Abstract
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) can lead to prominent nerve hypertrophy, which can mimic other forms of neuropathy radiologically. Neuro-ophthalmological complications can also occur in CIDP, either at presentation or chronically in the disorder. This can also cause diagnostic difficulties. We report three cases of neuro-ophthalmological complications of CIDP: two cases of papilloedema and one case of proptosis. In all three cases cranial nerve hypertrophy was present. CIDP should be considered in neuro-ophthalmological presentations associated with cranial/spinal nerve root hypertrophy.
Keywords: Chronic inflammatory demyelinating polyradiculoneuropathy, papilloedema, proptosis
INTRODUCTION
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) usually presents as a progressive or relapsing symmetrical tetraparesis with areflexia and peripheral sensory loss. It is predominantly a disease of the peripheral nervous system, although central nervous system involvement can occasionally occur. Diagnosis is based on the clinical presentation, supported by cerebrospinal fluid (CSF) examination and electrophysiological and histopathological findings.1
The CSF is usually acellular or paucicellular and CSF protein levels are usually raised, often considerably.2 Electrophysiologically there is a motor and sensory demyelinating polyradiculoneuropathy, which is often patchy, with evidence of conduction block or temporal dispersion that helps distinguish CIDP from hereditary demyelinating neuropathies.3
Histopathological findings are not specific for CIDP, but include endoneurial oedema, macrophage infiltration, demyelinated with some remyelinated nerve fibres, and endoneurial mononuclear cell infiltration. Fascicles may be variably involved within a single nerve. Ongoing cycles of demyelination and remyelination leads to concentric Schwann cell proliferation (“onion-bulb” formation), which can result in thickening of nerves.1 A magnetic resonance imaging (MRI) study of 14 cases of CIDP found cervical and brachial plexus hypertrophy in 8.4 These cases had significantly longer disease courses (mean 15.9 years) than those cases without hypertrophy (mean 3.3 years, p = 0.05). The phenomenon is most likely due to the chronic effects of the disease; however, two of the cases with hypertrophy had short disease courses (7 and 18 months), but both patients were moderately to severely disabled, suggesting that nerve hypertrophy may be a marker of disease activity. Recently, cases with massive nerve enlargement, including of the cranial nerves, have been described.5–8 Each thickened nerve may not be affected clinically.5 This has led to diagnostic confusion because a number of the hereditary neuropathies, such as hereditary motor and sensory neuropathy (HMSN) type I and type III, neurofibromatosis type 1, and schwannomatosis, can also have similar appearances.7,9–12
We report three cases of neuro-ophthalmological complications of CIDP, each in the presence of hypertrophic nerve enlargement and review the neuro-ophthalmological presentations of CIDP. In two cases (cases 1 and 3), the appearances led to doubt as to whether CIDP was the cause of the presenting problems, requiring histopathological confirmation.
CASE 1
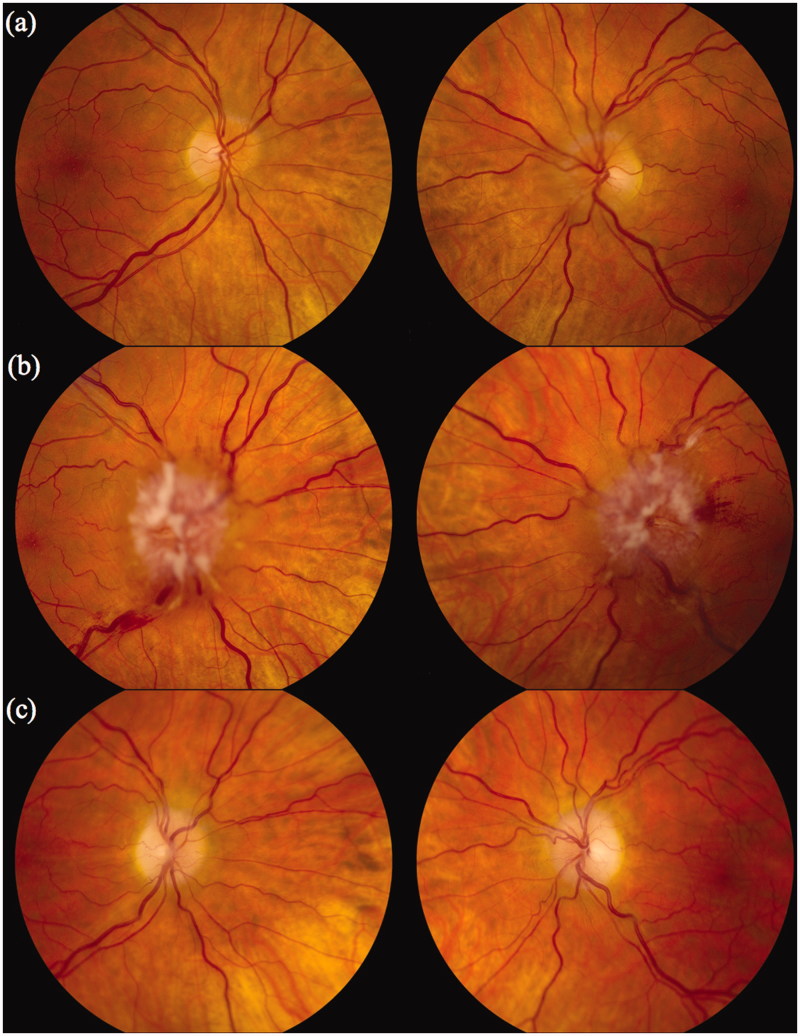
A 46-year-old white Caucasian woman presented with ocular irritation and dry eyes. Her visual acuity was 20/20 OD and 20/30 OS. Her visual fields were full, but she was found to have grade 1 papilloedema (Figure 1a).13
FIGURE 1.
Case 1. Fundi (a) at presentation; (b) 3 months after immunosuppression was stopped; and (c) 3 months after immunosuppression was restarted. Note: Figures 1, 3, 6 and 7 of this article are available in colour online at www.informahealthcare.com/oph.
