 1766–1776 [Google Scholar]
- De Micco V, Balzano A, Wheeler EA, Baas P (2016) Tyloses and gums: a review of structure, function and occurrence of vessel occlusions. IAWA J 37: 186–295 [Google Scholar]
- Demidchik V. (2015) Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ Exp Bot 109: 212–228 [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 1: 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire P, McCann MC, Roberts K (2007) Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dute RR, Duncan KM, Duke B (1999) Tyloses in abscission scars of loblolly pine. IAWA J 20: 67–74 [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG (2002) The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 14: 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Wang J, Rößler R, Kerp H, Wei H-B (2013) Complete tylosis formation in a latest Permian conifer stem. Ann Bot (Lond) 111: 1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, De Lorenzo G, Ferrari S (2008) The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol 148: 1695–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan V, Thomas G (2001) Salicylic acid response in rice: influence of salicylic acid on H2O2 accumulation and oxidative stress. Plant Sci 160: 1095–1106 [DOI] [PubMed] [Google Scholar]
- Gottwald HPJ. (1972) Tyloses in fibre tracheids. Wood Sci Technol 6: 121–127 [Google Scholar]
- Graham RC Jr., Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14: 291–302 [DOI] [PubMed] [Google Scholar]
- Hamann T. (2012) Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front Plant Sci 3: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Blanco C, Feng DX, Hu J, Sánchez-Vallet A, Deslandes L, Llorente F, Berrocal-Lobo M, Keller H, Barlet X, Sánchez-Rodríguez C, et al. (2007) Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 19: 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis WE. (1975) Ethylene and extraneous material formation in woody tissues. Phytochemistry 14: 2559–2562 [Google Scholar]
- Huang Z, Zhao P, Medina J, Meilan R, Woeste K (2013) Roles of JnRAP2.6-like from the transition zone of black walnut in hormone signaling. PLoS One 8: e75857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler BD, Showalter AM (2010) IRX14 and IRX14-LIKE, two glycosyl transferases involved in glucuronoxylan biosynthesis and drought tolerance in Arabidopsis. Mol Plant 3: 834–841 [DOI] [PubMed] [Google Scholar]
- Kitin P, Voelker SL, Meinzer FC, Beeckman H, Strauss SH, Lachenbruch B (2010) Tyloses and phenolic deposits in xylem vessels impede water transport in low-lignin transgenic poplars: a study by cryo-fluorescence microscopy. Plant Physiol 154: 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD. (2016) Cell wall-associated kinases and pectin perception. J Exp Bot 67: 489–494 [DOI] [PubMed] [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, Takei K, Kuroha T, Mizutani M, Ashikari M, Ueguchi-Tanaka M, Matsuoka M, Suzuki K, Sakakibara H (2009) Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant Cell Physiol 50: 1201–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J, Björklund S, Vahala J, Hertzberg M, Kangasjärvi J, Sundberg B (2009) Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc Natl Acad Sci USA 106: 5984–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe Y, Nafisi M, Verhertbruggen Y, Orfila C, Gille S, Rautengarten C, Cherk C, Marcus SE, Somerville S, Pauly M, et al. (2011) Loss-of-function mutation of REDUCED WALL ACETYLATION2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiol 155: 1068–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrone AJ, Grant JA, Kluepfel DA (2010) The role of tyloses in crown hydraulic failure of mature walnut trees afflicted by apoplexy disorder. Tree Physiol 30: 761–772 [DOI] [PubMed] [Google Scholar]
- Mishra P, Pramod S, Rao KS (2013) Effect of exogenous growth regulators on secondary vascular tissue differentiation in the twigs of Kigelia africana (Lam.). Benth Phyton 53: 133–149 [Google Scholar]
- Müller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B, Haughn G, Kermode AR (2013) Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol 161: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Funada R, Sano Y, Ohtani J (1999) The differentiation of contact cells and isolation cells in the xylem ray parenchyma of Populus maximowiczii. Ann Bot (Lond) 84: 429–435 [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V (2008) Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J 54: 43–55 [DOI] [PubMed] [Google Scholar]
- Pawar PM-A, Derba-Maceluch M, Chong S-L, Gómez LD, Miedes E, Banasiak A, Ratke C, Gaertner C, Mouille G, McQueen-Mason SJ, et al. (2016) Expression of fungal acetyl xylan esterase in Arabidopsis thaliana improves saccharification of stem lignocellulose. Plant Biotechnol J 14: 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Donoso AG, Greve LC, Walton JH, Shackel KA, Labavitch JM (2007) Xylella fastidiosa infection and ethylene exposure result in xylem and water movement disruption in grapevine shoots. Plant Physiol 143: 1024–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters WJ. (1974) Tylosis formation in Pinus tracheids. Bot Gaz 135: 126–131 [Google Scholar]
