Sorghum sucrose transporters may function to efflux sucrose from the phloem during early internode development and retrieve leaked sucrose to the phloem as internodes mature.
Abstract
How sucrose transporters (SUTs) regulate phloem unloading in monocot stems is poorly understood and particularly so for species storing high Suc concentrations. To this end, Sorghum bicolor SUTs SbSUT1 and SbSUT5 were characterized by determining their transport properties heterologously expressed in yeast or Xenopus laevis oocytes, and their in planta cellular and subcellular localization. The plasma membrane-localized SbSUT1 and SbSUT5 exhibited a strong selectivity for Suc and high Suc affinities in X. laevis oocytes at pH 5—SbSUT1, 6.3 ± 0.7 mm, and SbSUT5, 2.4 ± 0.5 mm Suc. The Suc affinity of SbSUT1 was dependent on membrane potential and pH. In contrast, SbSUT5 Suc affinity was independent of membrane potential and pH but supported high transport rates at neutral pH. Suc transport by the tonoplast localized SbSUT4 could not be detected using yeast or X. laevis oocytes. Across internode development, SUTs, other than SbSUT4, were immunolocalized to sieve elements, while for elongating and recently elongated internodes, SUTs also were detected in storage parenchyma cells. We conclude that apoplasmic Suc unloading from de-energized protophloem sieve elements in meristematic zones may be mediated by reversal of SbSUT1 and/or by uniporting SWEETs. Storage parenchyma localized SbSUT1 and SbSUT5 may accumulate Suc from the stem apoplasms of elongating and recently elongated internodes, whereas SbSUT4 may function to release Suc from vacuoles. Transiting from an apoplasmic to symplasmic unloading pathway as the stem matures, SbSUT1 and SbSUT5 increasingly function in Suc retrieval into metaphloem sieve elements to maintain a high turgor to drive symplasmic unloading by bulk flow.
In most herbaceous crop plants, photoassimilates fixed in source leaves are loaded into the collection phloem as Suc, which is translocated through the transport phloem by a pressure flow mechanism to supply carbon substrate for sink growth and/or storage (Münch, 1930). A portion of the translocated Suc is unloaded along the transport phloem linking collection phloem of leaf minor veins with release phloem in terminal sinks, such as shoot/root apices and developing tubers, fruits, and seeds. While there is a growing mechanistic understanding of phloem unloading into terminal sinks, unloading from the transport phloem has attracted less attention. This status particularly applies to stems of monocot species that accumulate Suc to high concentrations such as sugarcane (Saccharum officinarum) and sweet Sorghum (Slewinski, 2012; Grof et al., 2014), in which stem storage is the predominant sink, limited by its capacity to accumulate Suc (Watt et al., 2013).
Transport phloem of monocot stems traverses their intercalary meristems, located immediately above the basal node of each elongating internode, cell elongation and mature zones. Hence, Suc unloaded from the transport phloem supports stem growth (cell division and expansion) and storage (elongating and mature zones; Milne et al., 2015). Apoplasmic phloem unloading into the intercalary meristem occurs from symplasmically isolated protophloem sieve elements (PSEs) that extend into the elongation zone (Milne et al., 2015). Metaphloem sieve element-companion cell (SE-CC) complexes replace PSEs in the deaccelerating zone of cell elongation. Plasmodesmal interconnections between cells located along a radial unloading pathway from these SE-CC complexes to the surrounding storage parenchyma (SP) cells suggest a potential for symplasmic unloading (Walsh et al., 2005). Indeed, symplasmic phloem unloading of membrane-impermeant fluorochromes has been detected in mature stem zones of sugarcane (Rae et al., 2005) and sweet Sorghum cv Rio (Milne et al., 2015) as well as rice (Oryza sativa; Scofield et al., 2007) and wheat (Triticum aestivum; Aoki et al., 2004). In contrast, for another cultivar of sweet Sorghum, Wray, no evidence of symplasmic phloem unloading could be detected (Bihmidine et al., 2015). Interestingly, Wray stems exhibit a less pronounced thickening of the walls of bundle sheath and SP cells (Bihmidine et al., 2015) compared to Rio (Milne et al., 2015). This suggests these cultivar differences may arise from the degree to which a cell wall barrier develops to attenuate radial transport through the stem apoplasm from the vascular bundles to the SP cells.
The above analysis points to a central role played by plasma membrane sugar transporters in facilitating radial apoplasmic transfer of Suc or its hexose derivatives derived from hydrolysis of apoplasmic Suc by cell wall invertase (Grof et al., 2014), from SE lumens to SP cells in the intercalary meristems, elongating and transition zones of monocot stems. In some sweet Sorghum cultivars, this scenario could extend to mature stem zones (Bihmidine et al., 2015). Release of Suc across the plasma membranes of the PSEs or SE-CCs to the phloem apoplasm could occur by simple diffusion driven down transmembrane Suc concentration gradients (Patrick, 2013b) by reversal of de-energized Suc/proton symporters (Carpaneto et al., 2005) or possibly facilitated by the newly discovered family of Suc uniporters, SWEETs (e.g. Le Hir et al., 2015). In Arabidopsis (Arabidopsis thaliana) stems, phenotypes of knockout mutants of AtSUC2, a plasma membrane Suc/proton symporter localized to CCs (Stadler and Sauer, 1996), demonstrated that the transporter functions to retrieve Suc leaked to the phloem apoplasm (Srivastava et al., 2008; Gould et al., 2012). Similar roles might apply to SUT3 clade members localized to SE-CC complexes of mature stems of wheat and rice (Aoki et al., 2004; Scofield et al., 2007). Suc released to the stem apoplasm could be hydrolyzed by cell wall invertases active in the intercalary meristem and elongation zone of sugarcane stems (Grof et al., 2014) and taken up by hexose/proton symporters (Casu et al., 2003) or SWEETs expressed by SP cells (Sosso et al., 2015). Transitioning to a mature state, cell wall invertase activity decreases (Grof et al., 2014), and uptake from the stem apoplasm by SP cells is likely to be mediated by Suc/proton symporters. In this context, ShSUT1 was highly expressed in maturing internodes of sugarcane and was localized to cells surrounding vascular bundles (Rae et al., 2005). Since the Suc storage compartment in SP cells are their vacuoles (Grof et al., 2014), Suc loading of vacuoles could be a key element in determining levels to which Suc accumulates. Significantly, expression of Sorghum tonoplast sugar transporters (TSTs), but not that of SUTs or SWEETs, was found to be positively correlated with Suc accumulation in Sorghum stems (Bihmidine et al., 2016).
The primary focus of the reported study was on deducing the roles SUTs play in unloading Suc from the transport phloem during the key phases of internode development in sweet Sorghum by determining their functional transport properties, expression patterns, and cellular/subcellular localization. SbSUT1 was expressed preferentially in mature internode regions, whereas SbSUT5 was highly expressed midway through internode development. Both transporters localized to the plasma membrane and exhibited complementary functional properties—SbSUT5 demonstrated a high Suc affinity that was independent of pH and membrane potential, whereas SbSUT1 had a lower Suc affinity that was dependent on pH and membrane potential. SbSUTs immunolocalized to PSEs may function via reversal of transport direction to efflux Suc (Carpaneto et al., 2005), whereas those localized to mature SEs are likely to retrieve leaked Suc, maintaining high Suc concentrations within the SE lumen.
