Arabidopsis seed coat mucilage, an extracellular matrix composed of cell wall carbohydrates, contains a proteome functionally similar to that of cell wall but also includes proteins unique to mucilage.
Abstract
Plant cell wall proteins are important regulators of cell wall architecture and function. However, because cell wall proteins are difficult to extract and analyze, they are generally poorly understood. Here, we describe the identification and characterization of proteins integral to the Arabidopsis (Arabidopsis thaliana) seed coat mucilage, a specialized layer of the extracellular matrix composed of plant cell wall carbohydrates that is used as a model for cell wall research. The proteins identified in mucilage include those previously identified by genetic analysis, and several mucilage proteins are reduced in mucilage-deficient mutant seeds, suggesting that these proteins are genuinely associated with the mucilage. Arabidopsis mucilage has both nonadherent and adherent layers. Both layers have similar protein profiles except for proteins involved in lipid metabolism, which are present exclusively in the adherent mucilage. The most abundant mucilage proteins include a family of proteins named TESTA ABUNDANT1 (TBA1) to TBA3; a less abundant fourth homolog was named TBA-LIKE (TBAL). TBA and TBAL transcripts and promoter activities were detected in developing seed coats, and their expression requires seed coat differentiation regulators. TBA proteins are secreted to the mucilage pocket during differentiation. Although reverse genetics failed to identify a function for TBAs/TBAL, the TBA promoters are highly expressed and cell type specific and so should be very useful tools for targeting proteins to the seed coat epidermis. Altogether, these results highlight the mucilage proteome as a model for cell walls in general, as it shares similarities with other cell wall proteomes while also containing mucilage-specific features.
The plant cell wall plays key roles in structural support, cell-cell cohesion, and interaction of the cell with the environment. It is a dynamic structure and can be strengthened or loosened in response to environmental or developmental cues (Fry, 2000; Passardi et al., 2004). Plant cell walls typically contain cellulose and hemicellulose and may include pectin or lignin depending on the type of wall. In addition to these carbohydrate components, 5% to 10% of the cell wall biomass consists of proteins (Cassab and Varner, 1988; Burton et al., 2010). Despite being a relatively minor component in terms of cell wall biomass, these proteins are critical regulators of the cell wall architecture and, therefore, its physical properties. For example, structural proteins can cross-link various cell wall polysaccharides (Showalter, 1993), while carbohydrate-active enzymes modify polysaccharide structure.
Since cell wall proteins are generally difficult to extract and analyze, they remain a relatively poorly understood component of the cell wall. Several factors complicate the analysis of cell wall proteins. First, they often undergo extensive posttranslational modifications, such as Pro hydroxylation, glycosylation, and the addition of GPI anchors (Jamet et al., 2008b; Albenne et al., 2013). These modifications not only alter protein mass, thereby complicating protein identification, but they also can anchor the proteins in the apoplast by covalent or noncovalent interactions (Kieliszewski and Lamport, 1994; Spiro, 2002), which make cell wall protein extraction and identification more challenging. The extraction of cell wall proteins typically requires harsh conditions (Lee et al., 2004; Jamet et al., 2008b) that often lead to protein degradation and contamination with cytoplasmic proteins, with a resulting decrease in the quality of proteomic data. In addition, the cell wall resides in extracellular space and abuts the perimeters of adjacent cells. Since a variety of cell types with distinctive cell walls are found in most tissues and organs, it is common that cell wall extracts typically include carbohydrate and proteins derived from multiple cell types, and the relative contribution of specific cell types is difficult to assess. Despite these problems, several studies have characterized cell wall proteomes from different tissue types in various plant species, including the model plant Arabidopsis (Arabidopsis thaliana; for review, see Albenne et al., 2013). Out of the ∼5,000 Arabidopsis genes that encode a predicted signal peptide to allow a protein to enter the secretory pathway, 1,000 to 2,000 are thought to be cell wall proteins (Jamet et al., 2006). However, currently, most published cell wall proteomes contain less than 100 proteins each and are contaminated by cytoplasmic proteins to a variable extent, depending on the tissue type and extraction techniques (Albenne et al., 2013). This suggests that many cell wall proteins remain to be discovered and characterized and emphasizes the need for better models and more robust methodologies.
Arabidopsis seed coat mucilage is a specialized layer of the extracellular matrix composed of cell wall carbohydrates arranged in a distinct structure (for review, see Arsovski et al., 2010; Haughn and Western, 2012; Western, 2012; North et al., 2014, Voiniciuc et al., 2015c) that is used as a model to study cell wall structure and function. It contains cellulose and hemicellulose (Macquet et al., 2007a; Young et al., 2008; Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011; Griffiths et al., 2014; Yu et al., 2014; Voiniciuc et al., 2015a, 2015b; Hu et al., 2016a, 2016b) but is particularly rich in pectin, with unsubstituted rhamnogalacturonan I making up ∼85% of the total mucilage carbohydrate (Western et al., 2000, 2001; Willats et al., 2001; Dean et al., 2007; Macquet et al., 2007a; Young et al., 2008). Similar to cell walls, Arabidopsis seed coat mucilage also contains proteins. Forward and reverse genetics studies have identified several loci required for proper mucilage synthesis, secretion, and extrusion (for review, see Haughn and Chaudhury, 2005; Arsovski et al., 2010; Haughn and Western, 2012; Western, 2012; North et al., 2014; Francoz et al., 2015). Several of these gene products are believed to be secreted to the mucilage pocket or adjacent primary wall in the developing seed coat. For example, the mucilage-modifying enzyme MUCILAGE MODIFIED2 (MUM2) is secreted to the mucilage pocket during mucilage synthesis (Western et al., 2001; Dean et al., 2007; Macquet et al., 2007b). PEROXIDASE36 (PER36) has been shown to localize to the radial and tangential primary cell wall adjacent to the mucilage pocket (Kunieda et al., 2013). Two other genes that encode proteins needed for normal mucilage, SUBTILISIN-LIKE SERINE PROTEASE1.7 (SBT1.7; Rautengarten et al., 2008) and arabinofuranosidase β-XYLOSIDASE1 (BXL1; Arsovski et al., 2009), contain signal peptides and modify mucilage carbohydrates. However, a thorough analysis of mucilage proteins has not been described.
The deposition of seed coat mucilage is known as myxospermy and is common in angiosperms (Young and Evans, 1973; Grubert, 1974). During differentiation, Arabidopsis seed coat epidermal cells synthesize mucilage components and deposit them between the plasma membrane and the primary wall at the junction between the radial and tangential cell walls, forming a ring-shaped mucilage pocket surrounding a volcano-shaped cytoplasmic column (Western et al., 2000; Windsor et al., 2000). A cellulose-rich secondary cell wall, the columella, is subsequently deposited beneath the mucilage, gradually replacing the cytoplasm (Western et al., 2000; Windsor et al., 2000). Upon exposure of mature seeds to water, the pectin-rich mucilage swells rapidly, ruptures the primary wall, and extrudes to encapsulate the seed. The extruded Arabidopsis seed mucilage has at least two distinct layers, nonadherent and adherent (Western et al., 2000; Macquet et al., 2007a). The outermost layer (nonadherent layer) is amorphous in appearance, composed primarily of pectin and, as its name suggests, easily separated from the seed by gentle shaking. The layer of mucilage adjacent to the seed coat has a distinct ray-like structure, has cellulose and hemicellulose in addition to pectin, and is strongly adherent to the seed surface. Relative to cell wall preparations from most other tissue types, seed coat mucilage can be easily extracted in large amounts without contamination with cell wall material from other cell types (Haughn and Chaudhury, 2005; Haughn and Western, 2012; North et al., 2014). These advantages suggest that seed coat mucilage can yield cell wall proteomes that are potentially of higher quality than cell wall proteomes derived from other tissues. Here, we describe the extraction and proteomic analysis of the mature Arabidopsis seed coat mucilage and discuss the protein profiles of the mucilage in comparison with other cell wall proteomes. In addition, we characterize a family of unknown proteins that are particularly abundant in seed coat mucilage and strongly expressed in the developing seed coat.
RESULTS
Proteins Are a Component of Mucilage Extracted from Arabidopsis Seeds
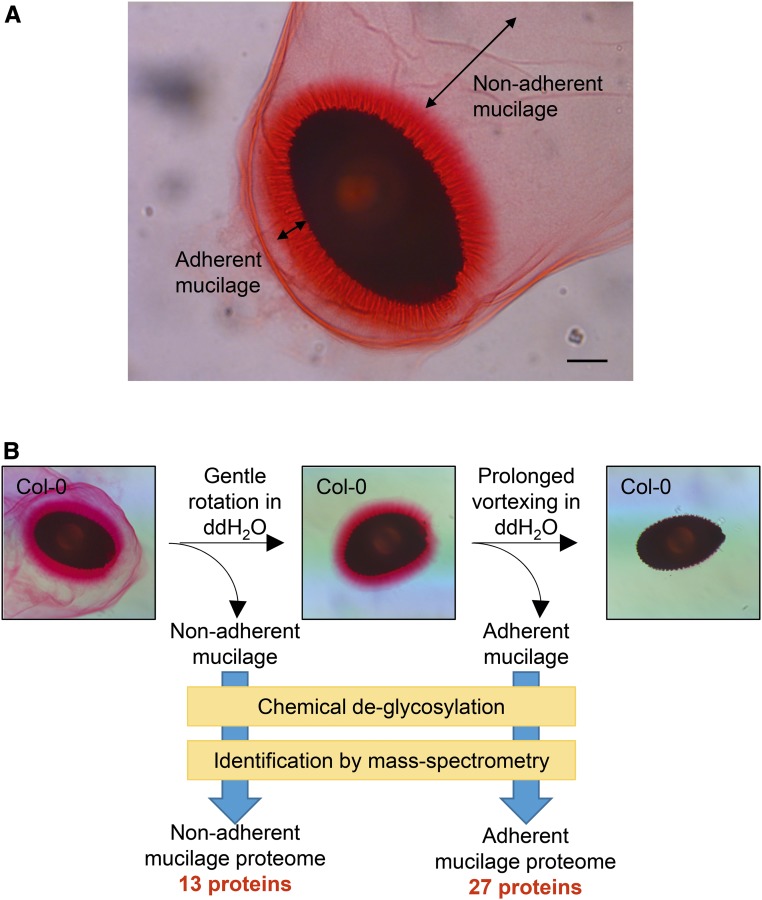
In order to identify and characterize proteins integral to the seed coat mucilage, a protocol was developed to extract seed coat mucilage for protein analyses (Fig. 1). Upon hydration, Arabidopsis seed coat epidermal cells extrude mucilage as two distinct layers: an outer nonadherent layer that detaches easily from the hydrated seed and a dense halo-like adherent layer that is bound tightly to the seed coat (Fig. 1A; Western et al., 2000). We took advantage of the different physical properties of these two layers to separate them by sequential extraction (Fig. 1B). Seeds imbibed in water were shaken gently to separate the nonadherent mucilage. The seeds were then shaken at high speed for several hours to remove the adherent mucilage (Fig. 1B). Ruthenium Red and Calcofluor White staining showed that the pectin and cellulosic components of seed coat mucilage were almost completely removed by the sequential extraction (Fig. 1B; Supplemental Fig. S1). The harvested adherent and nonadherent mucilage samples were chemically deglycosylated, trypsin digested, and analyzed by MS to identify mucilage-associated proteins in these samples (Fig. 1B; Supplemental Data Sets S1 and S2). Of those proteins detected by this process, only the ones identified in more than one biological replicate with MASCOT scores greater than 40 (where a score of 25 or greater corresponds to a 5% false discovery rate), and at least once with multiple peptides, were considered for further analyses. Based on these criteria, 30 proteins were considered to be robustly identified from mucilage, all containing predicted signal peptides (Supplemental Data Set S3). Cruciferin A1 and cruciferin C were discarded from further analyses, since they are not known to be secreted to the apoplast. This leaves a total 28 potential mucilage proteins identified (Table I; Supplemental Data Set S3). One protein was found only in the nonadherent layer, 15 only in the adherent layer, and the remaining 12 proteins were found in both mucilage layers (Table I).
