Abstract
Reactive nitrogen species (RNS) and reactive oxygen species (ROS) are important mediators of the bactericidal host response. We investigated the contribution of these two mediators to the control of infection with the facultative intracellular bacterium Francisella tularensis. When intradermally infected with the live vaccine strain F. tularensis LVS, mice deficient in production of RNS (iNOS−/− mice) or in production of ROS by the phagocyte oxidase (p47phox−/− mice) showed compromised resistance to infection. The 50% lethal dose (LD50) for iNOS−/− mice was <20 CFU, and the LD50 for p47phox−/− mice was 4,400 CFU, compared to an LD50 of >500,000 CFU for wild-type mice. The iNOS−/− mice survived for 26.4 ± 1.8 days, and the p47phox−/− mice survived for 10.1 ± 1.3 days. During the course of infection, the serum levels of gamma interferon (IFN-γ) and interleukin-6 were higher in iNOS−/− and p47phox−/− mice than in wild-type mice. Histological examination of livers of iNOS−/− mice revealed severe liver pathology. Splenocytes obtained 5 weeks after primary infection from antibiotic-treated iNOS−/− mice showed an in vitro recall response that was similar in magnitude and greater secretion of IFN-γ compared to cells obtained from wild-type mice. In summary, mice lacking expression of RNS or ROS showed extreme susceptibility to infection with F. tularensis LVS. The roles of RNS and ROS seemed to be distinct since mice deficient in production of ROS showed dissemination of infection and died during the early phase of infection, whereas RNS deficiency led to severe liver pathology and a contracted course of infection.
Francisella tularensis is a highly virulent bacterium that causes tularemia in many mammalian species. In humans, direct contact with infected animals or transmission by infected arthropods, such as ticks or mosquitoes, leads to the ulceroglandular form of the disease, whereas inhalation of F. tularensis results in the more rare respiratory form. The disease is characterized by flu-like symptoms and prominent enlargement of draining lymph nodes. Life-threatening manifestations, such as sepsis and rhabdomyolysis, may occur (29).
F. tularensis is a facultative intracellular bacterium, and the host protective mechanisms are similar to those that are active against listeriae and mycobacteria (24, 28). During the first few days of infection, T-cell-independent transient host resistance is evoked. During this phase, both gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) are crucial mediator molecules, primarily due to their ability to activate the antimicrobial mechanisms of mononuclear phagocytes (14, 18, 27). After the initial phase, T-cell-dependent long-term protective immunity to F. tularensis develops. The critical role of this cell-mediated immunity is demonstrated by the finding that SCID mice or mice lacking αβ T cells succumb to even the smallest inocula of F. tularensis (11, 13, 32).
Reactive nitrogen species (RNS) and reactive oxygen species (ROS) are intermediates that are involved in the host defense against various intracellular pathogens (4, 7, 8, 21). RNS can be generated by constitutive nitric oxide synthases, but high levels of RNS are produced only after the activation of inducible nitric oxide synthases (iNOS) (10). Production of ROS messengers (for example, superoxide) depends on the induction of phagocyte oxidase (phox) (2). Activation of iNOS and phox is induced in macrophages when they are exposed to proinflammatory cytokines, including IFN-γ or TNF-α (4, 22).
A role for RNS in the host resistance to tularemia has been suggested by in vitro studies of F. tularensis (1, 15) After activation with IFN-γ, macrophages are capable of arresting bacterial replication, an effect prevented by an inhibitor of iNOS. The role of macrophage-derived NO in the host defense against tularemia is, however, not completely understood, and its importance seems to vary with the organ localization of macrophages (23). No studies have directly assessed the role of RNS or ROS in vivo. The availability of mice deficient in expression of iNOS or phox provides models to directly assess the roles of these reactive molecular species in killing of F. tularensis.
We demonstrated in this study that mice lacking expression of RNS or ROS show extreme susceptibility to infection with the live vaccine strain F. tularensis LVS. However, RNS and ROS seem to be predominantly involved at different stages of the infection. Mice deficient in production of ROS died within 2 weeks, whereas RNS deficiency rapidly resulted in severe liver pathology but a contracted course of infection, and death occurred within 4 weeks.
MATERIALS AND METHODS
Bacterial strain.
The live vaccine strain F. tularensis LVS (= ATCC 29684) was supplied by the U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Md. It was grown to the logarithmic phase in modified Mueller-Hinton broth, harvested, and stored frozen at −70°C.
Mice.
Breeding stocks of iNOS gene-deficient mice (iNOS−/− mice) and wild-type C57BL/6 mice (designated iNOS+/+ mice) were obtained from Jackson Laboratories, Bar Harbor, Maine. p47phox−/− mice and wild-type mice (designated p47phox+/+ mice) were obtained through the courtesy of Steven Holland, National Institutes of Health,, Bethesda, Md. p47phox−/− mice were derived as described elsewhere (16) from heterozygous parents (C57BL/6 × 129 backcrossed to F5 in a C57BL/6 lineage). The wild-type mice also represented the F5 generation. Breeding stocks of leaky iNOS−/− mice were obtained through the courtesy of F. Y. Liew, Department of Immunology, University of Glasgow, Glasgow, United Kingdom (31). Strain 129 mice (designated iNOS+/+ 129 mice) were obtained from Harlan, Austerlitz, The Netherlands. All strains of mice were bred and housed at the Animal Facility of the Swedish Defense Research Agency, Umeå, Sweden, under conventional conditions and were given food and water ad libitum. The mice were 8 to 14 weeks old and age and sex matched when they were used in experiments. They were found to be free of specific pathogens. Permission for the experiments was obtained from the Ethical Committee, Umeå University, Umeå, Sweden.
Inoculation and enumeration of bacteria in mice.
For primary infection, mice were inoculated intradermally in the thoracic region with F. tularensis LVS cells suspended in 50 μl of saline. One day before the intradermal inoculation, the region was shaved, and at various times after injection, animals were killed, and a 1-cm2 sample of the region was excised, briefly washed in 70% ethanol, and added to a tube with saline. Spleens, livers, and lungs were also collected. Samples from each organ were homogenized, and 10-fold serial dilutions were cultured on agar plates. Peritoneal lavage was performed by injection and aspiration of 1.0 ml of saline with a Pasteur pipette. Blood (100 μl) was collected from the retroorbital vein. Serial dilutions of lavage and blood samples were plated for enumeration of bacteria. Bacterial numbers were expressed as the number of CFU per organ (lung, liver, or spleen), per 1.0 ml of peritoneal lavage fluid, or per 100 μl of blood.
The 50% lethal dose (LD50) was determined by the method of Reed and Muench as previously described (25).
Blood chemistry for organ function.
