Abstract
Enteropathogenic Escherichia coli (EPEC) specifically recognizes phosphatidylethanolamine (PE) on the outer leaflet of host epithelial cells. EPEC also induces apoptosis in epithelial cells, which results in increased levels of outer leaflet PE and increased bacterial binding. Consequently, it is of interest to investigate whether EPEC infection perturbs host cell phospholipid metabolism and whether the changes play a role in the apoptotic signaling. Our findings indicate that EPEC infection results in a significant increase in the epithelial cell PE level and a corresponding decrease in the phosphatidylcholine (PC) level. PE synthesis via both the de novo pathway and the serine decarboxylation pathway was enhanced, and de novo synthesis of phosphatidylcholine via CDP-choline was reduced. The changes were transitory, and the maximum change was noted after 4 to 5 h of infection. Addition of exogenous PC or CDP-choline to epithelial cells prior to infection abrogated EPEC-induced apoptosis, suggesting that EPEC infection inhibits the CTP-phosphocholine cytidylyltransferase step in PC synthesis, which is reportedly inhibited during nonmicrobially induced apoptosis. On the other hand, incorporation of exogenous PE by the host cells enhanced EPEC-induced apoptosis and necrosis without increasing bacterial adhesion. This is the first report that pathogen-induced apoptosis is associated with significant changes in PE and PC metabolism, and the results suggest that EPEC adhesion to a host membrane phospholipid plays a role in disruption of host phospholipid metabolism.
Enteropathogenic Escherichia coli (EPEC) is a gastrointestinal pathogen which causes severe infantile diarrhea and is responsible for up to one million deaths annually. EPEC initially adheres loosely to the intestinal epithelium and then consolidates its attachment through more intimate adhesion, causing characteristic attaching and effacing lesions (21, 22, 40). These interactions result in changes in the host cell plasma membrane architecture and cytoskeletal network, as well as the induction of complex signaling pathways (32, 37).
The plasma membrane phospholipid phosphatidylethanolamine (PE) has been identified as a receptor candidate for several pathogens, including EPEC (5, 13, 38, 42). EPEC recognizes PE in a specific and dose-dependent manner in both solid-phase and liposomal assays (5). It binds PE from human epithelial cells extracts, and bacterial adhesion to human epithelial cells can be inhibited with anti-PE (5).
Epithelial cell membranes contain considerable PE, and although the majority of it appears on the inner leaflets, up to 30% is presented on the outer leaflets of healthy cells (49) and even more is presented on the outer leaflets of dying cells (4, 23). Bacterial binding of host outer leaflet PE may lead to the sequestration of outer leaflet PE, which could disrupt host cell PE metabolism and possibly the metabolism of other phospholipids.
Disruption of phospholipid metabolism in the host cell, particularly metabolism of phosphatidylcholine (PC), is known to have profound effects on cell viability and function. Studies have indicated that PC biosynthesis is an important component of cell death and cell proliferation (16, 33, 34, 54). When quiescent cells are triggered to enter the cell cycle, an increase in PC biosynthesis occurs (31, 55). Several studies have shown that apoptosis induced by a number of ether-linked phospholipid analogs with anticancer properties is accompanied by inhibition of PC synthesis (2, 9, 47). On the other hand, relatively little is known about the impact of disrupted PE metabolism on cell function, but one study showed that reducing the PE content diminished cell death during simulated ischemia and reperfusion (50). It has also been shown that a PE-binding peptide induces reorganization of the lipid matrix, eliminating the typical bilayer structure (44). These findings suggest that disruptions in PE and PC levels and/or cellular localization through either sequestration events or augmentation-depletion treatments play a key role in cell viability and function.
Since EPEC adhesion to epithelial cells may sequester outer leaflet membrane PE and result in disruption of phospholipid metabolism, we examined the impact of bacterial adhesion on host phospholipid metabolism. Our studies have identified profound changes in PE and PC metabolism in EPEC-infected cells. Moreover, we found a new linkage between PE and PC biosynthesis in the preamble to apoptosis or necrosis or both.
MATERIALS AND METHODS
Materials.
Thin-layer chromatography (TLC) plates (SilG) were purchased from Polygram (Macherey-Nagel, Duren, Germany). Phospholipids, including PE from E. coli, lyso-PE, l-α-phosphatidylcholine from egg yolk, and phosphatidylserine (PS) from bovine liver, as well as cytidine 5′-diphosphocholine (CDP-choline), phosphocholine, choline, acridine orange, and ethidium bromide, were obtained from Sigma Chemical Co. (St. Louis, Mo.). Radiolabeled phospholipid precursors, including [2-14C]ethanolamine, [methyl-14C]choline, l-[3-14C]serine, [methyl-3H]choline, and l-[3-3H]serine, were purchased from Amersham Corp. Liquid scintillation fluid (Ready-Safe) was obtained from Beckman.
Bacterial strains and growth conditions.
The characteristics of bacterial strains used in this study are listed in Table 1. Strains 31-6-1(1), HB101(pMAR7), HB101(pCVD426), and JPN15 were generously provided by J. Kaper, University of Maryland School of Medicine, Baltimore. Bacteria were stored in 40% glycerol-5% citrate at −70°C. Prior to use, bacteria were cultured on Luria agar supplemented with the appropriate antibiotics, as shown in Table 1. For infection and binding assays, either overnight blood agar cultures or overnight cultures in Luria broth diluted 1:8 in Dulbecco modified Eagle medium (DMEM) (Gibco Laboratories, Grand Island, N.Y.) and grown to the mid-log phase (2 to 4 h at 37°C in 5%CO2) were used (51, 52). Both these culture protocols have been reported to enhance expression of EPEC virulence factors, including the bundle-forming pilus (BFP).
TABLE 1.
Bacterial strains used in this studya
| Strain | Description | Reference | bfpA Genotype |
|---|---|---|---|
| E2348/69 | Wild-type EPEC | 41 | + |
| JPN15 | EAF plasmid-cured E2348/69 | 35 | − |
| HB101 | Nonpathogenic laboratory strain | 43 | − |
| 31-6-1(1) | E2348/69 with mutation in bfpA, Kanr | 20 | − |
| HB101(pMAR7) | HB101 complemented with bfpA containing pMAR7 plasmid, Ampr | 25 | + |
| HB101(pCVD426) | HB101 complemented with LEE, Chlr | 46 | − |
EAF plasmid, EPEC adherence factor plasmid; Kanr, kanamycin resistant; Ampr, ampicillin resistant; Chlr, chloramphenicol resistant; LEE, locus of enterocyte effacement.
Cell culture and infection protocols.
The human epithelial cells, HEp-2 cells (human laryngeal cell line), and Caco-2 cells (human colonic cell line) were obtained from the American Type Culture Collection, Rockville, Md. HEp-2 cells were grown in minimum essential medium (Gibco Laboratories) supplemented with decomplemented 10% fetal calf serum (Cansera International Inc.), 0.5 l-glutamine (ICN Biomedicals Inc., Costa Mesa, Calif.), 0.1% sodium bicarbonate (ICN Biomedicals Inc.), and 0.1% gentamicin at 37°C in 5% CO2. The human colonic Caco-2 cells were grown in minimum essential medium with Earl's salts (Gibco BRL) supplemented with 0.5% l-glutamine, nonessential amino acids, 10% fetal calf serum, and 0.1% gentamicin (Gibco BRL) at 37°C in 5% CO2. Cells were infected with bacteria (multiplicity of infection [MOI], 100:1) for various periods of time, after which nonadherent bacteria were removed by washing. The total infection times (including the chase time with adherent bacteria) were varied in the pulse-chase experiments in order to monitor the phospholipid metabolic changes.