RESULTS
Functional Characterization of SbSUTs
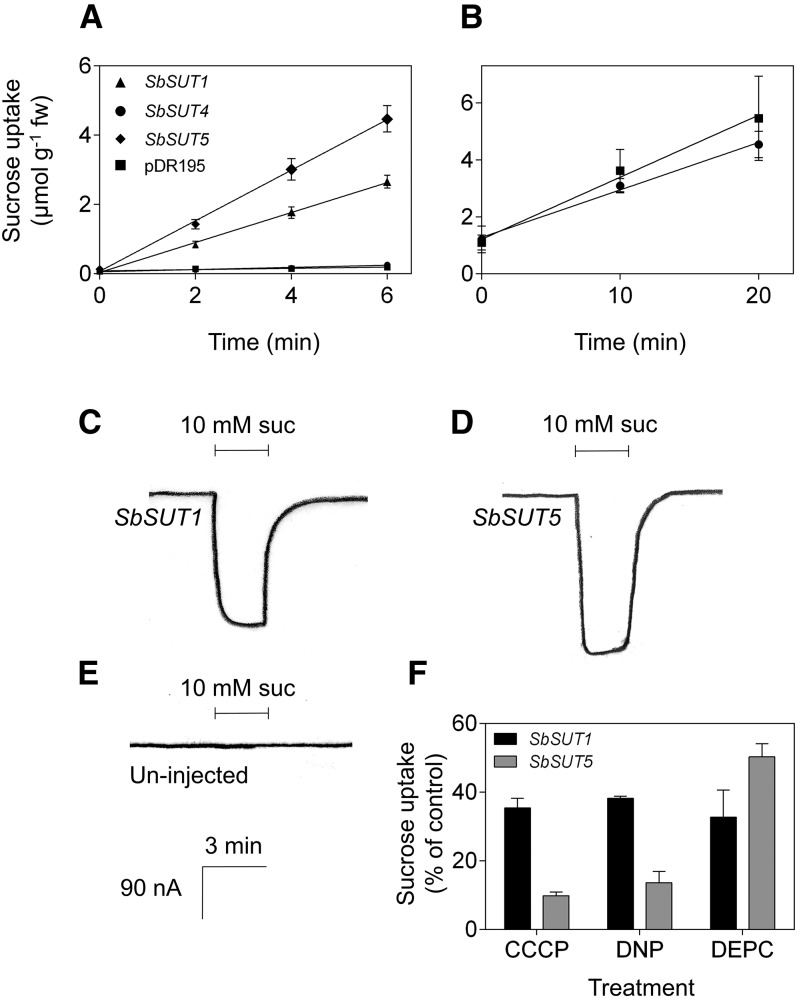
SbSUT1 and SbSUT5 were transport competent when their encoding genes were heterologously expressed in yeast cells and Xenopus laevis oocytes (Fig. 1, A, C, and D). In contrast, SbSUT4 did not transport Suc in yeast cells (Fig. 1, A and B) or oocytes (data not shown). SbSUT1 and SbSUT5 likely function as Suc/proton symporters as the protonophores, carbonyl cyanide m-chlorophenyl hydrazone (CCCP) and 2,4-dinitrophenol (DNP) reduced [14C]Suc uptake in transformed yeast (Fig. 1F). Additionally, Suc-induced currents in oocytes expressing SbSUT1 or SbSUT5 were consistent with Suc/proton symport activity (Fig. 1, C and D, versus Fig. 1E). DEPC negatively impacted Suc transport by SbSUT1 and SbSUT5 (Fig. 1F), presumably by binding to their conserved His-65 residue known to compromise Suc transport ability (Lu and Bush, 1998).
Figure 1.
Transport capability and energy coupling of SbSUTs after expression of their cDNAs in heterologous systems. A, Time course of Suc accumulation from a 1-mm Suc solution by the yeast strain SEY6210 transformed with SbSUT1, SbSUT4, SbSUT5, or empty pDR195 vector. B, Time course uptake from a 15-mm Suc solution by SEY6210 transformed with SbSUT4 or pDR195 empty vector. Uptake experiments were carried out at pH 5. Mean ± sd (vertical bars) of three biological replicates. C, D, and E, Current traces of X. laevis oocytes injected with (C) SbSUT1, (D) SbSUT5, or (E) uninjected, when exposed to a modified Na-Ringer solution at pH 5.6 containing 10 mm Suc. Traces shown are of single oocytes. Scale in E also applies to C and D. F, Changes in Suc uptake (expressed as proportion of control) by yeast transformed with SbSUT1 and SbSUT5 in the presence of the protonophores CCCP (10 µm) and DNP (50 µm) or the sulfhydryl reagent DEPC (1.5 mm). Mean ± sd (vertical bars) of three biological replicates.
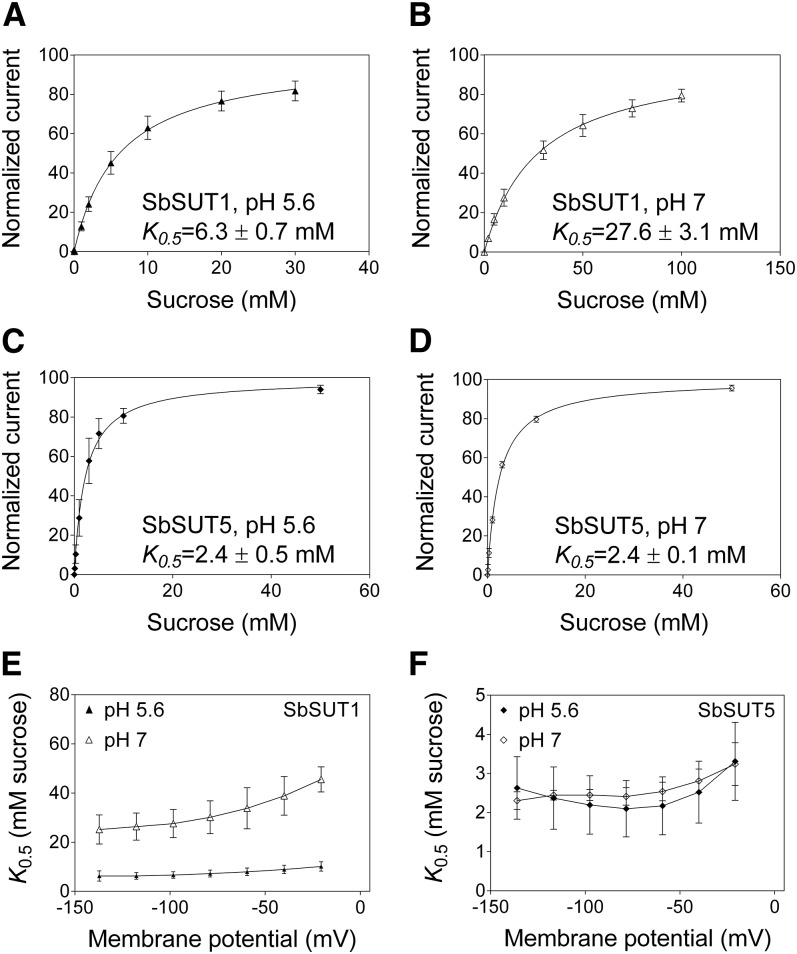
Suc transport by SbSUT1 was optimal at pH 5 in yeast and oocytes, whereas SbSUT5 exhibited transport optima at pH 7 in yeast and pH 5 in oocytes (Supplemental Fig. S1). In the yeast experiments, membrane potential was not controlled (it was clamped in the oocyte experiments). Membrane potential does affect transport rate and it may have been different in yeast at different pH values and influenced SbSUT5 transport rate. Suc affinity of SbSUT1 was strongly pH dependent (Fig. 2, A and B), while that of SbSUT5 was pH independent (Fig. 2, C and D).
Figure 2.
Voltage and pH dependences of the affinities of SbSUT1 and SbSUT5 for Suc. Transporters were expressed in X. laevis oocytes and recordings made by two-electrode voltage clamping. A to D, Concentration-dependent Suc transport. A, SbSUT1 at pH 5.6. B, SbSUT1 at pH 7. C, SbSUT5 at pH 5.6. D, SbSUT5 at pH 7. Currents were recorded under voltage-clamped conditions at a membrane potential of −117 mV. A Michaelis-Menten curve was fitted to each data set, which was then normalized to Vmax and plotted against the substrate concentration. E and F, Voltage dependence of SUT affinity for Suc. E, SbSUT1 Suc affinities at membrane potentials from −137 mV to −20 mV. F, SbSUT5 Suc affinities at membrane potentials from −137 mV to −20 mV. Mean ± sd (vertical bars) of three to five oocytes.
SbSUT1 and SbSUT5 showed linear current-voltage relations at both pH 5.6 and pH 7 (Supplemental Fig. S2), indicating that regulation of transport activity by membrane potential was not observed under these conditions. Suc affinity of SbSUT1 was voltage dependent at pH 7 but not pH 5.6 (Fig. 2E), while SbSUT5 was voltage independent at both pH 7 and 5.6 (Fig. 2F). Interestingly, unlike its affinity for Suc, the affinity of SbSUT5 for maltose was both pH and voltage dependent at pH 7 (Supplemental Fig. S3).
The SbSUTs were highly selective for Suc (Supplemental Fig. S4) similar to sugarcane and rice SUTs (Reinders et al., 2006; Sun et al., 2010). All sugars transported by SbSUTs were glucosides (although not all glucosides tested were transported), suggesting that their substrate-binding site requires Glc for recognition. SbSUT1 (Supplemental Fig. S4A) exhibited a slightly narrower substrate specificity compared to SbSUT5 (Supplemental Fig. S4B).
