Abstract
Inflammatory optic neuritis represents a frequent clinical situation in neurology and ophthalmology. In those parts of the world where multiple sclerosis is common, it is the condition most discussed as the cause of optic neuritis. However, the risk for conversion from optic neuritis to multiple sclerosis is evaluated at only around 50% after 15 years of follow-up. The risk is higher in cases in whom abnormalities typical of multiple sclerosis are found on magnetic resonance imaging of the brain and oligoclonal bands found on cerebrospinal fluid protein electrophoresis with no corresponding bands in serum. When these investigations are normal, optic neuritis is usually considered as “idiopathic” with a suspected viral aetiology, but in some cases, a systemic disease such as sarcoidosis, systemic lupus erythematosis, or Sjögren syndrome may be diagnosed. In rare cases, either recurrent optic neuritis or myelitis may occur without any evidence for multiple sclerosis. In the first case, it corresponds to a recently characterised disorder referred to as chronic relapsing inflammatory optic neuropathy and in the second case to a recently better identified entity, neuromyelitis optica. In the present paper, the differential diagnosis of inflammatory optic neuritis is presented from multiple sclerosis to infectious optic neuritis, systemic disease, and neuromyelitis optica.
Keywords: Multiple sclerosis, neuromyelitis optica, optic neuritis
Diagnosis of Inflammatory Optic Neuritis
Optic neuritis (ON) is an acute inflammatory demyelinating syndrome of the central nervous system and represents a common clinical presentation in both neurology and ophthalmology. In acute demyelinating ON, patients typically present with a short progressive unilateral vision loss of variable severity. Periocular and retro-orbital pain is observed in more than 90% and most patients show reduced contrast sensitivity, dyschromatopsia, and visual field defects.1 In those parts of the world where multiple sclerosis (MS) is common, it is also the most frequent single cause of ON and the Optic Neuritis Treatment Trial (ONTT) showed that 50% of ON patients had converted to clinically definite MS after a 15-year follow-up period.2 In this study, the risk of MS increased to around 80% in cases showing one or more typical brain magnetic resonance imaging (MRI) lesions and also in cases showing oligoclonal bands (OCBs) on cerebrospinal fluid (CSF) protein electrophoresis, although not independently of MRI findings.
When MRI, biological, and CSF analyses are normal, ON is usually considered as idiopathic with a suspected viral aetiology. However, the first step in the task of ON diagnosis is to be sure that the origin is inflammatory. Before confirming the diagnosis of inflammatory ON, there are several diagnoses to be considered, especially if there are certain “red flags” (lack of pain, age >50, vascular risk factors, atypical changes on fundus examination, etc.). Two common diagnoses in this situation are firstly meningioma,3 especially in women over 45–50 years of age with progressive visual loss, and secondly anterior ischaemic optic neuropathy (AION), especially in cases of very acute visual loss frequently associated with altitudinal visual field defect.4 In these two disorders, patients are frequently older than in inflammatory ON and pain is usually lacking.
Optic Neuritis in MS
The principal cause suspected during a first ON episode is the inaugural manifestation of MS, but ON may also occur in the setting of other pathologies such as neuromyelitis optica (NMO), infectious diseases, or autoimmune diseases. The 15-year follow-up report from the ONTT had previously considered a conversion rate of 50% (78% with an abnormal baseline brain MRI, 25% with a normal MRI) to MS after isolated ON in adults.2 With another look, this result also means that about 50% of patients will not develop MS after a first episode of ON, especially if brain MRI and CSF analyses are normal, even after a relatively long-term follow-up. Where no cause is found, the term “idiopathic ON” is often employed, which is probably a heterogeneous group of patients with “unknown” or “not yet known” diagnosis. Patients may be diagnosed as MS, NMO, or other diseases even after a very long time (10/15 years or more), but after such a long time period the number of patients who convert remains very low.