Effect of EPEC infection on PE metabolism.
Since bacterial binding to outer leaflet PE may inhibit PE hydrolysis, we designed a series of pulse-chase experiments to examine changes in PE hydrolysis in host cells after infection. By using a protocol similar to that described previously (39), Caco-2 cells in culture (1 × 106 cells, 80% confluence) were pulsed with [2-14C]ethanolamine (0.2 μCi/ml) for 48 h. The pulse was removed by three washes with phosphate-buffered saline (PBS), and the cells were infected with 108 bacteria in 0.015 M HEPES growth medium without fetal calf serum or gentamicin for a specified time at 37°C in 5% CO2. The nonadherent bacteria were removed, and the cells were incubated in fresh growth medium supplemented with fetal calf serum in HEPES and 5 mM unlabeled ethanolamine for various chase periods at 37°C. The cells were harvested by gentle scraping, collected by centrifugation, and resuspended in PBS. The cell number was standardized by counting with a Neubauer chamber. Cellular lipids were extracted into chloroform-methanol (1:1, vol/vol) with overnight shaking. After addition of 1 M KCl, the lower organic phase and upper aqueous phase were collected and dried under nitrogen. The lipid extracts were suspended in chloroform-methanol (2:1, vol/vol) and separated by thin-layer chromatography. The nonphospholipids in the lipid extracts were removed by chromatographing the lipid extracts in chloroform-methanol (98:2, vol/vol). The phospholipids were then separated by TLC in the same direction by using chloroform—methanol-0.88% KCl (60:35:8), which permits consistent separation of individual phospholipids (PE, lyso-PE, PC, and lyso-PC) (53). Commercial phospholipid standards were included to permit identification of the radiolabeled phospholipids based on mobility as detected by iodine and molybdenum blue. The radioactivity of the TLC-separated phospholipids was determined both by phosphorimaging with a Phosphorimager SI system laser scanner and by liquid scintillation. Quantitative data from phosphorimaged bands were analyzed by using the ImageQuant analysis software, version 1.2. For quantification by liquid scintillation counting, bands corresponding to specific phospholipids were excised, transferred into scintillation fluid, and counted with a Beckman Beta 5500 counter (95% counting efficiency for 14C). The data were expressed in decays per minute per 106 cells. Although prokaryotes do not utilize ethanolamine for PE synthesis, the pulse was always removed before infection. Furthermore, PE extracts from EPEC pulsed with radiolabeled ethanolamine for 2 h (the infection time) showed no radioactivity.
To examine changes in PE by PS decarboxylation (Fig. 1), a pathway known to contribute significantly to mammalian cell PE (18), l-[3-14C]serine was used as the pulse. The label position within the serine precluded loss during decarboxylation and ensured that the labeled PE resulted only from PS decarboxylation. Cells were pulsed with l-[3-14C]serine (0.2 μCi/ml), infected for times varying from 30 min to 2 h, and chased with 2.5 mM serine. PE and PS were extracted and quantified as described above. In order to minimize isotopic dilution, cells were cultured prior to pulsing in serine-free medium. Since prokaryotes use serine to synthesize PE, the serine pulse was always removed before infection.
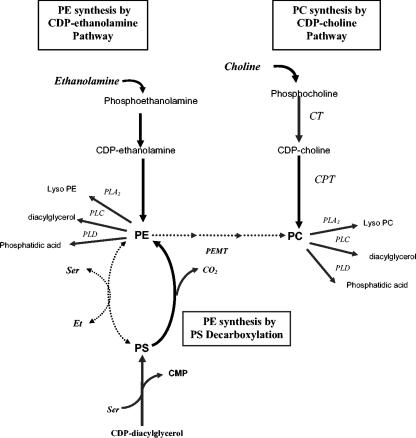
FIG. 1.
Metabolism of phosphatidylethanolamine and phosphatidylcholine in mammalian cells. De novo and salvage pathways for synthesis, as well as degradation, of PE and PC are shown. The solid lines indicate major pathways in epithelial cells, while the dotted lines indicate pathways that are less significant. Three different pulses (ethanolamine, choline, and serine) used in this study are indicated by boldface italics. Abbreviations for enzymes are in lightface italics. PLA2, phospholipase A2; PLC, phospholipase C; PLD, phospholipase D; CT, CTP-phosphocholine cytidylyltransferase; CPT, CDP-choline:diacylglycerol choline phosphotransferase; PEMT, phosphatidylethanolamine N-methyltransferase; Et, ethanolamine.
Role of BFP expression in the infection strain in host cell PE changes.
Since it has been shown previously that BFP specifically binds PE (38), we investigated the relationship between BFP expression and changes in PE metabolic levels. Cells were pulsed with [2-14C]ethanolamine and infected with E2348/69, HB101, HB101(pMAR7), 31-6-1(1), HB101(pCVD426), or JPN15 for 1 h; this was followed by removal of nonadherent bacteria and a 3-h chase with 5 mM ethanolamine as described previously. Phospholipids were extracted and separated by thin-layer chromatography, and the levels of radiolabeled PE were determined by autoradiography and phosphorimaging as described above.
Effect of EPEC infection on PC metabolism.
To examine the impact of EPEC infection on host phosphatidylcholine metabolism, we used a pulse-chase protocol similar to that described above for PE, in which cells were pulsed with [methyl-14C]choline (1 μCi/ml) for 48 h, infected, and chased with 250 μM choline. Prior to and during pulsing, the cells were grown in choline-free medium. PC, lyso-PC, and sphingomyelin levels were determined by using a protocol similar to the protocol described above and also described previously (9, 58). Since PC synthesis has been reported to be disrupted during apoptosis, we also pulsed the cells for only 2 h using [methyl-3H]choline and varied the chase times to monitor the changes in radioactivity through the pathway with the intent of focusing on changes associated with PC synthesis.
Assessment of apoptosis.
Prior to infection with either E2348/69 or the nonpathogenic strain E. coli HB101, HEp-2 cells were incubated for 1 h with exogenous metabolites (choline, phosphocholine, CDP-choline, or PC) in DMEM at 37°C in 5%CO2. In the case of PC, the cell culture medium was supplemented with an ethanolic solution of PC at the concentrations specified below, as previously described (2, 27). Control cells were treated with medium supplemented with equal volumes of ethanol alone. The cells were then washed to remove the remaining exogenous metabolite and infected for 1 h at a multiplicity of infection of 100:1. The nonadherent bacteria were removed after 1 h, and infection was continued for 4 to 6 h. The negative controls included addition of DMEM alone. Since verotoxin 1 has also been shown to induce apoptosis in certain epithelial cell lines, including HEp-2 cells, verotoxin 1 treatment (100 ng/ml for 8 h at 37°C in 5% CO2) was used as a positive control for induction of apoptosis. The concentrations of PC or PC precursors, including CDP-choline, phosphocholine, and choline, were varied over a range from 0 to 100 μM. Apoptosis and necrosis were determined by fluorescent dye staining as described previously (36). The cells were stained with acridine orange-ethidium bromide (100 μg/ml in PBS), and cell death was assessed by fluorescent microscopy. The percentages of apoptotic cells were determined by counting at least 200 cells in multiple randomly selected fields by using blind scoring.