Stem Expression Profiles of SbSUTs and SbTSTs
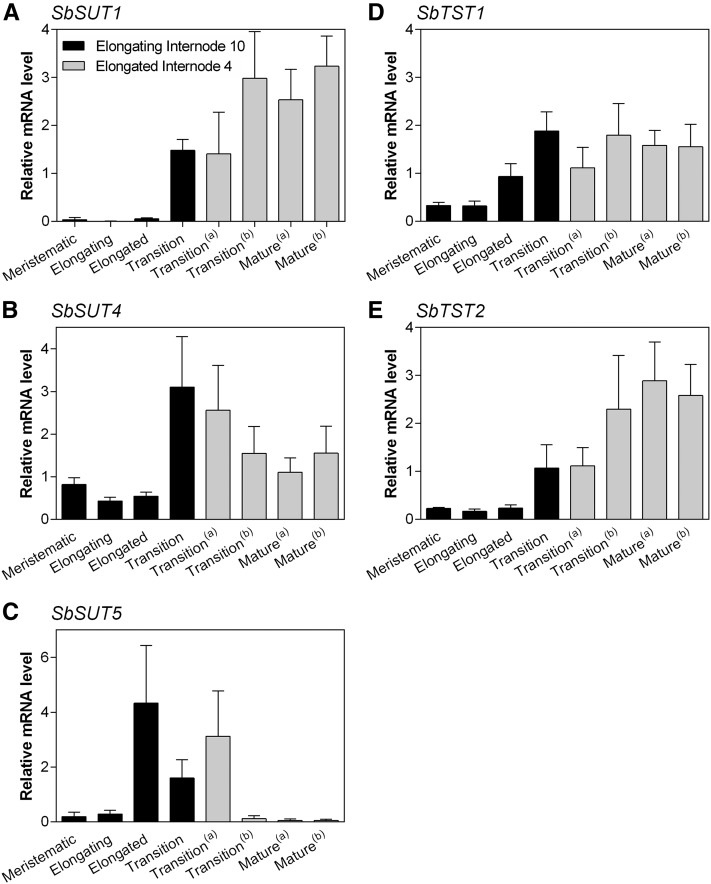
Transcript levels of SbSUTs and SbTSTs were quantified in developmental zones of elongating Internode 10 and fully elongated Internode 4 at booting (detailed in Supplemental Fig. S5). In elongating Internode 10, SbSUT1 expression was restricted to the transition zone and transcript levels doubled in the mature zones of fully elongated Internode 4 (Fig. 3A). In contrast, SbSUT4 was expressed throughout all internode zones with transcript levels being 3-fold higher within the transition zone of elongating Internode 10 and decreasing by 25% in the more mature zones of fully elongated Internode 4 (Fig. 3B). SbSUT5 was most highly expressed in the recently elongated zone of elongating Internode 10 and in the least mature zone of fully elongated Internode 4 (Fig. 3C).
Figure 3.
Expression of SbSUTs and SbTSTs in specified stem developmental zones of internodes at booting. Transporter transcript levels relative to SbEF-1α measured within specified developmental zones of elongating Internode 10 and fully elongated Internode 4 (Supplemental Fig. S5). A, SbSUT1; B, SbSUT4; C, SbSUT5; D, SbTST1; E, SbTST2. Columns with vertical bars respectively represent the mean ± sd of five biological replicates. “(a)” and “(b)” distinguish multiple transition and mature zones harvested at booting. The descriptors “transition” and “mature” refer to the extent of lignification/suberization predominantly in the inner regions of the internode (for further details, see Milne et al., 2015).
TSTs are capable of transporting Suc into rice, Arabidopsis, and sugar beet vacuoles (Cho et al., 2010; Schulz et al., 2011; Jung et al., 2015). Since TSTs were highly expressed in stem internodes of a sweet compared to a grain cultivar at anthesis (Bihmidine et al., 2016), their expression was quantified within developmental zones as candidates responsible for loading Suc into SP vacuoles of Sorghum stems. Transcript levels of SbTST1 were similar in the transition and mature zones of fully elongated Internode 4 (Fig. 3D), while SbTST2 exhibited maximal expression in mature regions (Fig. 3E). SbTST3 transcript levels were largely undetected (data not shown).
Subcellular and Cellular Localization of SbSUTs
Tobacco leaf protoplasts transformed with SbSUT-GFP fusion constructs were analyzed by confocal laser-scanning microscopy. Comparison of the position of fluorescence to that of chloroplasts was used to determine whether GFP was localized to the plasma membrane or tonoplast. For SbSUT1-GFP and SbSUT5-GFP, their GFP fluorescence was external to chloroplast position, suggesting a plasma membrane localization (Fig. 4, A and E). Additionally, endomembrane-localized fluorescence was evident for SbSUT1-GFP (Fig. 4A). In contrast, the subcellular localization of SbSUT4-GFP was on the tonoplast, as the GFP fluorescence signal was oriented inside of the position of chloroplasts (Fig. 4C). AtPTR1 (Dietrich et al., 2004) and AtPTR2 (Weichert et al., 2012) were used as positive controls and localized to the plasma membrane and tonoplast, respectively (Fig. 4, B and D).
Figure 4.
Localization of SUT-GFP-fusion proteins in tobacco leaf protoplasts. Confocal images of transformed protoplasts, chloroplasts false-colored magenta. A, C, and E, SUT-GFP merged with chloroplast fluorescence. A, SbSUT1-GFP (endomembrane localized fluorescence is also present; darts). C, SbSUT4-GFP. E, SbSUT5-GFP. Plasma membrane localization was observed in merged images A and E, where chloroplasts were positioned on the cytoplasmic side of the GFP fluorescence (arrows). Tonoplast localization was observed in C, where the GFP-fluorescent tonoplast was positioned on the cytoplasmic side of the chloroplast (arrow). B, AtPTR1-GFP plasma membrane control. D, AtPTR2-GFP tonoplast control. GFP excitation, 473 nm; emission, 487 to 521 nm. Chloroplast excitation, 559 nm; emission, 606 to 673 nm. Bar = 10 µm.
Immunolocalization of the dicot SUTs StSUT1 and LeSUT1 to either the SE (Kühn et al., 1997; Barker et al., 2000) or CC (Schmitt et al., 2008) plasma membranes alone led to the conclusion that aldehyde-based fixatives may negatively impact the antigenicity of membrane proteins (Schmitt et al., 2008). Hence, to assess any effects on antigenicity, two fixative solutions were tested on Sorghum tissue. Appropriate preimmune controls revealed no labeling of Sorghum sections (see Figs. 5 and 6 and Supplemental Fig. S6), indicating no cross-reactivity with unknown SE-specific epitopes as previously observed (Schmitt et al., 2008) and that immunolabeling by the antiserum was likely to be specific. Stronger immunolabeling with lower levels of background fluorescence within tissues fixed with ethanol-acetic acid, in comparison to those fixed with glutaraldehyde-paraformaldehyde (Supplemental Fig. S7), led to the former being used to fix tissue samples for subsequent experiments. This observation is in agreement with observations made by Schmitt et al. (2008).
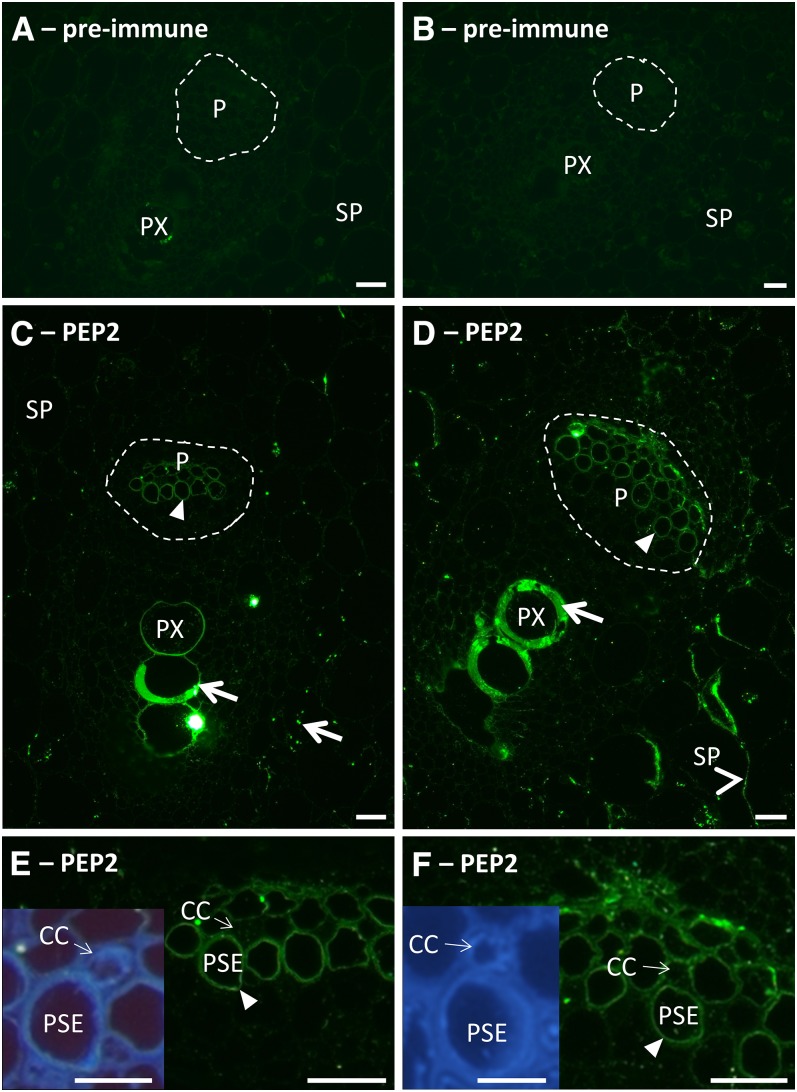
Figure 5.
