Abstract
Background
Platinum-based chemotherapy is the most effective regimen for nasopharyngeal carcinoma, which presents highly invasive and metastatic activity. However, the dose-related toxicity of chemotherapy agents limits the dose administration. Astragalus polysaccharide (APS) is the major active ingredient extracted from Chinese herb Radix Astragali and is proven to be active against carcinomas. We aimed to assess the chemosensitizing effects of Astragalus polysaccharides on nasopharyngeal carcinoma in vitro and in vivo and to explore the underlying mechanism.
Material/Methods
We used BALB/c nu/nu mice and human nasopharyngeal carcinoma cell lines CNE-1, CNE-2, and SUNE-1. MTT, Annexin V/PI, Western blot analysis, and TUNEL assay were carried out.
Results
APS significantly promoted anti-proliferative and apoptotic effects of cisplatin on nasopharyngeal carcinoma cells. APS also enhanced the anti-tumor effects and cisplatin-induced apoptosis in the xenograft model. The level of Bcl-2 decreased, while the levels of Bax, caspase-3, and caspase-9 increased in cisplatin combined with APS treatment compared to cisplatin only treatment. The ratio of Bax to Bcl-2 was significantly enhanced by the APS to cisplatin.
Conclusions
APS enhanced the anti-proliferative and apoptotic effect of cisplatin by modulating expression of Bax/Bcl-2 ratio and caspases on nasopharyngeal carcinoma cells and in the xenograft model.
MeSH Keywords: Apoptosis; Astragalus Membranaceus; bcl-2-Associated X Protein; Caspases; Genes, bcl-2; Nasopharyngeal Neoplasms
Background
Nasopharyngeal carcinoma (NPC) is a highly invasive and metastatic cancer type which is prevalent in East and South-East Asia [1]. Although traditional treatments including radiotherapy or chemotherapy achieved good therapeutic effects in the past decades and became the standard treatment, the limitations of these treatments keep them from completely curing the disease. About one-third of NPC patients achieve tumor relapse due to distant metastasis [2]. The overall survival of recurrent or metastatic patients is less than 2 years [3]. Cisplatin is regarded as the standard first-line chemotherapy agent for NPC, but the application is often limited by dose-related toxicity, and many patients cannot receive full-dose or complete chemotherapy cycles [4,5]. Therefore, the exploration of novel molecular therapeutic regimens to sensitizing chemotherapy with lower toxicity is urgently needed for NPC treatment [6]. Recently, bioactive components from natural sources have been developed and attracted a great deal of interest [7]. Anti-tumor agents from natural herbs have been studied and demonstrated to be effective in treating various carcinomas [8–15].
Astragalus polysaccharide (APS) is the major active ingredient extracted from Radix Astragali, which is a popular traditional Chinese medicine. It has been reported that APS possesses various biological activities, including antioxidant, anti-inflammation, immuno-modulation, anti-viral, and anti-tumor activities [16–19] Recent studies reported that APS had anti-tumor properties that can modulate various cancer signaling pathways and interact with specific transcription molecules, such as Wnt, p53, and NF-κB signaling pathway [20–24]. However, it was unknown whether APS has anti-tumor effects or synergistic reaction with chemotherapy agents on NPC, and the underlying mechanism is unclear. Therefore, the aim of present study was to explore the anti-tumor and chemosensitizing effect of APS on NPC and its underlying mechanism in vitro and in vivo. Our results suggest that APS may become an effective anti-tumor agent for sensitizing NPC cells to cisplatin, and the combination regimen may be helpful for NPC patients.
Material and Methods
Chemicals and reagents
Astragalus polysaccharide (C10H7ClN2O2S) was purchased from the Zhejiang Institute for Food and Drug Control (Hangzhou, China). Cisplatin was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). MTT and Annexin V Apoptosis Detection kits were obtained from BD Biosciences (Franklin Lakes, NJ, USA). Bcl-2, Bax, caspase-3, and caspase-9 antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-GAPDH was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). In Situ Cell Death Detection Kits were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Cell lines
Three nasopharyngeal carcinoma cell lines (CNE-1, CNE-2, and SUNE-1) were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). CNE-1, CNE-2, and SUNE-1 cell lines were cultured in RPMI 1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Logan, UT, USA) at 37°C with humidified 5% CO2.