On days 3, 6, and 9 of infection, blood was obtained from three mice per strain. Sera from mice (≥300 μl) were stored frozen until analysis. Alanine aminotransferase, aspartate aminotransferase, total bilirubin, amylase, and creatinine in serum were analyzed by using a Vitros 950 multianalyzer (Johnson-Johnson, Clinical Diagnostics).
Histological examination.
On days 3, 6 and 9, livers, spleens and lungs were collected from mice of each strain. The organs were prepared for histological staining by snap freezing them in liquid propane and were placed in OCT compound (EMS, Hatfield, Pa.). Samples were stored at −70°C until they were sectioned, and they were stained with Mayer's hematoxylin and eosin. Microscopy and photography were performed by using a Leica DMLB 100T microscope. The sections were assessed in a blind fashion.
Preparation of splenocytes.
Spleens from mice were homogenized in 10 ml of saline. Debris was removed by centrifugation at 200 × g for 30 s. The cells in the resulting supernatant were resuspended in 10 ml of saline, pelleted by centrifugation at 200 × g for 10 min, resuspended in 1 ml of 0.8% NH4Cl, and incubated for 10 min at room temperature to lyse erythrocytes. The cells were resuspended in 10 ml of ice-cold saline and centrifuged. This procedure was repeated once. The splenocytes were resuspended in 5 ml of cell culture medium (Dulbecco modified Eagle medium; Invitrogen, Paisley, United Kingdom) supplemented with 10% fetal bovine serum (Invitrogen), and enumeration was performed by using a Bürker chamber. Cells were stained by using May Grünwall Giemsa stain for differential counting of the splenocytes.
Assay of ROS production by splenocytes.
At different times, mice infected with 5 × 104 CFU of F. tularensis LVS were killed, and spleen cells were prepared as described above. ROS production by fresh splenocytes was measured by a luminol-based chemiluminescence method (12). In preliminary experiments, the method showed a good correlation with a colorimetric assay based on ROS-mediated reduction of cytochrome c. The kinetics of the chemiluminescence response were similar for all samples tested and were maximal within the 5-min length of the assay. Splenocytes (1 × 106 cells) were incubated at 37°C for 5 min in 1 ml of KRG buffer (Krebs-Ringer phosphate buffer containing 10 mM glucose, 1 mM Ca2+, and 1.5 mM Mg2+; pH 7.3) supplemented with 100 μM luminol (Sigma, Madison, Wis.). Immediately before measurement of the chemiluminescence response, 0.5 μl of phorbol myristate acetate (2 μg/ml) was added to the tube. In preliminary experiments, this concentration was found to be optimal. The chemiluminescence was measured with a luminometer (Luminometer 1250; LKB Wallac, Turku, Finland) for 5 min. The results were expressed in peak relative light units.
Assay of RNS production by splenocytes.
At different times, infected mice were killed, and spleen cells were prepared as described above. Cells were seeded in a 96-well cell culture plate at a density of 3 × 105 cells/well in 200 μl and incubated at 37°C in 5% CO2. Preliminary experiments indicated that measurable levels of the end metabolite NO2− were found only after incubation for at least 4 days. The concentration of NO2− was measured by the Griess reaction. Fifty microliters of the culture supernatant was mixed with 50 μl each of the Griess reagents p-aminobenzenesulfonamide (58 mM in 5% H3PO4) and 2,6,8-trihydroxypurine (3.9 mM) (Sigma). After 10 min of incubation at room temperature, the absorbance at 540 nm was recorded. The concentration of NO2− was determined by preparing a standard curve for sodium nitrite. The lower limit of detection of the assay was 2.5 μM.
Assay of serum cytokine levels.
All procedures for the cytokine enzyme-linked immunosorbent assay (OptEIA; BD Biosciences, San Diego, Calif.) were performed by following the instructions of the manufacturer. Dilutions of serum samples from LVS-infected mice were analyzed for the presence of IFN-γ, TNF-α, interleukin-6 (IL-6), IL-4, and IL-12. The level of detection for IL-12 and IFN-γ was 30 pg/ml, and the level of detection for IL-4, IL-6, and TNF-α was 15 pg/ml.
Assay of the T-cell response.
Mice were inoculated with 2 × 102 CFU of F. tularensis LVS, and 15 days later moxifloxacin was administered intraperitoneally at a dose of 0.1 mg/g of body weight once daily for 7 days. Mice were killed 5 weeks after inoculation, and spleen cell cultures were established. Each culture (200 μl) contained 6 × 105 splenocytes in RPMI (GIBCO BRL) supplemented with 15% inactivated fetal calf serum, 10 μg of gentamicin per ml, 5 × 10−5 M β-mercaptoethanol, and 2 mM l-glutamine (GIBCO BRL). Heat-killed F. tularensis LVS (106 cells/ml) or concanavalin A (10 μg/ml; Pharmacia, Uppsala, Sweden) was used as the stimulating agent. To estimate the proliferative response, splenocyte cultures were incubated at 37°C for 4 days, pulsed for 18 h with 0.5 μCi of [3H]thymidine (18 Ci/mmol), and harvested with an automated cell harvester (Inotech, Basel, Switzerland). For determination of secreted cytokines, parallel cultures were established, and after 2 days of incubation with antigen, 100 μl of supernatant was collected. The proliferative responses and cytokine levels have been found to be optimal at these times (26).
Statistical analysis.
Student's t test and Pearson's correlation coefficient test were used.
RESULTS
Numbers of bacteria in iNOS−/−, iNOS+/+, p47phox−/−, and p47phox+/+ mice after primary intradermal infection with F. tularensis.
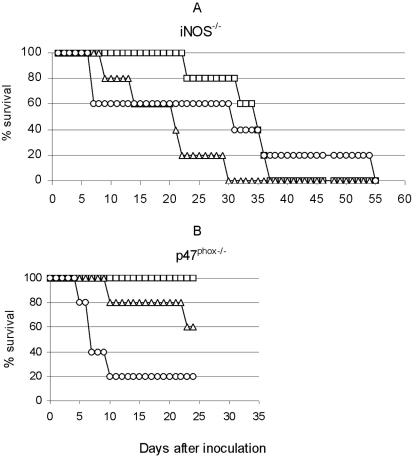
The LD50 for intradermal infection was found to be 4.4 × 103 CFU of F. tularensis LVS for p47phox−/− mice and less than 20 CFU for iNOS−/− mice (Fig. 1). The mean time to death was determined for all of the mice in the experiment, irrespective of the inoculum. In spite of a lower LD50, the mean time to death was significantly longer for the iNOS−/− mice (26.4 ± 1.8 days, compared with 10.1 ± 1.3 days for the p47phox−/− mice). For the iNOS+/+ mice the LD50 was 5.0 × 105 to 1.2 × 106 CFU and the mean time to death was 7.2 ± 1.2 days, and for the p47phox+/+ mice the LD50 was ∼8 × 105 CFU and the mean time to death was 6.9 ± 1.4 days.