Immunolocalization of SbSUTs with PEP2 antiserum in apoplasmic phloem unloading zones of elongating Internode 10. A, C, and E, Meristematic zone. A, Preimmune with secondary antibody control. C, Vascular bundle with the phloem outlined by a broken white line. E, Higher magnification of phloem shown in C; inset shows UV autofluorescent image of E. B, D, and F, Elongating zone. B, Preimmune with secondary antibody control. D, Vascular bundle with the phloem outlined by broken white line. F, Higher magnification of phloem shown in D; inset shows UV autofluorescent image of F. Immunolabeling by PEP2 antiserum was present on plasma membranes of PSEs in C to F (darts) and SP cells (arrowheads) in D. CCs did not appear to be labeled. Nonspecific labeling of protoxylem element walls in C and D, and starch grains in C (arrows). Enlarged preimmune control images shown in Supplemental Figure S6. Sections were viewed under the fluorescein isothiocyanate filter (excitation, 450 to 490 nm; emission, >515 nm) and the UV filter (excitation, 365 nm; emission, >420 nm). P, Phloem; PX, protoxylem element. Bars = 20 µm; inset bar = 10 µm.
Figure 6.
Immunolocalization of stem SbSUTs with PEP2 antiserum-symplasmic phloem unloading pathway in the mature zone of fully elongated Internode 4. A, Preimmune with secondary antibody control. B, Vascular bundle with the phloem outlined by broken white line. C, Higher magnification of phloem shown in B; inset shows enlarged UV autofluorescent image of C. Immunolabeling of SE plasma membranes in B and C (darts). Nonspecific labeling of metaxylem element walls in B (arrows). Sections were viewed under the fluorescein isothiocyanate filter (excitation, 450 to 490 nm; emission, >515 nm) and the UV filter (excitation, 365 nm; emission, >420 nm). MX, Metaxylem element; P, phloem. Bars = 20 µm, inset bar = 10 µm.
SbSUTs were immunolocalized within inner vascular bundles, consistent with their primary function in transport. In contrast, outer vascular bundles mainly provide structural support to the stem (Milne et al., 2015). Within the meristematic zone, SUTs were localized to the plasma membrane of PSEs (Fig. 5, C and E, for cell detail see inset in E). For the elongating and recently elongated zones, SUTs were localized to plasma membranes of PSEs (elongating), metaphloem SEs (recently elongated), and SP cells (elongating region shown in Fig. 5, D and F; recently elongated zone shown in Supplemental Fig. S8). In mature zones, where the apoplasmic pathway was restricted (Milne et al., 2015), SUTs were localized to SE plasma membranes alone (Fig. 6, B and C). Localization to CC plasma membranes was not evident in any of the sections examined. PEP2 labeling of starch granules in mesophyll cells (Fig. 5C) and cell walls of protoxylem (Fig. 5, C and D) and metaxylem (Fig. 6B) elements was considered to be nonspecific.
DISCUSSION
Overall, Suc transporter expression, and hence probably transporter abundance (Walley et al., 2016), tended to correlate with Suc accumulation in sweet Sorghum stems for SbSUT1, SbTST1, and SbTST2 (Fig. 3, A, D, and E; see Milne et al., 2015). In contrast, expression levels of SbSUT4 and SbSUT5 were more abundant in elongating internodes peaking during the internode transition period and thereafter decreasing across the major phase of Suc accumulation (Fig. 3, B and C). Here, a decrease in SbSUT4 expression is consistent with a shift to vacuolar accumulation of Suc (Milne et al., 2015). These broad developmental shifts in transporter expression provide a framework for evaluating the role of Suc transporters, and in particular the SbSUTs, in phloem unloading and storage of Suc across key development phases of sweet Sorghum internodes informed by their transport properties and cellular/subcellular localization patterns.
Functional Properties and Localization of SbSUTs
SbSUT1 and SbSUT5 fall into monocot-specific phylogenetic groups of the SUT family of Suc transporters (Milne et al., 2013). Both proteins were shown to function as Suc/proton symporters dependent on an inward-directed proton motive force (pmf; Bush, 1990), as both components of the pmf, membrane potential and ∆pH, affected SbSUT Suc transport rates in the heterologous systems (Fig. 1; Supplemental Fig. S2). Increased SbSUT transport rate in X. laevis oocytes at more negative membrane potentials, attenuated by a more alkaline external pH (Supplemental Fig. S2), indicates reliance on an inward directed pmf. Other monocot SUTs such as ShSUT1, HvSUT1, OsSUT1, and OsSUT5 also exhibited greater transport activity in oocytes at more negative membrane potentials (Sivitz et al., 2005; Reinders et al., 2006; Sun et al., 2010). Treatment of yeast harboring SbSUT1 and SbSUT5 with protonophores (CCCP and DNP), capable of dissipating ∆pH by reducing proton gradients (de la Peña et al., 1982; Gasková et al., 1998), reduced Suc uptake by SbSUT1 (∼65%) and SbSUT5 (∼90%), indicating Suc transport by both SUTs relies on proton coupling (Fig. 1F). Proton coupling has been observed for other SUTs (Lemoine, 2000).
Maximal transport activity of SbSUT1 occurred at pH 5 in yeast and oocytes; however, SbSUT1 activity in yeast was lower at pH 4 than at pH 5, even though the pmf is likely to be greater at pH 4 than pH 5. More acidic pH may physically affect outer face geometry of the SbSUT1 Suc-binding site, reducing the capability to bind and transport Suc. Reduction in activity from pH 5 to 4 was also observed for other SUTs such as AtSUT2 (Schulze et al., 2000), AtSUT4 (Weise et al., 2000), DcSUT1, and DcSUT2 from carrot (Daucus carota; Shakya and Sturm, 1998), GmSUT1 from soybean (Glycine max; Aldape et al., 2003), and PvSUT1 from French bean (Phaseolus vulgaris; Zhou et al., 2007). Few SUTs display maximal activity outside the pH 4 to 5 range and SbSUT5 fits into this category, as maximal uptake occurred at pH 7 in yeast (Supplemental Fig. S1). In oocytes, the greatest activity of SbSUT5 occurred at pH 5; however, SbSUT5 activity remained greater than SbSUT1 at more neutral pH values (pH 6 to 7; Supplemental Fig. S1). The closely related OsSUT5 also displayed somewhat similar pH dependence to SbSUT5 in oocytes (Sun et al., 2010). A broad pH optimum from pH 4 to 6 was reported for AtSUC1 and PmSUC1 (Sauer and Stolz, 1994; Gahrtz et al., 1996), and AtSUC9 showed highest activity above pH 6 (Sivitz et al., 2007). In the absence of regulation by pH, we expect proton-coupled transporter activity to be stimulated by low pH since the driving force for transport (pmf) is increased. The pH independence of SbSUT5 suggests that its activity is negatively regulated by low pH. In contrast, SbSUT1 activity showed an optimum at low pH.
Changes in external pH and membrane potential causing a reduction in pmf resulted in a reduction in Suc affinity of SbSUT1 (Fig. 2), similar to observations made for SUTs, such as ZmSUT1, HvSUT1, ShSUT1, and OsSUT1 (Carpaneto et al., 2005; Sivitz et al., 2005; Reinders et al., 2006; Sun et al., 2010). In contrast, the SbSUT5 Suc affinity was maintained independent of external pH and membrane potential, somewhat similar to observations made for OsSUT5 (Sun et al., 2010). SbSUT1 affinity may be more responsive to changing pH and membrane potentials in planta, whereas SbSUT5 may operate with similar affinity irrespective of pH and membrane potential. Interestingly, the SbSUT1 maltose affinity showed a similar voltage dependence profile to that of Suc (data not shown). However, the maltose affinity of SbSUT5 was affected by pH and membrane potential, indicating that this transporter may interact with maltose slightly differently than Suc (Supplemental Fig. S3). Since maltose and Suc consist of a Glc moiety linked to either a Glc or Fru moiety, respectively, it is likely that the SUT binding site interacts with both of the moieties, as different transport properties were observed for the two disaccharides.
The substrate specificity of SbSUT1 closely resembles that of other monocot SUT1s from rice (Sun et al., 2010), sugarcane (Reinders et al., 2006), and barley (Sivitz et al., 2005). Similarly, SbSUT5 exhibited the same substrate specificity as OsSUT5 (Sun et al., 2010). Substrates that were capable of being transported by SbSUTs contained a Glc moiety; however, the monosaccharide Glc was not transported (Supplemental Fig. S4). Specific amino acids of OsSUT1 contributing to its substrate specificity (Reinders et al., 2012) are conserved among SbSUT1, OsSUT1, ShSUT1, and HvSUT1. Significantly, these four transporters share almost identical substrate specificities. In contrast, four of these five amino acids differ between SbSUT1 and SbSUT5 sequences, possibly accounting for their slightly different substrate specificities.