Cell viability assay
MTT assay was used to detect the anti-proliferative effects of APS and cisplatin. CNE-1, CNE-2, and SUNE-1 cells were seeded into 96-well plates (1×104/well) and then treated with different concentrations of APS (10, 20, 40, 60, and 80 μg/ml) for 48 h. Further, the 3 cell lines were treated with cisplatin (from 0.5 to 4 μM) and APS (20, 40 and 60 μg/ml). Solution absorbance was measured at 490 nm by microplate reader. Three replica wells were prepared for each group.
Apoptosis assay
Annexin V/PI method was used to detect apoptosis induced by APS and cisplatin. CNE-1, CNE-2, and SUNE-1 cells were seeded (1×104/well) and then treated with cisplatin (0.5 to 4 μM) with or without APS (40 μg/ml) for 48 h. Cells were harvested, trypsinized, and then stained with annexin V and PI in the dark using an Apoptosis Detection kit prior to being detected by flow cytometry (Beckman Coulter, Inc., Brea, CA, USA). Three replica wells were prepared for each group.
Western blot analysis
CNE-1, CNE-2, and SUNE-1 cells were treated with cisplatin (2 μM) with or without APS (40 μg/ml) for 48 h. Cells were lysed and then total protein was extracted with cell lysis buffer. The proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA) electrophoretically. We used 5% non-fat milk in TBST to block the membranes, which then were incubated with primary antibodies (rabbit anti-rat Bcl-2, Bax, caspase-3, and caspase-9 antibodies and mouse anti-rat GAPDH monoclonal antibody (1: 1000) overnight at 4°C. Membranes then were reacted with secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit IgG) for 30 min at room temperature. Proteins were detected with ECL reagent and visualized using ImageQuant LAS 4000 (Pittsburg, PA, USA).
Animal treatment
BALB/c nu/nu mice were obtained from Zhejiang Academy of Medical Sciences (Hangzhou, China). CNE-2 cells (1×106 cells) were suspended in 0.1 ml of PBS, then subcutaneously injected into nude mice to establish a mouse model of xenograft tumor. We randomly divided 20 tumor-bearing nude mice (male, aged 4–5 weeks, weighing 18–20 g) into 4 groups (5 nude mice per group): the control group, receiving intraperitoneal injection with 0.1 ml normal saline twice a week; the APS group, receiving intraperitoneal injection with 0.1 ml APS (40 mg/kg) twice a week; the cisplatin group, receiving intraperitoneal injection with 0.1 ml cisplatin (5 mg/kg) twice a week; and the APS with cisplatin group, receiving intraperitoneal injection with 0.1 ml APS (40 mg/kg) and 0.1 ml cisplatin (5 mg/kg) twice a week. Tumor volume and tumor weight were evaluated after mice were sacrificed at 4 weeks. Tumor samples were obtained and fixed, then embedded in paraffin. All the animal experimental protocols were approved by the Hangzhou First People’s Hospital Research Ethics Committee.
TUNEL analysis
Terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) method was used to detect apoptosis induced by APS and cisplatin of tumor tissue with an in situ cell death detection kit according to the manufacturer’s instructions. An Olympus BX61 fluorescence microscope (Olympus Corporation, Tokyo, Japan) was used to examine the reactions.