She had a sensory peripheral neuropathy that had been diagnosed as CIDP 14 years previously on the basis of electrophysiological findings of a sensorimotor peripheral neuropathy with both demyelinating and axonal components and her CSF containing 2 white blood cells × 106/L and 6 g/L of protein. Papilloedema had not previously been noted despite an opening pressure of 31 cm CSF on this initial lumbar puncture. She had had only a partial response to treatment with corticosteroids, intravenous immunoglobulin (IVIg), and immunosuppression with azathioprine, then mycophenolate mofetil and, ultimately, methotrexate. She had become markedly cushingoid. She had global weakness of her limbs (Medical Research Council [MRC] grade 4/5) and walked with a stick. She had no skin lesions. She was areflexic and had pinprick sensory loss in her hands and feet.
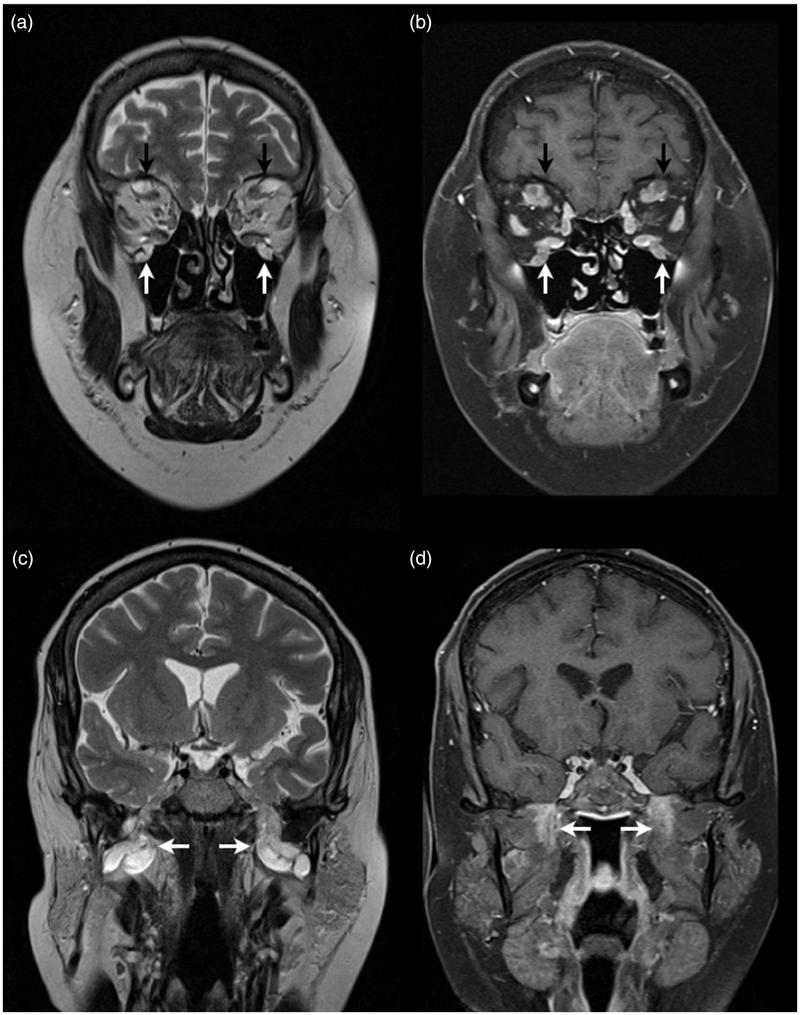
An MRI now demonstrated marked thickening of the cranial nerves (Figure 2), spinal nerve roots, and the brachial and lumbosacral plexi. This was felt to be more in keeping with neurofibromatosis type 1 than CIDP. Repeat nerve conduction studies (NCS) showed a severe symmetrical demyelinating and axonal peripheral neuropathy, more suggestive of an hereditary neuropathy, although there was no supporting family history and she tested negative for the Charcot-Marie-Tooth type 1 peripheral myelin protein 22 (PMP22), neurofibromatosis type 1, and the schwannomatosis INI1.SMARCB1 gene mutations. Given the lack of response to treatment and the profound delay on the NCS, it was felt that an hereditary neuropathy was now the most likely diagnosis and therefore her IVIg and other immunosuppression were discontinued.
FIGURE 2.
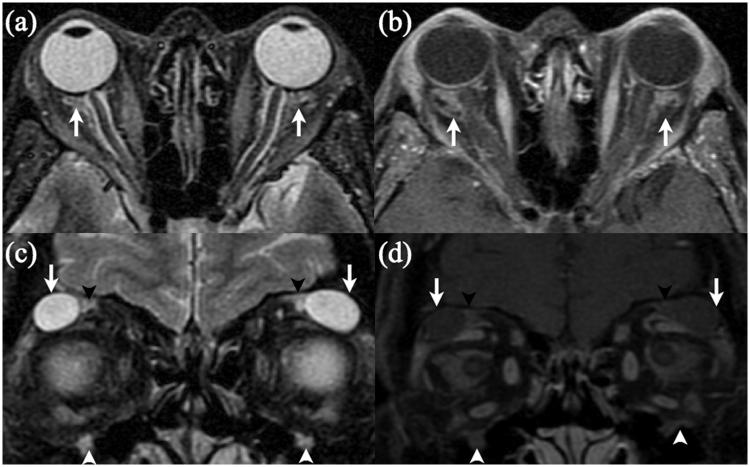
Case 1. Coronal T2- (a) and post-gadolinum fat-saturated T1- (b) weighted images demonstrating enlarged supra-orbital (black arrows) and infra-orbital (white arrows) nerves that also show gadolinium enhancement. Coronal T2- (c) and post-gadolinum fat-saturated T1- (d) weighted images demonstrating enlargement and gadolinium enhancement of both mandibular divisions of the trigeminal nerve (white arrows).
Over the next 3 months she developed transient visual obscurations and her papilloedema worsened considerably (Figure 1b). At repeat lumbar puncture, the opening pressure was 35 cm, with 2 × 106 white blood cells/L and 5.7 g/L protein. A biopsy of her lumbar plexus showed short sections of myelinated axons that had been widely dispersed by a loose, myxoid matrix and contained spindle cells with both plump and fusiform nuclei. There were scanty collagen fibres and a light chronic inflammatory infiltrate, including mast cells. Immunohistochemistry was not performed because of insufficient tissue. It was felt to be most consistent with an intraneural neurofibroma.