To test the effect of pretreatment with exogenous PE prior to infection, experiments similar to those described above were conducted by pretreating epithelial cells with PE at concentrations ranging from 20 to 100 μM (each cell culture was supplemented with a sonicated ethanolic solution of PE), washing the cells, and then infecting the cells as described above. The analysis of apoptosis and necrosis was performed using acridine orange-ethidium bromide. Exogenous PE is taken up rapidly by a cell, and within minutes at room temperature or above, the majority of PE is transferred to the inner leaflet (8). To ensure that the added PE was transferred to the inner leaflet and did not augment the outer leaflet or bacterial binding, cells were incubated with PE liposomes at 37°C for 1.5 h, after which very little remained in the outer leaflet, as determined in previous studies (4).
Adhesion assay.
To assess the effects of the metabolite pretreatment protocols on bacterial adhesion, we used a plate count assay described previously, in which 105 epithelial cells (adherent cells) were incubated with 107 bacteria for 2 h at 37°C, the nonadherent bacteria were removed, and the adherent bacteria were plated out and counted (4). Pretreatments with exogenous metabolites were conducted as described above.
Statistical analysis.
Results are expressed below as means ± standard deviations. Analysis of variance was used to compare both the percentages of radiolabeled phospholipid determined by scintillation counting and the percentages of apoptotic and necrotic cells determined by fluorescent dye staining.
RESULTS
Elevated levels of phosphatidylethanolamine in EPEC-infected epithelial cells.
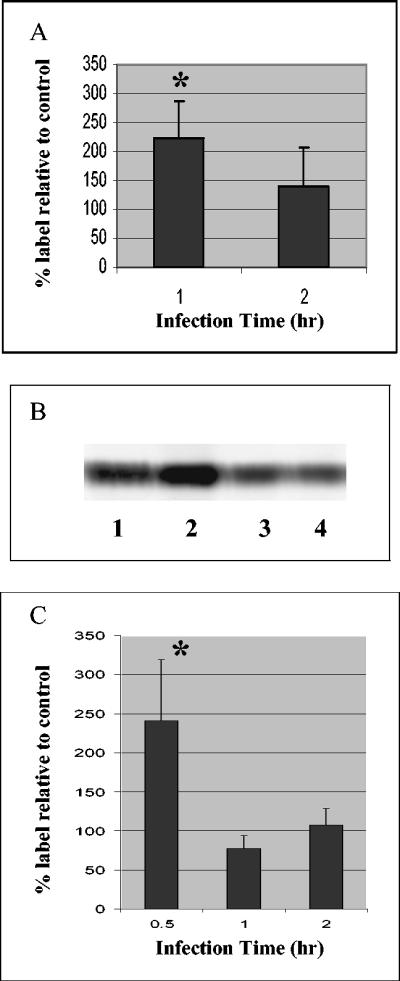
Pulse-chase studies with radiolabeled ethanolamine indicated that human epithelial cells infected with EPEC contained elevated levels of ethanolamine-derived labeled PE (Fig. 2A and B). The elevated levels of PE were transitory; the levels peaked at 1 h of infection (plus 3 h of chase) and returned to the control levels at 2 h. The levels of PE were significantly higher (range, 140 to 300%) in the EPEC-infected cells than in either uninfected cells or cells infected with the nonpathogenic HB101 strain (Fig. 2A). The results were similar for HEp-2 cells, as well as for colonic epithelial cell line Caco-2. The increased levels of ethanolamine-derived labeled PE indicated that either de novo PE synthesis was enhanced or PE degradation was inhibited or possibly both. Phorbol ester treatment has been shown to reduce PE levels by activating phospholipase D (PLD)-catalyzed hydrolysis of PE (39) (Fig. 1). Treatment with phorbol myristate acetate (PMA) at a concentration of 100 nM (a concentration known to stimulate PE hydrolysis) during the chase reduced the PE levels in both uninfected and infected cells to 49% of the PE levels in non-PMA-treated uninfected and infected cells, respectively (data not shown). However, the increase in the PE levels in PMA-treated EPEC-infected cells was still evident (185% of the level in PMA-treated uninfected cells), indicating that while PMA was able to stimulate PLD-catalyzed PE hydrolysis during EPEC infection, the PE levels were still higher in EPEC-infected cells than in uninfected cells. These results suggest that PLD hydrolysis is not inhibited during EPEC infection. There was also a significant increase in the ethanolamine-derived lyso-PE levels in EPEC-infected cells [and also in cells infected with HB101(pMAR7), the nonpathogenic strain complemented with a bfp-carrying plasmid]; the levels were 200% of the lyso-PE levels in HB101-infected cells (data not shown). There was, however, little or no change in the ethanolamine-derived PC or lyso-PC level after EPEC infection.
FIG. 2.
Phosphatidylethanolamine levels in EPEC-infected Caco-2 cells. (A and B) Cells were pulsed with [2-14C]ethanolamine, infected with E2348/69 (MOI, 100:1) for 1 to 2 h, and chased for 3 h with 5 mM ethanolamine. The nonadherent bacteria were removed after the infection time. (A) Results expressed as a percentage of the labeled PE in uninfected cells. The data are the means of three independent experiments. An asterisk indicates that the results are significantly different from the results for uninfected cells at the same time (P < 0.05). (B) Phosphorimaged PE bands (from Caco-2 cell extracts separated by thin-layer chromatography). Lane 1, treated with medium (1 h); lane 2, infected with EPEC (1 h); lane 3, treated with medium (2 h); lane 4, infected with EPEC (2 h). (C) Cells were pulsed with l-[3-14C]serine, infected with E2348/69 (MOI, 100:1) for the times indicated, and chased with 2.5 mM serine for 3 h. The nonadherent bacteria were removed after the infection time. The results are expressed as a percentage of the labeled PE in uninfected cells. The data are the means of three independent experiments. An asterisk indicates that the results are significantly different from the results for uninfected cells at the same time (P < 0.05).
Following pulsing with l-[3-14C]serine, the levels of serine-derived labeled PE were significantly elevated in EPEC-infected cells (241% ± 78% of the control levels) after only 30 min of infection (Fig. 2C). The PE levels returned to the level of control cells by 2 h of infection. Similarly, after 30 min of EPEC infection, the levels of radiolabeled phosphatidylserine were elevated in EPEC-infected cells (156% ± 33% of the control levels) and declined in a manner similar to the PE levels by 2 h of infection, suggesting that multiple pathways for PE synthesis are increased.
Elevated host PE levels are correlated with BFP expression in the infecting strain.
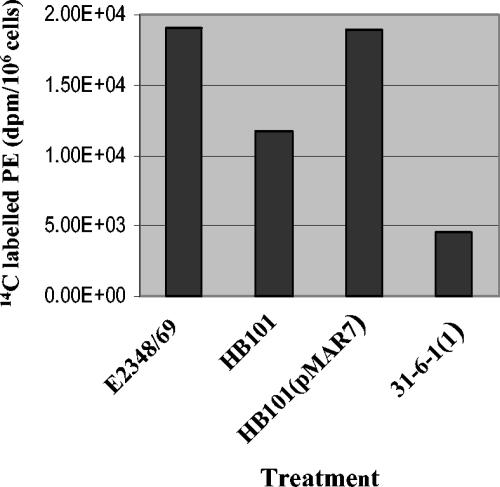
Since BFP has been shown to bind epithelial cell PE (38), it was of interest to determine whether PE changes in the infected cells correlated with BFP expression in the infecting strain. Pulse-chase studies with a variety of strains revealed that BFP expression correlated with elevated levels of ethanolamine-derived PE in infected epithelial cells (Fig. 3). When epithelial cells were infected with either E2348/69, wild-type EPEC, or HB101(pMAR7), an HB101 strain complemented with a bfp-carrying plasmid, the PE levels were significantly elevated relative to those of uninfected controls or controls infected with the nonpathogenic strain HB101. However, when 31-6-1(1), a bfp disruption mutant, was used to infect the epithelial cells, the PE levels were equivalent to those in cells infected with HB101. The PE levels in cells infected with HB101(pCVD426), a strain complemented with a plasmid containing the chromosomally encoded locus of enterocyte effacement pathogenicity island, and JPN15, a plasmid-cured strain, were 57 and 65%, respectively, of the PE levels in cells infected with the wild-type strain (the uninfected cell levels were 52% of the levels in cells infected with a wild-type strain). The lyso-PE levels were also elevated in cells infected with BFP-expressing strains relative to those in cells infected with non-BFP-expressing strains (data not shown).