Statistical analysis
Data are presented as the mean ± standard deviation. Student’s t-test was used to analyze 2 groups and one-way ANOVA was used to analyze multiple groups by using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
The inhibitory effect of APS combined with cisplatin on NPC cell lines
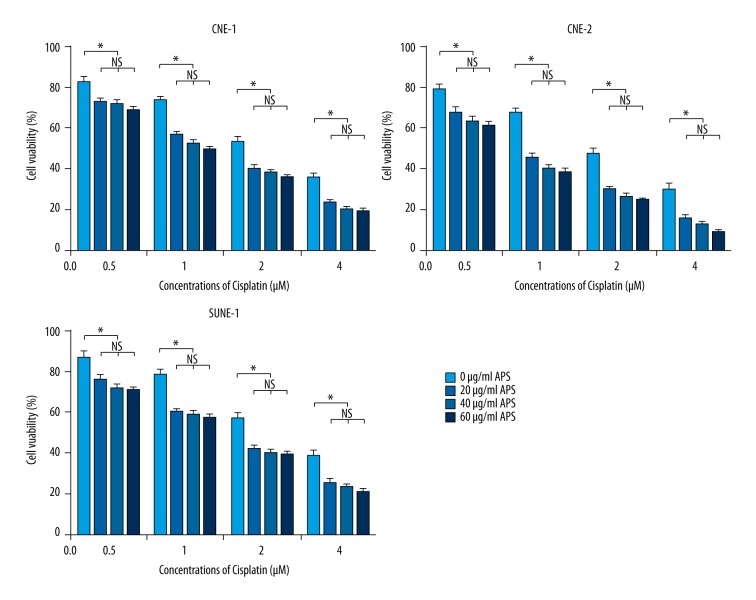
The CNE-1, CNE-2, and SUNE-1 cells were treated with 10, 20, 40, 60, and 80 μg/ml APS for 48 h, then different concentrations of APS (20, 40, and 60 μg/ml) were performed to combine with cisplatin. The results showed that the proliferation of CNE-1, CNE-2, and SUNE-1 cells were significantly inhibited by APS in a dose-dependent manner (Figure 1). Moreover, the inhibitory effect of cisplatin increased with the increasing concentration (from 0.5 to 4 μM), and the inhibitory effects were significantly enhanced when combined with different concentrations of APS. The inhibitory effects of different concentrations of APS had no significant difference (Figure 2).
Figure 1.
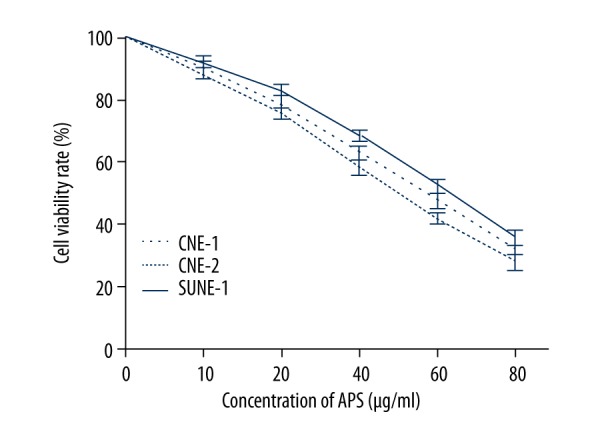
Anti-proliferative activity of APS on NPC cell lines CNE-1, CNE-2 and SUNE-1.
Figure 2.
APS enhanced cisplatin sensitivity on NPC cell lines CNE-1, CNE-2, and SUNE-1 with increasing doses. * Statistically significant difference (P<0.01) between the Cisplatin+APS groups and the cisplatin treatment group.
The apoptosis-inducing effect of APS combined with cisplatin on NPC cell lines
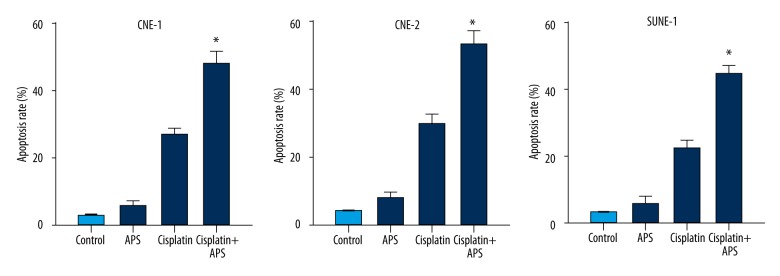
Annexin V-FITC assay was performed to evaluate the apoptosis-inducing effect of APS combined with cisplatin. The results showed that cisplatin induced a significant apoptosis compared to the control group. Moreover, the apoptosis rates increased significantly when combined with APS (40 μg/ml) at 48 h (Figure 3).
Figure 3.
APS enhanced cisplatin-induced apoptosis on NPC cell lines CNE-1, CNE-2 and SUNE-1. * Statistically significant difference (P<0.01) between the Cisplatin+APS group and the cisplatin treatment group.