Figure 1.
Strategy to isolate and identify seed coat mucilage proteins. A, Columbia-0 (Col-0) seed coat mucilage stained with Ruthenium Red. Double-headed arrows depict the two mucilage layers. Bar = 100 µm. B, Schematic depiction of the extraction and identification of mucilage proteins. The nonadherent mucilage and adherent mucilage were extracted sequentially. Proteins in each mucilage layer were identified by mass spectrometry (MS) after chemical deglycosylation and trypsin digestion. ddH2O, Distilled, deionized water.
Table I. Total proteins identified in mature Col-0 seed coat mucilage.
| Accession No. | ATG No. | Name | Location | Function | WallProtDB |
|---|---|---|---|---|---|
| O04575 | At1g62080 | TESTA ABUNDANT (TBA3) | Both | Unknown | Not detected |
| Q39168 | At1g62000 | TESTA ABUNDANT (TBA1) | Both | Unknown | Not detected |
| O04573 | At1g62060 | TESTA ABUNDANT (TBA2) | Both | Unknown | Not detected |
| Q9M8X3 | At3g04170 | RmlC-like cupin superfamily protein | Adherent | Oxidoreductase | Not detected |
| Q9FK75 | At5g45670 | GDSL motif esterase/acyltransferase/lipase | Adherent | Lipid metabolism | Not detected |
| Q9FGY1 | At5g49360 | β-XYLOSIDASE1 (BXL1) | Adherent | Carbohydrate-active enzyme | Multiple tissues |
| Q9LU14 | At3g16370 | GDSL motif esterase/acyltransferase/lipase | Adherent | Lipid metabolism | Leaves |
| O65351 | At5g67360 | SUBTILISIN-LIKE SERINE PROTEASE1.7 (Sbt1.7, ARA12) | Both | Protease | Multiple tissues |
| Q9LV33 | At3g18080 | B-S GLUCOSIDASE44 (BGLU44) | Both | Carbohydrate-active enzyme | Multiple tissues |
| Q9SD46 | At3g50990 | PEROXIDASE36 (PER36) | Both | Oxidoreductase | Hypocotyl |
| Q94CH6 | At1g75900 | GDSL motif esterase/acyltransferase/lipase | Adherent | Lipid metabolism | Not detected |
| Q9FFN4 | At5g63800 | MUCILAGE MODIFIED2 (MUM2) | Both | Carbohydrate-active enzyme | Multiple tissues |
| Q8L7S6 | At1g65590 | β-HEXOSAMINIDASE3 (HEXO3) | Both | Carbohydrate-active enzyme | Multiple tissues |
| Q9SCV4 | At2g28470 | β-GALACTOSIDASE8 (BGAL8) | Both | Carbohydrate-active enzyme | Leaves |
| Q9SUS0 | At4g23560 | GLYCOSYL HYDROLASE9B15 (GH9B15) | Adherent | Carbohydrate-active enzyme | Not detected |
| Q9M8X6 | At3g04200 | RmlC-like cupin superfamily protein | Both | Oxidoreductase | Not detected |
| Q9LLR6 | At5g59310 | LIPID TRANSFER PROTEIN4 (LTP4) | Adherent | Lipid metabolism | Not detected |
| Q9LS40 | At3g18490 | ASPARTIC PROTEASE IN GUARD CELL1 (ASPG1) | Both | Protease | Multiple tissues |
| Q8VY93 | At4g26790 | GDSL motif esterase/acyltransferase/lipase | Adherent | Lipid metabolism | Not detected |
| Q9FMK9 | At5g63140 | PURPLE ACID PHOSPHATASE29 (PAP29) | Adherent | Miscellaneous | Roots |
| Q9LDB4 | At3g08770 | LIPID TRANSFER PROTEIN6 (LTP6) | Adherent | Lipid metabolism | Roots |
| Q9LEY1 | At5g08260 | SERINE CARBOXYPEPTIDASE-LIKE35 (SCPL35) | Both | Protease | Not detected |
| Q94BT2 | At3g07390 | AUXIN-INDUCED IN ROOT CULTURES12 (AIR12) | Adherent | Oxidoreductase | Multiple tissues |
| Q9LZX4 | At3g60900 | FASCICLIN-LIKE ARABINOGALACTAN PROTEIN10 (FLA10) | Adherent | Arabinogalactan protein | Cell culture |
| Q9LHF1 | At3g24480 | Leu-rich repeat family protein | Adherent | Structural | Multiple tissues |
| P43297 | At1g47128 | RESPONSIVE TO DEHYDRATION21A (RD21a) | Adherent | Protease | Multiple tissues |
| Q9SVU5 | At4g28780 | GDSL motif esterase/acyltransferase/lipase | Adherent | Lipid metabolism | Not detected |
| Q66GR0 | At5g06390 | FASCICLIN-LIKE ARABINOGALACTAN PROTEIN17 (FLA17) | Nonadherent | Arabinogalactan protein | Not detected |
Mucilage Proteins Are Functionally Similar to Other Cell Wall Proteins
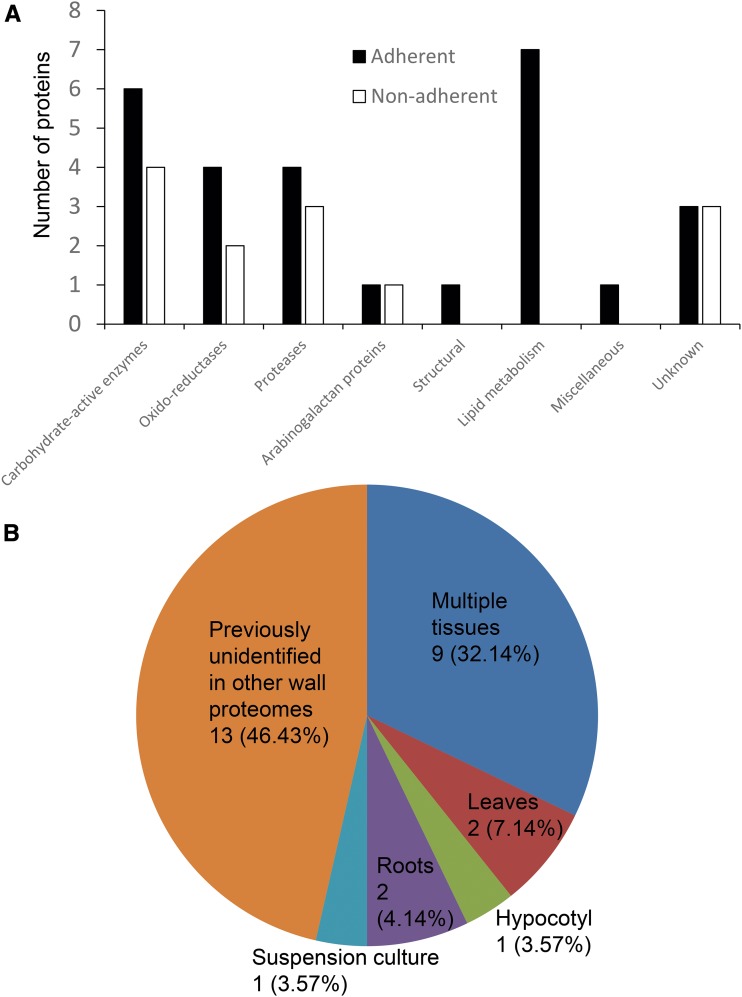
When proteins found in each mucilage layer were sorted by their predicted functions (Table I; Fig. 2A), most fell within the various functional categories of cell wall proteins as defined by Albenne et al. (2013). These categories include carbohydrate-active enzymes, oxidoreductases, proteases, proteins involved in lipid metabolism, arabinogalactan proteins, as well as miscellaneous proteins and proteins with unknown functions. The fact that seed coat mucilage-associated proteins appear to be functionally similar to proteins from other Arabidopsis cell wall proteomes reinforces the concept that seed coat mucilage is a specialized type of cell wall (Haughn and Western, 2012). On the other hand, nearly half of the specific mucilage-associated protein isoforms were unique to mucilage and not identified in other cell wall proteomes to date (Fig. 2B), including a family of unknown proteins (discussed below), RmlC-like cupin superfamily proteins, and GDSL lipases (Table I). Homologs of the RmlC-like cupin superfamily proteins and GDSL lipases are commonly found in other cell walls (Albenne et al., 2013), suggesting that these proteins may represent mucilage-specific isoforms.
Figure 2.
Mucilage proteins are functionally similar to other cell wall proteins. A, Numbers of seed coat mucilage proteins from each mucilage layer sorted by the cell wall protein functional categories. B, Proportions of mucilage proteins previously identified in cell walls from other tissue types as documented by WallProtDB. Numbers denote the number of proteins in each category, while percentages denote the proportion of proteins that occupy each category.
In general, the adherent layer displays a richer and more diverse protein profile compared with the nonadherent layer, including 15 proteins that are unique to the adherent layer (Table I). Interestingly, this adherent-specific group includes a number of proteins involved in lipid metabolism (Fig. 2A). Otherwise, the numbers of proteins that belong to each predicted functional class are more or less comparable between the two mucilage layers (Fig. 2A). This suggests that the types of protein-mediated biological processes associated with mucilage modification in the apoplast are comparable within the two layers.