She remained off immunosuppression and was started on acetazolamide 500 mg three times per day. Despite this, over the next 5 months her papilloedema remained and her visual acuity worsened to 20/25 OD and 20/60 OS, with constricted visual fields. Her neuropathy also worsened.
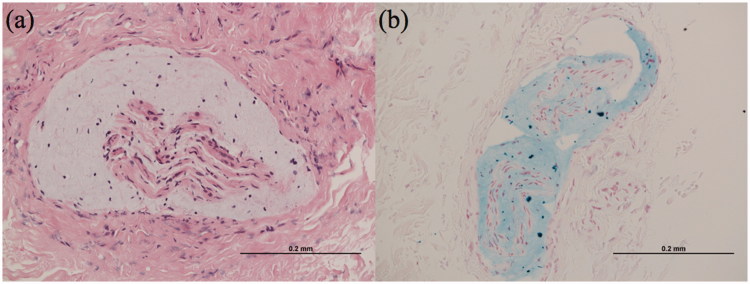
Due to this deterioration and the inadequate nature of the first biopsy, further biopsies were carried out of her sural nerve and a swelling of the occipital nerve. These showed evidence of nerve fibre loss, but also de/remyelinated fibres, suggesting a demyelinating process. This was associated with apparent Schwann cell hypertrophy and onion bulb formation, but also abundant mucopolysaccharide deposition, which had expanded the fascicles (Figure 3). This biopsy was felt to be supportive of a diagnosis of CIDP and therefore mycophenolate mofetil and regular IVIg were restarted.
FIGURE 3.
Case 1. Occipital nerve biopsy. (a) High-power haematoxylin and eosin–stained specimen demonstrating predominantly connective tissue surrounding nerve fascicles. The fascicles are permeated by mucopolysaccharide material, which accumulates beneath the perineurium. No inflammatory infiltrates are seen. (b) Alcian blue–stained specimen confirming the mucopolysaccharide deposition.
Over the next 8 months her mobility improved, as did her visual acuity to 20/20 OD and 20/30 OS. Her visual fields also improved and her papilloedema regressed, although she had residual optic disc pallor (Figure 1c).
CASE 2
A 23-year-old white Caucasian woman presented with a 6-month history of progressive numbness and weakness of her hands and feet. During this time she developed severe headaches, blurred vision, and orthostatic dizziness with several syncopal episodes. By admission, she was unable to stand independently and had developed facial weakness. She had no significant past medical history and there was no family history of hereditary neuropathy.
On examination, she had labile heart rate and arterial blood pressure. Because she was bed-bound and in severe pain from her headache formal visual acuity could not be measured. She had grade 2 papilloedema,13 facial weakness, and MRC grade 4/5 power in her proximal limbs, with 3/5 power in her hands and 2/5 power distally in her legs. She was areflexic. She had absent pinprick sensation distal to her wrists and knees, with absent proprioception and vibration sense in her hands and feet.
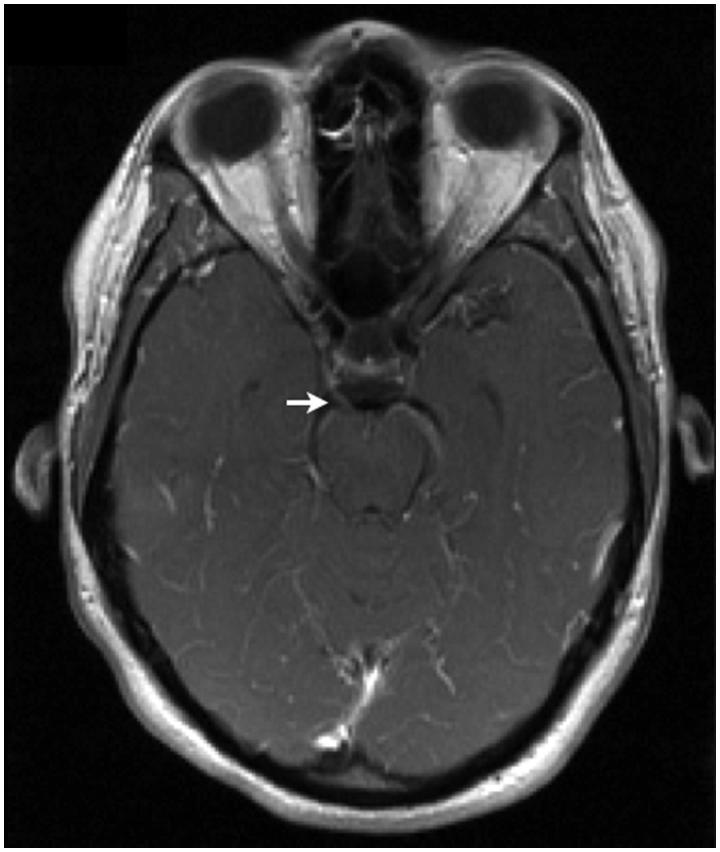
NCS revealed severely prolonged distal motor latencies and slowed conduction velocities. Partial conduction block was observed in several nerves. The sensory responses were uniformly absent. At lumbar puncture the opening pressure was >40 cm CSF. There were 6 × 106 white blood cells/L and the CSF protein was 6.05 g/L. She tested negative for both human immunodeficiency virus (HIV) and Borrelia. Serum ganglioside autoantibodies toward GM1, GD1a, GD1b, and GQ1b were absent, as were anti-myelin-associated glycoprotein (MAG) antibodies. She had normal serum and urine electrophoresis. An MRI demonstrated diffuse, uniform enlargement of the lumbosacral nerve roots and leptomeningeal enhancement along the right oculomotor nerves (Figure 4), the right abducens nerve, and both facial nerves.
FIGURE 4.
Case 2. Axial post-gadolinum T1-weighted image demonstrating enhancement of the right oculomotor nerve (white arrow).
A diagnosis of CIDP was made and she was initially treated with IVIg, but this was complicated by deranged liver function and haemolytic anaemia. Treatment was therefore changed to plasma exchange: initially five exchanges over 10 days, followed by weekly exchanges. Despite this, her headaches and blurred vision worsened and her papilloedema persisted. Insertion of a lumbar drain resulted in near-complete resolution of her headaches, but they returned upon its removal. A lumbar-peritoneal shunt was therefore inserted, which abolished her headaches.
