Abstract
INTRODUCTION
While there are many reported advantages to laparoscopic surgery compared to open surgery, the impact of a laparoscopic approach on postoperative morbidity in obese patients undergoing rectal surgery has not been studied. Our goal was to determine if obese patients undergoing laparoscopic rectal surgery experienced the same benefits as non-obese patients.
METHODS
We identified patients undergoing rectal resections using the National Surgical Quality Improvement Project (NSQIP) participant use data file. We performed multivariable analyses to determine the independent association between laparoscopy and postoperative complications.
RESULTS
26,437 patients underwent rectal resection. The mean age was 58.5 years, 32.6% were obese, and 47.2% had cancer. Laparoscopic procedures were slightly less common in obese patients compared to non-obese patients (36.0% vs. 38.2%, p=0.0006). In unadjusted analyses, complications were lower with the laparoscopic approach in both obese (18.9% vs. 32.4%, p<0.0001) and non-obese (15.6% vs. 25.3%, p<0.0001) patients. In a multivariable analysis controlling for potential confounders, the risk of postoperative complications increased as the degree of obesity worsened. The likelihood of experiencing a postoperative complication increased by 25%, 45%, and 75% for obese class I, obese class II, and obese class III patients respectively. A laparoscopic approach was associated with a 40% decreased odds of a postoperative complication for all patients (OR 0.60, 95% CI 0.56-0.64).
CONCLUSION
Laparoscopic rectal surgery is associated with fewer complications when compared to open rectal surgery in both obese and non-obese patients. Obesity was an independent risk factor for postoperative complications. In appropriately selected patients, rectal surgery outcomes may be improved with a minimally invasive approach.
Keywords: obesity, pelvic surgery, surgical outcomes, laparoscopic vs. open surgery, rectal surgery
INTRODUCTION
Greater than two-thirds of Americans are obese (body mass index (BMI) ≥ 30 kg/m2) or overweight (BMI 25.0 - 29.9 kg/m2).[1, 2] For patients undergoing surgery, obesity is known to be associated with increased postoperative complications and increased technical difficulty.[3] The increased adipose tissue hinders both adequate exposure and direct visualization and often adds to the surgical complexity, resulting in increased operative times and technical complications.[4] Obesity has been shown to independently increase the risk of atelectasis, thromboembolic events, colorectal anastomotic leakage, and surgical site infections in patients undergoing colorectal resection.[4, 5]
Compared to open surgery, laparoscopic surgery has been associated with fewer postoperative complications, earlier mobilization, and lower infection rates.[6] Compared to the open approach, laparoscopy facilitates visualization in a narrow pelvis, reduces hospital length of stay, and results in fewer postoperative complications.[7-10] As a result, laparoscopy has the potential to minimize surgical morbidity and mortality for obese patients. However, in obese patients laparoscopy is also more challenging due to larger amounts of intraabdominal/visceral fat and potential difficulty obtaining peritoneal access and maintaining pneumoperitoneum. As such, it is unknown whether obese patients undergoing laparoscopic rectal surgery derive the same benefits from a minimally invasive approach as non-obese patients.
The focus of this study was to characterize the outcomes of open and laparoscopic rectal surgery in obese patients and non-obese patients using data from the American College of Surgeons National Quality Improvement Program (ACS-NSQIP). We hypothesized that outcomes would be worse with increasing body mass index (BMI) and that laparoscopic rectal surgery compared to open surgery would improve outcomes for both obese and non-obese patients, with particular effect in the obese population.
METHODS
This study was submitted for review to the Institutional Review Board at the University of Texas Medical Branch and was deemed to be exempt.
Data Source
Data from the ACS-NSQIP Participant Use Data Files (PUF) was used. The ACS-NSQIP is a risk-adjusted, outcomes based, quality improvement program designed to prospectively collect 30-day morbidity and mortality for all major surgical procedures.[11] As a participating institution, the University of Texas Medical Branch was granted access to the data for research purposes.