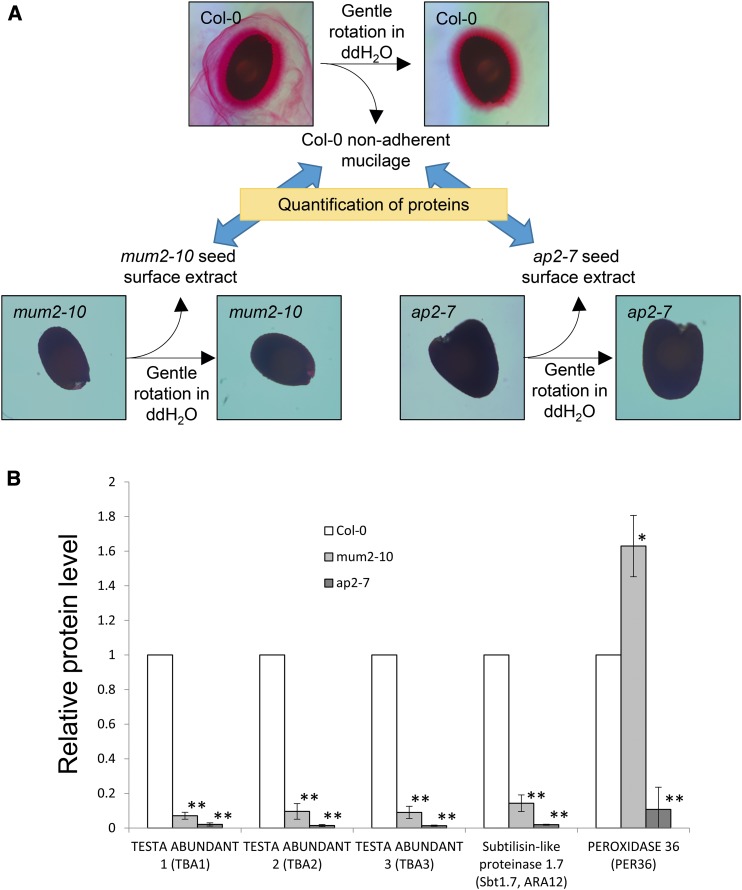
Since the seeds from which mucilage was obtained in these experiments had not been processed in any way prior to hydration and mucilage extraction, the possibility remains that the proteins we identified originate from sources other than the mucilage. In order to address this concern, the nonadherent mucilage protein profiles obtained from Col-0 seeds were compared with protein profiles obtained from the seed surface extracts from the mucilage mutants mum2-10 and apetala 2-7 (ap2-7; Fig. 3A). mum2-10 seeds synthesize mucilage but do not extrude it when hydrated (Dean et al., 2007; Macquet et al., 2007b), whereas ap2-7 seed coat epidermal cells fail to differentiate and, therefore, do not synthesize mucilage (Jofuku et al., 1994; Western et al., 2001; Dean et al., 2011). Since mucilage can only be extracted from hydrated Col-0 seeds, proteins that are significantly overrepresented in Col-0 nonadherent mucilage compared with mum2-10 and/or ap2-7 seed surface extracts would be predicted to be derived from the extruded mucilage. Several mucilage proteins identified were indeed found at much higher levels in Col-0 compared with mum2-10 and ap2-7 (Fig. 3B; Supplemental Data Sets S4 and S5). Overall, the recovery of mucilage-associated protein was reduced by ∼90% when mum2-10 seed was used and by ∼99% when ap2-7 seed was used compared with Col-0 seed (Fig. 3B). These data support the hypothesis that proteins identified in this study are derived from extruded mucilage of seed coat epidermal cells and not from the primary wall.
Figure 3.
Proteins identified are genuinely associated with mucilage. A, Schematic depiction of the mucilage protein quantification in mum2-10 and ap2-7 seed surface extracts relative to Col-0 nonadherent mucilage. ddH2O, Distilled, deionized water. B, Relative levels of mucilage proteins in Col-0 nonadherent mucilage, mum2-10 seed surface extract, and ap2-7 seed surface extract. Values are normalized to Col-0. Averages ± sd are shown; n = 3. *, P < 0.01; and **, P < 0.001.
The Identity of Many Mucilage Proteins Is Consistent with a Role in Mucilage/Cell Wall Modification
The collection of enzymes identified by our proteomics analyses includes all the secreted enzymes required for normal mucilage extrusion that have been identified previously by genetic analysis: MUM2 (At5g63800/Q9FFN4; Dean et al., 2007), BXL1 (At5g49360/Q9FGY1; Arsovski et al., 2009), PER36 (At3g50990/Q9SD46; Kunieda et al., 2013), and SBT1.7 (At5g67360/O65351; Rautengarten et al., 2008; Table I). Their identification here thus validates the robustness of the proteomic analysis.
In an attempt to determine the roles of other mucilage proteins we identified, plant lines with T-DNA insertions in the genes β-GLUCOSIDASE44 (At3g18080/Q9LV33), β-HEXOSAMINIDASE3 (HEXO3; At1g65590/Q8L7S6), ASPARTIC PROTEASE IN GUARD CELL1 (ASPG1; At3g18490/Q9LS40), AUXIN INDUCIBLE IN ROOTS12 (At3g07390/Q94BT2), RESPONSIVE TO DEHYDRATION1a (RD21a; At1g47128/P43297), and SERINE CARBOXYPEPTIDASE-LIKE35 (At5g08260/Q9LEY1) were characterized (Supplemental Fig. S2; Supplemental Table S1). These genes were chosen because they do not appear to have homologs that are also expressed in seed coat epidermal cells, thus decreasing the possibility of functional redundancy obscuring mutant phenotypes. In each case, the T-DNA insertion decreased or eliminated the steady-state levels of transcript in homozygous lines (Supplemental Fig. S2). Seeds of each insertional mutant were imbibed in water, 0.05 m EDTA, 0.05 m CaCl2, or 0.5 m Na2CO3, stained with Ruthenium Red, and examined for seed mucilage abnormalities. EDTA is believed to loosen mucilage by disrupting the homogalacturonan salt bridges through Ca2+ chelation (Western et al., 2001; Rautengarten et al., 2008; Saez-Aguayo et al., 2013; Voiniciuc et al., 2013). Na2CO3 treatment also loosens mucilage, possibly by cleaving cross-linking ester bonds between homogalacturonan polymers (Selvendran and Ryden, 1990; Fry, 2000; McCartney and Knox, 2002). In contrast, mucilage extruded in a CaCl2 solution is more compact and stains more intensely with Ruthenium Red than mucilage extruded in water, presumably by enhancing Ca2+ salt bridging between mucilage homogalacturonan molecules. However, no clear mucilage defects were found in any mucilage protein mutant lines under the conditions tested (Supplemental Fig. S3), suggesting that if the corresponding gene products have a role in mucilage modification, the mutant phenotype must be relatively subtle or conditional.
TBA Proteins Were Found to Be Highly Abundant in Seed Coat Mucilage
Among the mucilage proteins identified, three proteins of the unknown protein family 0540 (UPF0540), At1g62000/Q39168, At1g62060/O04573, and At1g62080/O04575, were of particular interest because they were consistently identified as the most abundant proteins in almost all samples (Table I; Supplemental Data Set S4). Consistent with these protein data, the corresponding genes were found to be expressed in the seed coat at very high levels (Supplemental Fig. S4; Schmid et al., 2005; Winter et al., 2007; Le et al., 2010; Dean et al., 2011). However, the proteins encoded by these genes do not contain known functional domains other than putative signal peptides, so no function has been ascribed to them to date. Members of the UPF0540 protein family are strongly conserved, as they share 79% amino acid sequence identity and 81% similarity with one another (Fig. 4). Furthermore, the loci that encode these proteins are tightly clustered on chromosome 1, suggesting that the gene family may have expanded through tandem duplication events. Due to the abundance of the UPF0540 proteins in the seed coat, these genes were named TESTA ABUNDANT1 (TBA1; At1g62000/Q39168), TBA2 (At1g62060/O04573), and TBA3 (At1g62080/O04575). Interestingly, a peptide from a fourth member of the UPF0540 family, At1g62220/O04587, also was detected in adherent mucilage (Supplemental Data Sets S1 and S3). However, At1g62220/O04587 was identified with only one peptide with a score below the cutoff for statistical significance. Due to the strong similarities in amino acid sequences and expression patterns between At1g62220/O04587 and the TBA proteins, At1g62220/O04587 was named TBA-LIKE (TBAL).
Figure 4.
Amino acid sequences of the TBA proteins. Amino acid sequence alignment is shown for TBA1, TBA2, and TBA3. Dark gray highlights amino acid residues that are identical, and light gray highlights amino acid residues that are similar. Underlined residues denote signal peptides predicted by SignalP (http://www.cbs.dtu.dk/services/SignalP/). Boldface S and T residues are predicted by NetOGlyc (http://www.cbs.dtu.dk/services/NetOGlyc/) to be O-glycosylated.
TBAs are small proteins (∼150 amino acids) with many conserved Ser and Thr residues predicted by NetOGlyc to be O-glycosylated (Steentoft et al., 2013; Fig. 4). These characteristics suggest that TBAs and TBAL may function as structural proteins that interact with various polysaccharides in seed coat mucilage.
TBA Proteins Are Synthesized in the Developing Seed Coat Epidermis and Secreted to the Apoplast
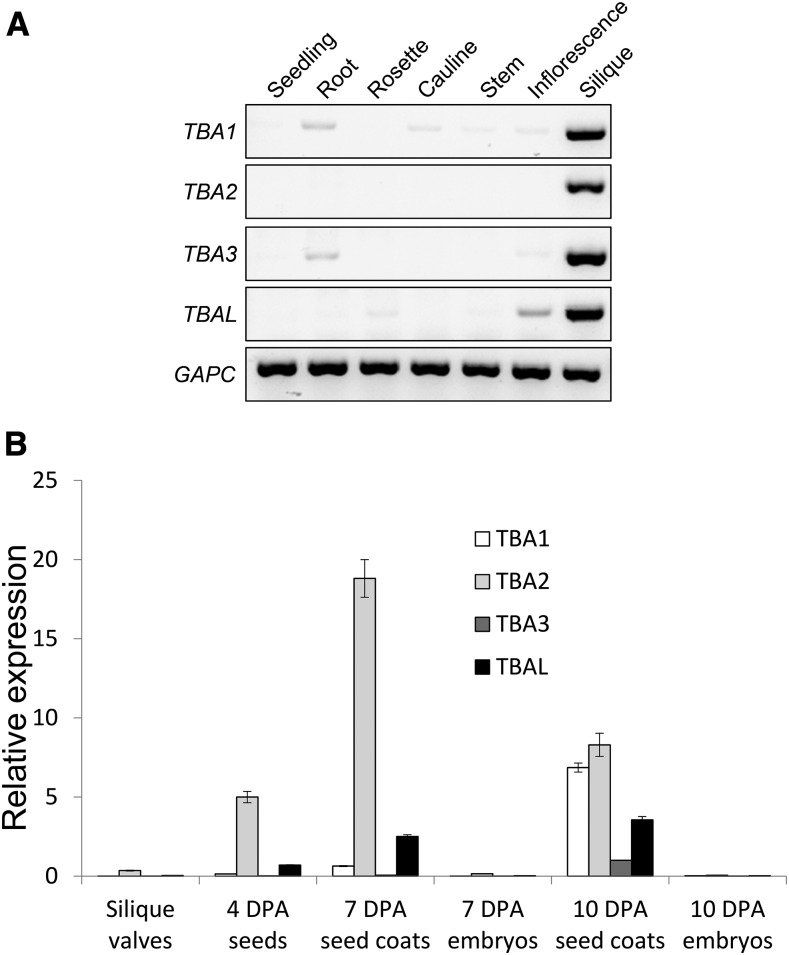
Public microarray data suggest that TBA and TBAL are expressed uniquely in the seed coat, and reverse transcription (RT)-PCR results are consistent with this pattern (Fig. 5). TBA and TBAL transcripts could only be detected in siliques (Fig. 5A) and, more specifically, in the 7- and 10-d post anthesis (DPA) seed coat (Fig. 5B). TBA2 expression levels are by far the highest and peaked at 7 DPA (coinciding with mucilage synthesis; Fig. 5B), whereas the expression levels of the remaining genes were lower and peaked at 10 DPA (coinciding with columella synthesis; Fig. 5B).
Figure 5.
TBA and TBAL transcripts are found predominantly in the developing seed coat. A, RT-PCR detection of TBA1, TBA2, TBA3, and TBAL transcripts in seedlings, roots, rosette and cauline leaves, stem, inflorescence, and siliques. CYTOSOLIC GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE (GAPC) transcripts are shown as cDNA loading controls. B, Quantitative RT-PCR results showing the relative expression levels of TBA1, TBA2, TBA3, and TBAL in empty silique valves, 4-DPA seeds, 7-DPA seed coats, 7-DPA embryos, 10-DPA seed coats, and 10-DPA embryos. Expression levels were relative to GAPC transcript levels. n = 3, and error bars denote sd. A second biological replicate was processed with similar results.