FIG. 3.
Phosphatidylethanolamine levels in cells infected with various strains. Cells were pulsed with [2-14C]ethanolamine and infected with a strain (MOI, 100:1) for 1 h, and this was followed by removal of the nonadherent bacteria and a 3-h chase with 5 mM ethanolamine. The results are expressed as the decays per minute per 106 cells and are representative of at least two independent experiments.
Reduced levels of phosphatidylcholine in EPEC-infected epithelial cells.
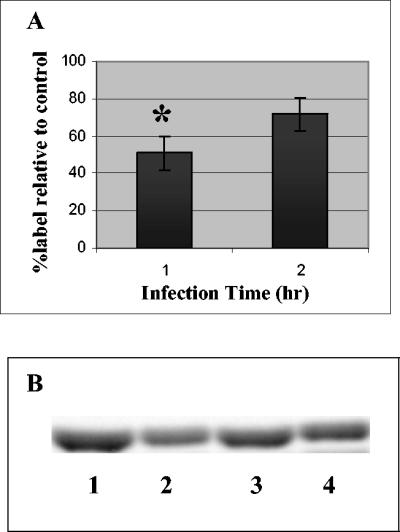
Pulse-chase studies with [methyl-14C]choline indicated that the levels of labeled PC were significantly reduced after 1 h of infection with EPEC (plus a 3-h chase) (Fig. 4). The levels of labeled PC were 50 to 70% of the levels in uninfected controls or controls infected with the nonpathogenic strain HB101. The results were consistent for both the Caco-2 and HEp-2 cell lines. The sphingomyelin and lyso-PC levels were also lower in EPEC-infected cells (total infection time, 4 h); these levels were 62% ± 0.5% and 76% ± 6% of the levels in HB101-infected controls, respectively, but were restored to control levels by 5 to 6 h of total infection time (data not shown). Experiments in which the pulse was shortened to 2 h and was followed by infection for 1 to 2 h and a chase from 2 to 4 h also resulted in equivalently decreased levels of PC in EPEC-infected cells, which supported the evidence that decreased levels of PC are due to inhibition of synthesis of PC. Very few counts were detected in PE derived from choline-pulsed cells, and there were no significant differences between uninfected and infected cells.
FIG. 4.
Phosphatidylcholine levels in EPEC-infected Caco-2 cells. Cells were pulsed with [methyl 14C]choline, infected with E2348/69 (MOI, 100:1), and chased for 3 h with 250 μM choline. The nonadherent bacteria were removed after the infection time. (A) Results expressed as a percentage of the labeled PC in cells infected with HB101. Uninfected cells were the same as cells infected with HB101. The data are the means of three independent experiments. An asterisk indicates that the results are significantly different from the results for uninfected cells at the same time (P < 0.05). (B) Phosphorimaged PC bands (from Caco-2 cell extracts separated by thin-layer chromatography). Lane 1, treated with medium (1 h); lane 2, infected with EPEC (1 h); lane 3, treated with medium (2 h); lane 4, infected with EPEC (2 h).
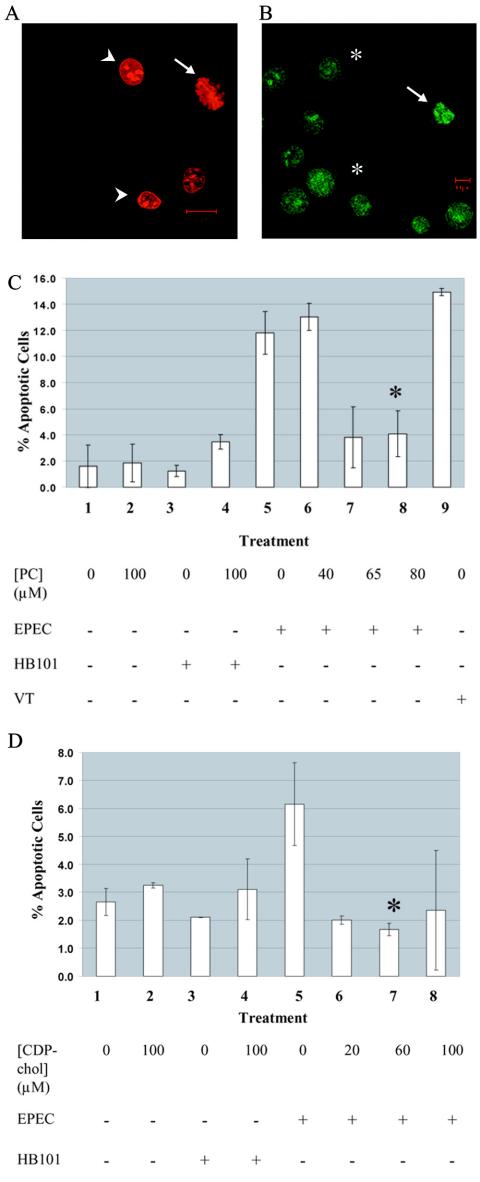
Exogenous phosphatidylcholine and CDP-choline protect against EPEC-induced apoptosis.
We and other workers have previously reported that EPEC induces apoptosis in epithelial cells (1, 15). It has also been established that PC biosynthesis is impaired during apoptosis induced by nonmicrobial agents, such as farnesol, chelerythrine, camptothecin, etoposide, or staurosporine, and that pretreatment with either PC or CDP-choline provided protection against apoptosis in those cases (2, 16, 33, 47). Therefore, since we observed a decrease in the levels of PC during EPEC infection, we wondered whether pretreatment of host cells with PC or PC biosynthetic precursors could protect against EPEC-induced apoptosis. Using an acridine-orange ethidium bromide stain to quantify both apoptosis and necrosis (Fig. 5), we found that pretreatment of epithelial cells with PC or CDP-choline provided significant protection against EPEC-induced apoptosis. Pretreatment with PC at concentrations of 65 to 80 μM reduced the level of apoptosis to that of either uninfected cells or cells infected with nonpathogenic strain HB101 (Fig. 5C). CDP-choline provided protection at concentrations of 20 to 60 μM (Fig. 5D). No protection against necrosis was provided by the metabolites. The metabolites did not affect the ability of EPEC to adhere to epithelial cells, as revealed by the results of a plate count adhesion assay (data not shown). Pretreatment with equivalent concentrations of either choline or phosphocholine did not reduce the level of apoptosis induced by EPEC infection (data not shown).
FIG. 5.