Within all examined developmental zones of the Sorghum stem, SUTs were localized to the SE plasma membrane (or PSE plasma membrane in meristematic/elongating regions) similar to observations in a wheat internode (Aoki et al., 2004). Only within elongating and recently elongated zones, transitory localization to SP cells was observed (Figs. 5 and 6; Supplemental Fig. S8). Rice SUTs were immunolocalized to both SE and CC plasma membranes along the pathway from source leaves to developing grains (Scofield et al., 2007). Sugarcane SUTs were immunolocalized to SE-CCs and cells surrounding vascular bundles, respectively, by two different antisera (Rae et al., 2005). It is likely SUTs localized to either SE or CC plasma membranes of the metaphloem will retrieve leaked Suc to maintain high concentrations within the phloem for long-distance transport. SUTs localized to cells surrounding vascular bundles in sugarcane are likely to reload leaked Suc to the radial symplasmic compartment (Rae et al., 2005). Depending on the cytoplasmic and apoplasmic Suc concentrations combined with membrane potential difference, PSE-localized SUTs may mediate Suc efflux to the phloem apoplasm via reversal of transport direction (as demonstrated for ZmSUT1; Carpaneto et al., 2005).
Functions of Sugar Transporters Where Apoplasmic Unloading May Occur in Stems
Within meristematic and elongating zones of sweet Sorghum stems, an apoplasmic continuity, and hence a potential apoplasmic phloem unloading pathway, extends from the phloem to SP cells (Milne et al., 2015; Fig. 7, A and B). For the meristematic zone, phloem unloading is confined to PSEs, some of which were accompanied by CCs similar to other monocot stems (e.g. Ervin and Evert, 1967). If the PSEs were symplasmically isolated, as found for PSEs in stems of P. vulgaris (Wood et al., 1998), then Suc will exit PSE lumens across their plasma membrane.
Figure 7.
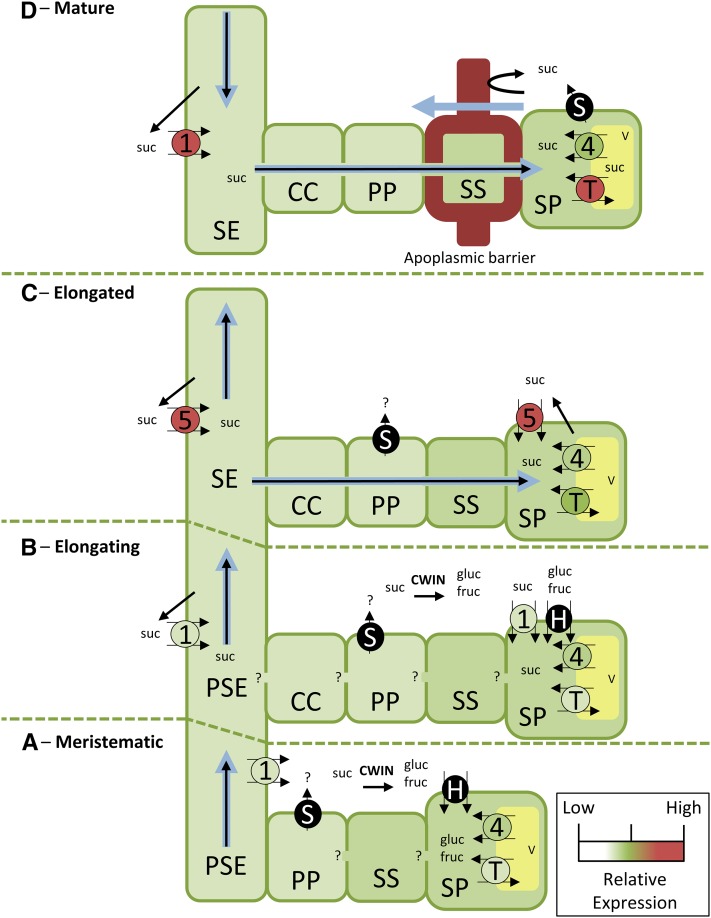
Predicted pathways and mechanisms of Suc unloading in elongating, recently elongated, and mature zones of Sorghum internodes. Bulk flow of photoassimilates is likely to be in an acropetal direction during internode elongation (Patrick, 1972). A and B, Unloading occurs through an apoplasmic pathway in meristematic and elongating zones. Suc is effluxed from the phloem by SUT reversal (where CCs are absent) or facilitated by SWEETs. Suc may be cleaved to hexoses by cell wall invertase (CWIN) and transported into SP cells by hexose transporters in A and B. Suc may also be imported into SP cells by SbSUTs in B. C, Unloading in the recently elongated zone may occur by a combination of symplasmic and apoplasmic transport, with SbSUTs localized on phloem and SP cell plasma membranes. Suc may be effluxed from phloem parenchyma (PP) by SbSWEETs. D, A symplasmic unloading pathway from SEs to SP cells is required to by-pass the apoplasmic barrier in the sclerenchyma sheath cell wall (Milne et al., 2015). Suc transporters on the SE-CCs are likely to retrieve leaked Suc. Suc leakage to the SP apoplasm may occur. In each developmental zone of the internode, tonoplast monosaccharide transporters (TSTs) and SbSUT4 may mediate Suc influx into and efflux from vacuoles. Heavy black arrows represent Suc movement, heavy blue arrows represent water movement. SbSUTs and SbTSTs are color coded according to their level of expression. 1, SbSUT1; 4, SbSUT4; 5, SbSUT5; gluc, Glc; fruc, Fru; CWIN, cell wall invertase; H, hexose transporter; PP, phloem parenchyma; S, SWEET; SS, sclerenchyma sheath; suc, Suc; T, tonoplast monosaccharide transporter; V, vacuole.
In the absence of energy-creating CCs (Lalonde et al., 2003), the membrane potential and hence pmf of the PSEs will be low (Carpaneto et al., 2005). Under these conditions, the outward-directed gradient in chemical potential energy generated by the transmembrane difference in Suc concentration across the PSE plasma membrane could exceed that of their pmf, thus reversing Suc/proton symport to an efflux mode (Carpaneto et al., 2005). Hence, the PSE localized SbSUTs may unload Suc by a reversed transport function (Fig. 7A; Carpaneto et al., 2005). The Suc concentration gradient could be maintained by extracellular invertase activity hydrolyzing apoplasmic Suc to Glc and Fru (Fig. 7A). In support of this assertion, cell wall invertase activity has been detected in intercalary meristems of sugarcane stems (Grof et al., 2014), and an extracellular invertase specifically localizes to cell walls of PSEs in tomato ovaries (Palmer et al., 2015). Additionally, acid invertase activity was greater in elongating Sorghum cv Rio compared to elongated internodes (Lingle, 1987).
When CCs are associated with PSEs in meristematic and elongation zones, the CC-localized proton-ATPases generate membrane potentials and pmfs in excess of −100 mV (Hafke et al., 2013) that drive PSE localized SUT-mediated Suc retrieval from the phloem apoplasm into the PSE lumen (Fig. 7B; Carpaneto et al., 2005; Srivastava et al., 2008; Gould et al., 2012). Under these conditions, energy-independent Suc transporters (SWEETs; Chen et al., 2012) could mediate Suc efflux to the stem apoplasm down the Suc concentration gradient (Fig. 7, A and B). In this context, SbSWEET13A was highly expressed in stem internodes (Bihmidine et al., 2016), and transcript profiling of a Setaria viridis internode revealed that SvSWEET15 (closely related to OsSWEET15) was highly expressed in the intercalary meristem and elongation zone (Martin et al., 2016). SvSWEET15, like SbSWEET13A, falls within the SWEET Clade III that preferentially transports Suc (Chen et al., 2012). Significantly, Clade III members AtSWEET11 and AtSWEET12 are expressed in phloem parenchyma cells of transport phloem in Arabidopsis stems where they could perform an unloading function (Le Hir et al., 2015). To avoid a futile cycle of Suc unloading from, and reloading into, the SE-CCs (Hafke et al., 2013), the released Suc could be hydrolyzed by a cell wall invertase (Fig. 7B; Grof et al., 2014; Palmer et al., 2015).
Hexose transporters are expressed in sugarcane stems (Casu et al., 2003) and throughout developmental zones of S. viridis stems (Martin et al., 2016). Since SbSUTs were not localized to SP cells of the meristematic zone, the hexoses accumulated in intercalary meristems of Sorghum stems (Milne et al., 2015) may result from hexose-mediated transport (Fig. 7, A and B). A portion of the SP accumulated hexoses may enter the Suc biosynthesis pathway as indicated by expression of sucrose-phosphate synthase and sucrose-phosphate phosphatase genes across internode development of S. viridis (Martin et al., 2016). The synthesized Suc could be transported into vacuoles of the SP mediated by TSTs (Fig. 7, A and B). Suc cleavage, followed by hexose transport from the apoplasm into storage cells and resynthesis of Suc in their cytoplasm is considered to be futile cycling (Rohwer and Botha, 2001; Uys et al., 2007). However, SbSUT4 was more highly expressed than SbTSTs in the meristematic zone. This could signify a greater Suc release from vacuole to cytosol, which may in turn be converted to hexoses to fuel stem growth.