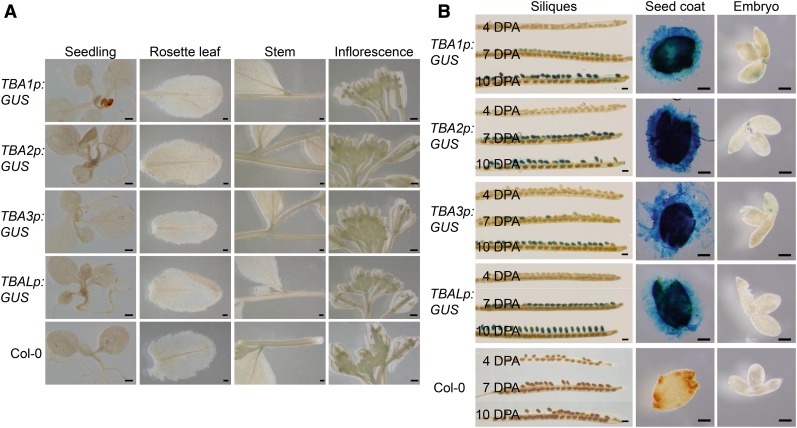
To verify the expression pattern of TBA and TBAL, reporter assays were performed on tissues of plants carrying chimeric genes encoding the GUS gene under the control of the TBA and TBAL native promoters. Consistent with RT-PCR data, GUS activity could be detected in developing seeds at 7 and 10 DPA (Fig. 6B) but not in seedlings, leaves, stems (Fig. 6A), and embryos (Fig. 6B). GUS under the control of TBA2p appeared earlier compared with other promoters, although in general, all TBA and TBAL promoters were active by 10 DPA (Fig. 6B). These data support the hypothesis that all four promoters are active primarily in the seed coat. The fact that the TBA promoter-GUS patterns mirror the presence of TBA transcripts implies that the expression of the TBA genes is largely regulated by their upstream cis-regulatory elements.
Figure 6.
TBA and TBAL promoters are active exclusively in the seed coat. A, TBA1p:GUS, TBA2p:GUS, TBA3p:GUS, TBALp:GUS, and Col-0 seedlings, rosette leaves, stems, and inflorescences stained for GUS activities. Bars = 500 µm. B, TBA1p:GUS, TBA2p:GUS, TBA3p:GUS, TBALp:GUS, and Col-0 siliques and developing seeds at 4, 7, and 10 DPA stained for GUS activities. The 10-DPA seed coats and embryos were dissected and stained separately, as shown in the two columns at right. Bars = 500 µm for siliques and 100 µm for dissected seed coats and embryos.
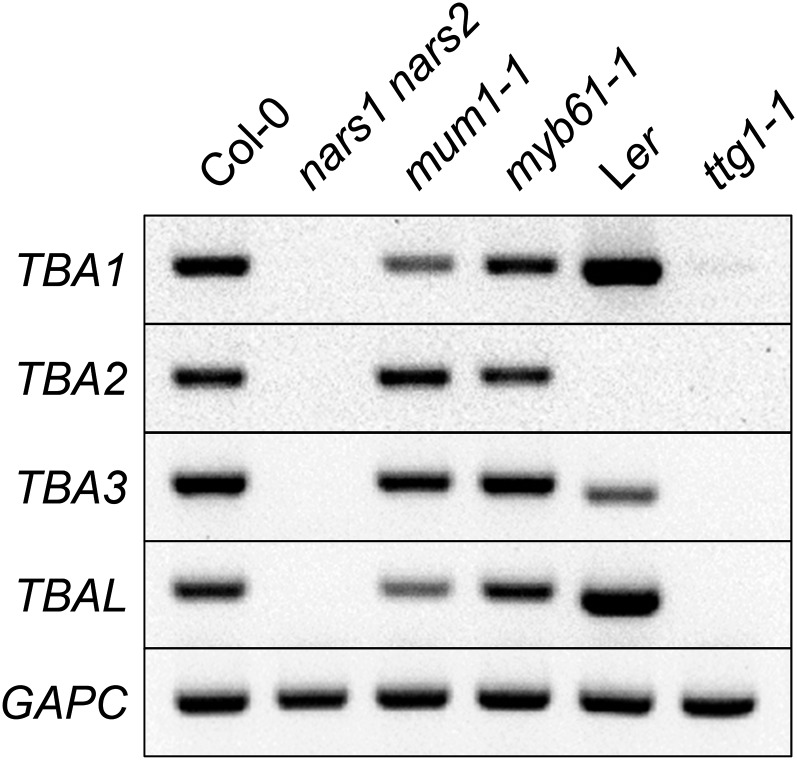
Several transcription factors are known to regulate the differentiation of seed coat epidermis and the synthesis of seed coat mucilage. Some of these master regulators include NAC-REGULATED SEED MORPHOLOGY1 (NARS1), NARS2, MUM1, MYELOBLASTOSIS61 (MYB61) and TRANSPARENT TESTA GLABRA1 (TTG1; Koornneef, 1981; Penfield et al., 2001; Kunieda et al., 2008; Huang et al., 2011). Since the TBA genes are expressed exclusively in the seed coat, we asked whether they are under the control of these transcription factors. RT-PCR results showed that TBA and TBAL transcripts were absent in developing seeds of both the nars1 nars2 double mutant and ttg1-1 (Fig. 7), which suggests that NARS1/NARS2 and TTG1 are all required for TBA and TBAL expression. Intriguingly, TBA2 transcripts appear to be absent in the Landsberg erecta ecotype, suggesting that there may be TBA expression variation among different natural accessions.
Figure 7.
TBA and TBAL expression requires NARS1, NARS2, and TTG1. RT-PCR detection is shown for TBA and TBAL transcripts in 7-DPA seeds of nars1 nars2, mum1-1, myb61-1, ttg1-1, and their respective ecotype backgrounds. GAPC transcripts are shown as cDNA loading controls. Ler, Landsberg erecta.
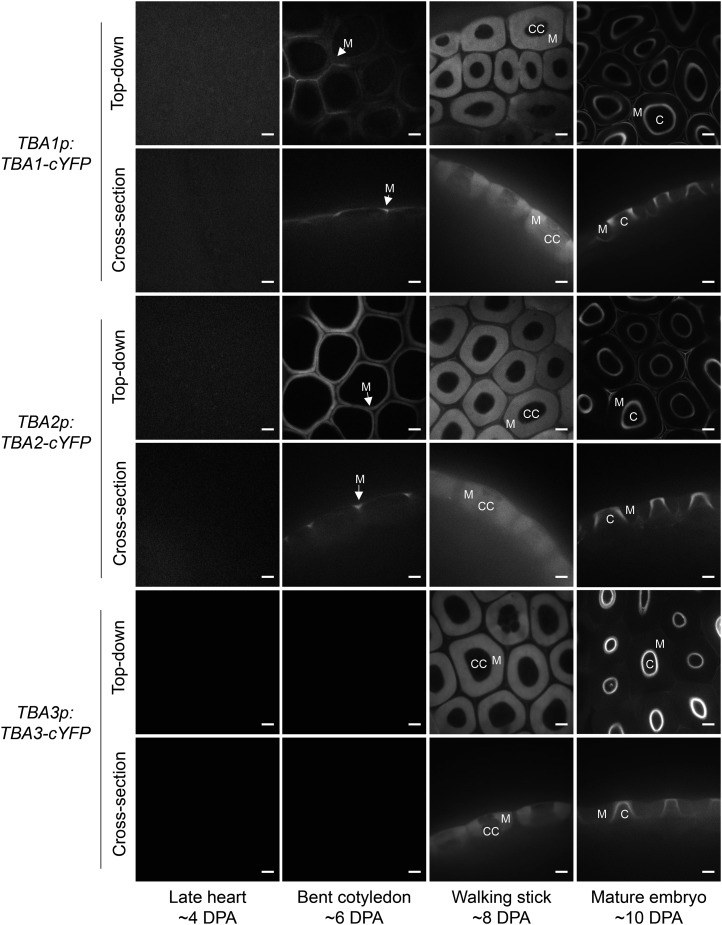
The subcellular localization of TBAs was characterized using C-terminally tagged citrine-yellow fluorescent protein (cYFP) TBA translational fusion constructs driven by their endogenous promoters. In agreement with the expression data, cYFP signals were detected in the developing seed coat. No cYFP signal was observed in 4-DPA seeds (Fig. 8). By 6 DPA, TBA1-cYFP and TBA2-cYFP could be detected in the seed coat epidermal lateral cell walls and the developing mucilage pockets. All three TBAs could be detected in the mucilage pockets by 8 DPA (Fig. 8). By 10 DPA, the cYFP signal was absent from the mucilage pocket but was observed in the developing columella (Fig. 8). This expression pattern coincides spatiotemporally with the TBA transcript and promoter activity patterns (Figs. 5 and 6) and reinforces the characterization of TBA proteins as mucilage proteins. Furthermore, cYFP fluorescence was detected only in the outer epidermal layer of the seed coat (Fig. 8), suggesting that TBA proteins may only be synthesized in mucilage-secretory cells.
Figure 8.
TBA proteins are secreted to the seed coat epidermis apoplast. Confocal microscopy images denote the localization of cYFP-tagged TBA1, TBA2, and TBA3 in developing seed coats driven by their respective endogenous promoters. C, Columella; CC, cytoplasmic column; M, mucilage pockets. Bars = 10 µm.
Despite the fact that TBAs were initially identified in mature mucilage, TBA-cYFP fluorescence was absent from mucilage pockets by 10 DPA, which raised the possibility that TBAs might be unstable proteins. To test this idea, immunoblot experiments were performed to try to detect TBA-cYFP extracted from developing siliques. Interestingly, full-length TBA-cYFP proteins were difficult to detect in the siliques of TBA-cYFP transgenic plants by immunoblotting. The abundance of TBA-cYFP was quite variable among different lines, and TBA3-cYFP appears to be partially insoluble (Supplemental Fig. S5). These results suggest that TBA-cYFP proteins are likely unstable and may undergo proteolysis or other posttranslational modification within the mucilage pocket.