Two months after presentation, weekly 1 g intravenous methylprednisolone was added to the weekly plasma exchange. She slowly improved, such that by 4 months after presentation her autonomic instability and facial weakness had resolved, although she could not yet sit unsupported. Her papilloedema had also improved and her visual acuity was measured at 20/20 OU. Follow-up brain MRI revealed near resolution of the cranial nerve enhancement.
CASE 3
A 56-year-old white Caucasian woman developed progressive proptosis during the last 2 years of a 16-year history of peripheral weakness and sensory loss. Serial NCS had shown lengthening distal motor latencies and decreasing conduction velocities. At her initial presentation, she had 4.5 g/L of protein in her CSF. Other investigations during her clinical course included positive IgG and IgM against sulphatide, β-tubulin, and GM1 ganglioside. She was negative for anti-MAG and PMP22. A sural nerve biopsy obtained at her initial presentation showed profound onion bulb formation, with reduced numbers of myelinated axons. There was no evidence of active axonal degeneration and a striking absence of inflammatory cells. She had been treated with intermittent glucocorticoids, plasmapheresis, and regular IVIg infusions, without noticeable effect.
On examination, she could see 20/20 OU with normal colour vision and normal automated visual fields. She measured 28 mm on the right and 26 mm on the left with a Hertel exophthalmometer. Her eye movements and alternate cover testing were normal and she did not complain of any diplopia. Her fundi were normal.
She had mild wasting of the intrinsic muscles of her hand and feet, with MRC 4/5 distal weakness. She was areflexic and had absent pinprick sensation in her hands and feet.
An MRI demonstrated bilateral cystic masses in the superior orbits and ill-defined T2 high signal, which enhanced after gadolinium administration surrounding the optic nerve sheaths. There was also T2 hyperintensity and gadolinium enhancement, which followed the course of several cranial nerves, including the supra- and infra-orbital nerves (Figure 5), the trigeminal nerves within Meckel’s cave, extending into the pterygopalatine fossa and foramen ovale, and the facial nerves in the internal auditory canal and temporal bone.
FIGURE 5.
Case 3. Axial fat-saturated T2- (a) and post-gadolinum T1- (b) weighted images demonstrating ill-defined high T2 signal with gadolinium enhancement within the intra-conal fat, primarily surrounding both optic nerve sheaths (white arrows). Coronal fat-saturated T2- (c) and post-gadolinum T1- (d) weighted images demonstrating round masses with high T2 signal and intermediate T1 signal in both lacrimal areas (white arrows), with enlarged and gadolinium-enhancing infra-orbital (white arrowheads) and supra-orbital (black arrowheads) nerves.
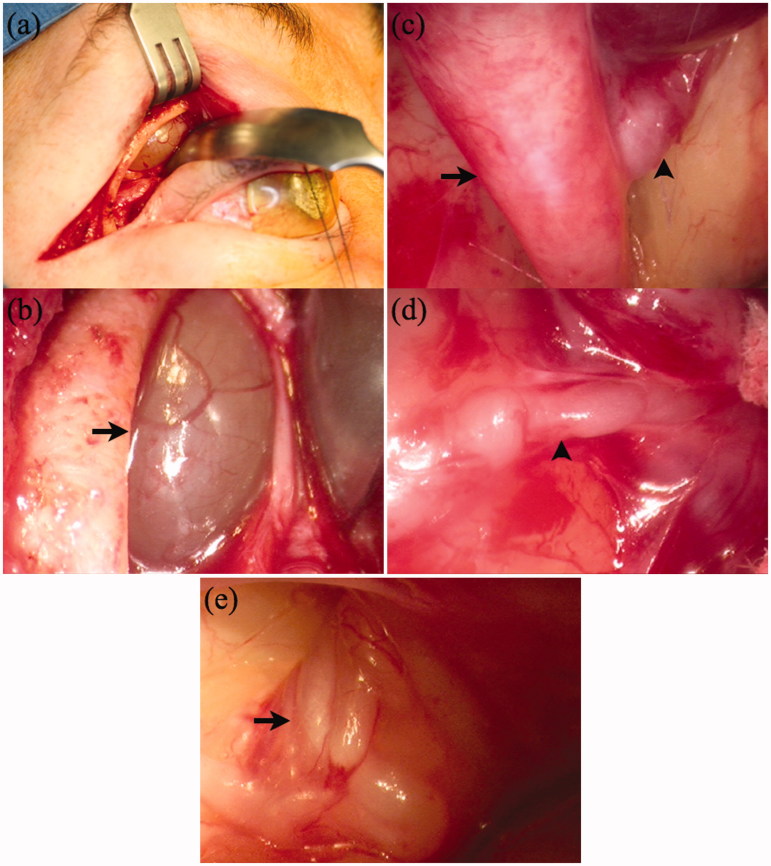
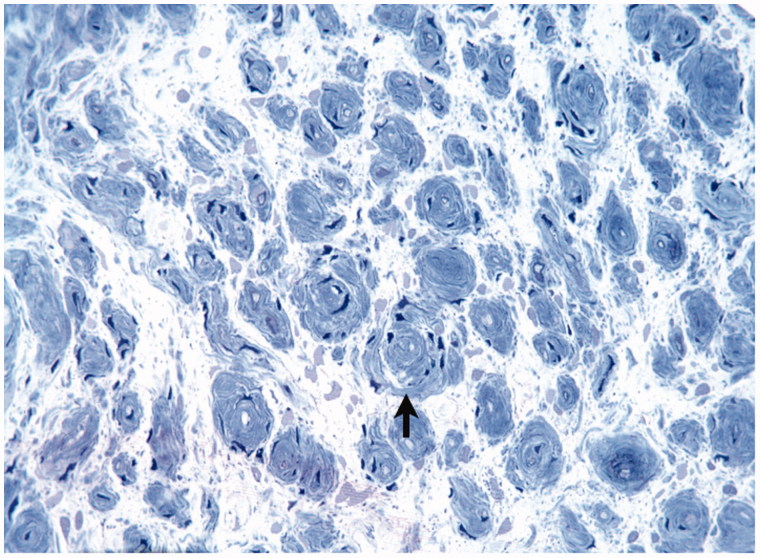
Given the lack of convincing response to treatment, the previous biopsy findings that had not shown classical findings for CIDP and now the unusual MRI appearances, surgical exploration of the orbit was undertaken (Figure 6). Histological examination of the upper lacrimal nerve showed an absence of myelinated axons and an increase in the number of Schwann cell nuclei, with proliferating Schwann cells and their processes forming onion bulbs (Figure 7). The specimen was again noted to be devoid of inflammatory cells. The process was marked by progressive and now complete loss of normal myelinated axons, compared with the earlier sural nerve biopsy. It was felt to be most consistent with CIDP.