Exogenous PC and CDP-choline protect against EPEC-induced apoptosis. (A and B) Cell death as assessed by acridine orange-ethidium bromide staining. Viable cells are uniformly green (asterisks in panel B). Apoptotic cells (arrows) are either green (panel B) (early apoptosis) or red (panel A) (late apoptosis) with evidence of fragmented and condensed chromatin. Necrotic cells having lost membrane integrity allow ethidium bromide to enter and intercalate DNA,which produces uniformly red cells (arrowhead in panel A). (C and D) HEp-2 cells were pretreated with exogenous PC or CDP-choline (medium supplemented with ethanolic solution of PC or CDP-choline) and then infected with E2348/69 or HB101(MOI, 100:1) or treated with medium as described in Materials and Methods. The cells were stained with acridine orange-ethidium bromide, and the viable, apoptotic, and necrotic cells were counted. The ordinate shows the percentage of apoptotic cells in a population of over 200 cells (averages for duplicate samples). (C) Treatments: 1, untreated HEp-2 cells; 2, HEp-2 cells treated with 80 μM PC; 3, untreated HB101-infected cells; 4, HB101-infected cells pretreated with 80 μM PC; 5, untreated EPEC-infected cells; 6, EPEC-infected cells pretreated with 40 μM PC; 7, EPEC-infected cells pretreated with 65 μM PC; 8, EPEC-infected cells pretreated with 80 μM PC; 9, treatment with 100 ng of verotoxin 1 (VT) per ml for 8 h, used as a positive control for induction of apoptosis. The data are representative of the results of three experiments. An asterisk indicates that the results are significantly different from the results for untreated cells infected with EPEC (P < 0.05). (D) Treatments: 1, untreated HEp-2 cells; 2, HEp-2 cells treated with 100 μM CDP-choline; 3, untreated HB101-infected cells; 4, HB101-infected cells pretreated with 100 μM CDP-choline; 5, untreated EPEC-infected cells; 6, EPEC-infected cells pretreated with 20 μM CDP-choline; 7, EPEC-infected cells pretreated with 60 μM CDP-choline; 8, EPEC-infected cells pretreated with 100 μM CDP-choline. The data are representative of the results of two experiments. An asterisk indicates that the results are significantly different from the results for untreated cells infected with EPEC (P < 0.05).
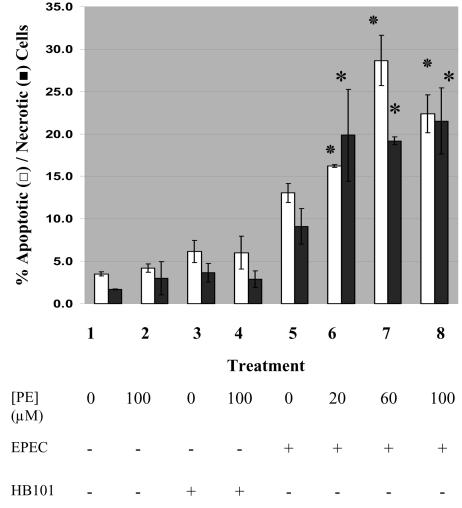
Exogenous phosphatidylethanolamine enhances EPEC-induced apoptosis and necrosis.
Since the PE levels were elevated in cells infected with EPEC, we also tested whether pretreatment with exogenous PE affected the extent of cell death induced by EPEC. Pretreatment of epithelial cells with 20 to 100 μM PE increased both apoptosis and necrosis induced by EPEC infection up to 200% compared with EPEC infection alone (Fig. 6). Pretreatment of cells with up to 100 μM PE followed by incubation with either medium or nonpathogenic strain HB101 for the same infection time did not result in significant levels of either apoptosis or necrosis. Pretreatment with PE did not affect the level of adhesion of EPEC to epithelial cells, as revealed by a plate count assay (data not shown). We also tested the effect of exogenous PE pretreatment on the level of cell death induced by camptothecin, a nonmicrobial apoptotic inducer which inhibits DNA topoisomerase I (45). Even at the highest concentration of PE (100 μM), there was no significant difference in the level of apoptosis induced by 86 μM camptothecin with HEp-2 cells (level of apoptosis with camptothecin alone, 79% ± 11% apoptosis; level of apoptosis with PE pretreatment and camptothecin, 75% ± 2%) (data not shown).
FIG. 6.
Exogenous PE augments EPEC-induced apoptosis and necrosis. HEp-2 cells were pretreated with exogenous PE and then infected with either E2348/69 or HB101 (MOI, 100:1) or treated with medium alone for 1 h at 37°C in 5% CO2. The nonadherent bacteria were removed, and incubation was continued for 5 more hours. The cells were stained with acridine orange-ethidium bromide, and the viable, apoptotic, and necrotic cells were counted. The ordinate shows the percentage of apoptotic cells (open bars) and the percentage of necrotic cells (solid bars) in a population of over 200 cells (averages for duplicate samples). Treatments: 1, untreated HEp-2 cells; 2, HEp-2 cells treated with 100 μM PE; 3, untreated HB101-infected cells; 4, HB101-infected cells pretreated with 100 μM PE; 5, untreated EPEC-infected cells; 6, EPEC-infected cells pretreated with 20 μM PE; 7, EPEC-infected cells pretreated with 60 μM PE; 8, EPEC-infected cells pretreated with 100 μM PE. An asterisk indicates that a PE treatment-EPEC infection group was significantly different from untreated EPEC infected cells (P < 0.05) with regard to apoptosis or necrosis.
DISCUSSION
The results presented here show that there is a profound, transitory change in both host cell PE and PC levels during EPEC infection. These changes occur after 4 to 5 h (1 to 2 h of infection plus 3 h of chase, during which adherent organisms are still present). The PE levels in the host cell are significantly enhanced after 4 h of infection, while the PC levels are significantly reduced during the same time period. The increased levels of ethanolamine-labeled PE may be due to either enhanced synthesis or decreased degradation. Since lyso-PE levels were also enhanced, inhibition of phospholipase A2 is unlikely, and in fact there are reports that phospholipase A2 is activated during apoptosis (19, 29). Phorbol ester PMA treatment after infection did reduce the levels of PE in both uninfected and infected cells, but the difference between the two was maintained, indicating that PLD is not inhibited during infection. The results do suggest that both de novo PE synthesis and synthesis via PS decarboxylation (Fig. 1) are enhanced. Clearly, when the cells were pulsed with radiolabeled serine, there were increases in both the labeled PS and PE levels within 30 min of infection compared to uninfected or HB101-infected controls, which indicated that there was elevated PE synthesis through PS synthase and PS decarboxylation (due to the position of the label on serine). The serine-derived labeled PS and PE levels returned to control values by 1 to 2 h of infection, indicating that the stimulated synthesis was brief. There was little or no change in the ethanolamine-derived labeled PC or lyso-PC, and this is consistent with the fact that PE N-methyltransferase, the enzyme which catalyzes conversion of PE to PC (as shown in Fig. 1), is largely liver specific (30).
The reduced levels of PC may be due to either inhibited de novo synthesis (from choline) or enhanced degradation of PC (as shown in Fig. 1). The results of the 2-h pulse-chase experiment suggest that de novo synthesis is inhibited, and this is consistent with reports that de novo synthesis of PC is inhibited during apoptosis (2, 16, 33). Furthermore, the fact that pretreatment with either CDP-choline or PC but not pretreatment with choline or phosphocholine provided protection against EPEC-induced apoptosis is consistent with inhibition of PC synthesis at the CTP-phosphocholine cytidylyltransferase (CT) step (Fig. 1). If this reaction is inhibited during apoptosis, only addition of the metabolites which follow the reaction should be able to alleviate the apoptosis. The CTP-phosphocholine cytidylyltransferase reaction is the rate-limiting step in PC synthesis and has been reported to be inhibited in nonmicrobially induced apoptosis (9, 10, 28, 56).