TBA Proteins Are Likely Functionally Redundant
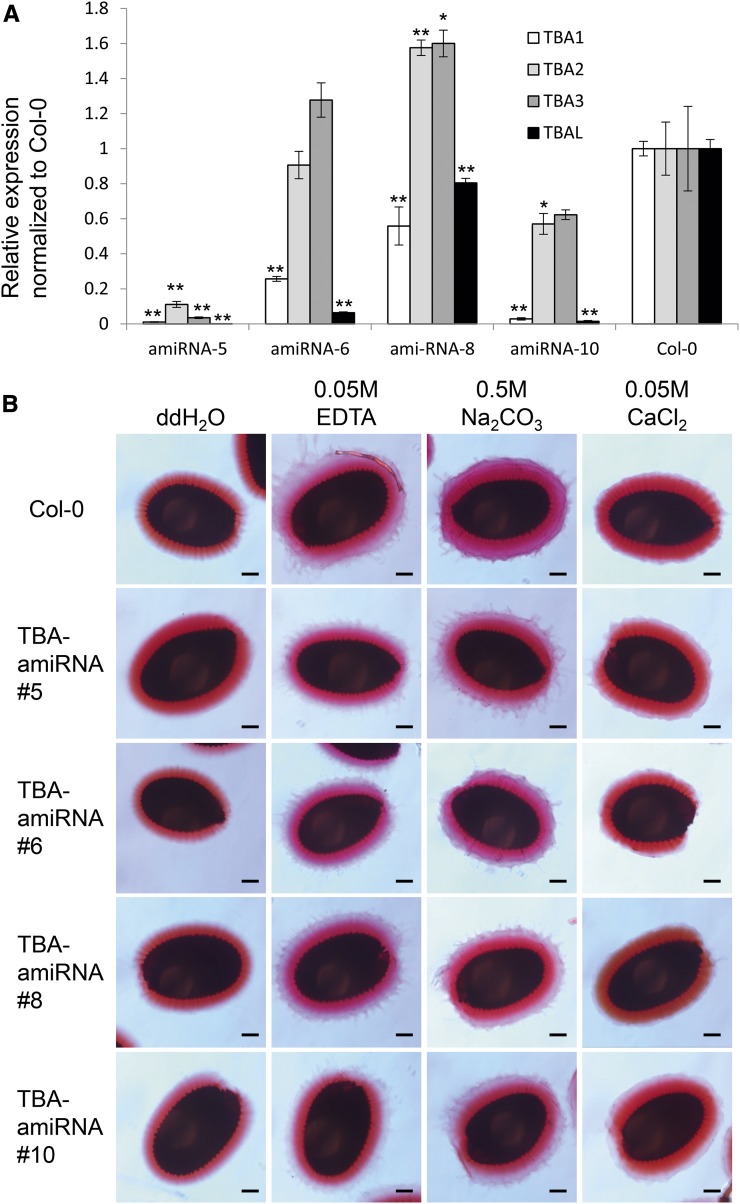
In an attempt to determine the function of the TBA proteins, we characterized tba loss-of-function mutants. Since the three TBA proteins are highly conserved and share a similar expression pattern, we anticipated that they might be functionally redundant. Furthermore, because all these genes are closely linked on the chromosome, the construction of double, triple, and quadruple tba mutants is relatively difficult. To overcome these problems, an artificial microRNA (amiRNA) driven by the UBIQUITIN EXTENSION PROTEIN1 promoter (UBQ1p) was designed to knock down all three TBA homologs and TBAL simultaneously. When TBA1 transcript levels in developing siliques were quantified in 12 UBQ1p:TBA-amiRNA transgenic lines, four lines showed significant down-regulation of TBA1, but only line 5 showed significant down-regulation in all three TBA genes and TBAL (Fig. 9A). However, none of the UBQ1p:TBA-amiRNA lines showed any mucilage defects when their seeds were imbibed in water, 0.05 m EDTA, 0.05 m CaCl2, or 0.5 m Na2CO3 followed by Ruthenium Red staining (Fig. 9B). These results suggest that either the TBA genes are functionally redundant, even at low levels of expression, or that the amiRNA knockdown lines possess mucilage phenotypes that are not clearly discernible with Ruthenium Red staining.
Figure 9.
Down-regulation of TBA and TBAL does not affect mucilage extrusion. A, Quantitative RT-PCR analysis of the expression of TBA and TBAL in 10-DPA siliques from four independent UBQ1p:TBA-amiRNA lines and Col-0. All expression levels were relative to GAPC and were then normalized to Col-0 expression levels. n = 3, and error bars denote sd. Asterisks denote transcript levels significantly different from the wild type at P < 0.05 (*) and P < 0.01 (**). B, Ruthenium Red-stained seed coat mucilage from the four independent UBQ1p:TBA-amiRNA lines shown in A. Seeds were imbibed in water, 0.05 m EDTA, 0.5 m Na2CO3, or 0.05 m CaCl2 prior to staining. Bars = 100 µm.
DISCUSSION
Proteins Are an Integral Part of the Arabidopsis Seed Coat Mucilage
Arabidopsis seed coat mucilage is a specialized layer of the extracellular matrix composed of cell wall carbohydrates arranged in a distinct structure in the apoplast of seed coat epidermal cells. Because of its accessibility and dispensability, mucilage has been used as a genetic model for studying the structure and function of the plant cell wall (Arsovski et al., 2009; Haughn and Western, 2012). Forward genetic analysis has enabled the identification of several proteins that are secreted with mucilage and required for normal mucilage structure. To more comprehensively define the array of proteins involved in mucilage structure and modification, we used proteomic analysis to examine mucilage extruded by mature Arabidopsis seeds. The mucilage extracted by extensive shaking yielded protein preparations that possessed a consistent array of secreted polypeptides relatively free of intracellular proteins. The 28 proteins identified in Col-0 seed coat mucilage by this approach may not be very numerous, but they represent the same classes of proteins found in the cell wall proteomes of other tissue types, which generally contain less than 100 proteins (Albenne et al., 2013). This suggests that the mucilage protein extraction protocol is at least comparable with other cell wall proteome studies in terms of protein recovery rate. Furthermore, the mucilage proteins identified include all proteins believed to be secreted to the mucilage pocket that are not membrane anchored: MUM2, SBT1.7, BXL1, and PER36 (Dean et al., 2007; Macquet et al., 2007b; Rautengarten et al., 2008; Arsovski et al., 2009; Kunieda et al., 2013). In addition, proteomic analysis of mucilage extracts from the seeds of ap2 mutants that fail to differentiate a seed coat epidermis showed decreases among the most abundant mucilage proteins with the exception of PER36 (see below), which, unlike the others, localizes to the primary cell wall surrounding the mucilage. Therefore, we believe that our method is sufficiently robust in characterizing the mature mucilage proteome.
Seed Coat Mucilage Is a Suitable Model for Cell Wall Protein Analyses
Most of the proteins of mature seed coat mucilage are functionally similar to proteins found in primary cell walls from other tissues (Albenne et al., 2013; Fig. 2), consistent with the idea that mucilage and cell walls share many biosynthetic and functional processes. Seed coat mucilage has the experimental advantage over other types of cell walls that it is actively extruded and can be extracted without tissue homogenization and associated cytoplasmic contamination (Supplemental Data Sets S1–S3). All of the mucilage proteins identified contain predicted signal peptides, while proteins without signal peptides detected in mucilage generally scored poorly (Supplemental Data Set S3), suggesting that this method indeed strongly favors apoplastic proteins. This reenforces mucilage as a strong model in which to study cell wall proteins, as it has markedly reduced cytoplasmic contamination compared with other cell wall proteomes while retaining a comparable protein recovery rate (Albenne et al., 2013). However, because the extraction of mucilage requires its hydration-induced extrusion, the proteomic profile established for mature mucilage may not include proteins that are normally present only in early developmental stages. Characterizing the mucilage proteins from developing seeds will require other analytical strategies.
Seed Coat Mucilage Proteome Is a Specialized Cell Wall Proteome
Carbohydrate-active enzymes identified in the seed mucilage proteome include two enzymes previously detected by molecular genetic analyses. The mucilage proteins MUM2 and BXL1 are required for the removal of arabinogalactan side chains of cell wall polysaccharides and for proper mucilage extrusion (Dean et al., 2007; Macquet et al., 2007b; Arsovski et al., 2009). Four other carbohydrate-active enzymes not previously known to be mucilage associated also were identified, including HEXO3 (At1g65590/Q8L7S6). HEXO3 has been shown to be involved in the removal of GlcNAc residues from glycoproteins and in the formation of paucimannosidic N-glycan (Gutternigg et al., 2007; Liebminger et al., 2011; this study). HEXO3 localizes primarily to the plasma membrane, although a minor fraction has been suggested to be soluble in the apoplast, which would be consistent with our findings (Liebminger et al., 2011). However, the overall biological role of HEXO3 and its ortholog HEXO1 remains unknown, as no defects in growth and stress responses were found in their respective mutants (Liebminger et al., 2011).
Proteases are commonly found in cell walls, and four were detected in the mucilage proteome. SBT1.7 has been identified previously by its mutant mucilage phenotype of defective extrusion and altered homogalacturonan methylation state. It has been suggested that SBT1.7 participates in the removal of the inhibitor domain from a pectin methylesterase (Rautengarten et al., 2008). Two other proteases detected in mucilage have been connected to roles in other tissues. Ectopic expression of ASPG1 (At3g18490/Q9LS40) enhances abscisic acid (ABA)-induced stomata closure, reactive oxygen species production, and drought resistance (Yao et al., 2012), and ASPG1 expression also is induced by ABA (Yao et al., 2012). RD21a (At1g47128/P43297) is up-regulated during drought stress, suggesting that it also may be connected to ABA-regulated processes (Koizumi et al., 1993). In addition, RD21a is known to facilitate apoptosis, which the outer seed coat epidermal cells eventually undergo at the end of seed development (Lampl et al., 2013). However, no obvious mucilage defects were observed in either aspg1 or rd21a seeds.
Oxidoreductases can potentially modify cell wall components either by regulating the production and turnover of reactive oxygen species or by participating in the oxidative modification of other cellular metabolites. Mucilage proteins of this category include PER36, which was shown previously to facilitate mucilage extrusion by weakening the primary cell wall (Kunieda et al., 2013). PER36 localizes to the radial and tangential primary walls of the mucilage pockets but was not detected in the mucilage itself (Kunieda et al., 2013). Therefore, it is likely that PER36 is not part of the mucilage proteome but, rather, a contaminant from the primary wall. Consistent with this, PER36 was the only protein found to be more abundant in mum2-10 seed surface extracts compared with Col-0 (Fig. 3B). Primary cell wall proteins such as PER36 would be expected to be overrepresented in the proteome extracted from mum2-10 seeds, since mucilage extrudes very poorly from this mutant.
Proteins involved in lipid metabolism also are common in cell wall proteomes (Albenne et al., 2013). These proteins are likely involved in the synthesis and modification of cuticles deposited outside the cell wall. In seed coat mucilage, proteins involved in lipid metabolism associate exclusively with the adherent layer, making it the only obvious distinction between the proteomes of the two mucilage layers (Fig. 2A). Since the seed coat epidermis likely has a cuticle (Watanabe et al., 2004; Panikashvili et al., 2009) and the primary wall remains attached to the top of the columella embedded in the adherent layer, these proteins may be involved in the synthesis/modification of a seed coat cuticle. The fact that proteins involved in lipid metabolism are found only in the adherent layer suggests that they are very strongly bound to the primary cell wall, either covalently cross-linked with other cell wall polymers or perhaps anchored by hydrophobic interactions with the cuticle. In contrast, PER36 was observed in the primary cell wall, but it was found in both adherent and nonadherent mucilage in our analysis, suggesting that it is less strongly bound to the cell wall than the proteins involved in lipid metabolism.
One difference between the mucilage and other cell wall proteomes is the apparent lack of structural proteins in mucilage. No extensins and Hyp-rich glycoproteins, typically major components of cell wall proteomes, were observed (Jamet et al., 2008a; Albenne et al., 2013), although the Leu-rich repeat family protein At3g24480/Q9LHF1 might play a structural role. Furthermore, the TBA protein structure (short, no identifiable protein domains, potential glycosylation sites) and abundance (see below) are characteristics consistent with those of structural proteins, although we have no direct evidence supporting this hypothesis. Arabinogalactan proteins also may function as structural proteins, as has been suggested for ARABINOXYLAN PECTIN ARABINOGALACTAN PROTEIN1 (Tan et al., 2013). Two fasciclin-like arabinogalactan proteins, FLA10 (At3g60900/Q9LZX4) and FLA17 (At5g06390/Q66GR0), were identified in mucilage, but their loss-of-function phenotypes did not provide any insight into a possible structural role. Surprisingly, SALT OVERLY SENSITIVE5 (SOS5/FLA4; At2g46550), the only FLA known to be required for normal mucilage structure (Harpaz-Saad et al., 2011; Griffiths et al., 2014), was not identified as a component of the mature mucilage proteome. It may be possible that SOS5 impacts mucilage structure indirectly, perhaps by facilitating matrix polysaccharide biosynthesis in the Golgi or acting as a GPI-anchored carrier of carbohydrates to the apoplast.