Study Sample
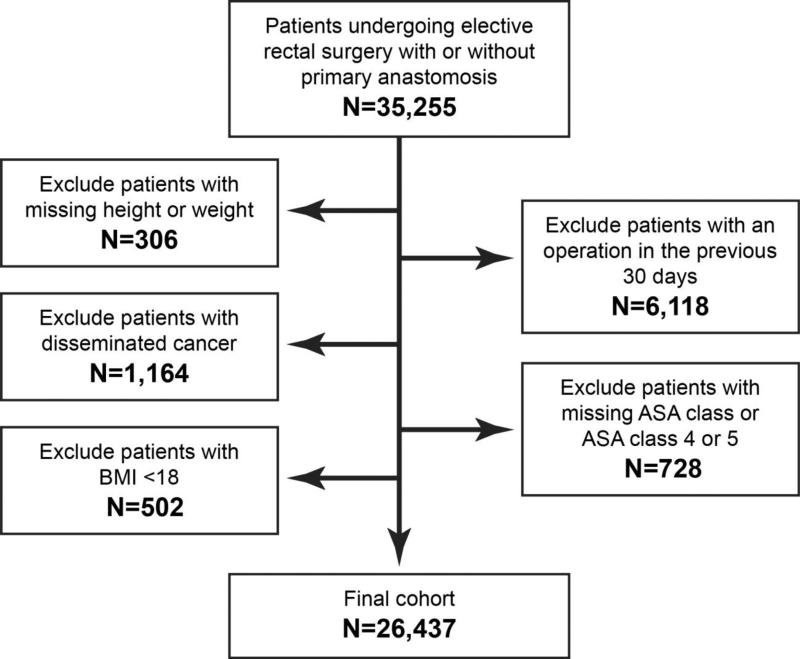
Using PUF data from 2005 through 2011, we identified patients with Current Procedural Terminology (CPT) codes for open and laparoscopic rectal resections with or without primary anastomosis (CPT codes: 45110, 45111, 45112, 45113, 45119, 45120, 44145, 44146, 44155, 44207, 44208, 44211, 44212, 44157, 44158, 45395, 45397). Only patients undergoing elective operations were included. Patients with a previous operation within 30 days, American Society of Anesthesiologists (ASA) class of 4 or 5, and patients with disseminated cancer were excluded. Patients with missing ASA class, height, or weight measurements, and patients with a BMI <18 were also excluded (Figure 1).
Figure 1.
ACS-NSQIP cohort selection 2005-2011. We identified patients with CPT codes for elective rectal resections with or without primary anastomosis. Patients who had had a previous operation within 30 days, those with an ASA class of 4 or 5, or were missing ASA class, height, or weight measurements, patients with disseminated cancer, and patients with a BMI <18 were excluded.
Outcome Measures
The outcomes studied included surgical site infections (SSI)/deep organ space infections or dehiscence, pulmonary complications (unplanned intubation, pneumonia, or failure to wean from the ventilator in 48 hours), acute renal failure, thromboembolic events (pulmonary embolism, DVT requiring therapy, or DVT/thrombophlebitis), sepsis, need for blood transfusions, return to the operating room, operative time, hospital length of stay, and 30-day mortality. Overall 30-day morbidity was defined as any of the complications listed above.
Covariates
Patient characteristics included age, BMI, sex, race, ASA class, tobacco use, alcohol use, presence of malignancy, and comorbid conditions. Operative factors included type of procedure (laparoscopic vs. open), wound classification, and creation of a primary bowel anastomosis without a diverting ostomy. BMI categories were defined as: normal: BMI 18 to < 25 kg/m2, overweight: BMI 25 to < 30 kg/m2, obese class I: BMI 30 to < 35 kg/m2, obese class II: BMI 35 to < 40 kg/m2, and obese class III: BMI ≥ 40 kg/m2. Malignancy was identified using postoperative diagnosis ICD-9 codes. Primary bowel anastomosis without a diverting stoma was defined using the following CPT codes: 45111, 45112, 45113, 45120, 44145, and 44207.
Statistical Analysis
Baseline characteristics of the study cohort were described using descriptive statistics. Bivariate analysis was performed to evaluate the patient factors associated with the operative approach for both obese and non-obese patients; chi-square tests were used for categorical variables and t-test for continuous variables. A multivariable logistic regression model was constructed for the entire cohort controlling for patient level factors and including the interaction between operative approach and obesity status to determine the association between operative approach and complications. The interaction was not significant in the multivariable model; therefore, it was not included in the final model. In the final multivariable logistic regression model, BMI was entered as a categorical variable.