FIG. 1.
Percentage of surviving mice after intradermal injection of F. tularensis LVS. Mice (10 mice per group) were inoculated intradermally with 2 × 101 CFU (□), 2 × 103 CFU (▵), or 2 × 104 CFU (○). p47phox−/− mice that survived for 28 days were sacrificed, and no bacteria were found in the livers or spleens. (A) iNOS −/− mice. (B) p47 phox−/− mice.
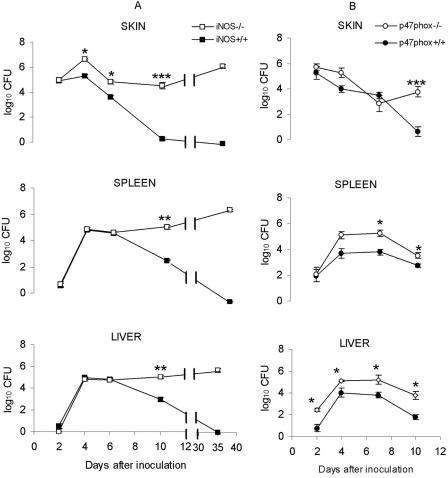
Bacterial counts in the skin, livers, and spleens of p47phox−/− and iNOS−/− mice and the corresponding wild-type mice were determined at various intervals after intradermal inoculation of 5 × 102 CFU of F. tularensis LVS, a dose at which all animals survived for ≥10 days. The data are summarized in Fig. 2. Compared to the wild-type mice, the iNOS−/− mice showed no significant differences in the numbers of bacteria in the liver and spleen during the first 6 days of infection (Fig. 2A). After this, the numbers of bacteria decreased in the iNOS+/+ mice but remained stationary in the iNOS−/− mice. In the skin, starting at day 4, the iNOS−/− mice showed higher numbers of bacteria than the wild-type mice. By day 35, the wild-type mice had cleared the infection, while the iNOS−/− mice still had more than 5 log10 CFU of bacteria in the liver, spleen, and skin (Fig. 2A).
FIG. 2.
Growth curves for F. tularensis LVS in the skin, spleens, and livers of iNOS−/− and iNOS+/+ mice (A) and p47phox−/− and p47phox+/+ mice (B). Mice were inoculated intradermally with 5 × 102 CFU of F. tularensis LVS, and the numbers of bacteria (CFU per organ sample) were determined at different times. The means ± standard errors of the means for five mice per group and time are shown. One asterisk indicates that the P value is <0.05, two asterisks indicate that the P value is <0.01, and three asterisks indicate that the P value is <0.001.
Compared to the wild-type mice, the p47phox−/− mice had higher numbers of bacteria in the liver and spleen at all times examined (Fig. 2B). In contrast, the numbers of bacteria in the skin of the p47phox−/− mice did not differ from the numbers of bacteria in the skin of the p47phox+/+ mice during the first 7 days of infection, but the numbers of bacteria were significantly higher on day 10 after inoculation.
We extended these experiments by monitoring the course of infection after inoculation of 5 × 104 CFU of F. tularensis LVS, a dose 10 times higher than the LD50 for p47phox−/− mice. Compared to liver samples of p47phox+/+ mice, liver samples of p47phox−/− mice contained significantly higher numbers of bacteria during the whole 9-day period of observation (Table 1). In lung, blood, and peritoneal lavage fluid samples of p47phox−/− mice, bacteria were present at detectable levels during the whole period, whereas in wild-type mice, bacteria were found only in samples from the lungs and peritoneum and only on day 3 (Table 1).
TABLE 1.
Viable counts in organs and tissues after intradermal inoculation of p47phox−/− or p47phox+/+ mice with F. tularensis LVSa
| Organ or tissue | Viable counts
|
|||||
|---|---|---|---|---|---|---|
| 3 Days after inoculation
|
5 Days after inoculation
|
9 Days after inoculation
|
||||
| p47phox+/+ mice | p47phox−/− mice | p47phox+/+ mice | p47phox−/− mice | p47phox+/+ mice | p47phox−/− mice | |
| Liver | 3.56 ± 0.61 | 4.79 ± 0.22 Ab | 4.76 ± 0.21 | 7.35 ± 0.29 C | 2.89 ± 0.20 | 4.60 ± 0.41 A |
| Lung | 3.16 ± 0.58 | 2.74 ± 0.52 | BDL | 5.38 ± 0.76 B | BDL | 3.10 ± 0.73 A |
| Bloodc | BDLd | 2.74 ± 0.52 A | BDL | 1.77 ± 0.08 A | NDc | ND |
| Peritoneumf | 2.08 ± 1.1 | 3.22 ± 0.48 | BDL | 3.77 ± 0.45 B | BDL | 1.78 ± 0.64 A |
The inoculum was 5 × 104 CFU of F. tularensis LVS per mouse, and the results are expressed as the mean log10 CFU ± standard error of the mean for three samples.
Significance is indicated as follows: A, P < 0.05 compared to p47phox+/+ mice; B, P < 0.01 compared to p47phox+/+ mice; C, P < 0.001 compared to p47phox+/+ mice.
Log10 CFU per 100 μl of blood.
BDL, below the detection limit.
ND, not determined.
Log10 CFU per milliliter of peritoneal lavage fluid.
When in similar experiments iNOS−/− mice were intradermally inoculated with 5 × 104 CFU, numbers of bacteria that were significantly higher than the numbers in wild-type mice were found in samples from livers on days 6 and 9 and in skin samples on day 3 and beyond (Table 2). In samples from the peritoneum, bacteria were detectable in iNOS−/− mice during the whole period, and in wild-type mice bacteria were detectable only on day 3. In lung samples, bacteria were isolated from iNOS−/− mice on days 6 and 9 and not at all from iNOS+/+ mice (Table 2). In blood samples, no bacteria were detectable, either in samples from iNOS−/− mice or in samples from iNOS+/+ mice.
TABLE 2.
Viable counts in organs and tissues after intradermal inoculation of iNOS−/− or iNOS+/+ mice with F. tularensis LVSa
| Organ or tissue | Viable counts
|
|||||
|---|---|---|---|---|---|---|
| 3 Days after inoculation
|
6 Days after inoculation
|
9 Days after inoculation
|
||||
| iNOS+/+ mice | iNOS−/− mice | iNOS+/+ mice | iNOS−/− mice | iNOS+/+ mice | iNOS−/− mice | |
| Skin | 6.91 ± 0.17 | 7.82 ± 0.26 Ab | 5.04 ± 0.01 | 7.27 ± 0.11 C | 1.31 ± 1.31 | 6.72 ± 0.24 C |
| Liver | 3.99 ± 0.33 | 3.91 ± 0.19 | 4.61 ± 0.21 | 5.36 ± 0.22 A | 2.72 ± 0.01 | 5.80 ± 1.13 A |
| Lung | BDLc | BDL | BDL | 2.34 ± 0.30 A | BDL | 2.00 ± 0.34 |
| Peritoneumd | 2.07 ± 0.47 | 2.93 ± 0.62 | BDL | 2.80 ± 0.48 B | BDL | 2.5 ± 0.18 C |
| Blood | BDL | BDL | BDL | BDL | BDL | BDL |
The inoculum was 5 × 104 CFU of F. tularensis LVS per mouse, and the results are expressed as the mean log10 CFU ± standard error of the mean for three samples.