These phospholipid changes correlated with induction of apoptosis. They were transitory, appearing within 4 to 5 h of infection (total time), the earliest time at which evidence of apoptosis has been reported in EPEC-infected cells (1). An early marker of apoptosis is the loss of phospholipid membrane asymmetry, which results in elevation of the phosphatidylserine level in the outer leaflet of the membrane bilayer. It has been shown by flow cytometry with Annexin V-FITC that externalization of PS can first be detected in epithelial cells after 5 h of EPEC infection, and interestingly, externalized PE is also detected in the outer leaflet at the same time (4). This is consistent with the loss of phospholipid bilayer asymmetry during apoptosis as a result of coordinated inhibition of the aminophospholipid translocase and activation of a calcium-induced bidirectional phospholipid scramblase (61, 62).
Enhanced bacterial binding to apoptotic cells in a manner consistent with elevation of the outer leaflet PE level in the apoptotic cells has been reported previously (4). The elevation of cellular PE levels after 4 h of EPEC infection found in this study correlates with the induction of apoptosis by EPEC and with the appearance of increased levels of outer leaflet host cell PE. The results suggest that initial bacterial binding to PE is required to trigger elevated host PE levels since only PE-binding BFP-expressing strains are able to trigger increased levels of PE in the host cell. Nonpathogenic strains, as well as bfp-negative mutants, are unable to do this. Yet, when HB101 is complemented with a bfp-carrying plasmid, it is able to trigger elevated host levels of PE similar to those produced by wild-type infection. It is also important that exogenous PE pretreatment of the cells enhances the level of EPEC-induced cell death. These results suggest that elevation of the PE level plays a role in the apoptosis program. It was surprising that necrosis induced by EPEC was also enhanced by pretreatment with exogenous PE. Yet, given that PE is a nonbilayer phospholipid associated with membrane bending events, PE supplementation may be associated with a loss of membrane integrity. Indeed, recent research has indicated the proapoptotic protein tBID can trigger liposomal leakage in liposomes enriched in PE (24). It has also been shown that the PE binding peptide Ro 09-0198 induces reorganization of the lipid matrix and eliminates the typical bilayer structure (44). Therefore, PE sequestration and changes in PE metabolism may be involved in both apoptotic events and the loss of membrane integrity associated with late apoptosis and necrosis.
This is the first report of altered PE and PC metabolism during bacterial infection. There have been numerous reports of inhibition of PC biosynthesis during nonmicrobially induced apoptosis (2, 16, 33). Apoptosis induced by antineoplastic ether-linked phospholipids is accompanied by inhibition of PC synthesis attributed to inhibition of either CT or CDP-choline:diacylglycerol choline phosphotransferase (CPT), both of which are required for de novo synthesis of PC, as indicated in Fig. 1 (2, 9, 10, 28, 47, 56). In another study the workers found that a mutation in the CT enzyme of Chinese hamster ovary cells resulted in significant apoptosis, the effects of which could be reversed by supplementation of the cell medium with saturated PC (16). Other exogenous PC metabolites, including CDP-choline as well as diacylglycerol, have also been shown to protect against nonmicrobially induced apoptosis (6, 47). Our results are consistent with inhibition at the CT step of PC synthesis during the early stages of EPEC infection and the onset of apoptosis. Clearly, PC levels decline during EPEC infection, and complementation with either CDP-choline or PC provides protection against apoptosis.
The relationship between the decrease in PC levels and apoptosis is not well understood. It has been suggested that since PC donates the choline headgroup to ceramide to generate sphingomyelin, a decrease in the PC level could lead to an increase in the ceramide level and a decrease in the sphingomyelin level (59). Since the decrease in the PC level occurs early in the apoptotic program and ceramide accumulation is a much later event, the link between the two events has not been substantiated (26). Gueguen et al. also demonstrated that the decrease in the PC level is upstream of caspase 3 activation, but again the link between the two events is not clear (26).
Relatively little is known about the impact of disrupted PE metabolism on cell viability. PE can be synthesized via the de novo pathway from ethanolamine, as well as via phosphatidylserine, either through decarboxylation or through base exchange; the latter mechanism is considered to be an insignificant source of mammalian cell PE (Fig. 1) (57). There has been no report of changes in PE metabolism associated with apoptosis or necrosis. One study did show that lowering the PE content of cells diminished the cell death during simulated ischemia and reperfusion (49). PE may play a role in controlling the apoptotic-mitogenic balance since it has also been shown to regulate various membrane-bound enzymes, such as the calcium pump (60), protein kinase C (7), and phospholipase D (48).
On the other hand, changes in the membrane distribution of PE can contribute to cell death by destabilizing the membrane. PE is a nonbilayer phospholipid that has a tendency to form an HII inverted micelle phase (17). Membranes rich in PE tend to promote membrane bending events, including endocytosis, cell division, and membrane blebbing (which is a characteristic feature of apoptosis) (14). Therefore, events which sequester membrane PE, including bacterial binding, may contribute to host cell architectural changes during apoptosis. Since EPEC specifically binds PE, the changes in PE levels seen in EPEC-infected cells, as well as the augmentation of EPEC induced-cell death with exogenous PE, may be due to PE sequestration by the bacteria. Whether there is a metabolic link to the decrease in PC levels seen in EPEC infection is not yet clear. Certainly, the events occur simultaneously. However, we saw no significant levels of choline-derived labeled PE in the choline pulse-chase studies, indicating that conversion of PC to PE does not explain the decreased levels of PC. Furthermore, the elevated PE levels seen in this study were derived from de novo synthesis and from PS decarboxylation rather than PC. There is obviously a need for further research to understand how these changes are associated with apoptotic signaling.
These metabolic changes may also depend on the differential enzyme expression in different cell types. In Jurkat cells pulsed with radioactive serine, CD-95-induced apoptosis resulted in a rapid and transient increase in PS radioactivity, primarily due to the inhibition of PS decarboxylation to PE (3). In thymocytes incubated with radioactive serine, dexamethasone-induced apoptosis triggered an increase in PS radioactivity with no change in PS decarboxylation (11). However, in macrophages pulsed with radioactive serine, apoptosis triggered by infection with group B Streptococcus decreased PS radioactivity but did not affect PS decarboxylation to PE (12). These conflicting results suggest that there is differential enzyme expression in different cell types. In the present study we examined epithelial cells undergoing apoptosis, for which there is little or no data on phospholipid metabolism. The studies to date linking changes in PC metabolism with apoptosis have largely been conducted with fibroblast and macrophage cell lines.
In conclusion, the results demonstrate that EPEC infection is associated with significant, transient changes in PE and PC levels in infected host cells and that these events are correlated with the induction of apoptosis. These findings provide important insight into the mechanism underlying EPEC-induced apoptosis and provide evidence of novel changes in host cell phospholipid metabolism during microbial infection.
Acknowledgments
B. Lau was the recipient of an NSERC summer student award. D. Barnett Foster was supported by NSERC operating grant 238684.
We acknowledge the technical support and advice provided by Beth Binnington-Boyd and Gyongi Vass.