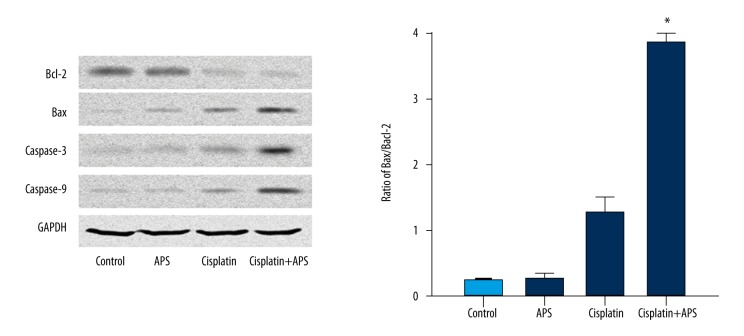
The effect of APS combined with cisplatin on the expressions of Bax/Bcl-2 ratio and caspases on NPC cell lines
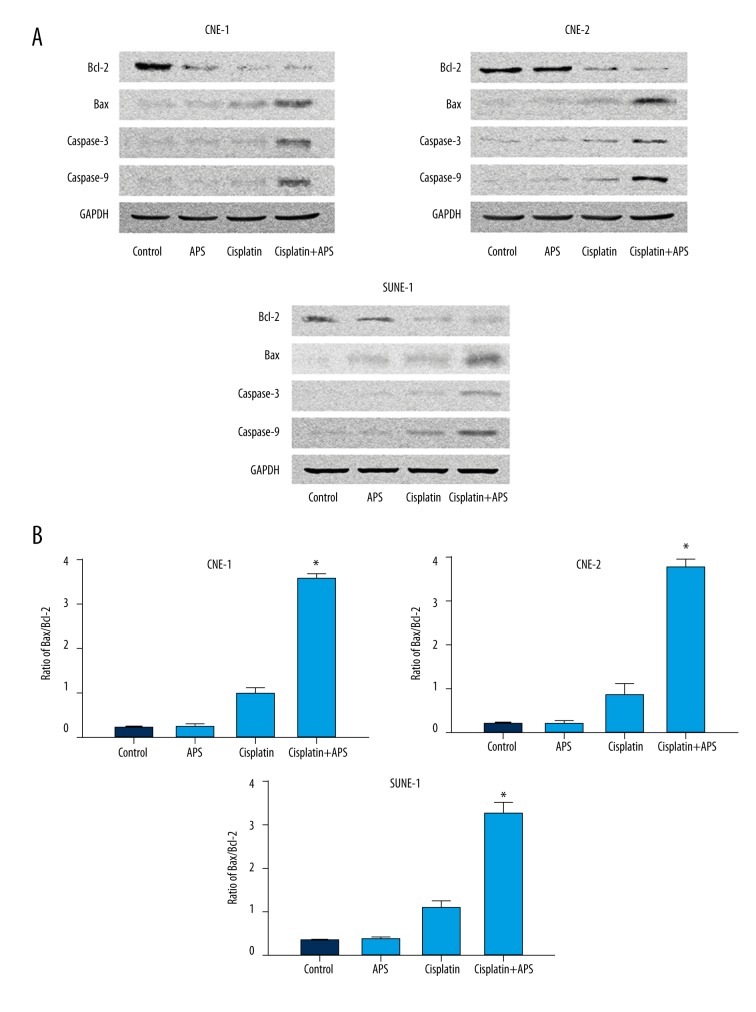
The expression levels of key regulator proteins, including Bax, Bcl-2, caspase-3, and caspase-9, were detected by Western blot analysis after treatment with APS (40 μg/ml) and/or cisplatin (2 μM) for 48 h on CNE-1, CNE-2, and SUNE-1 cells. The results showed that the level of Bcl-2 decreased, while the levels of Bax, caspase-3, and caspase-9 increased significantly (Figure 4A), and the ratio of Bax to Bcl-2 was significantly increased (Figure 4B).
Figure 4.
APS increased cisplatin-induced protein expressions of Bcl-2, Bax, caspase-3, and caspase-9 on NPC cell lines CNE-1, CNE-2, and SUNE-1. (A) Cisplatin combined with APS decreased the expressions of Bcl-2 and increased the expression of Bax, caspase-3, and caspase-9 compared to cisplatin only treatment and control, as determined by Western blot analysis. (B) The ratio of Bax/Bcl-2 of each group. * Statistically significant difference (P<0.01) between the Cisplatin+APS group and the cisplatin treatment group.