Significance is indicated as follows: A, P < 0.05 compared to iNOS+/+ mice; B, P < 0.01 compared to iNOS+/+ mice; C, P < 0.001 compared to iNOS+/+ mice.
BDL, below the detection limit.
Log10 CFU per milliliter of peritoneal lavage fluid.
In summary, the control of infection was found to be compromised in p47phox−/− mice throughout the course of infection, and when a large inoculum was used, dissemination of infection occurred, as shown by bacteremia and peritonitis. The iNOS−/− mice had increased numbers of bacteria in the skin even during the early phase and exhibited a loss of control also in the liver and spleen after the first week of infection. The iNOS−/− mice were unable to eradicate the infection, and after a protracted course of infection, they eventually succumbed to even the lowest challenge dose.
Histological examination of livers and assay of s-aminotransferase levels of infected mice.
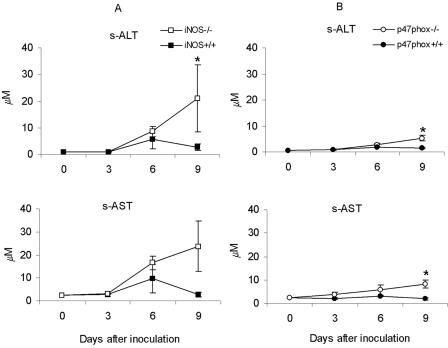
Histological examination of spleens, livers, and lungs from infected mice showed that the most pronounced differences were in livers, which became the focus of the comparative analysis. Examinations were performed on days 3, 6, and 9 after inoculation of 5 × 104 F. tularensis LVS cells, a dose found to be high enough to induce pathological changes. During the 9-day period, histological lesions developed in p47phox−/− and p47phox+/+ mice (Table 3). Increased serum levels of s-ALT and s-AST, sensitive markers for liver damage, were found in p47phox−/− mice on day 9 (Fig. 3). The iNOS−/− mice showed even more severe histological changes at all times (Table 3), and the enzyme levels on day 9 were higher than those in any other group of mice (Fig. 3). Representative histological sections from day 6 are shown in Fig. 4. Two indicators of kidney and pancreas function, s-creatinine and s-amylase, respectively, were also monitored, but no significant differences were observed between the gene-deficient and wild-type mice.
TABLE 3.
Histological examination of livers from mice infected with F. tularensis LVSa
| Days postinfection | Mouse strain | Liver damageb | Inflammatory response
|
|
|---|---|---|---|---|
| Extentc | Intensityd | |||
| 3 | iNOS+/+ | 1, 1, 1c | 1, 1, 1 | 1, 1, 1 |
| iNOS−/− | 1, 2, 2 | 1, 2, 2 | 1, 2, 2 | |
| p47phox+/+ | 1, 1, 1 | 1, 1, 1 | 1, 1, 1 | |
| p47phox−/− | 1, 1, 1 | 1, 1, 1 | 1, 1, 1 | |
| 6 | iNOS+/+ | 1, 1, 1 | 2, 2, 2 | 2, 2, 2 |
| iNOS−/− | 4, 4, 4 | 3, 4, 4 | 4, 4, 4 | |
| p47phox+/+ | 2, 2, 2 | 2, 2, 2 | 2, 2, 2 | |
| p47phox−/− | 2, 2, 2 | 2, 2, 2 | 2, 2, 2 | |
| 9 | iNOS+/+ | 1, 2, 3 | 1, 2, 3 | 1, 2, 3 |
| iNOS−/− | 4, 4, 4 | 4, 4, 4 | 4, 4, 4 | |
| p47phox+/+ | 2, 3, 3 | 2, 3, 3 | 2, 3, 3 | |
| p47phox−/− | 3, 3, 3 | 3, 3, 3 | 3, 3, 3 | |
The intradermal inoculum was 5 × 104 CFU of F. tularensis LVS per mouse.
Liver damage: 1, degeneration and necrosis of individual hepatocytes occasionally seen; 2, clusters or small aggregates of hepatocyte degeneration and necrosis; 3, medium to large aggregates of hepatocyte necrosis; 4, large areas of hepatocyte necrosis with loss of normal liver anatomic architecture.
Extent of the inflammatory response: 1, occasional inflammatory infiltrates (<1 foci/×100 field); 2, small numbers of inflammatory infiltrates (1 to 5 foci/×100 field); 3, moderate numbers of inflammatory infiltrates (>5 foci/×100 field); 4, large numbers of inflammatory infiltrates throughout the section.
Intensity of the inflammatory response: 1, small inflammatory infiltrates with a few inflammatory cells; 2, medium-size inflammatory infiltrates with small to moderate numbers of inflammatory cells; 3, large inflammatory infiltrates with moderate to large numbers of inflammatory cells; 4, extensive infiltration with large numbers of inflammatory cells. The values are averages for three individual mice.
Individual scores for liver samples for three mice for each day and group.
FIG. 3.
After inoculation of 5 × 104 CFU of F. tularensis LVS, the serum liver enzymes s-ALT and s-AST were monitored in iNOS−/− and iNOS+/+ mice (A) and in p47phox−/− and p47phox+/+ mice (B). The means ± standard errors of the means for five mice per group and time are shown. An asterisk indicates that the P value is <0.05.
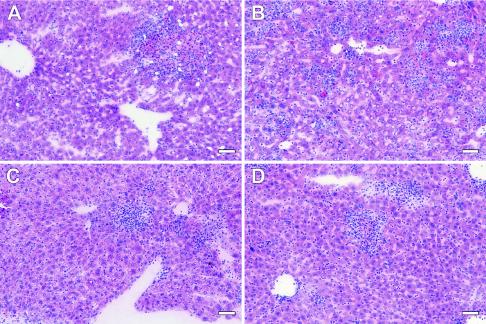
FIG. 4.