Editor: V. J. DiRita
REFERENCES
- 1.Abul-Milh, M., Y. Wu, B. Lau, C. A. Lingwood, and D. E. Barnett Foster. 2001. Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle-forming pili. Infect. Immun. 69:7356-7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony, M. L., M. Zhao, and K. M. Brindle. 1999. Inhibition of phosphatidylcholine biosynthesis following induction of apoptosis in HL-60 cells. J. Biol. Chem. 274:19686-19692. [DOI] [PubMed] [Google Scholar]
- 3.Aussel, C., C. Pelassy, and J. P. Breittmayer. 1998. CD-95 induces an increased phosphatidylserine synthesis that precedes its externalization during programmed cell death. FEBS Lett. 431:195-199. [DOI] [PubMed] [Google Scholar]
- 4.Barnett Foster, D., M. Abul-Milh, M. Huesca, and C. A. Lingwood. 2000. Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect. Immun. 68:3108-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett Foster, D. E., D. Philpott, M. Abul-Milh, M. Huesca, P. M. Sherman, and C. A. Lingwood. 1999. Phosphatidylethanolamine recognition promotes enteropathogenic and enterohemorrhagic Escherichia coli host cell attachment. Microb. Pathog. 27:289-301. [DOI] [PubMed] [Google Scholar]
- 6.Barrachina, M., J. Secades, R. Lozano, C. Gomez-Santos, S. Ambrosio, and I. Ferrer. 2002. Citicoline increases glutathione redox ratio and reduces caspase activation and cell death in staurosporine-treated SH-SY5Y human neuroblastoma cells. Brain Res. 957:84-90. [DOI] [PubMed] [Google Scholar]
- 7.Bazzi, M. D., M. A. Youakim, and G. L. Nelsestuen. 1992. Importance of phosphatidylethanolamine for association of protein kinase C and other cytoplasmic proteins with membranes. Biochemistry 31:1125-1134. [DOI] [PubMed] [Google Scholar]
- 8.Bevers, E. M., P. Comfurius, D. W. Dekkers, and R. F. Zwaal. 1999. Lipid translocation across the plasma membrane of mammalian cells. Biochim. Biophys. Acta 1439:317-330. [DOI] [PubMed] [Google Scholar]
- 9.Boggs, K., C. O. Rock, and S. Jackowski. 1995. Lysophosphatidylcholine and 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine inhibit the CDP-choline pathway of phosphatidylcholine synthesis at the CTP:phosphocholine cytidylyltransferase step. J. Biol. Chem. 270:7757-7764. [DOI] [PubMed] [Google Scholar]
- 10.Boggs, K., C. O. Rock, and S. Jackowski. 1995. Lysophosphatidylcholine attenuates the cytotoxic effects of the antineoplastic phospholipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine. J. Biol. Chem. 270:11612-11618. [DOI] [PubMed] [Google Scholar]
- 11.Buratta, S., G. Migliorati, C. Marchetti, R. Mambrini, C. Riccardi, and R. Mozzi. 2000. Dexamethasone increases the incorporation of [3H]serine into phosphatidylserine and the activity of serine base exchange enzyme in mouse thymocytes: a possible relation between serine base exchange enzyme and apoptosis. Mol. Cell. Biochem. 211:61-67. [DOI] [PubMed] [Google Scholar]
- 12.Buratta, S., K. Fettucciari, R. Mambrini, I. Fetriconi, P. Marconi, and R. Mozzi. 2002. Group B Streptococcus modifies macrophage phosphatidylserine metabolism during induction of apoptosis. FEBS Lett. 520:68-72. [DOI] [PubMed] [Google Scholar]
- 13.Busse, J., E. Hartmann, and C. A. Lingwood. 1996. Receptor affinity purification of a lipid-binding adhesin from Haemophilus influenzae. J. Infect. Dis. 175:77-83. [DOI] [PubMed] [Google Scholar]
- 14.Chernomordik, L., M. M. Kozlov, and Zimmerberg. 1995. Lipids in biological membrane fusion. J. Membr. Biol. 146:1-14. [DOI] [PubMed] [Google Scholar]
- 15.Crane, J. K., S. Majumdar, and D. F. Pickhardt III. 1999. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect. Immun. 67:2575-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui, Z., M. Houweling, M. H. Chen, M. Record, H. Chap, D. E. Vance, and F. Terce. 1996. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J. Biol. Chem. 271:14668-14671. [DOI] [PubMed] [Google Scholar]
- 17.Cullis, P. R., B. De Kruijff, M. J. Hope, A. J. Verkleij, R. Nagar, S. B. Farren, C. Tilcock, T. D. Madden, and M. B. Bally. 1983. Structural properties of lipids and their functional role in biological membranes., p. 39-81. In R. C. Aloia (ed.), Membrane fluidity in biology. Concepts of membrane structure, vol. I. Academic Press, New York, N.Y.
- 18.Dennis, E. A., and E. P. Kennedy. 1972. Intracellular sites of lipid synthesis and the biogenesis of mitochondria. J. Lipid Res. 13:263-267. [PubMed] [Google Scholar]
- 19.De Valck, D., D. Vercammen, W. Fiers, and R. Beyaert. 1998. Differential activation of phospholipases during necrosis or apoptosis: a comparative study using tumour necrosis factor and anti-Fas antibodies. J. Cell. Biochem. 71:392-399. [PubMed] [Google Scholar]
- 20.Donnenberg, M. S., J. A. Giron, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6:3427-3437. [DOI] [PubMed] [Google Scholar]
- 21.Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5:109-114. [DOI] [PubMed] [Google Scholar]
- 22.Ebel, F., T. Podzadel, M. Rohde, A. U. Kresse, S. Kramer, C. Diebel, C. A. Guzman, and T. Chakraborty. 1998. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol. Microbiol. 30:147-161. [DOI] [PubMed] [Google Scholar]
- 23.Emoto, K., N. Toyama-Sorimachi, H. Karasuyama, K. Inoue, and M. Umeda. 1997. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp. Cell Res. 232:430-434. [DOI] [PubMed] [Google Scholar]
- 24.Epand, R. F., J. Martinou, M. Fournallaz-Mulhauser, D. W. Hughes, and R. W. Epand. 2002. The apoptotic protein tBid promotes leakage by altering membrane curvature. J. Biol. Chem. 277:32632-32639. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gueguen, G., V. Granci, P. Rogalle, F. Briand-Mesage, M. Wilson, A. Klaebe, F. Terce, H. Chap, J. Salles, M. Simon, and F. Gaits. 2002. A lysophosphatidic acid analogue is revealed as a potent inhibitor of phosphatidylcholine synthesis, inducing apoptosis. Biochem. J. 368:447-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haug, J. S., C. M. Goldner, E. M. Yazlovitskaya, P. A. Voyizan, and G. Melnykovych. 1994. Directed cell killing (apoptosis) in human lymphoblastoid cells incubated in the presence of farnesol: effect of phosphatidylcholine. Biochim. Biophys. Acta 1223:133-140. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman, D. R., V. L. Thomas, and F. Snyder. 1992. Inhibition of cellular transport systems by alkyl phospholipid analogs in HL-60 human leukemia cells. Biochim. Biophys. Acta 1127:74-80. [DOI] [PubMed] [Google Scholar]
- 29.Hornfelt, M., A. Edstrom, and P. A. R. Ekstrom. 1999. Upregulation of cytosolic phospholipase A2 correlates with apoptosis in mouse superior cervical and dorsal root ganglia neurons. Neurosci. Lett. 265:87-90. [DOI] [PubMed] [Google Scholar]
- 30.Houweling, M., Z. Cui, and D. E. Vance. 1996. Expression of phosphatidylethanolamine N-methyltransferase-2 cannot compensate for an impaired CDP-choline pathway in mutant Chinese hamster ovary cells. J. Biol. Chem. 279:16277-16282. [DOI] [PubMed] [Google Scholar]