The anti-tumor enhancing effect of APS combined with cisplatin on the NPC xenograft model
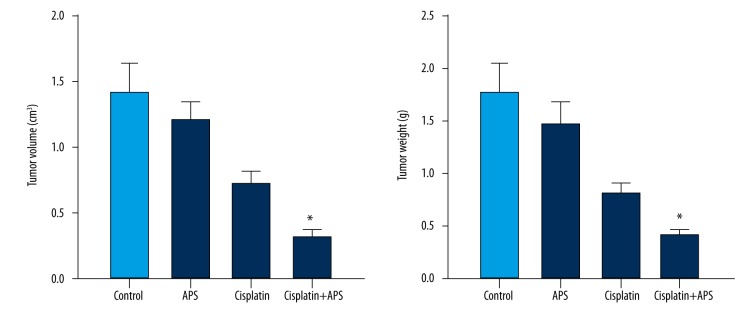
We evaluated the activity of APS combined with cisplatin on CNE-2 xenograft in vivo. The results showed that tumor volumes and weight were significantly reduced in the combination group compared to the cisplatin group and the APS group (Figure 5). TUNEL assay showed that the apoptotic index of the combination group significantly increased compared to the cisplatin group or APS group (Figure 6). Western blot analysis was performed to verify the expressions of Bax/Bcl-2 ratio and caspases in vivo. The level of Bcl-2 decreased, while the levels of Bax, caspase-3, and caspase-9 increased significantly, and the ratio of Bax to Bcl-2 was significantly enhanced (Figure 7).
Figure 5.
APS enhanced cisplatin sensitivity on tumor volumes and weight of CNE-2 xenograft. * Statistically significant difference (P<0.01) between the Cisplatin+APS group and the cisplatin treatment group.
Figure 6.
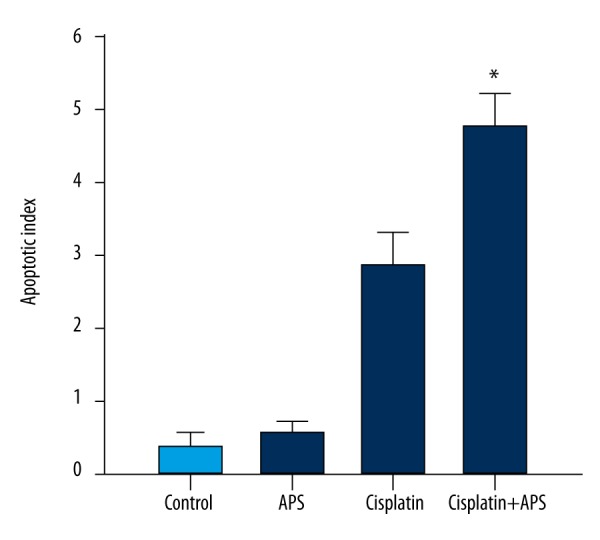
APS enhanced cisplatin-induced apoptosis of CNE-2 xenograft. * Statistically significant difference (P<0.01) between the Cisplatin+APS group and the cisplatin treatment group.
Figure 7.
The effects of cisplatin combined with APS on the levels of Bcl-2, Bax, caspase-3, and caspase-9 expressions and the ratio of Bax/Bcl-2 in tumor tissues. * Statistically significant difference (P<0.01) between the Cisplatin+APS group and the cisplatin treatment group.
Discussion
At present, there is no anti-tumor agent that can achieve complete remission of cancers without adverse effects. It has been demonstrated that traditional Chinese medicine has great advantages as effective chemotherapeutics or adjuvant agents through increasing chemo- or radio-sensitivity and reducing the side effects, leading to improved quality of life and patient survival. Natural products, especially Chinese herbs, have demonstrated promising anti-cancer effects with minimal toxicity. Among them, Radix Astragali has shown a potential therapeutic value in cancer treatment. It has been demonstrated that Astragali extract can inhibit the growth of various cancer cells in vitro and in vivo, including gastric cancer cells, renal carcinoma cells, urinary carcinoma cells, and hepatoma cells [25–28]. Randomized trials showed that Astragalus-based formula increased the effectiveness of a platinum-based chemotherapy regimen for advanced non-small-cell lung cancer (NSCLC) [29].