SbSUTs Retrieve Suc during Symplasmic Unloading in Maturing Internode Zones
Phloem unloading becomes symplasmic in maturing Sorghum stems (Fig. 7D), as heavy lignification and suberization of sclerenchyma sheath cell walls surrounding vascular bundles form a barrier to radial apoplasmic solute movement (Milne et al., 2015). Based on the assumption that similar turgor pressures are present in stem SEs to those measured in barley roots (∼1.0 to 1.3 MPa; Gould et al., 2004), unloading by bulk flow could be supported by a hydrostatic pressure difference between SEs and SP (Moore and Cosgrove, 1991; Fig. 7, C and D).
Transporters such as SUTs likely play a key role in turgor homeostasis to maintain unloading by bulk flow (Patrick, 2013a). A strong pmf of the metaphloem SE-CC complexes (Hafke et al., 2013) in both the recently elongated zone and maturing zone would drive SUT-mediated retrieval of Suc leaked to the phloem apoplasm. Higher SbSUT transcript levels compared to those in the meristematic and elongating zones (Fig. 3), along with SbSUT localization to SEs (Supplemental Fig. S8), suggest a developmentally programmed increase in Suc retrieval by SE-CC complexes (Fig. 7, C and D). Based on their relative transcript levels, SbSUT5 is predicted to be the predominant transporter supporting Suc retrieval in the recently elongated stem zone to be replaced by SbSUT1 in the mature stem zone. In addition, their high Suc affinities suggest that these SUTs function effectively in Suc retrieval from the phloem apoplasm predicted to contain low Suc concentrations (Welbaum et al., 1992). Interestingly, SbSUT5 expression was greater in the higher Suc storing stems of sweet versus grain Sorghum cultivars (cv Rio and cv BTx623, respectively; Milne et al., 2013). Hence, SbSUT5 expression levels may correlate with stem Suc storage capacity (Milne et al., 2015), but this may not be universal (Bihmidine et al., 2015).
SbSUTs immunolocalized to SP plasma membranes in the recently elongated zone (Supplemental Fig. S8) may function to retrieve leaked Suc for vacuolar storage (Fig. 7C). Plasma membrane localization of SbSUTs in SP cells was not observed beyond this developmental point (Fig. 7C versus Fig. 7D). Their absence could elevate Suc leakage to the SP apoplasm underpinning the observed SP turgor homeostasis during the major phase of Suc accumulation (Moore and Cosgrove, 1991).
Cellular localization of the tonoplast SbSUT4 has not been definitively demonstrated; however, the most likely cellular localization for SbSUT4 would be the SP cells. High and matching transcript levels of SbSUT4 compared to combined SbTST1 and SbTST2 levels in maturing stems (Fig. 3) are consistent with an elevated Suc turnover between vacuole and cytosol (Fig. 7, C and D). This may be part of a futile cycle of Suc as observed in sugarcane stems (Rohwer and Botha, 2001). As development proceeds, SbSUT4 transcripts decline and SbTST transcripts increase (Fig. 3) in step with Suc accumulation (Milne et al., 2015; Martin et al., 2016). Preferential SbTST1 and SbTST2 expression in sweet Sorghum over grain Sorghum putatively contribute to higher sugar accumulation (Bihmidine et al., 2016). A similar scenario applies to sugar beet taproots, where expression of BvTST2.1 increased with Suc content (Jung et al., 2015). It remains to be determined whether SbTSTs are capable of transporting Suc.
CONCLUSION
SbSUT1 may reverse to efflux Suc from the de-energized PSEs in the stem intercalary meristem and during the accelerating phase of stem elongation. Subsequently developed metaphloem SE-CC complexes contain a higher abundance of SbSUT5, or SbSUT1 when approaching maturity, which function to retrieve leaked Suc back into SE-CC complexes, thus maintaining the turgor pressure differential to drive symplasmic unloading by bulk flow.
MATERIALS AND METHODS
Plant Growth
Sorghum cultivar Rio seeds were germinated and grown in 10-L pots under glasshouse conditions (Milne et al., 2013). Tobacco (Nicotiana tabacum cv Xanthi), used for subcellular localization studies, was grown under the same environmental conditions as Sorghum. Here 20 to 30 tobacco seeds were germinated in 10-cm square pots. Three weeks postgermination, seedlings were thinned to two plants per pot.
Yeast [14C]Suc Uptake Procedures
Saccharomyces cerevisiae strain SEY6210 (Robinson et al., 1988) was transformed with the full-length coding sequence from SbSUT1, SbSUT4, or SbSUT5, cloned by PCR (Milne et al., 2013), and ligated into the yeast expression vector pDR196 (Rentsch et al., 1995). SEY6210 transformed with the empty pDR196 vector served as a negative control.
Transformed yeast were cultured in minimal medium (1.72 g/L yeast nitrogen base, 5 g/L ammonium sulfate, 2% w/v Glc, 0.02 g/L His, 0.3 g/L Lys, 0.1 g/L Leu, and 0.03 g/L Trp) to early logarithmic phase (OD600 of 0.8 to 1.0), washed in 25 mm MES-HEPES buffered at specified pH values (pH 4, 5, 6, 7, and 8) and resuspended to an OD600 of 20. Three technical replicates of yeast were kept in suspension by orbital shaking at 150 rpm for 10 min at 30°C. Thereafter, the cells were energized with Glc (final concentration 10 mm) or ethanol (final concentration 100 mm), with specified metabolic inhibitors 30 s later. Following a further 30 s, 0.5 µCi of [14C]Suc (Perkin-Elmer), of known specific activity in a specified concentration of carrier Suc buffered with 25 mm MES-HEPES, was added. After incubation with shaking for specified times, 100 µL of yeast cells were collected onto Whatman GF/C glass fiber filters (ThermoFisher) under vacuum. Filters were rapidly washed three times with 5 mL of ice-cold Suc solution, placed into scintillant (Optima Gold; Perkin-Elmer) and incubated overnight in the dark. Thereafter, disintegrations per minute, and hence Suc content, of each sample was determined using a Packard Tri-Carb 2100TR scintillation counter (Perkin Elmer).
Functional Characterization of SbSUTs Expressed in Xenopus laevis Oocytes
SbSUT1, SbSUT4, and SbSUT5 were amplified from the pGEM-T Easy vector (Promega; constructs described in Milne et al., 2013) with specified primers (Supplemental Table S1) and cloned into pENTR1A (Life Technologies) at the BamHI and EcoRI restriction sites. Each SUT gene was then recombined into the destination oocyte expression vector, pOO2/GW (Sun et al., 2010), using LR clonase recombinase according to the manufacturer’s protocol (Life Technologies). SUT sequences were confirmed by Sanger sequencing. Thereafter, plasmids were linearized with MluI restriction enzyme (New England Biolabs) to transcribe SUT complimentary RNA driven by the SP6 promoter using the Ambion mMessage mMachine kit according to the manufacturer’s protocol (Life Technologies).
Oocytes were harvested, prepared, and injected with complimentary RNA (Sivitz et al., 2005). Two to four days postinjection, oocytes were placed in a recording bath and submerged in modified Na-Ringer solution (MES-Tris Ringer; 115 mm NaCl, 1 mm KCl, 1.8 mm CaCl2, 1 mm CaCl2, and 5 mm MES-Tris at appropriate pH). Recording pipettes, filled with 1 m KCl with resistances between 1 and 3 megaohms, measured currents using the two-electrode voltage-clamp technique (Sivitz et al., 2005). Substrate-dependent currents were obtained by subtracting an averaged background current before and after the provision of substrate.
Quantifying Transcript Levels of SbSUTs and SbTSTs
Tissue samples were harvested from Internodes 4 and 10 at booting (Supplemental Fig. S5 and Supplemental Materials and Methods) and snap-frozen in liquid nitrogen. Tissue disruption, RNA isolation, genomic DNA digestion, cDNA synthesis, and SbSUT expression quantification were performed as described by Milne et al. (2013). Primers for SbTST expression quantification are shown in Supplemental Table S1.
Subcellular Localization
C-terminal SUT-GFP fusions in the pMDC85 vector (Curtis and Grossniklaus, 2003) were isolated using the Plasmid Maxi Kit (Qiagen) in accordance with the manufacturer’s protocol and transformed into tobacco mesophyll protoplasts using PEG-mediated transformation (see Supplemental Materials and Methods for details). AtPTR1-GFP (Dietrich et al., 2004) and AtPTR2-GFP (Weichert et al., 2012) were kindly provided by Prof. D. Rentsch (University of Bern) to serve as positive controls for plasma membrane and tonoplast localization, respectively. Protoplasts were imaged with an Olympus FV1000 confocal laser-scanning microscope equipped using a 60×/1.2 NA UPLSAPO oil immersion objective lens (Olympus). GFP fluorescence was observed by excitation with the 473-nm laser and emission between 487 and 521 nm. Chloroplast autofluorescence was observed by excitation with the 559-nm laser and emission between 606 and 673 nm.