Representative liver histopathology for different strains of mice killed on day 6 after intradermal infection with 5 × 104 CFU of F. tularensis LVS. (A) Liver from an iNOS+/+ mouse, showing a medium-size focal accumulation of mixed inflammatory cells and hepatic necrosis (the scores were 1, 1, and 1, as shown in Table 3). (B) Liver from an iNOS−/− mouse, showing the presence of numerous small to medium-size inflammatory infiltrates throughout the entire liver section (the scores were 4, 4, and 4). (C and D) Livers from p47phox+/+ (C) and p47phox−/− (D) mice, showing the presence of small to medium-size focal accumulations of mixed inflammatory cells and occasional hepatic necrosis of some severity (in both cases the scores were 2, 2, and 2). Hematoxylin and eosin staining was used. Bars = 20 μm.
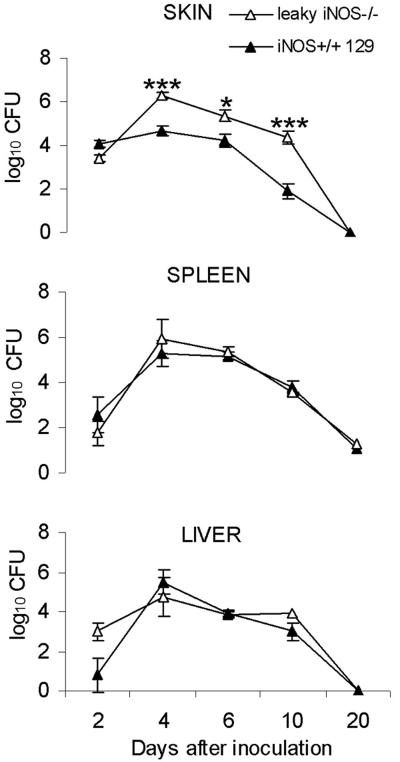
Numbers of bacteria in skin, spleens, and livers of leaky iNOS−/− and iNOS+/+ mice after primary infection with F. tularensis.
To obtain more information about the role of RNS in control of F. tularensis, we monitored the growth of F. tularensis LVS in mice with a partially defective iNOS gene, which were designated leaky mice. These mice have been shown to exhibit NO-dependent killing of Leishmania, although they display no measurable serum levels of NO (22).
At various times after intradermal injection of 5 × 102 CFU of F. tularensis LVS, a dose that was sublethal for the mice, bacterial counts in the skin, livers, and spleens were determined. In samples from skin obtained on day 4, 6, or 10, the numbers of bacteria were consistently 1 to 2 log10 higher in iNOS−/− mice than in iNOS+/+ 129 mice (Fig. 5). In each of five separate experiments, the numbers of bacteria in the skin were higher in iNOS−/− mice on days 4 to 7 of infection, and the bacteria were eradicated within 3 weeks. In livers and spleens, there were no significant differences between the two strains of mice (Fig. 5). After peak numbers of bacteria were reached on day 4, complete eradication occurred within 3 weeks postinfection. The data indicate that low levels of NO are not sufficient for control of LVS infection in the skin, although they are sufficient for control of LVS infection in the liver and spleen.
FIG. 5.
Growth curves for F. tularensis LVS in the skin, livers, and spleens of leaky iNOS−/− mice and iNOS+/+ 129 mice after primary intradermal infection with 5 × 102 F. tularensis LVS cells. The means ± standard errors of the means for five mice per group and time are shown. One asterisk indicates that the P value is <0.05, and three asterisks indicate that the P value is <0.001.
Production of RNS and superoxide ex vivo by splenocytes of mice infected with F. tularensis LVS.
After intradermal injection of 5 × 104 CFU of F. tularensis LVS (the same dose that was used in the histological examinations and liver enzyme and cytokine assays), mice were killed on days 3 to 9 in order to determine the capacity of splenocytes to produce RNS and ROS. The ROS assay was highly sensitive and allowed detection of superoxide-dependent chemoluminescence after only minutes of incubation, whereas RNS had to be accumulated by in vitro incubation of the splenocytes for 4 days before quantifiable amounts were present. Although these differences in the assay time cannot be reliably translated to time of occurrence in vivo, both assays were nonetheless thought to reflect the in vivo ability of the splenocytes to produce the corresponding mediators.
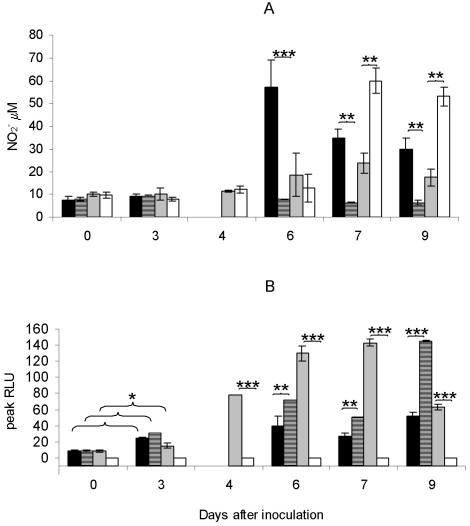
Splenocytes harvested from infected iNOS+/+ mice produced significant levels of NO2− only when they were obtained on day 6 of infection or later (Fig. 6A). Similarly, splenocytes obtained from p47phox+/+ and p47phox−/− mice started to produce significant levels of NO2− only when they were obtained ≥7 days after inoculation. On days 7 and 9, the levels were significantly higher for p47phox−/− mice than for p47phox+/+ mice. Splenocytes derived from iNOS−/− mice showed no production of NO2− at any time.
FIG. 6.
iNOS+/+ mice (solid bars), iNOS−/− mice (striped bars), p47phox+/+ mice (gray bars), and p47phox−/− mice (open bars) were inoculated with 5 × 104 CFU of F. tularensis LVS. On different days after inoculation mice were sacrificed, and splenocytes were prepared and analyzed for NO production (A) and ROS production (B). The values are the means ± standard errors of the means for three mice per group and time. Samples were analyzed in triplicate. No values are given for iNOS+/+ and iNOS−/− mice on day 4. One asterisk indicates that the P value is <0.05, two asterisks indicate that the P value is <0.01, and three asterisks indicate that the P value is <0.001. RLU, relative light units.
Splenocytes from wild-type (iNOS+/+ and p47phox+/+) and iNOS−/− mice harvested on day 3 produced ROS at levels that were significantly higher than the levels produced by splenocytes from noninfected mice (Fig. 6B). At this time, the level of ROS produced by cells from iNOS−/− mice was similar to the level of ROS produced by cells from iNOS+/+ mice. After this, the capacity of cells from iNOS−/− mice increased significantly; on days 6 and 7 the levels were almost twice as high as the levels produced by cells from iNOS+/+ mice, and on day 9 the levels were more than three times as high as the levels produced by cells from iNOS+/+ mice (Fig. 6B). Splenocytes from p47phox−/− mice did not produce ROS at any time.