FIGURE 6.
Case 3. Intra-operative photographs. (a) A right-sided extended superior-lateral eyelid crease incision was performed, to allow access to the superior lesion and lateral optic nerve without removing the lateral orbital rim. (b) A translucent mass was encounter immediately deep to the superior rim (black arrow). A fine-needle aspiration was performed but intra-operative cytological analysis was unrevealing. Therefore an incisional biopsy was performed. (c) Images c-e are photographed using a standard rigid 30-degree endoscope. After incision, the mass partially “deflated” and is visible in the upper right hand corner of the picture as a deeper red structure. Now the underlying frontal branch of the trigeminal nerve is visible as a massively enlarged nerve, projecting deep towards the orbital apex (black arrow). An acutely branching trunk, just deep to the deflated cyst, projects to the bony orbital roof and probably represents either the supra-orbital or supra-trochlear nerve (black arrowhead). (d) A magnified view of this branch is seen in this figure (black arrowhead), with the deflated deeper red cystic structure in the upper right hand corner of the image. (e) A tangle of nerves embedded in orbital fat, surrounding the optic nerve was encountered (black arrow). A smaller nerve was grasped, and a segment excised for biopsy. The anatomical location of these nerves is consistent with and likely represents enlarged short posterior ciliary nerves.
FIGURE 7.
Case 3. Right upper lacrimal nerve biopsy specimen showing severe loss of axons, a complete absence of normal myelinated axons, and clumps of proliferating Schwann cells with processes forming onion bulbs (black arrow). Again, there is a striking absence of inflammatory cells.
Her treatment was subsequently changed to rituximab. Her peripheral weakness and sensory loss have persisted but the right proptosis remained reduced 2 years after surgical decompression of the cystic nerve lesion, with no recurrence of the right cystic mass on follow-up MRI. The optic nerve sheath/ciliary nerve bundles have continued to show enhancement.
DISCUSSION
Neuro-ophthalmological complications of CIDP have been reported in a small percentage of patients in large case series of the disease (Table 1)14–21 or as single case reports or smaller series (Table 2).6,8,22–34 The neuro-ophthalmological presentation usually occurs in the context of a long history of CIDP (Tables 1 and 2). It may represent peripheral or central effects of the disease and also may precede the onset of limb involvement.27,35–37 Therefore, the clinician has to be aware of the possibility of CIDP, especially if neuro-imaging reveals cranial nerve hypertrophy, or if raised CSF protein is detected. A further diagnostic difficulty in case 1 was in distinguishing the root hypertrophy of CIDP from that of neurofibromatosis type 1, which it may mimic.7 Distinguishing the cause of nerve thickening histologically may also be difficult in biopsies that may be small due to the anatomical difficulty of obtaining biopsies of nerve roots. Myxoid expansion of the stroma may occur in both disorders, whilst the onion-bulb formations and demyelination that favour CIDP may be difficult to detect if samples do not allow for good orientation and the use of special stains.
TABLE 1.
Case series of CIDP in adults that have reported the frequency of neuro-ophthalmological complications.
| Neuro-ophthalmological finding | Frequency reported (%) | Case series |
|---|---|---|
| Papilloedema | 4/53 (7%) | Dyck et al., 197514 |
| 1/92 (1%) | McCombe et al., 198715 | |
| 2/60 (3%) | Barohn et al., 198916 | |
| 1/45 (2%) | do Nascimento, 199517 | |
| Ptosis | 1/53 (2%) | Dyck et al., 197514 |
| As part of Horner’s syndrome | ||
| 1/103 (1%) | Simmons et al., 199318 | |
| Diplopia or ophthalmoparesis | 2/53 (4%) | Dyck et al., 197514 |
| 4/92 (4%) | McCombe et al., 198715 | |
| 2/60 (3%) | Barohn et al., 198916 | |
| 5/103 (5%) | Simmons et al., 199318 | |
| Reported as blurred or double vision | ||
| 5/93 (5%) | Maisonabe et al., 199619 | |
| 2/67 (3%) | Gorson et al., 199720 | |
| 8/100 (8%) | Bouchard et al., 199921 |
TABLE 2.
Single case reports or small case series describing other neuro-ophthalmological complications of CIDP.