Analyses were performed with SAS version 9.3 (SAS Inc., Cary, NC, USA). Statistical significance was accepted at the p<0.05 level. All p-values were from two-sided tests.
RESULTS
We identified 26,437 patients undergoing elective rectal resections with or without primary bowel anastomosis. Their characteristics are summarized in Table 1. The mean age of the cohort was 58.5 ± 14.9 years and the mean BMI was 28.3 ± 6.2 kg/m2. Thirty-two percent of patients (N=8,613) were classified as obese (5,247 patients obese class I, 2,134 patients obese class II, and 1,232 patients obese class III). Cancer was present in 47.2% of the cohort and diabetes was documented in 12.2%. Open procedures were more commonly performed, with 62.6% of the cohort having an open operation. A primary bowel anastomosis without a protective diverting stoma was performed in 16,878 patients (63.8%). Postoperative complications occurred in 6,221 patients (23.5%) and 30-day operative mortality was 0.7% for the overall cohort.
Table 1.
Overall cohort and bivariate analysis comparing patient demographic factors in non-obese and obese patients.
| Factor | Overall cohort N=26,437 | Normal N=8,622 | Overweight N=9,202 | Class I Obesity N=5,247 | Class II Obesity N=2,134 | Class III Obesity N=1,232 | p-value |
|---|---|---|---|---|---|---|---|
| Age (mean; years) | 58.5 ± 14.9 | 58.5 ± 17.1 | 59.3 ± 14.4 | 58.5 ± 13.1 | 56.7 ± 12.8 | 55.1 ± 12.3 | <0.0001 |
| BMI (mean; kg/m2) | 28.3 ± 6.2 | 22.3 ± 1.8 | 27.4 ± 1.4 | 32.2 ± 1.4 | 37.1 ± 1.4 | 45.1 ± 5.6 | NA |
| Obesity (yes) | 8,613 (32.6%) | NA | NA | NA | NA | NA | NA |
| BMI category (kg/m2) | NA | ||||||
| Normal: < 25 | 8,622 (32.6%) | 8,622 (100%) | NA | NA | NA | NA | |
| Overweight: 25 to < 30 | 9,202 (34.8%) | NA | 9,202 (100%) | NA | NA | NA | |
| Class I Obesity: 30 to < 35 | 5,247 (19.8%) | NA | NA | 5,247 (100%) | NA | NA | |
| Class II Obesity: 35 to < 40 | 2,134 (8.1%) | NA | NA | NA | 2,134 (100%) | NA | |
| Class III Obesity: ≥ 40 | 1,232 (4.7%) | NA | NA | NA | NA | 1,232 (100%) | |
| Gender | |||||||
| Female | 12,846 (48.7%) | 4,739 (36.9%) | 3,758 (29.2%) | 2,410 (18.8%) | 1,165 (9.1%) | 774 (6.0%) | <0.0001 |
| Race | <0.0001 | ||||||
| White | 21,524 (89.6%) | 6,922 (32.2%) | 7,518 (34.9%) | 4,336 (20.1%) | 1,755 (8.2%) | 993 (4.6%) | |
| Black | 1,628 (6.8%) | 463 (28.4%) | 532 (32.7%) | 343 (21.1%) | 171 (10.5%) | 119 (7.3%) | |
| Hispanic | 190 (0.8%) | 65 (34.2%) | 67 (35.3%) | 37 (19.5%) | 16 (8.4%) | 5 (2.6%) | |
| Other | 666 (2.8%) | 371 (55.7%) | 196 (29.4%) | 68 (10.2%) | 21 (3.2%) | 10 (1.5%) | |
| ASA class | <0.0001 | ||||||
| 1 | 916 (3.5%) | 399 (43.5%) | 372 (40.6%) | 107 (11.7%) | 31 (3.4%) | 7 (0.8%) | |
| 2 | 14,868 (56.2%) | 4,974 (33.4%) | 5,433 (36.5%) | 2,966 (20.0%) | 1,039 (7.0%) | 456 (3.1%) | |
| 3 | 10,653 (40.3%) | 3,249 (30.5%) | 3,397 (31.9%) | 2,174 (20.4%) | 1,064 (10.0%) | 769 (7.2%) | |
| Cancer (yes) | 12,488 (47.2%) | 4,107 (32.9%) | 4,412 (35.3%) | 2,427 (19.4%) | 938 (7.5%) | 604 (4.9%) | 0.0042 |
| Tobacco use (yes) | 4,782 (18.1%) | 1,852 (38.7%) | 1,573 (32.9%) | 850 (17.8%) | 325 (6.8%) | 182 (3.8%) | <0.0001 |
| Alcohol use (yes) | 938 (3.6%) | 315 (33.6%) | 358 (38.2%) | 193 (20.6%) | 49 (5.2%) | 23 (2.4%) | <0.0001 |
| Diabetes (yes) | 3,220 (12.2%) | 523 (16.2%) | 995 (30.9%) | 865 (26.9%) | 481 (14.9%) | 356 (11.1%) | <0.0001 |
Unadjusted Analysis-Non-obese versus Obese Patients
Obese patients had a mean BMI of 35.2 ± 5.2 kg/m2. They were more likely to be younger, female, black, diabetic, and have a higher ASA class than non-obese patients and less likely to use alcohol and tobacco (Table 1).