To investigate whether the increase in the capacity of splenocytes from iNOS−/− mice to produce ROS was associated with changes in the proportion of phagocytes in the spleen, differential counts were obtained on days 3, 6, and 9 of infection. Infection resulted in increased proportions of neutrophils and monocytes in the spleens of all four strains of mice at all times compared to noninfected mice. However, no marked differences were found between the gene-deficient mouse strains and the corresponding wild-type strains (Table 4). Thus, the enhanced production in cultures from infected mice compared with cultures from noninfected mice may have been related to the increased numbers of ROS-producing neutrophils and monocytes in the former mice.
TABLE 4.
Spleen cell composition in spleens of mice infected with F. tularensis LVSa
| Cell type | Mouse strain | No. of cells (×106) in spleens on:
|
|||
|---|---|---|---|---|---|
| Day 0a,b | Day 3c | Day 6c | Day 9c | ||
| Neutrophils | p47phox+/+ | 0.3 (1.1) | 7.8 ± 0.6 (14) | 14.9 ± 2.9 (18) | 6.4 ± 2.2 (7.3) |
| p47phox−/− | 0.3 (1.1) | 4.9 ± 2.7 (9.8) | 12.0 ± 2.7 (16) | 10.3 ± 1.9 (14) | |
| iNOS+/+ | 0.6 (2.0) | 13.5 ± 2.3 (17) | 12.6 ± 4.6 (15) | 6.7 ± 4.3 (6.6) | |
| iNOS−/− | 0.6 (2.0) | 14.9 ± 2.9 (21) | 5.9 ± 2.9 (9.3) | 11.0 ± 0.9 (15) | |
| Monocytes | p47phox+/+ | 0 | 1.7 ± 0.3 (3.0) | 4.8 ± 1.6 (5.8) | 1.0 ± 0.6 (1.1) |
| p47phox−/− | 0 | 0.5 ± 0.3 (1.0) | 4.8 ± 1.3 (6.5) | 1.0 ± 0.8 (1.4) | |
| iNOS+/+ | 0 | 1.9 ± 0.6 (2.4) | 4.2 ± 0 (5.0) | 3.3 ± 1.5 (3.2) | |
| iNOS−/− | 0 | 2.1 ± 1.1 (2.9) | 2.6 ± 1.6 (4.1) | 3.7 ± 1.0 (5.0) | |
The intradermal inoculum was 5 × 104 CFU of F. tularensis LVS per mouse.
The values are the numbers of cells before inoculation of F. tularensis LVS. The numbers in parentheses are the percentages of the total numbers of cells in the spleens.
The values are means ± standard errors of the means for three samples. The numbers in parentheses are the percentages of the total numbers of cells in the spleens.
In conclusion, p47phox−/− mice seemed to compensate for the lack of the phagocyte oxidase by increased expression of NO2−, and in contrast, iNOS−/− mice seemed to compensate for the lack of iNOS by an increased ability to produce ROS. The increased ROS levels observed in the latter mice was likely related to an enhanced cellular capability for ROS production, since there were no significant differences in the proportion of phagocytes between the iNOS−/− mice and the iNOS+/+ mice.
Cytokine levels in sera from iNOS−/−, iNOS+/+, p47phox−/−, and p47phox+/+ mice after infection with F. tularensis.
At various times after intradermal inoculation of 5 × 104 CFU of F. tularensis LVS, blood samples were collected for an assay of cytokines. Both p47phox−/− mice and iNOS−/− mice had much higher serum levels of IFN-γ than the corresponding wild-type mice (Table 5). On day 10, the levels in the gene-deficient mice were >2,000 pg/ml, compared to <500 pg/ml in the wild-type strains. Leaky iNOS−/− mice showed down-regulation of IFN-γ levels in the same manner as wild-type mice, and the level on day 10 was <200 pg/ml. In serum from noninfected animals, IFN-γ was not detectable (concentration, <30 pg/ml). Based on the values for individual animals on days 3, 6, 9, and 10, the levels of IFN-γ showed a correlation coefficient of 0.5 or higher with the numbers of bacteria in spleens and livers irrespective of the mouse strain.
TABLE 5.
IFN-γ in sera of mice infected with F. tularensis LVSa
| Mouse strain | IFN-γ concn in serum (pg/ml)
|
||||
|---|---|---|---|---|---|
| Day 0b | Day 3c | Day 6c | Day 9c | Day 10c | |
| p47phox+/+ | <30 | 1,070 ± 390 | 4,340 ± 2,590 | 220 ± 25 | 280 ± 15 |
| p47phox−/− | <30 | 2,088 ± 418d | 6,190 ± 2,510 | 3,600 ± 640 C | 2,010 ± 75 B |
| iNOS+/+ | <30 | 1,530 ± 620 | 3,243 ± 1,641 | NDe | <30 |
| iNOS−/− | <30 | 2,790 ± 1,500 | 12,080 ± 575 A | 6,490 ± 1,220 | 2,020 ± 710 A |
| iNOS+/+ 129 | <30 | 1,190 ± 440 | 1,650 ± 240 | ND | 220 ± 120 |
| Leaky iNOS−/− | <30 | 1,530 ± 525 | 4,000 B | ND | 150 ± 45 |
The intradermal inoculum was 5 × 104 CFU of F. tularensis LVS per mouse.
IFN-γ serum levels before inoculation of F. tularensis LVS.
Means ± standard errors of the means for three samples.
Significance is indicated as follows: A, P < 0.05 compared to wild-type mice; B, P < 0.01 compared to wild-type mice; C, P < 0.001 compared to wild-type mice.
ND, not determined.
Also, the IL-6 levels were generally higher in sera from the iNOS−/− and p47phox−/− mice than in the sera from the corresponding wild-type mice (Table 6). The IL-12p40 levels were <2,000 pg/ml in all serum samples and were not different in gene-deficient and wild-type mice. TNF-α and IL-4 were not detected in any serum sample from any strain of mice.
TABLE 6.
IL-6 levels in sera of mice infected with F. tularensis LVSa
| Mouse strain | IL-6 concn in serum (pg/ml)
|
||||
|---|---|---|---|---|---|
| Day 0b | Day 3c | Day 6c | Day 9c | Day 10c | |
| p47phox+/+ | <15 | 419 ± 137 | 115 ± 50 | 52 ± 10 | NDd |
| p47phox−/− | <15 | 1,260 ± 588 Ae | 1,007 ± 891 | 203 ± 25 A | ND |
| iNOS+/+ | <15 | 522 ± 145 | 140 ± 81 | 57 ± 11 | 102 ± 9 |
| iNOS−/− | <15 | 625 ± 43 | 1450 ± 86 B | 1,480 ± 420 B | 684 ± 5 C |
The intradermal inoculum was 5 × 104 CFU of F. tularensis LVS per mouse.
IL-6 serum levels before inoculation of F. tularensis LVS.
Means ± standard errors of the means for three samples.
ND, not determined.