| Neuro-ophthalmological finding | Case description | Reference |
|---|---|---|
| Proptosis | 28-Year-old woman with a 13-year history of CIDP, who developed vertical and horizontal diplopia. Examination revealed bilateral proptosis along with a right pseudo-internuclear ophthalmoparesis. An MRI revealed enhancement of hypertrophied trigeminal nerves extending from the Gasserian ganglia to the terminal branches of all three divisions, with cavernous sinus enlargement and displacement of both optic nerves and superior recti. | Guibord et al., 199822 |
| 19-Year-old woman with a 15-year history of CIDP, who developed marked proptosis and horizontal diplopia on left lateral gaze. A left miotic pupil, which was unreactive to light, had been noted 3 years previously. An orbital CT scan showed generalised marked thickening of the orbital segments of the oculomotor nerves. | Duarte et al., 19996 | |
| Proptosis (continued) | Two patients with CIDP, a woman of 23 and a man of 21, who presented with proptosis and ocular palsies. MRI showed hypertrophy of cranial nerves III and V in both cases. | Aidi et al., 200223 |
| 43-Year-old woman with a 15-year history of CIDP, who developed progressive proptosis, eyelid retraction and diplopia. She had twice previously had ptosis correction surgery. An MRI 10 years later showed bilateral enhancement and enlargement of oculomotor nerves extending from the subarachnoid space into the orbits. | Alwan and Mejico, 20078 | |
| Pupillary abnormalities | A patient with hypertrophic neuropathy with clinical features suggesting possible CIDP. The patient had 3-mm regular pupils that were unreactive to light, but did reactive to accommodation. | Garcin et al., 196024 |
| 31-Year-old woman with an 11-year history of CIDP, who developed a painful pupil-involving right oculomotor palsy. MRI and MRA were normal. The palsy completely resolved after corticosteroid therapy. | Arroyo and Horton, 199525 | |
| 39-Year-old man with a 1-year history of CIDP, who was noted to have 5-mm pupils bilaterally that were unreactive to light. The near reaction was not reported. Administration of 0.125 pilocarpine produced 1.5 mm of constriction, suggestive of pupillary denervation. | Midroni and Dyck, 199626 | |
| Brainstem involvement | In a case series of six patients with both CIDP and central nervous system lesions on MRI, one patient had ocular flutter and an INO, one patient had an INO, and two patients had bilateral INO. | Thomas et al., 198727 |
| In a case series of 13 patients with CIDP, one had a history of nystagmus with a lesion seen in the pons on MRI. | Ohtake et al., 199028 | |
| 50-Year-old man with a 2-year history of CIDP, who developed a left abducens nerve palsy at a time when his CIDP was in remission. An MRI showed a high signal lesion in the proximal part of the fila radicularia of the left abducens nerve within the pons as well as other white matter lesions. The diplopia settled spontaneously three months after onset. | Wokke et al., 199629 | |
| 14-Year-old girl with a 10-year history of CIDP, who developed WEBINO, although no brain lesions were visible on MRI. She remained on her normal immunosuppressive therapy and her eye movements improved gradually. | Korkmaz et al., 200230 | |
| Optic neuropathy | In a case series of six patients with both CIDP and central nervous system lesions on MRI, three had attacks of optic neuritis with typical clinical courses. | Thomas et al., 198727 |
| In a case series of six patients with both CIDP and central nervous system lesions on MRI, three had attacks of optic neuritis but clinical details were not reported. | Mendell et al., 198731 | |
| In a case series of 13 patients with CIDP, one had a history of optic neuritis, with delay on VEP from one eye, but swelling of both optic nerves on MRI | Ohtake et al., 199028 | |
| Two cases: The first was a 57-year-old woman with a 1-year history of CIDP, who developed chronic optic neuritis with visual acuities of 20/25 OD and 20/30 OS. An MRI showed gadolinium enhancement of the right optic nerve. The second was in a 52-year-old woman with a 5-year history of CIDP, who developed acute bilateral sequential optic neuritis with spontaneous incomplete recovery. Brain MRI was normal in both cases. | Lee et al., 199932 | |
| 32-Year-old man with a 1-year history of CIDP, who developed relapsing corticosteroid responsive bilateral optic neuritis. Brain MRI was normal but optic nerve MRI on one occasion showed a high signal lesion in the left optic nerve. | Tsai et al., 200033 | |
| 46-Year-old man with a 20-year history of CIDP, who developed bilateral visual loss, which was thought to be due to bilateral corticosteroid-induced cataracts. The loss of vision worsened to less than hand movement perception following cataract extraction. Four months later he died of pneumonia. At autopsy, optic nerve demyelination was seen with axonal preservation. There was also prominent infiltration of the optic nerves by macrophages and microglial cells. | Holtkamp et al., 200134 |
INO = internuclear ophthalmoplegia; MRI = magnetic resonance imaging; MRA = magnetic resonance angiography; VEP = visual evoked potentials; WEBINO = wall-eyed bilateral internuclear ophthalmoplegia.
Papilloedema in association with polyneuritis was first reported by Gilpin et al. in 1936.38 It can occur in association with a number of inflammatory neuropathies apart from CIDP, including Guillain-Barré syndrome and POEMS syndrome (Polyneuropathy, Organomegaly, Endocrinopathy, M-protein, Skin changes).39,40 It occurs due to an unknown mechanism. It was originally felt that CSF protein occluded the arachnoid villi.41 On the basis of biopsy findings in one patient, Joynt proposed that the papilloedema might be due to intracellular cerebral oedema.42 Further theories have proposed that there is increased functional resistance to CSF flow across the absorptive channels,43 or that there is increased effective venous outflow resistance at sites of CSF absorption.44 A raised CSF protein appears to be a prerequisite for its development, with levels ranging from 2.14 to 11.52 g/L, with raised opening pressures (where reported) at lumbar puncture in all cases.15,26,36,45–49 However, cases with papilloedema represent only a small percentage of cases of CIDP with raised CSF protein. The opening pressure in these papilloedema-negative high–CSF protein cases has not been stated though.16,17,19,20 The occurrence of papilloedema with hypertrophic nerve enlargement in CIDP has not previously been reported, even though it is a marker of active disease. This may be because most of the cases series of CIDP with papilloedema were in the pre-MRI era. It is interesting that despite having a raised opening pressure and CSF protein on her original lumbar puncture, the patient in the first case only developed frank papilloedema and visual loss on stopping her immunosuppression. This suggests that, although there was an ongoing inflammatory process that had led to her developing nerve hypertrophy, the immunosuppression had prevented the progression of papilloedema, because it resolved when the immunosuppression was restarted.
Diplopia in CIDP may result from single cranial nerve palsies or as part of a more complex ophthalmoparesis. It is not clear, in the latter case, to what extent this results from a Fisher syndrome–like relapse of CIDP, although in a case where anti-GQ1b antibodies were measured these turned out to be negative.8,37,50 Some of the cases may also be contributed to by mechanical restriction secondary to proptosis cause by hypertrophic nerve enlargement (Table 2),6,8,22,23 although the proptosis in our third case was not accompanied by any degree of ophthalmoparesis.
The best treatment for neuro-ophthalmological complications of CIDP is to treat the underlying condition with appropriate immunosuppression, with reports of resolution of papilloedema,36 diplopia,51,52 and optic neuritis,33 although proptosis tends to persist despite treatment.22 Other treatment options for refractory papilloedema include acetazolamide, optic nerve sheath fenestration, and CSF diversion surgery.27,46,49
Declaration of interest: authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Note: Figures 1, 3, 6 and 7 of this article are available in colour online at www.informahealthcare.com/oph.