Optic Neuritis due to Infection
One important differential diagnosis of ON due to MS is infectious ON. This is a relatively rare condition and corresponds more frequently to neuroretinitis rather than typical ON. The ophthalmologist frequently makes the diagnosis due to macular oedema associated with macular star, which is pathognomonic of infection, especially Bartonella.5 However, in several situations (high-risk population for sexually transmissible diseases; high-risk region for Lyme disease; immunosuppression), the clinician must consider infectious or parainfectious ON. For example, although relatively rare, we recently report typical cases of ON secondary to Lyme disease.6 Cerebrospinal analysis frequently shows lymphocytic meningitis and a high intrathecal index for Borrelia, which is a very highly specific but only 70% sensitive test for “neurolyme.”7 Recently, we also observed in our department an increased number of cases with ON secondary to syphilis or human immunodeficiency virus (HIV), but the majority of these cases were a neuroretinitis syndrome rather than typical ON without signs of cerebral inflammation on MRI or latency delay on visual evoked potentials (unpublished data). Also observed is a post-infectious ON, isolated after various infections or vaccination or as a component of a larger syndrome such as Guillain-Barré syndrome or acute disseminated encephalomyelitis (ADEM). In this group of disorders, ON is frequently bilateral and associated with other neurological symptoms, including severe deficit and alteration of consciousness.8 In cases of post-infectious ON, intravenous corticosteroid treatment may be used as for other inflammatory ON, but the clinician has to be sure that infection is eradicated before employing such therapy.
Optic Neuritis due to NMO
ON may be the first symptom of NMO in about 60% of cases,9,10 as ophthalmological symptoms alone (40–50%) or associated with myelitis (10–20%). However, the frequency of this inflammatory disease is relatively low, evaluated around 1/100 compared with MS in Europe and North America.11 In Japan and South America, including the Caribbean region, the prevalence of the disease may be higher and may be as frequent as MS.12 From a clinical point of view, ON secondary to MS or NMO may be significantly different. The initial presentation may be similar but NMO is believed to cause very severe and often bilateral, visual disability and optic nerve damage. In MS, attacks tend to be less severe and have a better visual prognosis.13,14 These data suggest that a greater retinal nerve fibre layer (RNFL) thickness reduction (<15 µm) measured by optical coherence tomography (OCT) can be helpful in distinguishing MS from NMO. However, this is significant at the group level but an important overlap is observed between the two groups of disease.
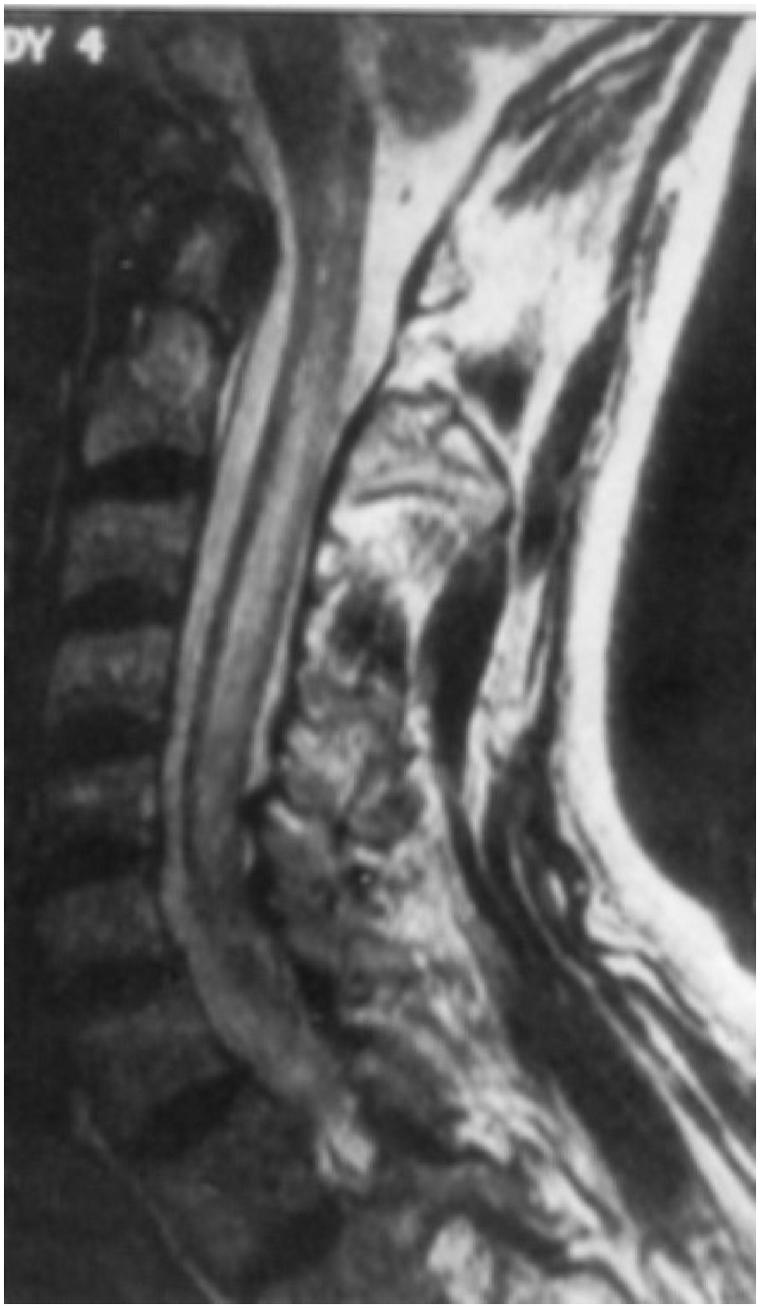
Brain MRI is frequently normal in NMO but can show brainstem or periventricular (particularly periependymal) lesions, especially in young patients. Spinal cord MRI may show extended T2 hypersignal, encompassing three vertebral segments or more (Figure 1). However, spinal cord episodes may occur many years after one or more ON episodes. This finding is usually observed only in patients with spinal cord symptoms, contrary to what is observed in MS where asymptomatic spinal cord lesions are frequent).15 CSF analysis shows OCB in about 20% only compared with 90% in MS. There is also frequently a greater leukocytosis CSF, especially polymorphonuclear cells.16
FIGURE 1.