Immunolocalization
Tissue segments were harvested from fully elongated Internode 4 and elongating Internode 10 at booting (Supplemental Fig. S5), fixed in ethanol-acetic acid (3:1 v/v) and vacuum infiltrated. Samples were dehydrated before infiltration with LR white resin (ProSciTech) and polymerization at 60°C (full details in Supplemental Materials and Methods). Serial sections (1 µm) were cut on glass knives and transferred to drops of Milli-Q H2O on 0.5% (w/v) gelatin-coated slides and dried at 40°C. Chloroform was waved over the top to flatten the sections.
For immunolocalization, stem sections were probed with polyclonal PEP2 antiserum provided by Dr. R. Furbank (CSIRO; Bagnall et al., 2000), raised against a highly conserved region of the first cytoplasmic loop of StSUT1 (87–106: GYYSDNCSSRFGRRRPFIAA) that possessed 65% sequence identity to SbSUT1, 56% sequence identity to SbSUT4, and 80% sequence identity to SbSUT5. Hybridization of PEP2 antibody with SbSUTs was verified by detecting a band corresponding with the estimated SbSUT size of ∼50 kD in a microsomal protein fraction isolated from yeast cells separately harboring SbSUT1, SbSUT4, and SbSUT5, but not yeast transformed with the empty vector pDR196 (Supplemental Fig. S9). Additionally, PEP2 antibody hybridized with ∼65 kD band in a western blot of isolated Sorghum microsomal proteins (Supplemental Fig. S10). The slightly larger molecular size compared to that detected in yeast may be attributable to different posttranslational modifications in plant versus yeast cells. Sections were blocked with Tris-buffered saline plus Tween 20 (TBST)-1% (w/v) BSA at room temperature for 1.5 h prior to exposure to primary antibody PEP2 (diluted 1:50 with TBST-BSA) at 4°C for 18 h. Sections were rinsed four times, 10 min for each wash, with TBST-BSA. Thereafter, secondary antibody (dilution 1:100 goat anti-rabbit fluorescein isothiocyanate in TBST-BSA) was applied for 2 h at room temperature. Washing was performed as previously described, followed by two additional washes in TBST. Sections were mounted in antifade medium (nine parts Mowiol, one-part p-phenylene diamine) and viewed under fluorescence optics of an Axioscope A1 with the Filter Set 09 (Carl Zeiss; excitation 450 to 490 nm, long-pass emission above 515 nm). UV autofluorescence was observed with the Filter Set 02 (Carl Zeiss; excitation 365 nm, long pass emission above 420 nm).
Accession Numbers
Sequence data from this article can be found in the GenBank/Phytozome data libraries under the following accession numbers: SbSUT1, KY287229; SbSUT4, KY287232; SbSUT5, KY287233; SbTST1, Sb01g030430; SbTST2, Sb04g008150; SbTST3, Sb10g031000; StSUT1, CAA48915; AtPTR1, At3g54140; and AtPTR2, At2g02040.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. pH dependence of Suc uptake rates mediated by SbSUTs after expression of their cDNAs in yeast strain SEY6210 and X. laevis oocytes.
Supplemental Figure S2. Current-voltage relation of Suc-induced currents into X. laevis oocytes.
Supplemental Figure S3. The affinity of SbSUT5 for maltose was dependent on pH and membrane potential as measured in X. laevis oocytes.
Supplemental Figure S4. Substrate specificity of SbSUTs.
Supplemental Figure S5. Diagrammatic representation of developmental zones of specified internodes sampled at booting.
Supplemental Figure S6. Preimmune controls in apoplasmic phloem unloading zones of elongating Internode 10.
Supplemental Figure S7. Comparison of tissue fixatives used for immunolocalization.
Supplemental Figure S8. Immunolocalization of stem SbSUTs with PEP2 antiserum—elongated zone of elongating Internode 10.
Supplemental Figure S9. Immunoblot of yeast microsomal proteins with PEP2 antiserum.
Supplemental Figure S10. Immunoblot of Sorghum microsomal proteins with PEP2 antiserum.
Supplemental Table S1. Primer sets used for PCR amplification of SbSUTs and SbTSTs.
Supplemental Materials and Methods. Internode sampling, protoplast isolation and transformation, tissue fixation trials and immunolocalization, and protein isolation and western blotting
Supplementary Material
Acknowledgments
We thank J. Enright for maintenance of healthy plant material; T. Tiew, D. Pugh, S. McGaughey, and H. Osborn for technical assistance; and Dr. X.-D. Wang for assistance with sectioning and immunolabeling experiments.
Glossary
- PSE
protophloem sieve element
- SE-CC
sieve element-companion cell
Footnotes
This work was supported by the Australian Research Council (project no. LP0883808) and the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the United States Department of Energy (DE-FG02-10ER15886). R.J.M. was supported by an Australian Postgraduate Award scholarship.
Articles can be viewed without a subscription.
References
- Aldape MJ, Elmer AM, Chao WS, Grimes HD (2003) Identification and characterization of a sucrose transporter isolated from the developing cotyledons of soybean. Arch Biochem Biophys 409: 243–250 [DOI] [PubMed] [Google Scholar]
- Aoki N, Scofield GN, Wang XD, Patrick JW, Offler CE, Furbank RT (2004) Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 219: 176–184 [DOI] [PubMed] [Google Scholar]
- Bagnall N, Wang XD, Scofield GN, Furbank RT, Offler CE, Patrick JW (2000) Sucrose transport-related genes are expressed in both maternal and filial tissues of developing wheat grains. Aust J Plant Physiol 27: 1009–1020 [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihmidine S, Baker RF, Hoffner C, Braun DM (2015) Sucrose accumulation in sweet sorghum stems occurs by apoplasmic phloem unloading and does not involve differential Sucrose transporter expression. BMC Plant Biol 15: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihmidine S, Julius BT, Dweikat I, Braun DM (2016) Tonoplast Sugar Transporters (SbTSTs) putatively control sucrose accumulation in sweet sorghum stems. Plant Signal Behav 11: e1117721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR. (1990) Electrogenicity, pH-dependence, and stoichiometry of the proton-sucrose symport. Plant Physiol 93: 1590–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R (2005) Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem 280: 21437–21443 [DOI] [PubMed] [Google Scholar]
- Casu RE, Grof CPL, Rae AL, McIntyre CL, Dimmock CM, Manners JM (2003) Identification of a novel sugar transporter homologue strongly expressed in maturing stem vascular tissues of sugarcane by expressed sequence tag and microarray analysis. Plant Mol Biol 52: 371–386 [DOI] [PubMed] [Google Scholar]
- Chen L-Q, Qu X-Q, Hou B-H, Sosso D, Osorio S, Fernie AR, Frommer WB (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335: 207–211 [DOI] [PubMed] [Google Scholar]
- Cho JI, Burla B, Lee DW, Ryoo N, Hong SK, Kim HB, Eom JS, Choi SB, Cho MH, Bhoo SH, et al. (2010) Expression analysis and functional characterization of the monosaccharide transporters, OsTMTs, involving vacuolar sugar transport in rice (Oryza sativa). New Phytol 186: 657–668 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña P, Barros F, Gascón S, Ramos S, Lazo PS (1982) The electrochemical proton gradient of Saccharomyces. The role of potassium. Eur J Biochem 123: 447–453 [DOI] [PubMed] [Google Scholar]
- Dietrich D, Hammes U, Thor K, Suter-Grotemeyer M, Flückiger R, Slusarenko AJ, Ward JM, Rentsch D (2004) AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis. Plant J 40: 488–499 [DOI] [PubMed] [Google Scholar]
- Ervin EL, Evert RF (1967) Aspects of sieve element ontogeny and structure in Smilax rotundifolia. Bot Gaz 128: 138–144 [Google Scholar]
- Gahrtz M, Schmelzer E, Stolz J, Sauer N (1996) Expression of the PmSUC1 sucrose carrier gene from Plantago major L. is induced during seed development. Plant J 9: 93–100 [DOI] [PubMed] [Google Scholar]
- Gásková D, Brodská B, Herman P, Vecer J, Malínský J, Sigler K, Benada O, Plásek J (1998) Fluorescent probing of membrane potential in walled cells: diS-C3(3) assay in Saccharomyces cerevisiae. Yeast 14: 1189–1197 [DOI] [PubMed] [Google Scholar]
- Gould N, Minchin PEH, Thorpe MR (2004) Direct measurements of sieve element hydrostatic pressure reveal strong regulation after pathway blockage. Funct Plant Biol 31: 987–993 [DOI] [PubMed] [Google Scholar]
- Gould N, Thorpe MR, Pritchard J, Christeller JT, Williams LE, Roeb G, Schurr U, Minchin PEH (2012) AtSUC2 has a role for sucrose retrieval along the phloem pathway: evidence from carbon-11 tracer studies. Plant Sci 188-189: 97–101 [DOI] [PubMed] [Google Scholar]
- Grof CPL, Byrt CS, Patrick JW (2014) Phloem transport of resources. In Moore P, Botha F, eds, Sugarcane: Physiology, Biochemistry & Functional Biology. John Wiley & Sons, Somerset, NJ, pp 267–305 [Google Scholar]