Significance is indicated as follows: A, P < 0.05 compared to wild-type mice; B, P < 0.01 compared to wild-type mice; C, P < 0.001 compared to wild-type mice.
In vitro recall response of splenocytes from iNOS−/− and iNOS+/+ mice.
To determine whether the high level of susceptibility of the iNOS−/− mice was related to an impaired ability to respond to F. tularensis antigen with T-cell proliferation and IFN-γ production, mice were primed by intradermal inoculation of a low dose of F. tularensis LVS, treated with moxifloxacin on days 15 to 22, and killed on day 35 for preparation of splenocyte cultures. Naïve iNOS−/− and iNOS+/+ mice showed low or nonsignificant proliferative responses in vitro to heat-killed F. tularensis (stimulatory indices, ≤2.4), whereas primed mice from both groups displayed a strong response, with stimulatory indices of ≥10.5 (Table 7). In response to heat-killed F. tularensis, splenocytes from immune iNOS−/− mice secreted much higher levels of IFN-γ than cells from primed iNOS+/+ mice secreted (Table 7). Irrespective of immunization, the spleen cells showed vigorous proliferation and a high level of secretion of IFN-γ in response to the T-cell mitogen concanavalin A. Thus, the immune iNOS−/− mice showed recall responses that were as strong as those of immune iNOS+/+ mice and produced much higher levels of the Th1 cytokine IFN-γ than immune iNOS+/+ mice produced.
TABLE 7.
In vitro proliferation and IFN-γ production in splenocytes from naïve or F. tularensis-primed iNOS−/− or iNOS+/+ mice after stimulation with heat-killed F. tularensisa
| Assay | Mice
|
|||
|---|---|---|---|---|
|
F. tularensis immuneb
|
Nonimmune
|
|||
| iNOS+/+ | iNOS−/− | iNOS+/+ | iNOS−/− | |
| Proliferative response (SI)c | 10.5 ± 2.7 | 12.3 ± 1.5 | 2.4 ± 0.5 | 1.9 ± 0.2 |
| IFN-γ (ng/ml) | 7.5 ± 0.4 | 24.5 ± 2.1 | <0.03 | <0.03 |
To estimate the proliferative response, spleen cell cultures were stimulated for 4 days with 106 heat-killed cells of F. tularensis LVS and pulsed with [3H]thymidine. Cytokine levels were determined in cell supernatants after 2 days of stimulation. The values are means ± standard errors of the means for five cultures.
Mice were subjected 5 weeks previously to intradermal infection with 5 × 103 CFU of F. tularensis LVS and given moxifloxacin on days 15 to 22.
Stimulation index (SI) = mean counts per minute for five cultures containing antigen/mean counts per minute for five cultures lacking antigen. Stimulation with concanavalin A resulted in a stimulation index of >28.7. Cultures without antigen gave 1,400 to 1,800 cpm.
DISCUSSION
F. tularensis is a potent pathogen, and the principal virulence mechanism seems to be intracellular survival. As observed for other facultative intracellular bacteria, the mechanisms of host protection are critically dependent on cell-mediated immunity (30) (i.e., the activation of macrophages by T cells to kill intracellularly located bacteria). The T-cell-dependent activation requires the involvement of IFN-γ, and previous in vitro studies demonstrated that a release of nitric oxide by the macrophages might contribute to killing (1, 15). By using p47phox−/− and iNOS−/− mice, we demonstrated here that both ROS and RNS play an essential role in vivo for the control of murine tularemia. Mutant mice were significantly more susceptible to death caused by F. tularensis LVS. The LD50 for the iNOS−/− mice was <20 CFU, the LD50 for the p47phox−/− mice was 4,400 CFU, and the LD50 for the wild-type mice was >500,000 CFU. In p47phox−/− mice the infection was exacerbated on day 4, whereas in iNOS−/− mice exacerbation occurred in the second week of infection. In iNOS−/− mice there was a prolonged course of disease until death after 26.4 days of infection (mean value) and there was severe liver damage, whereas in p47phox−/− mice the disease was more acute, the mean time to death was 10.1 days, and there was less pronounced liver damage.
Thus, our results indicate that ROS have a critical role during the first few days of infection, whereas RNS seemed to be critical at a subsequent phase. Few previous studies have assessed the role of ROS in the control of tularemia. One study demonstrated that superoxide and hydrogen peroxide are produced during the course of experimental tularemia but did not analyze if this production correlated with a bactericidal effect (17). The high level of susceptibility of p47phox−/− mice may be related to a lack of macrophage- and neutrophil-mediated bactericidal mechanisms. Neutrophils have been shown to play a crucial role in the control of murine tularemia (26), and human neutrophils display bactericidal activity against F. tularensis by formation of hypochloric acid (19). This acid is produced as a result of the reaction between hydrogen peroxide and chloride anions and is thus not formed in neutrophils from p47phox−/− mice. Although killing of F. tularensis by murine neutrophils has not been demonstrated, the important role of these cells in the control of infection may be one explanation for why in the present experiments p47phox−/− mice showed such a high level of susceptibility to tularemia. During the interval from day 4 to 7, when exacerbation was observed in p47phox−/− mice, splenocytes derived from wild-type mice produced high levels of superoxide, indirectly supporting the hypothesis that ROS production plays an important role during this stage. Collectively, our data indicate that ROS have an important role in control of the early phase of the F. tularensis infection when innate immune mechanisms are operative. However, since small challenge inocula were controlled in p47phox−/− mice, ROS-independent mechanisms are apparently operative as well.
In contrast to the role of ROS, the absence of RNS did not affect the numbers of bacteria in livers and spleens during the first week of infection. After this, the numbers of bacteria increased in the iNOS−/− mice, which developed severe liver pathology and showed high serum levels of liver-specific enzymes and IFN-γ. Together, these results indicate that RNS have both immunoregulatory and bactericidal effects. Besides their lack of possible NO-mediated antimicrobial mechanisms, the iNOS−/− mice may be afflicted with dysregulation of the immune response, leading to liver damage and persistent, high serum levels of IFN-γ. This is in line with extensive evidence concerning the important immunoregulatory role of NO (5, 6, 9). Since iNOS−/− mice with a leaky phenotype displayed no measurable serum levels of NO but showed no signs of exacerbation of systemic infection, no liver damage, and normal serum levels of IFN-γ on day 10, such regulatory mechanisms might depend on the presence of very low serum levels of NO. Previous studies have demonstrated that immune mice are effectively protected by a number of overlapping, compensatory mechanisms (11, 14, 26).
The role of RNS in killing of F. tularensis in skin was evident even during the early phase of infection in both iNOS−/− mice and leaky iNOS−/− mice and persisted throughout the course of infection. Thus, in skin, low levels of NO did not appear to be sufficient for host control of an F. tularensis infection. The skin is an important barrier for controlling both murine and human tularemia. The data suggest that NO-dependent, dermal killing mechanisms may be important for control of tularemia.