Unadjusted operative factors and operative outcomes are shown in Table 2. Laparoscopic procedures were performed more commonly in non-obese patients (38.2% vs. 36.0%; p=0.0006). There was no difference in laparoscopic surgery rates versus open surgery rates between obese men and women. Laparoscopic surgery was performed in 17.7% of men and 18.2% of women (P=NS). While wound classification was similar, obese patients were more likely to undergo primary bowel anastomosis without a diverting stoma. The absence of a protective stoma in this population did not lead to a higher rate of overall complications, deep space infections, or sepsis in these patients. Obese patients with a primary bowel anastomosis without a diverting stoma had a postoperative complication rate of 24.2% compared to a rate of 34.4% for patients with a diverting stoma (p<0.0001). The deep or organ space infection rate for this group was 6.5% vs. 4.1% for patients with and without a stoma respectively (p<0.0001). Similarly, the rate of sepsis was higher in patients with a stoma (8.8% vs. 5.5%; p<0.0001). Operative times were longer in the obese group. Obese patients had a higher 30-day morbidity rate (27.5% vs. 21.6%; p<0.0001) and a higher rate of wound, pulmonary, renal, thromboembolic, and septic complications compared to non-obese patients. Obese patients also had more reoperations within the 30-day postoperative period and more blood transfusions (Table 2). Despite the increased complication rates, mean lengths of stay were similar between the two groups (7.7 vs. 7.8 days).
Table 2.
Bivariate analysis comparing operative factors and morbidity and mortality in obese and non-obese patients.
| Factor | Normal N=8,622 | Overweight N=9,202 | Class I Obesity N=5,247 | Class II Obesity N=2,134 | Class III Obesity N=1,232 | p-value |
|---|---|---|---|---|---|---|
| Procedure type | <0.0001 | |||||
| Open | 5,405 (62.7%) | 5,620 (61.1%) | 3,239 (61.7%) | 1,423 (66.7%) | 853 (69.2%) | |
| Laparoscopic | 3,217 (37.3%) | 3,582 (38.9%) | 2,008 (38.3%) | 711 (33.3%) | 379 (30.8%) | |
| Wound classification | NS | |||||
| Clean | 19 (0.2%) | 18 (0.2%) | 16 (0.3%) | 4 (0.2%) | 7 (0.65%) | |
| Clean contaminated | 7,150 (82.9%) | 7,605 (82.6%) | 4,315 (82.2%) | 1,735 (81.3%) | 1,006 (81.67%) | |
| Contaminated | 1,026 (11.9%) | 1,105 (12.0%) | 650 (12.4%) | 291 (13.6%) | 144 (11.7%) | |