- Hafke JB, Höll S-R, Kühn C, van Bel AJE (2013) Electrophysiological approach to determine kinetic parameters of sucrose uptake by single sieve elements or phloem parenchyma cells in intact Vicia faba plants. Front Plant Sci 4: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B, Ludewig F, Schulz A, Meißner G, Wöstefeld N, Flügge U-I, Pommerrenig B, Wirsching P, Sauer N, Koch W, et al. (2015) Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat Plants 1: 14001. [DOI] [PubMed] [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298–1300 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26: 37–56 [Google Scholar]
- Le Hir R, Spinner L, Klemens PA, Chakraborti D, de Marco F, Vilaine F, Wolff N, Lemoine R, Porcheron B, Géry C, et al. (2015) Disruption of the sugar transporters AtSWEET11 and AtSWEET12 affects vascular development and freezing tolerance in Arabidopsis. Mol Plant 8: 1687–1690 [DOI] [PubMed] [Google Scholar]
- Lemoine R. (2000) Sucrose transporters in plants: update on function and structure. Biochim Biophys Acta 1465: 246–262 [DOI] [PubMed] [Google Scholar]
- Lingle SE. (1987) Sucrose metabolism in the primary culm of sweet sorghum during development. Crop Sci 27: 1214–1219 [Google Scholar]
- Lu JMY, Bush DR (1998) His-65 in the proton-sucrose symporter is an essential amino acid whose modification with site-directed mutagenesis increases transport activity. Proc Natl Acad Sci USA 95: 9025–9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AP, Palmer WM, Brown C, Abel C, Lunn JE, Furbank RT, Grof CPL (2016) A developing Setaria viridis internode: an experimental system for the study of biomass generation in a C4 model species. Biotechnol Biofuels 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RJ, Byrt CS, Patrick JW, Grof CPL (2013) Are sucrose transporter expression profiles linked with patterns of biomass partitioning in Sorghum phenotypes? Front Plant Sci 4: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RJ, Offler CE, Patrick JW, Grof CPL (2015) Cellular pathways of source leaf phloem loading and phloem unloading in developing stems of Sorghum bicolor in relation to stem sucrose storage. Funct Plant Biol 42: 957–970 [DOI] [PubMed] [Google Scholar]
- Moore PH, Cosgrove DJ (1991) Developmental changes in cell and tissue water relations parameters in storage parenchyma of sugarcane. Plant Physiol 96: 794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch E. (1930) Material Flow in Plants. Translated 2003 by Milburn JA and Kreeb KH, Gustav Fischer Verlag Jena; University of Bremen, Germany [Google Scholar]
- Palmer WM, Ru L, Jin Y, Patrick JW, Ruan Y-L (2015) Tomato ovary-to-fruit transition is characterized by a spatial shift of mRNAs for cell wall invertase and its inhibitor with the encoded proteins localized to sieve elements. Mol Plant 8: 315–328 [DOI] [PubMed] [Google Scholar]
- Patrick J. (1972) Distribution of assimilate during stem elongation in wheat. Aust J Biol Sci 25: 455–467 [Google Scholar]
- Patrick JW. (2013a) Does Don Fisher’s high-pressure manifold model account for phloem transport and resource partitioning? Front Plant Sci 4: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JW. (2013b) Fundamentals of phloem transport physiology. In Thompson GA, van Bel AJE, eds, Phloem: Molecular Cell Biology, Systemic Communication, Biotic Interactions. Wiley-Blackwell Publishing, London, pp 30–59 [Google Scholar]
- Rae AL, Perroux JM, Grof CPL (2005) Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: a potential role for the ShSUT1 sucrose transporter. Planta 220: 817–825 [DOI] [PubMed] [Google Scholar]
- Reinders A, Sivitz AB, Hsi A, Grof CPL, Perroux JM, Ward JM (2006) Sugarcane ShSUT1: analysis of sucrose transport activity and inhibition by sucralose. Plant Cell Environ 29: 1871–1880 [DOI] [PubMed] [Google Scholar]
- Reinders A, Sun Y, Karvonen KL, Ward JM (2012) Identification of amino acids important for substrate specificity in sucrose transporters using gene shuffling. J Biol Chem 287: 30296–30304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch D, Laloi M, Rouhara I, Schmelzer E, Delrot S, Frommer WB (1995) NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett 370: 264–268 [DOI] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol 8: 4936–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer JM, Botha FC (2001) Analysis of sucrose accumulation in the sugar cane culm on the basis of in vitro kinetic data. Biochem J 358: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N, Stolz J (1994) SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker’s yeast and identification of the histidine-tagged protein. Plant J 6: 67–77 [DOI] [PubMed] [Google Scholar]
- Schmitt B, Stadler R, Sauer N (2008) Immunolocalization of solanaceous SUT1 proteins in companion cells and xylem parenchyma: new perspectives for phloem loading and transport. Plant Physiol 148: 187–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A, Beyhl D, Marten I, Wormit A, Neuhaus E, Poschet G, Büttner M, Schneider S, Sauer N, Hedrich R (2011) Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J 68: 129–136 [DOI] [PubMed] [Google Scholar]
- Schulze W, Weise A, Frommer WB, Ward JM (2000) Function of the cytosolic N-terminus of sucrose transporter AtSUT2 in substrate affinity. FEBS Lett 485: 189–194 [DOI] [PubMed] [Google Scholar]
- Scofield GN, Hirose T, Aoki N, Furbank RT (2007) Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J Exp Bot 58: 3155–3169 [DOI] [PubMed] [Google Scholar]
- Shakya R, Sturm A (1998) Characterization of source- and sink-specific sucrose/H+ symporters from carrot. Plant Physiol 118: 1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Johnson ME, Krentz AD, Grof CPL, Perroux JM, Ward JM (2007) Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol 143: 188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Ward JM (2005) Analysis of the transport activity of barley sucrose transporter HvSUT1. Plant Cell Physiol 46: 1666–1673 [DOI] [PubMed] [Google Scholar]
- Slewinski TL. (2012) Non-structural carbohydrate partitioning in grass stems: a target to increase yield stability, stress tolerance, and biofuel production. J Exp Bot 63: 4647–4670 [DOI] [PubMed] [Google Scholar]
- Sosso D, Luo D, Li Q-B, Sasse J, Yang J, Gendrot G, Suzuki M, Koch KE, McCarty DR, Chourey PS, et al. (2015) Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat Genet 47: 1489–1493 [DOI] [PubMed] [Google Scholar]
- Srivastava AC, Ganesan S, Ismail IO, Ayre BG (2008) Functional characterization of the Arabidopsis AtSUC2 Sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol 148: 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Sauer N (1996) The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Bot Acta 109: 299–306 [Google Scholar]
- Sun Y, Reinders A, LaFleur KR, Mori T, Ward JM (2010) Transport activity of rice sucrose transporters OsSUT1 and OsSUT5. Plant Cell Physiol 51: 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys L, Botha FC, Hofmeyr J-HS, Rohwer JM (2007) Kinetic model of sucrose accumulation in maturing sugarcane culm tissue. Phytochemistry 68: 2375–2392 [DOI] [PubMed] [Google Scholar]
- Walley JW, Sartor RC, Shen Z, Schmitz RJ, Wu KJ, Urich MA, Nery JR, Smith LG, Schnable JC, Ecker JR, et al. (2016) Integration of omic networks in a developmental atlas of maize. Science 353: 814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Sky RC, Brown SM (2005) The anatomy of the pathway of sucrose unloading within the sugarcane stalk. Funct Plant Biol 32: 367–374 [DOI] [PubMed] [Google Scholar]
- Watt DA, McCormick AJ, Cramer MD (2013) Source and sink physiology. In Moore P, Botha F, eds, Sugarcane: Physiology, Biochemistry, and Functional Biology. John Wiley & Sons, Hoboken, NJ, pp 483–520 [Google Scholar]
- Weichert A, Brinkmann C, Komarova NY, Dietrich D, Thor K, Meier S, Suter Grotemeyer M, Rentsch D (2012) AtPTR4 and AtPTR6 are differentially expressed, tonoplast-localized members of the peptide transporter/nitrate transporter 1 (PTR/NRT1) family. Planta 235: 311–323 [DOI] [PubMed] [Google Scholar]
- Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum GE, Meinzer FC, Grayson RL, Thornham KT (1992) Evidence for and consequences of a barrier to solute diffusion between the apoplast and vascular bundles in sugarcane stalk tissue. Aust J Plant Physiol 19: 611–623 [Google Scholar]
- Wood RM, Offler CE, Patrick JW (1998) The cellular pathway of short-distance transfer of photosynthates and potassium in the elongating stem of Phaseolus vulgaris L. A structural assessment. Ann Bot (Lond) 82: 337–345 [Google Scholar]
- Zhou Y, Qu H, Dibley KE, Offler CE, Patrick JW (2007) A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J 49: 750–764 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.