Typical extended T2-weighted hypersignal in the spinal cord of a NMO patient).
The most informative test for distinguishing MS and NMO, along with brain and spinal cord MRI, is the titre of anti-aquaporin 4 (AQP-4) antibodies discovered in 2004.17 This test is very specific for NMO but sensitivity is between 50% and 80% depending upon methodology and population.18 In 2006, new diagnostic criteria for NMO, including ON and myelitis, were proposed, plus two of the following three criteria: normal brain MRI, positive AQP-4 antibodies, and a longitudinally extensive lesion on spinal cord MRI.19
Because of the high frequency of ON, the question should be asked whether it is necessary to test all cases of ON for this antibody (problem of cost and also possible false positive, although this situation remains rare). In first-line investigation of ON, we propose to test severe, recurrent, or bilateral ON only.
The differentiation between NMO and MS is essential for therapeutic and management decisions because the treatments are quite different. It was demonstrated that immunosuppressor rather than immunomodulatory therapy (such as interferon-β) are more effective in NMO.20,21 In several cases it was demonstrated that interferon-β may increase disease activity.22 Rituximab seems to be one of the most promising drug in NMO and this treatment may therefore be considered in severe ON, associated with positive AQP-4 antibodies.23
Optic Neuritis due to Systemic Diseases
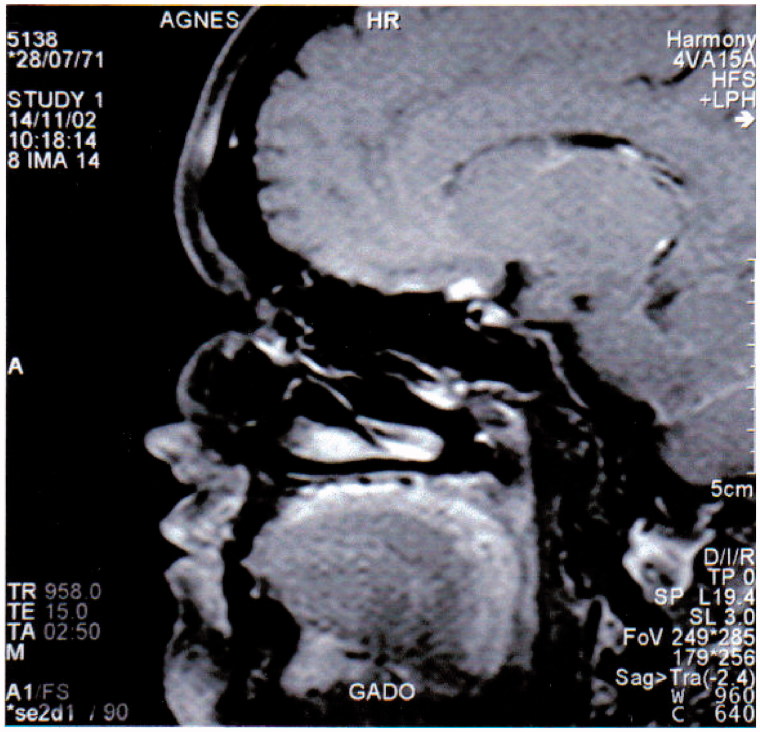
ON may also be a part of a systemic disease such as systemic lupus erythematosus, sarcoidosis, or Sjögren syndrome. However, in our experience, and when a large screening of the literature is made, the frequency of these pathologies is very low. The most frequent findings may be Sjögren syndrome,24–26 sometimes associated with myelitis leading to a diagnosis of NMO, this association being also observed with lupus.27 Another possible diagnosis, in the field of systemic disease, is sarcoidosis. In this case, we frequently observed perineuritis with granuloma or infiltration of the perioptic or chiasmal region (Figure 2). Salivary gland biopsy may be of major interest for these two last diagnoses (Sjögren and sarcoidosis).