Despite the identification of numerous new mucilage proteins, in addition to the four proteins (MUM2, BXL1, PER36, and SBT1.7) previously known to regulate mucilage extrusion and structure (Dean et al., 2007; Macquet et al., 2007b; Rautengarten et al., 2008; Arsovski et al., 2009; Kunieda et al., 2013), the biological roles of the new proteins in mucilage remain unknown. Analysis of loss-of-function mutants for many of the genes encoding these proteins failed to identify mucilage extrusion and morphology defects. This may reflect functional redundancy. Alternatively, it may indicate that these mucilage proteins play roles that do not directly impact the extrusion, adherence, or structure of the mature mucilage and that, therefore, do not generate a loss-of-function phenotype readily detectable by Ruthenium Red staining and light microscopy.
TBA Proteins Are Seed Coat-Specific Proteins with Potential Roles in Seed Coat Differentiation
Among the mucilage proteins most frequently identified in this study are a family of three uncharacterized proteins designated TBA, which are highly and specifically expressed in the seed coat epidermis during late seed development (Figs. 5 and 6). The expression of TBAs requires the seed coat differentiation master regulators NARS1, NARS2, and TTG1 (Fig. 7) and coincides temporally and spatially with mucilage synthesis and secretion, while ectopically expressed TBAs were shown to localize to the seed coat mucilage and the columella (Fig. 8). Since TBAs are abundant and are predicted to be heavily glycosylated while lacking known functional domains, they may function as structural proteins and cross-link other cell wall polysaccharides (Fig. 4). However, our attempts to use amiRNA to knock down all of the TBA homologs simultaneously failed to produce a Ruthenium Red-stained mucilage mutant phenotype (Fig. 9), so this hypothesis remains to be validated. It is interesting that, following the completion of mucilage synthesis during columella formation, cYFP fluorescence in the mucilage pocket abruptly disappears (Fig. 8; 10 DPA), leaving fluorescence only in the columella. These observations suggest that the TBA-cYFP proteins may be actively degraded, although we cannot rule out the possibility that the fluorescence of the intact protein is being quenched due to changes in the chemical environment of the mucilage pocket.
The seed coat specificity and relative strength of the TBA promoters make them valuable tools for seed coat mucilage studies. Since the seed coat mucilage is not essential to plant fitness under laboratory conditions, it can tolerate genetic perturbation, which has made it a powerful genetic model for cell wall analysis. The specificity of the TBA promoters makes them suitable tools to genetically manipulate the seed coat epidermis in general and seed mucilage specifically. Another Arabidopsis seed coat-specific promoter, from the DIRIGENT PROTEIN1 gene (DP1), differs from expression of the TBA promoters in that DP1 is expressed in both the epidermal and palisade cell layers and peaks in expression during midseed development (Esfandiari et al., 2013). Since the TBA promoters are active only in the epidermis late in seed development, the TBA and DP1 promoters complement each other by covering different temporal and spatial domains.
In summary, a novel method was developed to extract and detect proteins integral to the Arabidopsis seed coat mucilage. A total of 28 proteins were identified in mature seed coat mucilage, mostly with predicted functions consistent with a cell wall proteome. The protein profiles are largely similar between the adherent and nonadherent mucilage, with the exception of lipid metabolism proteins that occur exclusively in the adherent layer mucilage. Three homologous, previously undescribed proteins we named TBA were highly abundant in seed coat mucilage. Although their functions remain to be determined, their seed coat epidermis-specific promoters should prove to be useful tools for targeted gene expression.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Most Arabidopsis (Arabidopsis thaliana) plants used in this study were derived from the Col-0 ecotype, except for ttg1-1, which is derived from the Landsberg erecta ecotype. Seeds were germinated on plates with Arabidopsis medium (Haughn and Somerville, 1986) at 7% agar and transferred to soil SunshineMix 4 (SunGro). Plants were grown with continuous fluorescent illumination of 80 to 140 µE m−2 s−1 at 20°C to 22°C. T-DNA insertion lines used in this study were obtained from the ABRC and are listed in Supplemental Table S1. T-DNA insertion lines were selected with a PCR-based assay using primers listed in Supplemental Table S2.
Seed Coat Mucilage Extraction
Dry seeds (40 mg) were imbibed with 800 µL of double-distilled water in a microcentrifuge tube. The seeds were gently shaken on a tabletop shaker for 1 h at 120 rpm. Supernatants that contain the nonadherent mucilage were collected. The seeds were washed once with 200 µL of double-distilled water, which was pooled with the supernatant to form the nonadherent mucilage fraction. To obtain the adherent mucilage, 800 µL of double-distilled water was added to the seeds after extracting the nonadherent mucilage, and the seeds were secured horizontally to a tabletop vortex and shaken at top speed for 3 h. The supernatants containing the adherent mucilage were collected. The seeds were washed once with 200 µL of double-distilled water, which was pooled with the supernatant as the adherent layer fraction. The mucilage samples were freeze dried overnight and then chemically deglycosylated as described by Edge et al. (1981). Briefly, 15 µL of anisole (Sigma-Aldrich) and 135 µL of trifluoromethanesulfonic acid (Sigma-Aldrich) were added to one freeze-dried mucilage sample in a Reacti-vial. The samples were sealed and incubated at 4°C for 2 h. Four microliters of 0.2% Bromophenol Blue was added to each sample, and 60% pyridine (Sigma-Aldrich) was added drop wise to each mucilage sample on ice until the solution turned light blue. The neutralized mucilage samples were dialyzed overnight in double-distilled water with dialysis tubing pore sizes of 3,500 to 5,000 D and then freeze dried overnight.
MS
Mucilage protein samples were resuspended in SDS-PAGE sample buffer and separated briefly on a 10% SDS-PAGE gel until all of the Mr markers just entered the resolving gel. The proteins were stained with blue silver (Candiano et al., 2004), and the entire lane was excised from the gel as one gel slice. Protein samples were analyzed by tandem mass spectrometry (MS/MS) at the Centre for High-Throughput Biology Proteomics Core Facility at the University of British Columbia. In brief, samples were subjected to reduction/alkylation with DTT/iodoacetamide followed by digestion with trypsin essentially as described by Shevchenko et al. (1996). The resulting peptides were desalted and concentrated with STAGE tips (Rappsilber et al., 2003) and analyzed by liquid chromatography-MS/MS on a linear-trapping quadrupole-Orbitrap mass spectrometer (LTQ Orbitrap Velos) online coupled to an Agilent 1290 series HPLC device using a nanospray ionization source (ThermoFisher Scientific) including a 2-cm-long 100-μm i.d. fused silica trap column, a 20-cm-long 50-μm i.d. fused silica fritted analytical column, and a 20-μm i.d. fused silica gold-coated spray tip (6-μm-diameter opening, pulled on a P-2000 laser puller from Sutter Instruments, coated on a Leica EM SCD005 Super Cool Sputtering Device). The trap column is packed with 5-μm-diameter Aqua C-18 beads (Phenomenex; www.phenomenex.com), while the analytical column is packed with 1.9 μm-diameter Reprosil-Pur C-18-AQ beads (Dr. Maisch; www.dr-maisch.com). Standard 90-min gradients were run from 10% to 32% buffer B (0.5% acetic acid and 80% acetonitrile) over 51 min, then from 32% to 40% in the next 5 min, then increased to 100% over a 2-min period, held at 100% for 2.5 min, and then dropped to 0% for another 20 min. The HPLC system included an Agilent 1290 series pump and autosampler with thermostat. The thermostat temperature was set at 6°C. The sample was loaded on the trap column at 5 μL min−1, and the analysis was performed at 0.1 μL min−1. The LTQ-Orbitrap was set to acquire a full-range scan at 60,000 resolution from 350 to 1,600 Th in the Orbitrap to simultaneously fragment the top ten peptide ions by CID and the top five peptide ions by HCD (resolution, 7,500) in each cycle in the LTQ (minimum intensity, 1,000 counts). Parent ions were then excluded from MS/MS for the next 30 s. Singly charged ions were excluded, since in electrospray ionization (ESI) mode, peptides usually carry multiple charges. The Orbitrap was continuously recalibrated using lock-mass function. For mass accuracy, the error of mass measurement is typically within 5 ppm and is not allowed to exceed 10 ppm.
For quantitative analyses, Col-0 mucilage along with mum2-10 and ap2-7 seed surface extracts were prepared from 80 mg of seeds using the protocol described above for nonadherent mucilage. All protein samples were reduced/alkylated and digested as described above and then dimethylated with light, medium, and heavy formaldehyde. Col-0 samples were labeled with light formaldehyde for all replicates. mum2-10 samples were labeled with medium formaldehyde for replicates 1 and 2 and with heavy formaldehyde for replicate 3. ap2-7 samples were labeled with heavy formaldehyde for replicates 1 and 2 and with medium formaldehyde for replicate 3. Samples from all three genotypes were pooled before the MS analysis. For replicates 1 and 2, samples were analyzed using the LTQ Orbitrap Velo as described above. For replicate 3, samples were analyzed using the Impact II quadrupole-time of flight mass spectrometer (Bruker Daltonics) online coupled to an Easy nano LC 1000 HPLC device (ThermoFisher Scientific) using a captive spray nanospray ionization source (Bruker Daltonics) including a 2-cm-long 100-μm i.d. fused silica fritted trap column and a 75-μm i.d. fused silica analytical column with an integrated spray tip (6–8-μm-diameter opening pulled on a P-2000 laser puller from Sutter Instruments). The trap column is packed with 5-μm Aqua C-18 beads (Phenomenex; www.phenomenex.com), while the analytical column is packed with 1.9-μm-diameter Reprosil-Pur C-18-AQ beads (Dr. Maisch; www.dr-maisch.com). The analytical column was held at 50°C by an in-house constructed column heater. Samples were resuspended and loaded in buffer A (0.1% aqueous formic acid). Standard 45-min gradients were run from 10% to 60% buffer B (0.1% formic acid and 80% acetonitrile) over 28 min, then increased to 100% over 2 min, and held at 100% for 15 min. The liquid chromatograph thermostat temperature was set at 7°C. The sample was loaded on the trap column at 850 Bar, and the analysis was performed at a flow rate of 0.25 μL min−1. The Impact II quadrupole-time of flight mass spectrometer was set to acquire in a data-dependent auto-MS/MS mode with inactive focus fragmenting the 20 most abundant ions (one at the time at a rate of 18 Hz) after each full-range scan from m/z 200 to 2,000 Th (at a rate of 5 Hz). The isolation window for MS/MS was 2 to 3 Th depending on parent ion mass-to-charge ratio, and the collision energy ranged from 23 to 65 eV depending on ion mass and charge. Parent ions were then excluded from MS/MS for the next 0.4 min and reconsidered if their intensity increased more than 5 times. Singly charged ions were excluded, since in ESI mode, peptides usually carry multiple charges. Strict active exclusion was applied. For mass accuracy, the error of mass measurement is typically within 5 ppm and is not allowed to exceed 10 ppm. The nano ESI source was operated at 1,700 V capillary voltage, 0.20 Bar nano buster pressure, 3 L min−1 drying gas, and 150°C drying temperature.