References
- 1.Joint Task Force of the EFNS and the PNS Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst 2005;10:220–228 [DOI] [PubMed] [Google Scholar]
- 2.Tackenberg B, Lunemann JD, Steinbrecher A, Rothenfusser-Korber E, Sailer M, Brück W, Schock S, Zschenderlein R, Zipp F, Sommer N. Classifications and treatment responses in chronic immune-mediated demyelinating polyneuropathy. Neurology 2007;68:1622–1629 [DOI] [PubMed] [Google Scholar]
- 3.Lunn MPT, Willison HJ. Diagnosis and treatment in inflammatory neuropathies. J Neurol Neurosurg Psychiatry 2009;80:249–258 [DOI] [PubMed] [Google Scholar]
- 4.Duggins AJ, McLeod JG, Pollard JD, Davies L, Yang F, Thompson EO, Soper JR. Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain 1999;122:1383–1390 [DOI] [PubMed] [Google Scholar]
- 5.Niino M, Tsuji S, Tashiro K. Chronic inflammatory demyelinating polyneuropathy with multiple hypertrophic nerves in intracranial, and intra- and extra-spinal segments. Int Med 1999;38:445–449 [DOI] [PubMed] [Google Scholar]
- 6.Duarte J, Martinez AC, Rodriguez F, Mendoza A, Sempere AP, Claveria LE. Hypertrophy of multiple cranial nerves and spinal roots in chronic inflammatory demyelinating neuropathy. J Neurol Neurosurg Psychiatry 1999;67:685–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pytel P, Rezania K, Soliven B, Frank J, Wollmann R. Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) with hypertrophic spinal radiculopathy mimicking neurofibromatosis. Acta Neuropathol 2003;105:185–188 [DOI] [PubMed] [Google Scholar]
- 8.Alwan AA, Mejico LJ. Ophthalmoplegia, proptosis, and lid retraction caused by cranial nerve hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy. J Neuro-Ophthalmol 2007;27:99–103 [DOI] [PubMed] [Google Scholar]
- 9.Aho TR, Wallace RC, Pitt AM, Sivakumar K. Charcot-Marie-Tooth disease: extensive cranial nerve involvement on CT and MR imaging. AJNR Am J Neuroradiol 2004;25:494–497 [PMC free article] [PubMed] [Google Scholar]
- 10.Yener GG, Guiochon-Mantel A, Obuz F, Baklan B, Öztürk V, Kovanlikaya I, Çakmur R, Genç A. Phe 84 deletion of the PMP22 gene associated with hereditary motor and sensory neuropathy HMSN III with multiple cranial neuropathy: clinical, neurophysiological and magnetic resonance imaging findings. J Neurol 2001;248:193–196 [DOI] [PubMed] [Google Scholar]
- 11.Ferner RE, Hughes RAC, Hall SM, Upadhyaya M, Johnson MR. Neurofibromatous neuropathy in neurofibromatosis 1 (NF1). J Med Genet 2004;41:837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai W, Kassarjian A, Bredell MA, Harris GJ, Yoshida H, Mautner VF, Wenzel R, Plotkin SR. Tumor burden in patients with neurofibromatosis types 1 and 2 and schwannomatosis: determination on whole-body MR images. Radiology 2009;250:665–673 [DOI] [PubMed] [Google Scholar]
- 13.Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982;45:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyck PJ, Lais AC, Ohta M, Bastron JA, Okazaki H, Groover RV. Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc 1975;50:621–637 [PubMed] [Google Scholar]
- 15.McCombe PA, Pollard JD, McLeod JG. Chronic inflammatory demyelinating polyradiculoneuropathy: a clinical and electrophysiological study of 92 cases. Brain 1987;110:1617–1630 [DOI] [PubMed] [Google Scholar]
- 16.Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy: clinical characteristics, course, and recommendations for diagnostic criteria. Arch Neurol 1989;46:878–884 [DOI] [PubMed] [Google Scholar]
- 17.do Nascimento OJM. Chronic inflammatory demyelinating polyradiculoneuropathy (Austin-Dyck syndrome): study of 45 cases. Arq Neuropsiquatr 1995;53:530–531 [Google Scholar]
- 18.Simmons Z, Albers JW, Bromberg MB, Feldman EL. Presentation and initial clinical course in patients with chronic inflammatory demyelinating polyradiculoneuropathy: comparison of patients without and with monoclonal gammopathy. Neurology 1993;43:2202–2209 [DOI] [PubMed] [Google Scholar]
- 19.Maisonabe T, Chassande B, Vérin M, Jouni M, Léger J-M, Bouche P. Chronic dysimmune demyelinating polyneuropathy: a clinical and electrophysiological study of 93 patients. J Neurol Neurosurg Psychiatry 1996;61:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorson KC, Allam G, Ropper AH. Chronic inflammatory demyelinating polyneuropathy: clinical features and response to treatment in 67 consecutive patients with and without a monoclonal gammopathy. Neurology 1997;48:321–328 [DOI] [PubMed] [Google Scholar]
- 21.Bouchard C, Lacroix C, Planté V, Adams D, Chedru F, Guglielmi J-M, Said G. Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology 1999;52:498–503 [DOI] [PubMed] [Google Scholar]
- 22.Guibord N, Chalk C, Wein F, Richardson J, Snipes GJ, Del Carpio R. Trigeminal nerve hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 1998;51:1459–1462 [DOI] [PubMed] [Google Scholar]
- 23.Aidi S, El Alaoui Faris M, Amarti A, Belaïdi H, Jiddane M, Guezzaz M, Medjel A, Chkili T. Chronic inflammatory demyelinating polyradiculoneuropathy with hypertrophy of spinal roots, brachial plexus and cranial nerves (in French). Rev Neurol (Paris) 2002;158:819–823 [PubMed] [Google Scholar]
- 24.Garcin R, Gruner J, Man HX. Documents pour servir a l’etude pathogenique du signe Argyll-Robertson dans la nevrite hypertrophique de Dejerine-Sottas. Bull Acad Natl Med 1959;143:499–508 [PubMed] [Google Scholar]