FIGURE 2.
Bilateral optic neuritis in a patient with neurosarcoidosis. MRI post-gadolinimum injection T1-weighted image showing a chiasmal granuloma.
Recurrent Optic Neuritis
Although ON is frequently limited to a single episode, 3–5% of patients experience recurrent episodes (affecting either or both eyes, sequentially or simultaneously) with a negative workup for MS, NMO, or other causes.28,29 That disorder has been recently named relapsing inflammatory ON (RION) but remains poorly described in view of the lack of large cohorts.30–32 Medical literature describes two forms of RION. The first one is a chronic form named CRION,33 which is unilateral or bilteral progressive ON frequently relapsing after withdrawal of corticosteroids and with a frequent progression of visual loss between episodes. The second one is RION,30,31,34 which is a non-progressive relapsing ON, frequently without steroids dependence. However, these two conditions are frequently overlapping for both aetiologies and outcome, and studies in larger populations are needed for a better classification of each group. As for isolated ON, RION may progress to demyelinating central nervous system (CNS) diseases, including MS, NMO, or systemic diseases. Study of their natural history has demonstrated a global risk of progression to MS considerably lower than in the ONNT. In a previous study, the combined conversion rate to MS or NMO without differentiation was 27% at 5 years and 42% at 10 years.29 The discovery of AQP-4 antibodies, specific for patients with NMO, has also changed our understanding of RION.17 This antibody is a valuable tool to define an extended spectrum of NMO disorder, distinguishing, among inflammatory demyelinating diseases of the CNS, several features corresponding to NMO spectrum with similar epidemiology, immunopathology, disease course, and prognosis than NMO.17,18 Recent studies report that about 20–25% of the patients with RION converted to NMO within 5 years, with a higher rate (50%) in positive AQP-4 antibodies group, than in the seronegative group (10%).30,35 To date, only six clinical studies have been published. Although these studies provide useful data, they have several limitations. The first two studies were processed before the identification of AQP-4 antibodies, making difficult any interpretation regarding NMO diagnosis.29,31 The other four studies mainly explored the association between RION and the frequency of AQP-4 antibodies without focusing on clinical and paraclinical data.30,34–36 In addition, description of RION by these studies expressed a disagreement about several features. There is no consensus on the existence of two forms of RION, one recurrent (RION) and one chronic and/or corticosteroid dependent (CRION), nor is there a clear RION nosology (expanding spectrum of NMO, atypical MS, or a new autoimmune disease).
We recently performed a study on 62 patients with relapsing ON and we showed that about 70% correspond to RION and 30% to CRION (submitted). After 8 years of follow-up, we distinguished three groups: 20% of patients with a high risk of MS (few MRI lesions not fulfilling MS criteria and/or oligoclononal bands), 10% with a high risk of NMO (positive anti-AQP4 antibodies), and 10% associated with a systemic disease. The 60% remaining patients seems to correspond to a subgroup of “idiopathic” RION that could be classified as a separated autoimmune entity, but we cannot exclude that several patients will convert later to one of the three other subgroups during a longer follow-up. In this study, we also individualized two groups of patients with a poor prognosis (high risk of NMO and CRION patients) that may be treated early with immunosuppressive treatments (oral or intravenous immunosuppressive drugs depending on the severity of the disease).
Conclusion
ON is a frequent symptom, mainly (50%) associated with MS, but there are also many other conditions that may be evoked especially when some red flags are present. In a number of cases of ON, aetiology remains “unknown” or “not yet known” and follow-up of patients is of importance regarding the possible evolution to MS, NMO, or vasculitis. However, in other cases, ON remains idiopathic even after a large workup and a long follow-up and a viral cause is suggested but usually without serological proof. Finally, several ON are recurrent, corresponding to RION or CRION, two new entities recently identified in the literature that seem to be autoimmune entities possibly separate from other aetiologies.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
Note: Figure 2 of this article is available in colour online at www.informahealthcare.com/oph.