MS Data Analyses
For all qualitative analyses and replicates 1 and 2 of the quantitative analyses, liquid chromatography-MS/MS data were processed with Proteome Discoverer version 1.2 (ThermoFisher Scientific) and then searched against the Uniprot-Swissprot Arabidopsis database using the MASCOT algorithm version 2.4 (Perkins et al., 1999; http://www.matrixscience.com). The database contains 12,069 sequences; no contaminants were added in the search space. The following parameters were applied: peptide mass accuracy, 10 ppm; fragment mass accuracy, 0.6 D; trypsin enzyme specificity, fixed modifications, carbamidomethyl, variable modifications, Met oxidation; deamidated N, Q, and N-acetyl peptides, ESI-TRAP fragment characteristics. Only those peptides with IonScores exceeding the individually calculated 99% confidence limit (as opposed to the average limit for the whole experiment) were considered as accurately identified. Proteome Discoverer parameters were as follows: event detector, mass precision of 4 ppm (corresponds to extracted ion chromatograms at ±12 ppm max error); S/N threshold, 1; quantitation method, ratio calculation; replace missing quantitation values with minimum intensity, yes; use single peak quantitation channels, yes; protein quantification, use all peptides, yes. In order for a protein to be considered a true mucilage protein in qualitative analysis, it must be identified in at least two out of the three biological replicates with MASCOT protein scores >40 (a score of ≥25 corresponds to a false discovery rate of ≤5%), and identified in at least one out of the three biological replicates with two or more unique peptides.
For replicate 3 of the quantitative analysis, data analysis was performed using MaxQuant 1.5.3.30 (Cox and Mann, 2008) with the Arabidopsis protein sequence database plus common contaminants. The search was performed using the following parameters: peptide mass accuracy, 10 ppm; fragment mass accuracy, 0.05 D; trypsin enzyme specificity, fixed modifications, carbamidomethyl, variable modifications, Met oxidation; and N-acetyl proteins. Only those peptides exceeding the individually calculated 99% confidence limit (as opposed to the average limit for the whole experiment) were considered as accurately identified. Relative protein levels from mum2-10 and ap2-7 seed surface extracts were normalized to Col-0 nonadherent mucilage. Proteins that could be detected and quantified in all three replicates were analyzed. A two-tailed Student’s t test was used to determine the statistical significance of relative protein level differences between mum2-10, ap2-7, and Col-0.
Expression Analyses
RNA was extracted from various plant tissues using Trizol reagent (Life Technologies), except that siliques were processed with the RNAqueous Total RNA Isolation Kit (Ambion) while developing seed coats and embryos were processed with the RNAqueous-Micro Total RNA Isolation Kit (Ambion) according to the manufacturer’s instructions. cDNA synthesis was carried out using SuperScript II reverse transcriptase (Life Technologies) according to the manufacturer’s instructions. Quantitative PCR (qPCR) was performed using iQ SYBR Green Supermix (Bio-Rad) and the primers listed in Supplemental Table S3. The qPCRs were assayed with the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). GAPC transcripts were used as an internal control. A two-tailed Student’s t test was used to determine the statistical significance of differences in TBA and TBAL expression levels between the amiRNA lines and the wild type.
For gene expression analysis of TBAs in transcription factor mutants, RNA extraction and cDNA synthesis were performed as described previously (Kunieda et al., 2013). PCR was performed using Mango-Taq polymerase (Bioline) and the same primers that were used for qPCR.
Generation of Transgenic Plants
The TBAp:GUS constructs were generated using the pBI101 vector, with the promoter fragments amplified from Col-0 genomic DNA. The TBAp:TBA-cYFP translational fusion constructs were assembled in the citrine-pCambia1300 vector as described by Debono et al. (2009), with the promoter and coding region fragments amplified from Col-0 genomic DNA. The TBA amiRNA constructs were designed and built as described by Schwab et al. (2006) using the UBQ1-pCambia1300 vector as described by Ambrose et al. (2011). Primers used for these constructs are listed in Supplemental Table S3. The TBA and TBAL promoters were defined as the DNA sequence extending upstream of the TBA start codon to the next annotated gene, not including pseudogenes, to capture as much of the promoter region as possible without introducing another gene. TBA1p is ∼0.5 kb, TBA2p is ∼1.3 kb, TBA3p is ∼1.8 kb, and TBALp is ∼1.4 kb long. Col-0 Arabidopsis plants were transformed using the floral dip method (Clough and Bent, 1998); at least 20 independent transgenic lines were selected for each construct. Results from at least three representative lines are shown.
Microscopy
For seed coat mucilage staining with Ruthenium Red, ∼20 dry seeds were imbibed in 1 mL of distilled, deionized water, 0.05 m EDTA, 0.05 m CaCl2, or 0.5 m Na2CO3 for 1 h and washed twice with double-distilled water. The seeds were then stained with 0.01% (w/v) Ruthenium Red (Sigma-Aldrich) for 1 h and washed once with double-distilled water. Seeds were imaged with a DFC450 C camera (Leica) on an Axioskop 2 upright light microscope (Carl Zeiss).
Histochemical GUS assays were performed essentially as described by Esfandiari et al. (2013). Tissue samples were vacuum infiltrated with GUS staining solution (0.5 mm potassium ferricyanide, 0.5 mm potassium ferrocyanide, 20 mm Na2EDTA, and 0.1% [v/v] Triton X-100, supplemented with 1 mg mL−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronide [Gold BioTechnology] in 100 mm phosphate buffer [pH 7]), incubated at 37°C for 16 h, and then washed several times with 75% ethanol. Tissues were imaged with a DP72 camera (Olympus) mounted on an SZX10 stereomicroscope (Olympus).
All confocal images were acquired from an Ultraview VoX Spinning Disk Confocal System (PerkinElmer).
For cellulose staining, seeds after mucilage extraction were stained with 0.1% (w/v) Calcofluor White for 5 min and then washed twice with double-distilled water. The seeds were inspected under UV light with the Axioskop 2 microscope.
Immunoblot Analyses
Developing siliques were ground in liquid nitrogen and added to extraction buffer containing 50 mm Tris-HCl (pH 8), 150 mm NaCl, 1 mm EDTA, 10% (v/v) glycerol, 1% Triton X-100, 1 mm PMSF, and protease inhibitor cocktail (Roche). The homogenates were centrifuged at 15,000 rpm for 10 min at 4°C, and the supernatant was collected. Protein concentrations were determined by Bradford assay (Bio-Rad), and 100-µg protein samples were electrophoretically resolved by 12% SDS-PAGE. cYFP fusion proteins were detected using a mouse anti-GFP polyclonal antibody (Roche) and horseradish peroxidase-conjugated goat anti-mouse polyclonal antibody (Santa Cruz Biotechnology). ECL Prime western-blotting detection reagent (GE Healthcare) was used for target detection.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: At1g65590/Q8L7S6, HEXO3; At5g63800/Q9FFN4, MUM2; At5g49360/Q9FGY1, BXL1; At3g50990/Q9SD46, PER36; At5g67360/O65351, SBT1.7; At3g18080/Q9LV33, BGLU44;At1g65590/Q8L7S6, HEXO3; At3g18490/Q9LS40, ASPG1; At3g07390/Q94BT2, AIR12; At1g47128/P43297, RD21a; At5g08260/Q9LEY1, SCPL35; At1g62000/Q39168, TBA1; At1g62060/O04573, TBA2; At1g62080/O04575, TBA3; At5g63140/Q9FMK9, PAP29; At5g06390/Q66GR0, FLA17; At3g60900/Q9LZX4, FLA10; At2g28470/Q9SCV4, BGAL8, At4g23560/Q9SUS0, GH9B15; At5g59310/Q9LLR6, LTP4; At3g08770/Q9LDB4, LTP6; At3g04170/Q9M8X3, RmlC-like cupin superfamily protein; At5g45670/Q9FK75, GDSL motif esterase/acyltransferase/lipase; At3g16370/Q9LU14, GDSL motif esterase/acyltransferase/lipase; At1g75900/Q94CH6, GDSL motif esterase/acyltransferase/lipase; At4g26790/Q8VY93, GDSL motif esterase/acyltransferase/lipase; At4g28780/Q9SVU5, GDSL motif esterase/acyltransferase/lipase; At3g24480/Q9LHF1, Leu-rich repeat family protein.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Mucilage extraction removes pectin and cellulose from the seed coat.
Supplemental Figure S2. Expression (RT-PCR) of genes mutated by T-DNA insertion.
Supplemental Figure S3. Imbibed seeds from plants homozygous for a T-DNA insertion in genes encoding proteins found in seed mucilage.
Supplemental Figure S4. TBA and TBAL expression patterns in various plant tissues.
Supplemental Figure S5. Detection TBA-cYFP by immunoblotting.
Supplemental Table S1. T-DNA insertion lines used in this study.
Supplemental Table S2. Primers used for mucilage protein T-DNA line analyses in this study.
Supplemental Table S3. Primers used for TBA and TBAL constructs in this study.
Supplemental Data Set S1. Col-0 adherent mucilage protein MS data.
Supplemental Data Set S2. Col-0 nonadherent mucilage protein MS data.
Supplemental Data Set S3. Summary of Col-0 mucilage protein identification.
Supplemental Data Set S4. mum2-10 and ap2-7 mucilage protein quantification MS data relative to Col-0.
Supplemental Data Set S5. Summary of mum2-10 and ap2-7 mucilage protein quantification relative to Col-0.
Supplementary Material
Acknowledgments
We thank Suzanne Perry, Jamie Hackworth, and Jenny Hyung-Mee Moon of the University of British Columbia Centre for High-Throughput Biology Proteomics Core Facility for technical assistance, data analyses, and equipment use; the University of British Columbia Bioimaging Facility for technical assistance and equipment use; and Ikuko Hara-Nishimura and Dr. Tomoo Shimada (Department of Botany, Kyoto University) for providing the nars1 nars2 mutant.
Glossary
- Col-0
Columbia-0
- RT
reverse transcription
- DPA
days post anthesis
- amiRNA
artificial microRNA
- ABA
abscisic acid
- MS/MS
tandem mass spectrometry
- ESI
electrospray ionization
- MS
mass spectrometry
- qPCR
quantitative PCR
Footnotes
This work was supported by the National Sciences and Engineering Research Council (NSERC; Discovery Grants to B.E.E. and G.W.H.), a British Columbia Proteomic Network Graduate/Postdoctoral Training Grant (to A.Y.-L.T.), the NSERC Collaborative Research and Training Experience Program Working on Walls (to A.Y.-L.T., G.W.H., and B.E.E.), and the Japan Society for the Promotion of Science (Postdoctoral Fellowship for Research Abroad to T.K.).
Articles can be viewed without a subscription.