| Dirty/Infected | 427 (5.0%) | 474 (5.2%) | 266 (5.1%) | 104 (4.9%) | 75 (6.1%) | |
| Anastomosis w/o stoma (yes) | 5,118 (59.4%) | 5,938 (64.5%) | 3,513 (67.0%) | 1,473 (69.0%) | 836 (67.9%) | <0.0001 |
| Operative time (mean; min) | 203.3 ± 98.5 | 211.9 ± 101.7 | 218.0 ± 103.7 | 225.3 ± 106 | 234.3 ± 109.2 | <0.0001 |
| Any complication | 1,848 (21.4%) | 2,003 (21.8%) | 1,331 (25.4%) | 616 (28.9%) | 423 (34.3%) | <0.0001 |
| Specific Complications | ||||||
| Superficial wound infections | 708 (8.2%) | 986 (10.7%) | 691 (13.2%) | 367 (17.2%) | 279 (22.7%) | <0.0001 |
| Deep organ Space Infections | 429 (5.0%) | 433 (4.7%) | 248 (4.7%) | 99 (4.6%) | 71 (5.8%) | NS |
| Pulmonary | 280 (3.3%) | 250 (2.7%) | 167 (3.2%) | 78 (3.7%) | 44 (3.6%) | NS |
| Acute renal failure | 32 (0.4%) | 59 (0.6%) | 27 (0.5%) | 14 (0.7%) | 13 (1.1%) | 0.01 |
| Thromboembolic | 126 (1.5%) | 108 (1.2%) | 97 (1.9%) | 41 (1.9%) | 22 (1.8%) | 0.006 |
| Sepsis/Septic shock | 461 (5.4%) | 501 (5.4%) | 317 (6.0%) | 150 (7.0%) | 100 (8.1%) | 0.0001 |
| Transfusion | 355 (4.1%) | 292 (3.2%) | 196 (3.7%) | 96 (4.5%) | 47 (3.8%) | 0.005 |
| Return to the OR | 486 (5.6%) | 472 (5.1%) | 298 (5.7%) | 137 (6.4%) | 85 (6.9%) | 0.03 |
| Length of stay (mean; days) | 7.8 ± 7.0 | 7.6 ± 6.5 | 7.5 ± 5.8 | 7.9 ± 7.7 | 8.6 ± 8.8 | <0.0001 |
| 30-day mortality | 76 (0.9%) | 51 (0.6%) | 32 (0.6%) | 12 (0.6%) | 9 (0.7%) | NS |
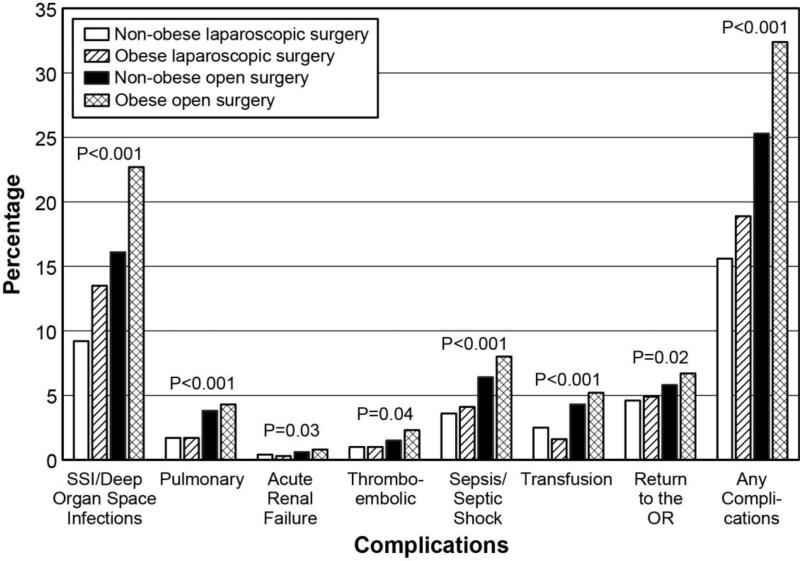
Complication rates were higher for obese patients, regardless of surgical approach. Laparoscopy benefited both groups with lower rates of complications in comparison to open procedures (Figure 2). When compared to non-obese patients undergoing laparoscopic rectal resection, obese patients undergoing laparoscopic rectal resection had higher overall complication rates and a higher incidence of SSI, deep or organ space infections, wound dehiscence, and blood transfusions (Figure 2). Length of stay was lower with laparoscopy in both obese (mean=5.8 vs. 8.8 days, p<0.0001) and non-obese (mean=6.0 vs. 8.7 days, p<0.0001) patients. While all complication rates were higher, open rectal surgery demonstrated similar patterns of morbidity in obese patients compared to non-obese patients (Figure 2). Obese patients had higher rates of SSI, deep or organ space infections, wound dehiscence, thromboembolic events, sepsis, blood transfusions, and reoperations.