References
- 1.Beck RW, Cleary PA, Anderson MM, Jr, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992;326:581–588 [DOI] [PubMed] [Google Scholar]
- 2.ONTT Group Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol 2008;65:727–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouyon M, Blanc F, Ballonzoli L, Fleury M, Zaenker C, Speeg-Schatz C, de Seze J. Optic neuropathy and meningioma: a diagnostic trap. J Fr Ophtalmol 2013;36:221–229 [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS, Zimmerman B. Visual field abnormalities in nonarteritic anterior ischemic optic neuropathy: their pattern and prevalence at initial examination. Arch Ophthalmol 2005;123:1554–1562 [DOI] [PubMed] [Google Scholar]
- 5.Chi SL, Stinnett S, Eggenberger E, Foroozan R, Golnik K, Lee MS, Bhatti MT. Clinical characteristics in 53 patients with cat scratch optic neuropathy. Ophthalmology 2012;119:183–187 [DOI] [PubMed] [Google Scholar]
- 6.Blanc F, Ballonzoli L, Marcel C, De Martino S, Jaulhac B, de Seze J. Lyme optic neuritis. J Neurol Sci 2010;295:117–119 [DOI] [PubMed] [Google Scholar]
- 7.Blanc F, Jaulhac B, Fleury M, de Seze J, de Martino SJ, Remy V, Blaison G, Hansmann Y, Christmann D, Tranchant C. Relevance of the antibody index to diagnose Lyme neuroborreliosis among seropositive patients. Neurology 2007;69:953–958 [DOI] [PubMed] [Google Scholar]
- 8.de Seze J, Debouverie M, Zephir H, Lebrun C, Blanc F, Bourg V, Wiertlewski S, Pittion S, Laplaud D, Le Page E, Deschamps R, Cabre P, Pelletier J, Malikova I, Clavelou P, Jaillon V, Defer G, Labauge P, Gout O, Boulay C, Edan G, Vermersch P. Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol 2007;64:1426–1432 [DOI] [PubMed] [Google Scholar]
- 9.Collongues N, Marignier R, Zéphir H, Papeix C, Blanc F, Ritleng C, Tchikviladzé M, Outteryck O, Vukusic S, Fleury M, Fontaine B, Brassat D, Clanet M, Milh M, Pelletier J, Audoin B, Ruet A, Lebrun-Frenay C, Thouvenot E, Camu W, Debouverie M, Créange A, Moreau T, Labauge P, Castelnovo G, Edan G, Le Page E, Defer G, Barroso B, Heinzlef O, Gout O, Rodriguez D, Wiertlewski S, Laplaud D, Borgel F, Tourniaire P, Grimaud J, Brochet B, Vermersch P, Confavreux C, de Seze J. Neuromyelitis optica in France: A multicenter study of 125 patients. Neurology 2010;74:736–742 [DOI] [PubMed] [Google Scholar]
- 10.Jarius S, Ruprecht K, Wildemann B, Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, Kleiter I, Kleinschnitz C, Berthele A, Brettschneider J, Hellwig K, Hemmer B, Linker RA, Lauda F, Mayer CA, Tumani H, Melms A, Trebst C, Stangel M, Marziniak M, Hoffmann F, Schippling S, Faiss JH, Neuhaus O, Ettrich B, Zentner C, Guthke K, Hofstadt-van Oy U, Reuss R, Pellkofer H, Ziemann U, Kern P, Wandinger KP, Bergh FT, Boettcher T, Langel S, Liebetrau M, Rommer PS, Niehaus S, Münch C, Winkelmann A, Zettl U UK, Metz I, Veauthier C, Sieb JP, Wilke C, Hartung HP, Aktas O, Paul F. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflamm 2012;19:14–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabre P. Environmental changes and epidemiology of multiple sclerosis in the French West Indies. J Neurol Sci 2009;286:58–61 [DOI] [PubMed] [Google Scholar]
- 12.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol 2012;69:1176–1178 [DOI] [PubMed] [Google Scholar]
- 13.Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, Calabresi PA, Kerr DA. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 2009;73:302–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Seze J, Blanc F, Jeanjean L, Zephir H, Labauge P, Bouyon M, Zéphir H, Labauge P, Bouyon M, Ballonzoli L, Castelnovo G, Fleury M, Defoort S, Vermersch P, Speeg C. Optical coherence tomography in neuromyelitis optica. Arch Neurol 2008;65:20–23 [DOI] [PubMed] [Google Scholar]
- 15.Traboulsee AL, Li DK. The role of MRI in the diagnosis of multiple sclerosis. Adv Neurol. 2006;98:125–146 [PubMed] [Google Scholar]