References
- Albenne C, Canut H, Jamet E (2013) Plant cell wall proteomics: the leadership of Arabidopsis thaliana. Front Plant Sci 4: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Allard JF, Cytrynbaum EN, Wasteneys GO (2011) A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat Commun 2: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovski AA, Haughn GW, Western TL (2010) Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signal Behav 5: 796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovski AA, Popma TM, Haughn GW, Carpita NC, McCann MC, Western TL (2009) AtBXL1 encodes a bifunctional β-D-xylosidase/α-L-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells. Plant Physiol 150: 1219–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Gidley MJ, Fincher GB (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6: 724–732 [DOI] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25: 1327–1333 [DOI] [PubMed] [Google Scholar]
- Cassab GI, Varner JE (1988) Cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 39: 321–353 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26: 1367–1372 [DOI] [PubMed] [Google Scholar]
- Dean G, Cao Y, Xiang D, Provart NJ, Ramsay L, Ahad A, White R, Selvaraj G, Datla R, Haughn G (2011) Analysis of gene expression patterns during seed coat development in Arabidopsis. Mol Plant 4: 1074–1091 [DOI] [PubMed] [Google Scholar]
- Dean GH, Zheng H, Tewari J, Huang J, Young DS, Hwang YT, Western TL, Carpita NC, McCann MC, Mansfield SD, et al. (2007) The Arabidopsis MUM2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 19: 4007–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debono A, Yeats TH, Rose JK, Bird D, Jetter R, Kunst L, Samuels L (2009) Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell 21: 1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge AS, Faltynek CR, Hof L, Reichert LE Jr, Weber P (1981) Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem 118: 131–137 [DOI] [PubMed] [Google Scholar]
- Esfandiari E, Jin Z, Abdeen A, Griffiths JS, Western TL, Haughn GW (2013) Identification and analysis of an outer-seed-coat-specific promoter from Arabidopsis thaliana. Plant Mol Biol 81: 93–104 [DOI] [PubMed] [Google Scholar]
- Francoz E, Ranocha P, Burlat V, Dunand C (2015) Arabidopsis seed mucilage secretory cells: regulation and dynamics. Trends Plant Sci 20: 515–524 [DOI] [PubMed] [Google Scholar]
- Fry SC. (2000) The Growing Plant Cell Wall: Chemical and Metabolic Analysis. Blackburn Press, Caldwell, NJ [Google Scholar]
- Griffiths JS, Tsai AY, Xue H, Voiniciuc C, Sola K, Seifert GJ, Mansfield SD, Haughn GW (2014) SALT-OVERLY SENSITIVE5 mediates Arabidopsis seed coat mucilage adherence and organization through pectins. Plant Physiol 165: 991–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubert M. (1974) Studies on the distribution of myxospermy among seeds and fruits of Angiospermae and its ecological importance. Acta Biol Venez 8: 315–551 [Google Scholar]
- Gutternigg M, Kretschmer-Lubich D, Paschinger K, Rendić D, Hader J, Geier P, Ranftl R, Jantsch V, Lochnit G, Wilson IB (2007) Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated mechanisms in nematodes, plants, and insects. J Biol Chem 282: 27825–27840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz-Saad S, McFarlane HE, Xu S, Divi UK, Forward B, Western TL, Kieber JJ (2011) Cellulose synthesis via the FEI2 RLK/SOS5 pathway and cellulose synthase 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J 68: 941–953 [DOI] [PubMed] [Google Scholar]
- Haughn G, Chaudhury A (2005) Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci 10: 472–477 [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Haughn GW, Western TL (2012) Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Front Plant Sci 3: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Li J, Wang X, Zhao X, Yang X, Tang Q, He G, Zhou G, Kong Y (2016a) Xylan synthesized by Irregular Xylem 14 (IRX14) maintains the structure of seed coat mucilage in Arabidopsis. J Exp Bot 67: 1243–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Li J, Yang X, Zhao X, Wang X, Tang Q, He G, Zhou G, Kong Y (2016b) Irregular xylem 7 (IRX7) is required for anchoring seed coat mucilage in Arabidopsis. Plant Mol Biol 92: 25–38 [DOI] [PubMed] [Google Scholar]
- Huang J, DeBowles D, Esfandiari E, Dean G, Carpita NC, Haughn GW (2011) The Arabidopsis transcription factor LUH/MUM1 is required for extrusion of seed coat mucilage. Plant Physiol 156: 491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet E, Albenne C, Boudart G, Irshad M, Canut H, Pont-Lezica R (2008a) Recent advances in plant cell wall proteomics. Proteomics 8: 893–908 [DOI] [PubMed] [Google Scholar]
- Jamet E, Boudart G, Borderies G, Charmont S, Lafitte C, Rossignol M, Canut H, Pont-Lezica R (2008b) Isolation of plant cell wall proteins. Methods Mol Biol 425: 187–201 [DOI] [PubMed] [Google Scholar]
- Jamet E, Canut H, Boudart G, Pont-Lezica RF (2006) Cell wall proteins: a new insight through proteomics. Trends Plant Sci 11: 33–39 [DOI] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6: 1211–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski MJ, Lamport DTA (1994) Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J 5: 157–172 [DOI] [PubMed] [Google Scholar]
- Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K (1993) Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene 129: 175–182 [DOI] [PubMed] [Google Scholar]
- Koornneef M. (1981) The complex syndrome of ttg mutants. Arabidopsis Information Server 18: 45–51 [Google Scholar]
- Kunieda T, Mitsuda N, Ohme-Takagi M, Takeda S, Aida M, Tasaka M, Kondo M, Nishimura M, Hara-Nishimura I (2008) NAC family proteins NARS1/NAC2 and NARS2/NAM in the outer integument regulate embryogenesis in Arabidopsis. Plant Cell 20: 2631–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunieda T, Shimada T, Kondo M, Nishimura M, Nishitani K, Hara-Nishimura I (2013) Spatiotemporal secretion of PEROXIDASE36 is required for seed coat mucilage extrusion in Arabidopsis. Plant Cell 25: 1355–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl N, Alkan N, Davydov O, Fluhr R (2013) Set-point control of RD21 protease activity by AtSerpin1 controls cell death in Arabidopsis. Plant J 74: 498–510 [DOI] [PubMed] [Google Scholar]
- Le BH, Cheng C, Bui AQ, Wagmaister JA, Henry KF, Pelletier J, Kwong L, Belmonte M, Kirkbride R, Horvath S, et al. (2010) Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc Natl Acad Sci USA 107: 8063–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Saravanan RS, Damasceno CM, Yamane H, Kim BD, Rose JK (2004) Digging deeper into the plant cell wall proteome. Plant Physiol Biochem 42: 979–988 [DOI] [PubMed] [Google Scholar]
- Liebminger E, Veit C, Pabst M, Batoux M, Zipfel C, Altmann F, Mach L, Strasser R (2011) Beta-N-acetylhexosaminidases HEXO1 and HEXO3 are responsible for the formation of paucimannosidic N-glycans in Arabidopsis thaliana. J Biol Chem 286: 10793–10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Kronenberger J, Marion-Poll A, North HM (2007a) In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol 48: 984–999 [DOI] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Loudet O, Kronenberger J, Mouille G, Marion-Poll A, North HM (2007b) A naturally occurring mutation in an Arabidopsis accession affects a β-D-galactosidase that increases the hydrophilic potential of rhamnogalacturonan I in seed mucilage. Plant Cell 19: 3990–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney L, Knox JP (2002) Regulation of pectic polysaccharide domains in relation to cell development and cell properties in the pea testa. J Exp Bot 53: 707–713 [DOI] [PubMed] [Google Scholar]
- Mendu V, Griffiths JS, Persson S, Stork J, Downie AB, Voiniciuc C, Haughn GW, DeBolt S (2011) Subfunctionalization of cellulose synthases in seed coat epidermal cells mediates secondary radial wall synthesis and mucilage attachment. Plant Physiol 157: 441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North HM, Berger A, Saez-Aguayo S, Ralet MC (2014) Understanding polysaccharide production and properties using seed coat mutants: future perspectives for the exploitation of natural variants. Ann Bot (Lond) 114: 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Shi JX, Schreiber L, Aharoni A (2009) The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol 151: 1773–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9: 534–540 [DOI] [PubMed] [Google Scholar]
- Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW (2001) MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 13: 2777–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Rappsilber J, Ishihama Y, Mann M (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem 75: 663–670 [DOI] [PubMed] [Google Scholar]
- Rautengarten C, Usadel B, Neumetzler L, Hartmann J, Büssis D, Altmann T (2008) A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J 54: 466–480 [DOI] [PubMed] [Google Scholar]
- Saez-Aguayo S, Ralet MC, Berger A, Botran L, Ropartz D, Marion-Poll A, North HM (2013) PECTIN METHYLESTERASE INHIBITOR6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell 25: 308–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37: 501–506 [DOI] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvendran RR, Ryden P (1990) Isolation and analysis of plant cell walls. In Dey PM, Harbourne JB, eds, Methods in Plant Biochemistry. Vol. 2. Carbohydrates. Academic Press, San Diego, pp 549–575 [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858 [DOI] [PubMed] [Google Scholar]
- Showalter AM. (1993) Structure and function of plant cell wall proteins. Plant Cell 5: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro RG. (2002) Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12: 43R–56R [DOI] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, et al. (2013) Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J 32: 1478–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Ralet MC, Berger A, Diatloff E, Bischoff V, Gonneau M, Marion-Poll A, North HM (2011) CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol 156: 1725–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Eberhard S, Pattathil S, Warder C, Glushka J, Yuan C, Hao Z, Zhu X, Avci U, Miller JS, et al. (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25: 270–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Dean GH, Griffiths JS, Kirchsteiger K, Hwang YT, Gillett A, Dow G, Western TL, Estelle M, Haughn GW (2013) Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell 25: 944–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Guenl M, Schmidt MH, Usadel B (2015a) Highly branched xylan made by IRREGULAR XYLEM14 and MUCILAGE-RELATED21 links mucilage to Arabidopsis seeds. Plant Physiol 169: 2481–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Schmidt MH, Berger A, Yang B, Ebert B, Scheller HV, North HM, Usadel B, Günl M (2015b) MUCILAGE-RELATED10 produces galactoglucomannan that maintains pectin and cellulose architecture in Arabidopsis seed mucilage. Plant Physiol 169: 403–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Yang B, Schmidt MH, Günl M, Usadel B (2015c) Starting to gel: how Arabidopsis seed coat epidermal cells produce specialized secondary cell walls. Int J Mol Sci 16: 3452–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Tanaka H, Watanabe D, Machida C, Machida Y (2004) The ACR4 receptor-like kinase is required for surface formation of epidermis-related tissues in Arabidopsis thaliana. Plant J 39: 298–308 [DOI] [PubMed] [Google Scholar]
- Western TL. (2012) The sticky tale of seed coat mucilages: production, genetics and role in seed germination and dispersal. Seed Sci Res 22: 1–25 [Google Scholar]
- Western TL, Burn J, Tan WL, Skinner DJ, Martin-McCaffrey L, Moffatt BA, Haughn GW (2001) Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol 127: 998–1011 [PMC free article] [PubMed] [Google Scholar]
- Western TL, Skinner DJ, Haughn GW (2000) Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol 122: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Knox JP (2001) In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213: 37–44 [DOI] [PubMed] [Google Scholar]
- Windsor JB, Symonds VV, Mendenhall J, Lloyd AM (2000) Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J 22: 483–493 [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Xiong W, Ye T, Wu Y (2012) Overexpression of the aspartic protease ASPG1 gene confers drought avoidance in Arabidopsis. J Exp Bot 63: 2579–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Evans RA (1973) Mucilaginous seed coats. Weed Sci 21: 52–54 [Google Scholar]
- Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL (2008) Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell 20: 1623–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Shi D, Li J, Kong Y, Yu Y, Chai G, Hu R, Wang J, Hahn MG, Zhou G (2014) CELLULOSE SYNTHASE-LIKE A2, a glucomannan synthase, is involved in maintaining adherent mucilage structure in Arabidopsis seed. Plant Physiol 164: 1842–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.