- 25.Arroyo JG, Horton JC. Acute, painful, pupil-involving third nerve palsy in chronic inflammatory demyelinating polyneuropathy. Neurology 1995;45:846–847 [DOI] [PubMed] [Google Scholar]
- 26.Midroni G, Dyck PJ. Chronic inflammatory demyelinating polyradiculoneuropathy: unusual clinical features and therapeutic responses. Neurology 1996;46:1206–1212 [DOI] [PubMed] [Google Scholar]
- 27.Thomas PK, Walker WH, Rudge P, Morgan-Hughes JA, King RHM, Jacobs JM, Mills KR, Ormerod IEC, Murray NMF, McDonald WI. Chronic demyelinating neuropathy associated with multifocal central nervous system demyelination. Brain 1987;110:53–76 [DOI] [PubMed] [Google Scholar]
- 28.Ohtake T, Komori T, Hirose K, Tanabe H. CNS involvement in Japanese patients with chronic inflammatory demyelinating polyradiculoneuropathy. Acta Neurol Scand 1990;81:108–112 [DOI] [PubMed] [Google Scholar]
- 29.Wokke JHJ, van den Berg LH, van Shaik JPJ. Sixth nerve palsy from a CNS lesion in chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 1996;60:696–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korkmaz A, Topaloglu H, Kansu T. Wall eyed bilateral internuclear ophthalmoplegia in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol 2002;9:687–702 [DOI] [PubMed] [Google Scholar]
- 31.Mendell JR, Kolkin S, Kissel JT, Weiss KL, Chakeres DW, Rammohan KW. Evidence for central nervous system demyelination in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 1987;37:1291–1294 [DOI] [PubMed] [Google Scholar]
- 32.Lee AG, Galetta SL, Lepore FE, Appel SH. Optic atrophy and chronic acquired polyneuropathy. J Neuro-Ophthalmol 1999;19:67–69 [DOI] [PubMed] [Google Scholar]
- 33.Tsai D-C, Lin P-K, Lin K-P, Kao K-P, Liu J-H. Optic neuropathy in a patient with chronic inflammatory demyelinating polyneuropathy. Eye (Lond) 2000;14:911–912 [DOI] [PubMed] [Google Scholar]
- 34.Holtkamp M, Zschenderlein R, Brück W, Weber JR. Chronic inflammatory demyelinating polyradiculoneuropathy with histologically proven optic neuritis. Acta Neuropathol 2001;101:529–531 [DOI] [PubMed] [Google Scholar]
- 35.Donaghy M, Earl CJ. Ocular palsy preceding chronic relapsing polyneuropathy by several weeks. Ann Neurol 1985;17:49–50 [DOI] [PubMed] [Google Scholar]
- 36.Morrison KE, Davies PTG. Chronic inflammatory demyelinating polyneuropathy presenting with headache and papilledema. Headache 1999;39:299–300 [DOI] [PubMed] [Google Scholar]
- 37.Pieh C, Rossillion B, Heritier-Barras AC, Chofflon M, Landis T, Safran AB. Isolated unilateral adduction deficit and ptosis as the presenting features of chronic inflammatory demyelinating polyradiculoneuropathy. J Neuro-Ophthalmol 2002;22:92–94 [DOI] [PubMed] [Google Scholar]
- 38.Gilpin SF, Moersch FP, Kernohan JW. Polyneuritis: a clinical and pathologic study of special group of cases frequently referred to as instances of neuronitis. Arch Neurol Psychiatry 1936;35:937–963 [Google Scholar]
- 39.Morley JB, Reynolds JH. Papilloedema and the Landry-Guillain-Barré syndrome. Brain 1966;89:205–222 [DOI] [PubMed] [Google Scholar]
- 40.Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TT, Larson DR, Greipp PR, Witzig TE, Basu R, Suarez GA, Fonseca R, Lust JA, Gertz MA. POEMS syndrome: definitions and long-term outcome. Blood 2003;101:2496–2506 [DOI] [PubMed] [Google Scholar]
- 41.Denny-Brown DE. The changing pattern of neurologic medicine. N Engl J Med 1952;246:839–846 [DOI] [PubMed] [Google Scholar]
- 42.Joynt RJ. Mechanism of production of papilledema in the Guillain-Barré syndrome. Neurology 1958;8:8–12 [DOI] [PubMed] [Google Scholar]
- 43.Morley JB, Reynolds EH. Papilloedema and the Landry-Guillain-Barré syndrome. Brain 1996;89:205–222 [DOI] [PubMed] [Google Scholar]
- 44.Ropper AH, Marmarou A. Mechanism of pseudotumour in Guillain-Barré syndrome. Arch Neurol 1984;41:259–261 [DOI] [PubMed] [Google Scholar]
- 45.Gross ML, Thomas PK. The treatment of chronic relapsing and chronic progressive idiopathic inflammatory polyneuropathy by plasma exchange. J Neurol Sci 1981;52:69–78 [DOI] [PubMed] [Google Scholar]
- 46.Fantin A, Feist RM, Reddy CV. Intracranial hypertension and papilloedema in chronic inflammatory demyelinating polyneuropathy. Br J Ophthalmol 1993;77:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Zunni SA, Prakash PS, Saitti KM, El-Zunni MA. Chronic inflammatory demyelinating polyneuropathy with papillodema and response to immunoglobulins. Neurosciences 2000;5:69–71 [PubMed] [Google Scholar]
- 48.Sinha UK, James A. Visual disturbance in a patient with progressive limb weakness. Postgrad Med J 2001;77:e1 (http://www.postgradmedj.com/cgi/content/full/77/909/e1) [Google Scholar]
- 49.Thomas S, Tan J, Lawden M, Sampath R. Optic nerve sheath fenestration for intracranial hypertension associated with chronic inflammatory demyelinating polyneuropathy. Ophthal Plast Reconstr Surg 2004;20:325–327 [DOI] [PubMed] [Google Scholar]
- 50.Ryo M, Saito T, Kunii N, Hasegawa H, Kowa H. A case of chronic inflammatory demyelinating polyneuropathy with recurrent ophthalmoplegia, persistent conduction block, antibody activity against gangliosides GM1. Rinsho Shinkeigaku 1994;34:702–706 [PubMed] [Google Scholar]
- 51.Brar AS, Lee AG. Ophthalmoplegia in an adult with chronic inflammatory demyelinating polyradiculoneuropathy. Strabismus 1997;5:1–4 [DOI] [PubMed] [Google Scholar]
- 52.Chalmers AC, Miller RG. Chronic inflammatory demyelinating polyradiculoneuropathy with ophthalmoplegia. J Clin Neuro-ophthalmol 1986;6:166–168 [PubMed] [Google Scholar]