- Plavcová L, Hacke UG (2011) Heterogeneous distribution of pectin epitopes and calcium in different pit types of four angiosperm species. New Phytol 192: 885–897 [DOI] [PubMed] [Google Scholar]
- Pogorelko G, Lionetti V, Fursova O, Sundaram RM, Qi M, Whitham SA, Bogdanove AJ, Bellincampi D, Zabotina OA (2013) Arabidopsis and Brachypodium distachyon transgenic plants expressing Aspergillus nidulans acetylesterases have decreased degree of polysaccharide acetylation and increased resistance to pathogens. Plant Physiol 162: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi S, Ferrarini A, Delledonne M, Dunand C, Ranocha P, De Lorenzo G, Cervone F, Ferrari S (2015) The Arabidopsis class III peroxidase AtPRX71 negatively regulates growth under physiological conditions and in response to cell wall damage. Plant Physiol 169: 2513–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, Cervone F, Bellincampi D (2011) Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant Microbe Interact 24: 432–440 [DOI] [PubMed] [Google Scholar]
- Rioux D, Nicole M, Simard M, Ouellette GB (1998) Immunocytochemical evidence the secretion of pectin occurs during gel gum and tylosis formation in trees. Phytopathology 88: 494–505 [DOI] [PubMed] [Google Scholar]
- Saitoh T, Ohtani J, Fukazawa K (1993) The occurrence and morphology of tyloses and gums in the vessels of Japanese hardwoods. IAWA J 14: 359–371 [Google Scholar]
- Scheckler SE, Galtier J (2003) Tyloses and ecophysiology of the early carboniferous progymnosperm tree Protopitys buchiana. Ann Bot (Lond) 91: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Liese W (1993) Response of xylem parenchyma by suberization in some hardwoods after mechanical injury. Trees (Berl) 8: 23–30 [Google Scholar]
- Scott PC, Miller LW, Webster BD, Leopold AC (1967) Structural changes during bean leaf abscission. Am J Bot 54: 730–734 [Google Scholar]
- Sexton R. (1976) Some ultrastructural observations on the nature of foliar abscission in Impatiens sultani. Planta 128: 49–58 [DOI] [PubMed] [Google Scholar]
- Shi J, Mueller WC, Beckman CH (1992) Vessel occlusion and secretory activities of vessel contact cells in resistant or susceptible cotton plants infected with Fusarium oxysporum f. sp. vasinfectum. Physiol Mol Plant Pathol 40: 133–147 [Google Scholar]
- Siedlecka A, Wiklund S, Péronne MA, Micheli F, Leśniewska J, Sethson I, Edlund U, Richard L, Sundberg B, Mellerowicz EJ (2008) Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol 146: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SD, Ashford DA, Harvey DJ, Bowles DJ (1998) Short chain oligogalacturonides induce ethylene production and expression of the gene encoding aminocyclopropane 1-carboxylic acid oxidase in tomato plants. Glycobiology 8: 579–583 [DOI] [PubMed] [Google Scholar]
- Skrzypek E, Miyamoto K, Saniewski M, Ueda J (2005) Identification of jasmonic acid and its methyl ester as gum-inducing factors in tulips. J Plant Res 118: 27–30 [DOI] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Matthews MA (2008) Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): Tyloses in summer and gels in winter1. Am J Bot 95: 1498–1505 [DOI] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Matthews MA (2006) Pruning-induced tylose development in stems of current-year shoots of Vitis vinifera (Vitaceae). Am J Bot 93: 1567–1576 [DOI] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Reid MS, Matthews MA (2007) Ethylene and not embolism is required for wound-induced tylose development in stems of grapevines. Plant Physiol 145: 1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Gartner BL, Morrell JJ (2002) Heartwood formation and natural durability-a review. Wood Fiber Sci 34: 587–611 [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Tsang DL, Edmond C, Harrington JL, Nühse TS (2011) Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol 156: 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Yordanov L, Edreva A (2000) Oxidative stess and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 151: 59–66 [Google Scholar]
- Vogel JP, Raab TK, Schiff C, Somerville SC (2002) PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14: 2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Somerville CR, Somerville SC (2004) Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J 40: 968–978 [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot (Lond) 111: 1021–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Mravec J, Greiner S, Mouille G, Höfte H (2012) Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol 22: 1732–1737 [DOI] [PubMed] [Google Scholar]
- Wolf S, van der Does D, Ladwig F, Sticht C, Kolbeck A, Schürholz AK, Augustin S, Keinath N, Rausch T, Greiner S, et al. (2014) A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc Natl Acad Sci USA 111: 15261–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang Z, Wang M, Wei J, Chen H, Gao Z, Sui C, Luo H, Zhang X, Yang Y, Meng H, Li W (2013) Identification of genes related to agarwood formation: transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genomics 14: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Shen L, Fan B, Zhao D, Zheng Y, Sheng J (2009) The effect of MeJA on ethylene biosynthesis and induced disease resistance to Botrytis cinerea in tomato. Postharvest Biol Technol 54: 153–158 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.