- 16.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999;53:1107–1114 [DOI] [PubMed] [Google Scholar]
- 17.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112 [DOI] [PubMed] [Google Scholar]
- 18.Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol 2010;6:383–392 [DOI] [PubMed] [Google Scholar]
- 19.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 20.Collongues N, de Seze J. Current and future treatment approaches for neuromyelitis optica. Ther Adv Neurol Disord 2011;4:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B, Lebrun C, Moreau T, Vermersch P, Fontaine B, Lyon-Caen O, Gout O. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007;13:256–259 [DOI] [PubMed] [Google Scholar]
- 22.Shimizu J, Hatanaka Y, Hasegawa M, Iwata A, Sugimoto I, Date H, Goto J, Shimizu T, Takatsu M, Sakurai Y, Nakase H, Uesaka Y, Hashida H, Hashimoto K, Komiya T, Tsuji S. IFNbeta-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology 2010;75:1423–1427 [DOI] [PubMed] [Google Scholar]
- 23.Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 2005;64:1270–1272 [DOI] [PubMed] [Google Scholar]
- 24.Tesar JT, Mcmillan V, Molina R, Armstrong J. Optic neuropathy and central nervous system disease associated with primary Sjögren's syndrome. Am J Med 1992;92:686–692 [DOI] [PubMed] [Google Scholar]
- 25.Delalande S, de Seze J, Fauchais AL, Hachulla E, Stojkovic T, Ferriby D, Dubucquoi S, Pruvo JP, Vermersch P, Hatron PY. Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Medicine (Baltimore) 2004;83:280–291 [DOI] [PubMed] [Google Scholar]
- 26.Govoni M, Padovan M, Rizzo N, Trotta F. CNS involvement in primary Sjögren’s syndrome: prevalence, clinical aspects, diagnosis assessment and therapeutic approach. CNS Drugs 2001;15:597–607 [DOI] [PubMed] [Google Scholar]
- 27.Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, Lucchinetti CF, Zéphir H, Moder K, Weinshenker BG. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol 2008;65:78–83 [DOI] [PubMed] [Google Scholar]
- 28.Lucchinetti CF, Kiers L, O'Duffy A, Gomez MR, Cross S, Leavitt JA, O'Brien P, Rodriguez M. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology 1997;49:1413–1418 [DOI] [PubMed] [Google Scholar]
- 29.Pirko I, Blauwet LA, Lesnick TG, Weinshenker BG. The natural history of recurrent optic neuritis. Arch Neurol 2004;61:1401–1405 [DOI] [PubMed] [Google Scholar]
- 30.Matiello M, Lennon VA, Jacob A, Pittock SJ, Lucchinetti CF, Wingerchuk DM, Weinshenker BG. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology 2008;70:2197–2200 [DOI] [PubMed] [Google Scholar]
- 31.Arndt C, Labauge P, Speeg-Schatz C, Jeanjean L, Fleury M, Castelnovo G, Ballonzolli L, Blanc F, Carlander B, de Sèze J. Recurrent inflammatory optic neuropathy. J Fr Ophtalmol 2008;31:363–367 [DOI] [PubMed] [Google Scholar]
- 32.de Seze J, Arndt C. Recurrent inflammatory optic neuritis and neuromyelitis optica. Rev Neurol 2010;166:966–969 [DOI] [PubMed] [Google Scholar]
- 33.Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION). Brain 2003;126:276–284 [DOI] [PubMed] [Google Scholar]
- 34.Petzold A, Pittock S, Lennon V, Maggiore C, Weinshenker BG, Plant GT. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. J Neurol Neurosurg Psychiatry 2011;81:109–111 [DOI] [PubMed] [Google Scholar]
- 35.de Seze J, Arndt C, Jeanjean L, Zephir H, Blanc F, Labauge P, Bouyon M, Ballonzoli L, Fleury M, Vermersch P, Speeg C. Relapsing inflammatory optic neuritis: is it neuromyelitis optica? Neurology 2008;70:2075–2076 [DOI] [PubMed] [Google Scholar]
- 36.Jarius S, Frederikson J, Waters P, Paul F, Akman-Demir G, Marignier R, Franciotta D, Ruprecht K, Kuenz B, Rommer P, Kristoferitsch W, Wildemann B, Vincent A. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci 2010;298:158–162 [DOI] [PubMed] [Google Scholar]