The major active constituents of Radix Astragali are polysaccharides, triterpene saponins, flavonoids, fatty acids, sterols, betaine, and choline [30]. Recently, APS has been widely studied for its immunopotentiating properties and anti-tumor activity. Several studies have indicated that APS has anti-tumor activity in various tumor types, including gastric cancer, liver cancer, colon cancer, breast cancer, lung cancer, and renal and bladder tumors [31–35]. APS inhibited the growth of Lewis lung cancer cells and enhanced the therapeutic effect when combined with cisplatin [34]. APS also can enhance the chemosensitivity of H22/ADM cells [35]. APS was reported to increase tumor response, improve patient performance status, and reduce toxicity of chemotherapy. Clinical study has also confirmed that APS integrated with vinorelbine and cisplatin significantly improved quality of life (QOL) in advanced NSCLC patients [36]. It was reported that the potential mechanism of the anti-tumor activity of APS was related to apoptosis, cell cycle arrest, downregulation of Akt phosphorylation, and upregulation of p53 and PTEN [37]. APS could reverse multidrug resistance by downregulation of MDR1 mRNA expression, inhibition of P-GP efflux pump function, and decreasing the expression of MDR1 protein [35].
In the present study, we evaluated the anti-tumor and chemosensitizing activity of APS on NPC cells and the potential mechanism was explored. The results showed that the proliferation of NPC cells was significantly inhibited by APS in a dose-dependent manner, and the inhibitory effects of cisplatin were significantly enhanced when combined with different concentrations of APS in vitro and in vivo. Studies reported that APS could enhance the chemosensitivity of the most common chemotherapy drugs in vitro, such as Cyclophosphamide, Adriamycin, 5-Fluorouracil, cisplatin, Etoposide, and Vincristine [35]. The present results are supported by previous studies.
Apoptosis is important for cell proliferation and tumor growth. The apoptosis signaling pathway is mediated by caspases, and among them, caspase-3 and caspase-9 are crucial mediators in the apoptosis procedure. The apoptosis pathway can be initiated through caspase-9, and trigger apoptosis through the cleavage of the downstream executioner caspase-3 [38,39]. As 2 typical proteins of the Bcl-2 family, Bcl-2 and Bax play key roles in caspase-dependent apoptosis. Bcl-2 is an anti-apoptotic protein mainly located in the nuclear and mitochondrial membrane, but the family member Bax that promotes apoptosis is mainly located in the cytoplasm. Bcl-2 had a strong anti-apoptosis effect in inhibiting the Bax-induced caspase-dependent apoptosis. Therefore, a high ratio of Bcl-2/Bax is considered to be resistant to apoptosis [40]. The present study showed that APS increased the rate of cisplatin-induced apoptosis on NPC cells and in the xenograft model. Further, the Western blotting analysis showed that the level of Bcl-2 expression decreased, while cisplatin combined with APS increased the levels of Bax, caspase-3, and caspase-9 expression, and the ratio of Bax to Bcl-2 was significantly enhanced.
Conclusions
In summary, the results of the present study indicate that APS enhanced the anti-proliferative and apoptotic effect of cisplatin by modulating expression of Bax/Bcl-2 ratio and caspases on NPC cells and a xenograft model. APS appears to have potential as a good chemosensitizing agent for NPC treatment, but further study is needed to elucidate the precise mechanisms.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Rotolo F, Pignon JP, Bourhis J, et al. Surrogate end points for overall survival in loco-regionally advanced nasopharyngeal carcinoma: An individual patient data meta-analysis. J Natl Cancer Inst. 2016;109(4) doi: 10.1093/jnci/djw239. pii: djw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L, Hong S, Wang Y, et al. Development and external validation of nomograms for predicting survival in nasopharyngeal carcinoma patients after definitive radiotherapy. Sci Rep. 2015;5:15638. doi: 10.1038/srep15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin Y, Shi YX, Cai XY, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138(10):1717–25. doi: 10.1007/s00432-012-1219-x. [DOI] [PubMed] [Google Scholar]