Figure 2.
Overall complication rate and specific complications in patients undergoing rectal surgery stratified by operative approach and presence of obesity.
Adjusted Analyses
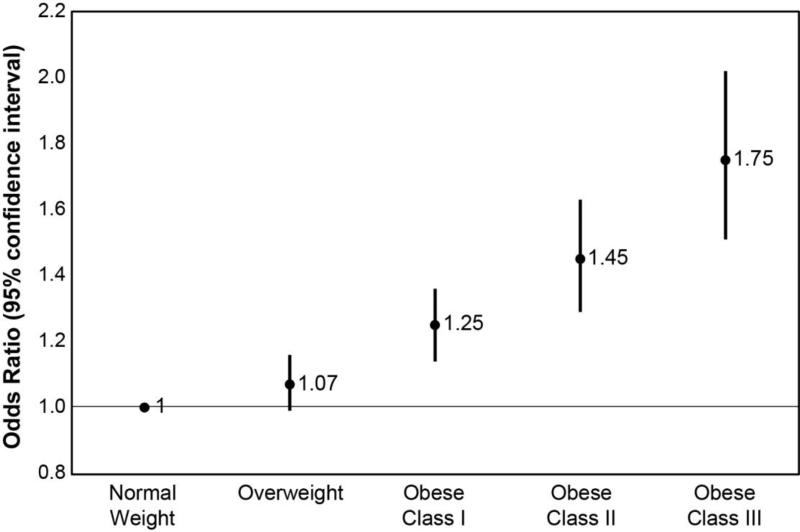
Table 3 reports the results for final multivariable model. We evaluated the interaction between obesity and operative approach (open vs. laparoscopic). The interaction was not statistically significant (p-value of 0.16), indicating that both obese and non-obese patients had similar benefit from the laparoscopic approach. Therefore, the interaction was not included in the final model. After controlling for gender, race, ASA class, primary bowel anastomosis, and patient comorbidity, a laparoscopic approach was associated with 40% lower odds of postoperative complications (OR, 0.60; 95% CI, 0.56-0.64). The likelihood of postoperative morbidity increased as the degree of obesity worsened. Compared to normal BMI patients, obese class I patients (BMI 30 to < 35 kg/m2) had 25% higher odds of developing a postoperative complication, obese class II patients (BMI 35 to < 40 kg/m2) had 45% higher odds of developing a postoperative complication, and obese class III patients (BMI ≥ 40 kg/m2) had a 75% higher odds of developing a postoperative complication.
Table 3.
Multivariable logistic regression models modeling the odds of developing a postoperative complication in non-obese and obese patients.
| Factor (Ref) | OR (95% CI) |
|---|---|
| Laparoscopic Procedure Type (Open) | 0.60 (0.56-0.64) |
| BMI category (Normal) | |
| Overweight | 1.07 (0.99-1.16) |
| Obese Class I | 1.25 (1.14-1.36) |
| Obese Class II | 1.45 (1.29-1.63) |
| Obese Class III | 1.75 (1.51-2.02) |
Model also controlled for age, sex, race, ASA class, creation of an anastomosis, diabetes, dyspnea, COPD, esophageal varices, congestive heart failure, history of MI, previous coronary stent, angina, hypertension, peripheral vascular disease, dialysis, hemiparesis, cerebrovascular accidents, presence of preoperative wound, chronic steroid use, > 10% weight loss in 6 mos prior to surgery, bleeding disorder, cancer diagnosis, chemotherapy, radiation, and preoperative sepsis.
DISCUSSION
Our study demonstrates that laparoscopic rectal surgery is associated with lower complication rates when compared to open rectal surgery in both obese and non-obese patients. Controlling for patient factors, the risk of developing a postoperative complication in patients undergoing rectal surgery increased as BMI increased. For patients with a BMI ≥ 40, the risk of postoperative morbidity was 75% higher even after controlling for operative approach.