- 31.Houweling, M., L. B. M. Tjiburg, H. Jamil, D. E. Vance, C. B. Nyathi, W. J. Vaartjes, and L. M. Van Golde. 1991. Phosphatidylcholine metabolism in rat liver after partial hepatectomy. Evidence for increased activity and amount of CTP:phosphocholine cytidylyltransferase. Biochem. J. 278:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ismaili, A., D. Philpott, M. T. Dytoc, and P. M. Shermann. 1995. Signal transduction responses following adhesion of verotoxin-producing Escherichia coli. Infect. Immun. 63:3316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackowski, S. 1996. Cell cycle regulation of membrane phospholipid metabolism. J. Biol. Chem. 271:20219-20222. [DOI] [PubMed] [Google Scholar]
- 34.Jackowski, S. 1994. Coordination of membrane phospholipid synthesis with the cell cycle. J. Biol. Chem. 269:3858-3867. [PubMed] [Google Scholar]
- 35.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones, N. L., A. Islur, R. Haq, M. Mascarenhas, M. Karmali, M. H. Perdue, B. W. Zanke, and P. M. Sherman. 2000. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G811-G819. [DOI] [PubMed] [Google Scholar]
- 37.Kenny, B., and B. B. Finlay. 1997. Intimin-dependent binding of enteropathogenic Escherichia coli host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ 1. Infect. Immun. 65:2528-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khursigara, C., M. Abul-Milh, B. Lau, J. Giron, C. A. Lingwood, and D. E. Barnett Foster. 2001. Enteropathogenic Escherichia coli virulence factor bundle forming pilus binds to phosphatidylethanolamine. Infect. Immun. 69:6573-6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiss, Z., and W. B. Anderson. 1989. Phorbol ester stimulates the hydrolysis of phosphatidylethanolamine in leukemic HL-60, NIH 3T3, and baby hamster kidney cells. J. Biol. Chem. 264:1483-1487. [PubMed] [Google Scholar]
- 40.Knutton, S. 1995. Cellular responses to enteropathogenic Escherichia coli infection. Biosci. Rep. 15:469-479. [DOI] [PubMed] [Google Scholar]
- 41.Levine, M. M., J. Berquist, D. R. Nalen, D. H. Waterman, R. B. Hornich, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119-1122. [DOI] [PubMed] [Google Scholar]
- 42.Lingwood, C. A., M. Huesca, and A. Kuksis. 1992. The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect. Immun. 60:2470-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louie, M., J. DeAzavedo, R. Clarke, and J. Brunton. 1994. Serotype distribution and sequence heterogeneity of eae gene in verotoxin-producing Escherichia coli. Epidemiol. Infect. 112:449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machaidze, G., A. Ziegler, and J. Seelig. 2002. Specific binding of Ro 09-0198 (cinnamycin) to phosphatidylethanolamine: a thermodynamic analysis. Biochemistry 41:1965-1971. [DOI] [PubMed] [Google Scholar]
- 45.Mattern, M. R., S. M. Mong, H. F. Bartus, C. K. Mirabelli, S. T. Crooke, and R. K. Johnson. 1987. Relationship between the intracellular effects of camptothecin and the inhibition of DNA topoisomerase I in cultured L1210 cells. Cancer Res. 47:1793-1798. [PubMed] [Google Scholar]
- 46.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 47.Miquel, K., A. Pradines, F. Terce, S. Selmi, and G. Favre. 1998. Competitive inhibition of choline phosphotransferase by geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis and induces apoptosis in human lung adenocarcinoma. J. Biol. Chem. 273:26179-26186. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura, S., Y. Kiyohara, H. Jinnai, T. Hitoma, C. Ogino, K. Yoshida, and Y. Nishizuka. 1996. Mammalian phospholipase D: phosphatidylethanolamine as an essential component. Proc. Natl. Acad. Sci. USA 93:4300-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Op den Kamp, J. A. F. 1979. Lipid asymmetry in membranes. Annu. Rev. Biochem. 48:47-71. [DOI] [PubMed] [Google Scholar]
- 50.Post, J. A., J. J. M. Bijvelt, and A. J. Verkleij. 1995. The role of phosphatidylethanolamine in sarcolemmal damage of cultured heart myocytes during simulated ischemia and metabolic inhibition. Am. J. Physiol. 268:H773-H780. [DOI] [PubMed] [Google Scholar]
- 51.Puente, J., D. Bieber, S. Ramer, W. Murray, and G. Schoolnik. 1996. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol. Microbiol. 20:87-100. [DOI] [PubMed] [Google Scholar]
- 52.Rosenshine, I., S. Ruschowski, and B. B. Finlay. 1996. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect. Immun. 64:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross, B. M., K. Eder, A. Moszczynska, N. Mamalias, J. Lamarche, M. Ang, M. Pandolfo, G. Rouleau, M. Kirchgessner, and S. Kish. 2000. Abnormal activity of membrane phospholipid synthetic enzymes in the brain of patients with Friedreich's ataxia and spinocerebellar atrophy type-I. Movement Disorders 15:294-300. [DOI] [PubMed] [Google Scholar]
- 54.Terce, F., H. Brun, and D. E. Vance. 1994. Requirement of phosphatidylcholine for normal progression through the cell cycle in C3H/10T1/2 fibroblasts. J. Lipid Res. 35:2130-2142. [PubMed] [Google Scholar]
- 55.Tessner, T. G., C. O. Rock, G. B. Kalmar, R. B. Cornell, and S. Jackowski. 1991. Colony-stimulating factor 1 regulates CTP:phosphocholine cytidylyltransferase mRNA levels. J. Biol. Chem. 266:16261-16264. [PubMed] [Google Scholar]
- 56.Tronchere, H., F. Terce, M. Record, G. Ribbes, and H. Chap. 1991. Modulation of CTP:phosphocholine cytidylyltransferase translocation by oleic acid and the antitumoral alkylphospholipid in HL-60 cells. Biochem. Biophys. Res. Commun. 176:157-165. [DOI] [PubMed] [Google Scholar]
- 57.Vance, J. E., E. J. Aasman, and R. Szarko. 1991. Brefeldin A does not inhibit the movement of phosphatidylethanolamine from its sites of synthesis to the cell surface. J. Biol. Chem. 266:8241-8247. [PubMed] [Google Scholar]
- 58.Walkley, C. J., G. B. Kalmar, and R. B. Cornell. 1994. Overexpression of rat liver CTP:phosphocholine cytidylyltransferase accelerates phosphatidylcholine synthesis and degradation. J. Biol. Chem. 269:5742-5749. [PubMed] [Google Scholar]
- 59.Wieder, T., C. E. Orfanos, and C. Geilen. 1998. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hecadecylphosphocholine. J. Biol. Chem. 273:11025-11031. [DOI] [PubMed] [Google Scholar]
- 60.Yeagle, P. L., and A. Sen. 1986. Hydration and the lamellar to hexagonal II phase transition of phosphatidylethanolamine. Biochemistry 25:7518-7522. [DOI] [PubMed] [Google Scholar]
- 61.Zachowski, A. 1993. Phospholipids in animal eukarytotic membranes: transverse asymmetry and movement. Biochem. J. 294:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zwaal, R. F. A., and A. J. Schroit. 1997. Pathophysiological implications of membrane phospholipid asymmetry in blood cells. Blood 89:1121-1132. [PubMed] [Google Scholar]