- 4.Marcato PD, Fávaro WJ, Durán N. Cisplatin properties in a nanobiotechnological approach to cancer: A mini-review. Curr Cancer Drug Targets. 2014;14(5):458–76. doi: 10.2174/1568009614666140508154020. [DOI] [PubMed] [Google Scholar]
- 5.Yue Z, Cao Z. Current strategy for cisplatin delivery. Curr Cancer Drug Targets. 2016;16(6):480–88. doi: 10.2174/1568009616666160328113006. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton G. Cyclophilin A as a target of Cisplatin chemosensitizers. Curr Cancer Drug Targets. 2014;14(1):46–58. doi: 10.2174/15680096113136660109. [DOI] [PubMed] [Google Scholar]
- 7.Hu XQ, Sun Y, Lau E, et al. Advances in synergistic combinations of Chinese herbal medicine for the treatment of cancer. Curr Cancer Drug Targets. 2016;16(4):346–56. doi: 10.2174/1568009616666151207105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng R, Rao Y, Jiang H, et al. Therapeutic potential of Ginsenoside Rg3 via inhibiting Notch/HES1 pathway in lung cancer cells. Translational Cancer Research. 2016;5(4):464–69. [Google Scholar]
- 9.Zhu X, Jiang H, Li J, et al. Anticancer effects of Paris saponins by apoptosis and PI3K/AKT pathway in gefitinib-resistant non-small cell lung cancer. Med Sci Monit. 2016;22:1435–41. doi: 10.12659/MSM.898558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao PJ, Song Sc, Du LW, et al. Paris saponins enhance radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line by inducing apoptosis and G2/M cell cycle phase arrest. Mol Med Rep. 2016;13(3):2878–84. doi: 10.3892/mmr.2016.4865. [DOI] [PubMed] [Google Scholar]
- 11.Song S, Du L, Jiang H, Zhu X, Li J, Xu J. Paris saponin I sensitizes gastric cancer cell lines to Cisplatin via cell cycle arrest and apoptosis. Med Sci Monit. 2016;22:3798–803. doi: 10.12659/MSM.898232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao P, Jiang H, Su D, et al. Inhibition of cell proliferation by mild hyperthermia at 43 C with Paris saponin I in the lung adenocarcinoma cell line PC-9. Mol Med Rep. 2015;11(1):327–32. doi: 10.3892/mmr.2014.2655. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Zhao PJ, Su D, et al. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9(6):2265–72. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Zhao P, Feng J, et al. Effect of Paris saponin I on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett. 2014;7(6):2059–64. doi: 10.3892/ol.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng A, Li H, Wang X, et al. Anticancer effect of a curcumin derivative B63: ROS production and mitochondrial dysfunction. Curr Cancer Drug Targets. 2014;14(2):156–66. doi: 10.2174/1568009613666131126115444. [DOI] [PubMed] [Google Scholar]
- 16.Fan W, Zhang S, Hao P, et al. Structure characterization of three polysaccharides and a comparative study of their immunomodulatory activities on chicken macrophage. Carbohydr Polym. 2016;153:631–40. doi: 10.1016/j.carbpol.2016.07.116. [DOI] [PubMed] [Google Scholar]
- 17.Pu X, Ma X, Liu L, et al. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr Polym. 2016;137:154–64. doi: 10.1016/j.carbpol.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Bian F, Yue L, et al. Selenium-dependent antitumor immunomodulating activity of polysaccharides from roots of A. membranaceus. Int J Biol Macromol. 2014;69:64–72. doi: 10.1016/j.ijbiomac.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Gao FF, Li Q, et al. Protective effect of astragalus polysaccharides on liver injury induced by several different chemotherapeutics in mice. Asian Pac J Cancer Prev. 2014;15(23):10413–20. doi: 10.7314/apjcp.2014.15.23.10413. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Zhang J, Li M, et al. Inhibition of HepG2 cell proliferation by ursolic acid and polysaccharides via the downregulation of cyclooxygenase-2. Mol Med Rep. 2014;9(6):2505–11. doi: 10.3892/mmr.2014.2059. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Zheng K, Zhao H, et al. Regulatory effect of astragalus polysaccharides on intestinal intraepithelial γδT cells of tumor bearing mice. Molecules. 2014;19(9):15224–36. doi: 10.3390/molecules190915224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian QE, Li HD, Yan M, et al. Astragalus polysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice. World J Gastroenterol. 2012;18(47):7079–86. doi: 10.3748/wjg.v18.i47.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med. 2016;44(1):1–22. doi: 10.1142/S0192415X16500014. [DOI] [PubMed] [Google Scholar]