In mice, the lack of IFN-γ leads to lethal exacerbation of tularemia even with the smallest challenge inocula (18, 27). In the present study, a paradoxical relationship between the serum levels of IFN-γ and the numbers of bacteria in the liver and spleen was demonstrated and was evident especially during protracted infection in iNOS−/− mice. The simplest explanation for the paradox is the occurrence of frustrated overproduction of IFN-γ, which is not sufficient to control infection in the absence of complementary effector molecules generated directly or indirectly by iNOS and phox. The present data and previous studies (14, 18, 27) together show that although IFN-γ is absolutely required in vivo for control of tularemia, high, excessive levels of the cytokine do not necessarily lead to more effective killing of F. tularensis.
The host protective mechanisms for murine tularemia seem to resemble those that are operative in other experimental models of intracellular infections. For example, in cutaneous leishmaniasis, RNS were needed for killing of the parasite in the skin and draining lymph nodes, whereas the activity of phox was dispensable for resolution of the acute skin infection but essential for clearance of the parasites in the spleen (3). Resistance to virulent Salmonella enterica biovar Typhimurium in the mouse model was dependent on ROS, because phox-deficient mice showed dramatic exacerbation and succumbed to infection within 5 days. In contrast, iNOS−/− mice contained increased numbers of bacteria only after the first week of infection (20). Thus, in these two infection models there is RNS- and ROS-dependent organ- and stage-specific control, and the roles of the two classes of effector molecules appear to be quite distinct.
Our results demonstrate that both RNS and ROS play important roles in the control of experimental tularemia. Exacerbation was observed in p47phox−/− mice during the early phase of infection, at a stage when a lack of NO resulted in no systemic exacerbation. However, iNOS−/− mice subsequently succumbed to the infection, and they succumbed to even the lowest dose. The extreme susceptibility of iNOS−/− mice might be in line with a complex regulatory role for NO in intracellular infections, and death may be an effect of dysregulation of the cytokine response and resulting severe liver damage.
Acknowledgments
Grant support was obtained from the Swedish Medical Research Council, Samverkansnämnden, Norra Sjukvårdsregionen, Umeå, Sweden, and the Medical Faculty, Umeå University, Umeå, Sweden.
We thank Steven Holland for supplying the p47phox−/− mice and F. Y. Liew for supplying leaky iNOS−/− mice.
Editor: A. D. O'Brien
REFERENCES
- 1.Anthony, L. S., P. J. Morrissey, and F. E. Nano. 1992. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from l-arginine metabolism. J. Immunol. 148:1829-1834. [PubMed] [Google Scholar]
- 2.Babior, B. M., J. D. Lambeth, and W. Nauseef. 2002. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397:342-344. [DOI] [PubMed] [Google Scholar]
- 3.Blos, M., U. Schleicher, F. J. Soares Rocha, U. Meissner, M. Rollinghoff, and C. Bogdan. 2003. Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase. Eur. J. Immunol. 33:1224-1234. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907-916. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 7.Brunet, L. R. 2001. Nitric oxide in parasitic infections. Int. Immunopharmacol. 1:1457-1467. [DOI] [PubMed] [Google Scholar]
- 8.Chakravortty, D., and M. Hensel. 2003. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 5:621-627. [DOI] [PubMed] [Google Scholar]
- 9.Cifone, M. G., S. Ulisse, and A. Santoni. 2001. Natural killer cells and nitric oxide. Int. Immunopharmacol. 1:1513-1524. [DOI] [PubMed] [Google Scholar]
- 10.Coleman, J. W. 2001. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 1:1397-1406. [DOI] [PubMed] [Google Scholar]
- 11.Conlan, J. W., A. Sjöstedt, and R. J. North. 1994. CD4+ and CD8+ T-cell-dependent and -independent host defense mechanisms can operate to control and resolve primary and secondary Francisella tularensis LVS infection in mice. Infect. Immun. 62:5603-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlgren, C., and A. Karlsson. 1999. Respiratory burst in human neutrophils. J. Immunol. Methods 232:3-14. [DOI] [PubMed] [Google Scholar]
- 13.Elkins, K. L., T. Rhinehart-Jones, C. A. Nacy, R. K. Winegar, and A. H. Fortier. 1993. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect. Immun. 61:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, S. H., J. I. Gallin, and S. M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovarova, H., A. Macela, and J. Stulik. 1990. The production of oxygen metabolites and their possible regulatory role in the course of tularemia infection. Folia Microbiol. 35:413-422. [DOI] [PubMed] [Google Scholar]
- 18.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löfgren, S., A. Tärnvik, M. Thore, and J. Carlsson. 1984. A wild and an attenuated strain of Francisella tularensis differ in susceptibility to hypochlorous acid: a possible explanation of their different handling by polymorphonuclear leukocytes. Infect. Immun. 43:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedbala, W., X. Q. Wei, D. Piedrafita, D. Xu, and F. Y. Liew. 1999. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 29:2498-2505. [DOI] [PubMed] [Google Scholar]
- 23.Polsinelli, T., M. S. Meltzer, and A. H. Fortier. 1994. Nitric oxide-independent killing of Francisella tularensis by IFN-gamma-stimulated murine alveolar macrophages. J. Immunol. 153:1238-1245. [PubMed] [Google Scholar]
- 24.Portnoy, D. A., V. Auerbuch, and I. J. Glomski. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sjöstedt, A., G. Sandström, and A. Tärnvik. 1992. Humoral and cell-mediated immunity in mice to a 17-kilodalton lipoprotein of Francisella tularensis expressed by Salmonella typhimurium. Infect. Immun. 60:2855-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöstedt, A., J. W. Conlan, and R. J. North. 1994. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect. Immun. 62:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjöstedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369-1374. [DOI] [PubMed] [Google Scholar]
- 28.Stenger, S., and R. L. Modlin. 1999. T cell mediated immunity to Mycobacterium tuberculosis. Curr. Opin. Microbiol. 2:89-93. [DOI] [PubMed] [Google Scholar]
- 29.Tärnvik, A., and L. Berglund. 2003. Tularaemia. Eur. Respir. J. 21:361-373. [DOI] [PubMed] [Google Scholar]
- 30.Tärnvik, A., M. Ericsson, I. Golovliov, G. Sandström, and A. Sjöstedt. 1996. Orchestration of the protective immune response to intracellular bacteria: Francisella tularensis as a model organism. FEMS Immunol. Med. Microbiol. 13:221-225. [DOI] [PubMed] [Google Scholar]
- 31.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 32.Yee, D., T. R. Rhinehart-Jones, and K. L. Elkins. 1996. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J. Immunol. 157:5042-5048. [PubMed] [Google Scholar]