Obesity presents a unique set of challenges in surgery. The increased subcutaneous adipose tissue often mandates larger incisions. The excess visceral adipose tissue can limit the ability to visualize relevant anatomy, makes retraction difficult, and may necessitate more extensive tissue dissection.[3] Limited visualization is particularly challenging for a surgeon operating in the confines of the pelvis.[12] Furthermore, increased adiposity and higher intraabdominal pressures may cause difficulty achieving and maintaining sufficient pneumoperitoneum for a laparoscopic approach.[13] All of these factors place obese patients at higher risk for perioperative complications. Obesity has been shown to be an independent risk factor for numerous postoperative complications including infections, thromboembolic events, sepsis, anastomotic leaks, and incisional hernias.[4, 5, 14] In the study by Pikarsky and colleagues, conversion to an open procedure occurred with dramatically higher frequency in obese patients (39% vs. 13.4%; p=0.01) undergoing segmental colorectal resection.[14]
While obese patients generally have worse outcomes regardless of operative approach, our study suggests that laparoscopy is equally beneficial in this population relative to non-obese patients. Previous studies have confirmed that laparoscopy has the added potential benefits of improved visualization, smaller incision size, decreased wound complications, and quicker mobilization in comparison to open surgery.[6, 10] Early ambulation after laparoscopic surgery contributes to faster resolution of postoperative ileus, reduces the risk for thromboembolic events and pulmonary complications, and subsequently leads to shorter length of stay.[6, 15, 16] In addition, the smaller incisions used in minimally invasive surgery produce less pain, allowing for a reduction in the use of narcotics and contribute to earlier return of bowel function.[6, 17] Our study confirms the benefits of laparoscopy specifically for the obese population, in whom we observed fewer infectious, pulmonary, and thromboembolic complications with a laparoscopic approach. As a result, our data suggest that the increased difficulty of laparoscopy in the setting of obesity does not negate the benefits.
There are several limitations associated with a retrospective analysis. A significant limitation of our study is the inability to determine the laparoscopic to open conversion rate, and many obese patients who are categorized as having undergone an open procedure may have initially started with a minimally invasive approach. It is well known that conversions are associated with increased morbidity.[18] Increasing BMI and distal colon/rectal procedures are independent risk factors for laparoscopic conversions.[19-21] Since we are unable to analyze patients on an intent-to-treat basis based on the initial surgical approach, it is possible that the higher complication rate seen with open procedures is partly a reflection of this limitation in both groups. Likewise, the reason for selection of an open vs. laparoscopic approach is not known. There are patient factors, such as previous abdominal surgery, and surgeon factors, such as limited MIS experience, that are relative contraindications to a laparoscopic approach. Therefore, there is likely significant selection bias; however, we would anticipate this bias to be similar in both obese and non-obese patients. Lastly, this analysis relies on the coding of procedures and diagnoses, which is dependent on accurate coding by the surgical team. Discrepancies between the diagnosis and the procedure performed were encountered. For example, we noted that some patients diagnosed with sigmoid cancers or diverticulitis were coded as having undergone low anterior resections rather than a segmental colectomy. Although a coloproctostomy is performed for both procedures, the two operations pose different operative challenges and are likely to have different complication profiles. Despite these limitations, our study offers novel insight into the optimal treatment of rectal pathology in the setting of obesity.
In conclusion, laparoscopic rectal surgery is associated with fewer complications when compared to open rectal surgery for both obese and non-obese patients. Given the increased risk of postoperative morbidity with increasing BMI, minimally invasive rectal surgery may be of greater benefit to the obese patient. As obesity rates increase, establishing safe surgical approaches to rectal pathology in the setting of associated comorbidities and increased adiposity will be of paramount importance. In appropriately selected obese patients, rectal surgery outcomes may be improved with a minimally invasive approach.
Figure 3.
Odds ratios of developing a post-operative complication based on the type of procedure performed and BMI category. All odds ratios (OR) are compared to patients with normal BMI undergoing laparoscopic procedure.
Acknowledgments
Funding: Clinical and Translational Science Award #UL1TR000071, NIH T-32 Grant #5T32DK007639, and AHRQ grant #1R24HS022134.
Footnotes
DISCLOSURES
The authors have no disclosures.