- 24.Jiao R, Liu Y, Gao H, et al. The anti-oxidant and antitumor properties of plant polysaccharides. Am J Chin Med. 2016;44(3):463–88. doi: 10.1142/S0192415X16500269. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Dong HF, Oppenheim JJ, Howard OM. Effects of astragali radix on the growth of different cancer cell lines. World J Gastroenterol. 2003;9(4):670–73. doi: 10.3748/wjg.v9.i4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau BH, Ruckle HC, Botolazzo T, Lui PD. Chinese medicinal herbs inhibit growth of murine renal cell carcinoma. Cancer Biother. 1994;9(2):153–61. doi: 10.1089/cbr.1994.9.153. [DOI] [PubMed] [Google Scholar]
- 27.Kurashige S, Akuzawa Y, Endo F. Effects of astragali radix extract on carcinogenesis, cytokine production, and cytotoxicity in mice treated with a carcinogen, N-butyl-N′-butanolnitrosoamine. Cancer Invest. 1999;17(1):30–35. doi: 10.1080/07357909909011714. [DOI] [PubMed] [Google Scholar]
- 28.Cui R, He J, Wang B, et al. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother Pharmacol. 2003;51(1):75–80. doi: 10.1007/s00280-002-0532-5. [DOI] [PubMed] [Google Scholar]
- 29.McCulloch M, See C, Shu XJ, et al. Astragalus-based Chinese herbs and platinum – based chemotherapy for advanced non-small-cell lung cancer: Meta-analysis of randomized trials. J Clin Oncol. 2006;24(3):419–30. doi: 10.1200/JCO.2005.03.6392. [DOI] [PubMed] [Google Scholar]
- 30.Ma XQ, Shi Q, Duan JA, et al. Chemical analysis of Radix astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50(17):4861–66. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Yang Y, Zhang X, et al. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell invasion by modulating transforming growth factor-beta/Smad in HepG2 cell. J Gastroenterol Hepatol. 2010;25(2):420–26. doi: 10.1111/j.1440-1746.2009.05981.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu XN, Zhang CY, Jin XD, et al. Inhibitory effect of schisandrin B on gastric cancer cells in vitro. World J Gastroenterol. 2007;13(48):6506–11. doi: 10.3748/wjg.v13.i48.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou RF, Liu PX, Tan M. Effect of Astragalus mongholicus injection on proliferation and apoptosis of hormone sensitive (MCF-7) breast cancer cell lines with physiological dose E2. Zhong Yao Cai. 2009;32(5):744–47. [PubMed] [Google Scholar]
- 34.Ming H, Chen Y, Zhang F, et al. Astragalus polysaccharides combined with cisplatin decreases the serum levels of CD44 and collagen type IV and hyaluronic acid in mice bearing Lewis lung cancer. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(7):909–13. [PubMed] [Google Scholar]
- 35.Tian QE, De Li H, Yan M, et al. Effects of Astragalus polysaccharides on P-glycoprotein efflux pump function and protein expression in H22 hepatoma cells in vitro. BMC Complement Altern Med. 2012;12:94. doi: 10.1186/1472-6882-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Bai SP, Zhao L, Wang XH. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: Effects on quality of life and survival. Med Oncol. 2012;29(3):1656–62. doi: 10.1007/s12032-011-0068-9. [DOI] [PubMed] [Google Scholar]
- 37.Ye MN, Chen HF, Zhou RJ, Liao MJ. Effects of Astragalus polysaccharide on proliferation and Akt phosphorylation of the basal-like breast cancer cell line. Zhong Xi Yi Jie He Xue Bao. 2011;9(12):1339–46. doi: 10.3736/jcim20111210. [DOI] [PubMed] [Google Scholar]
- 38.Wu MH, Jin XK, Yu AQ, et al. Caspase-mediated apoptosis in crustaceans: Cloning and functional characterization of EsCaspase-3-like protein from Eriocheir. Fish Shellfish Immunol. 2014;41(2):625–32. doi: 10.1016/j.fsi.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Mondal A, Bennett LL. Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer MCF7 cells through ROS, cell cycle inhibition, caspase 3 and PARP cleavage. Biomed Pharmacother. 2016;84:1906–14. doi: 10.1016/j.biopha.2016.10.096. [DOI] [PubMed] [Google Scholar]
- 40.Akyol Z, Çoker-Gürkan A, Arisan ED, et al. DENSpm overcame Bcl-2 mediated resistance against Paclitaxel treatment in MCF-7 breast cancer cells via activating polyamine catabolic machinery. Biomed Pharmacother. 2016;84:2029–41. doi: 10.1016/j.biopha.2016.11.016. [DOI] [PubMed] [Google Scholar]