REFERENCES
- 1.Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obesity Reviews. 2005;6:5–7. doi: 10.1111/j.1467-789X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Y, Zhou Y, Li Z, Xiang J, Chen Z. Surgical outcome of laparoscopic colectomy for colorectal cancer in obese patients: A comparative study with open colectomy. Oncol Lett. 2013;6:1057–62. doi: 10.3892/ol.2013.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hourigan JS. Impact of obesity on surgical site infection in colon and rectal surgery. Clin Colon Rectal Surg. 2011;24:283–90. doi: 10.1055/s-0031-1295691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senagore AJ, Delaney CP, Madboulay K, Brady KM, Fazio VW. Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg. 2003;7:558–61. doi: 10.1016/s1091-255x(02)00124-5. [DOI] [PubMed] [Google Scholar]
- 6.Burden C, Vyas S. Laparoscopic Surgery in Obese Women. Obesity A ticking time bomb for reproductive health. 2012:670. [Google Scholar]
- 7.Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212:844–54. doi: 10.1016/j.jamcollsurg.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson MZ, Hollenbeak CS, Stewart DB. Laparoscopic colectomy is associated with a lower incidence of postoperative complications than open colectomy: a propensity score-matched cohort analysis. Colorectal Dis. 2014;16:382–9. doi: 10.1111/codi.12537. [DOI] [PubMed] [Google Scholar]
- 9.Kiran RP, El-Gazzaz GH, Vogel JD, Remzi FH. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: data from national surgical quality improvement program. J Am Coll Surg. 2010;211:232–8. doi: 10.1016/j.jamcollsurg.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Lai JH, Law WL. Laparoscopic surgery for colorectal cancer. Br Med Bull. 2012;104:61–89. doi: 10.1093/bmb/lds026. [DOI] [PubMed] [Google Scholar]
- 11.ACS NSQIP [Google Scholar]
- 12.Scheidbach H, Benedix F, Hugel O, Kose D, Kockerling F, Lippert H. Laparoscopic approach to colorectal procedures in the obese patient: risk factor or benefit? Obes Surg. 2008;18:66–70. doi: 10.1007/s11695-007-9266-0. [DOI] [PubMed] [Google Scholar]
- 13.Hasson AM. Risk of Pneumoperitoneum in Obese: Old Myths and New Realities. World Journal of Laparoscopic Surgery. 2011;4:97–102. [Google Scholar]
- 14.Pikarsky AJ, Saida Y, Yamaguchi T, Martinez S, Chen W, Weiss EG, et al. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc. 2002;16:855–8. doi: 10.1007/s004640080069. [DOI] [PubMed] [Google Scholar]
- 15.Kitching AJ, O'Neil SS. Fast-Track Surgery and Anaesthesia. Continuing Education in Anaesthesia. Critical Care & Pain. 2009:9. [Google Scholar]
- 16.Martin ST, Stocchi L. Laparoscopic colorectal resection in the obese patient. Clin Colon Rectal Surg. 2011;24:263–73. doi: 10.1055/s-0031-1295690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones OM, Lindsey I, Cunningham C. Laparoscopic Colorectal Surgery. BMJ. 2011 doi: 10.1136/bmj.d8029. [DOI] [PubMed] [Google Scholar]
- 18.Belizon A, Sardinha CT, Sher ME. Converted laparoscopic colectomy: what are the consequences? Surg Endosc. 2006;20:947–51. doi: 10.1007/s00464-005-0553-3. [DOI] [PubMed] [Google Scholar]
- 19.Gervaz P, Pikarsky A, Utech M, Secic M, Efron J, Belin B, et al. Converted laparoscopic colorectal surgery. Surg Endosc. 2001;15:827–32. doi: 10.1007/s004640080062. [DOI] [PubMed] [Google Scholar]
- 20.Tekkis PP, Senagore AJ, Delaney CP. Conversion rates in laparoscopic colorectal surgery: a predictive model with, 1253 patients. Surg Endosc. 2005;19:47–54. doi: 10.1007/s00464-004-8904-z. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez R, Smith CD, Mason E, Duncan T, Wilson R, Miller J, et al. Consequences of conversion in laparoscopic colorectal surgery. Dis Colon Rectum. 2006;49:197–204. doi: 10.1007/s10350-005-0258-7. [DOI] [PubMed] [Google Scholar]