Abstract
Polysaccharide-protein conjugates as vaccines have proven to be very effective in preventing Haemophilus influenzae type b infections in industrialized countries. However, cost-effective technologies need to be developed for increasing the availability of anti-H. influenzae type b vaccines in countries from the developing world. Consequently, vaccine production with partially synthetic antigens is a desirable goal for many reasons. They may be rigidly controlled for purity and effectiveness while at the same time being cheap enough that they may be made universally available. We describe here the antigenicity and immunogenicity of several H. influenzae type b synthetic oligosaccharide-protein conjugates in laboratory animals. The serum of H. influenzae type b-immunized animals recognized our synthetic H. influenzae type b antigens to the same extent as the native bacterial capsular polysaccharide. Compared to the anti-H. influenzae type b vaccine employed, these synthetic versions induced similar antibody response patterns in terms of titer, specificity, and functional capacity. The further development of synthetic vaccines will meet urgent needs in the less prosperous parts of the world and remains our major goal.
The advent of conjugate vaccines against Haemophilus influenzae type b-associated diseases has opened a new era in vaccinology (15). These vaccines, which demonstrate excellent immunogenicity, safety, and efficacy in infants, have been obtained by conjugating the capsular polysaccharide polyribosylribitolphosphate from H. influenzae type b to carrier proteins (1, 3, 8, 12). They have been particularly useful in preventing infection with H. influenzae type b in high-risk infant populations. In fact, the almost complete disappearance of H. influenzae type b disease in the developed world together with the corresponding reduction in H. influenzae type b pharyngeal carriage testify to the usefulness of these conjugate vaccines.
However, it is extremely difficult to accomplish the progress brought by these commercial vaccines in many poor countries because their high cost reduces both their acquisition and their availability. More than 118 million children are without protection, and only ≈2% of cases of H. influenzae type b disease are actually prevented worldwide (15).
Given this, H. influenzae type b vaccination in developing countries is urgent but limited by cost and the availability of vaccines. The availability of vaccines depends on producing them with improved technologies and making them affordable to even the poorest societies. In 1989, we embarked on a project to produce a new conjugate anti-H. influenzae type b vaccine from a fully synthetic fragment of the capsular polysaccharide. We have now successfully completed the production, preclinical, and clinical development stages for this new vaccine (17). Here we describe the main preclinical studies that have shown the potential of this new vaccine. Several synthetic H. influenzae type b oligosaccharide-protein conjugates were prepared and their immunological properties (e.g., antigenicity and inmunogenicity) were evaluated with laboratory animals.
MATERIALS AND METHODS
HbO-HA antigen.
Plates coated with H. influenzae type b conjugated to human albumin (HA) (HbO-HA antigen) (lot 17) was supplied by the National Institute for Biological Standards and Control, London, United Kingdom. The H. influenzae type b conjugates were obtained by controlled periodic oxidation to degree of polymerization of 30 followed by reductive amination to human serum albumin.
Licensed vaccines.
Polyribosylribitolphosphate conjugated to tetanus toxoid (PRP-TT; Hiberix, lot Hib 900A11) was from Smith Kline Beecham. The vaccine is composed of the capsular polysaccharide activated by cyanogen bromide and coupled to tetanus toxoid through an adipic acid hydrazide spacer. PRP-CRM197 (cross-reacting mutant 197; Vaxem-Hib Lot 3204) was from Chiron. The vaccine is composed of oligosaccharide fragments obtained from the capsular polysaccharide by acid hydrolysis (DP 10) and coupled to CRM197 through adipic acid hydrazide.
Synthetic polyribosylribitolphosphate.
The synthetic oligosaccharides that we used were produced in our facilities on a multigram scale under good manufacturing practice conditions. The chemical synthesis process starts from d-ribose (18). As shown in Fig. 1, the synthetic oligosaccharides (sPRP) contained between six and nine repeating units of the Haemophilus influenzae type b capsular polysaccharide. The synthetic compound was made with a spacer arm with a terminal maleimido function ready for conjugation to protein carriers.
FIG. 1.
Structure of synthetic Haemophilus influenzae type b oligosaccharides (synthetic polyribosylribitolphosphate).
Carrier proteins.
Tetanus toxoid (TT; lot P-007′00) and Neisseria meningitidis outer membrane protein complex (OMP; lot 0006B) were produced as components of licensed vaccines by the Finlay Institute for Serum and Vaccines, Havana, Cuba. Human serum albumin (HSA) and bovine serum albumin (BSA) were from Sigma.
Native polyribosylribitolphosphate.
The capsular polysaccharide polyribosylribitolphosphate (lot 00-Hib350-01) was supplied by RIVM, Biltjoven, The Netherlands.
Protein conjugates.
Several conjugates containing the synthetic antigens described below were obtained with a two-step procedure (7).
(i) Step 1.
To a solution of human serum albumin (24.5 mg, 0.36 μmol) in phosphate-buffered saline (PBS; pH 8, with EDTA at 1.86 g/liter, 5.0 ml), a solution of N-hydroxysuccinimide dithiopropionate (2.9 mg, 7.2 μmol) in dimethyl sulfoxide (50 μl) was added under N2 atmosphere. After 2 h, dithiothreitol (19.3 mg, →25 mmol/liter) was added (under N2 gas) and the mixture was stirred at 4°C for 1 h. The resulting solution was diafiltered with N2 as the pressure source (pH 7.2, regenerated cellulose membrane, 30-kDa cutoff). Protein and SH content were analyzed by the methods of Lowry (11) and Ellman (6), respectively. A 20 to 25% molar substitution with the oligosaccharide was usually attained on human serum albumin.
When the Neisseria meningitidis outer membrane protein complex was used, the procedure was modified at the step of diafiltration. The reaction mixture was precipitated twice with 80% ethanol (in purified water) followed by centrifugation (1,500 rpm, 4°C, 10 min).
(ii) Step 2.
To a solution of thiolated human serum albumin (20.8 mg) in PBS (pH 7.2, EDTA 1.86 g/liter, 5 ml) a solution of synthetic polyribosylribitolphosphate (16.8 mg) previously dissolved in PBS (pH 7.2, 0.4 ml) was added under an N2 atmosphere. The conditions used with the other proteins may be seen in Table 2. The resulting solution was gently stirred for several hours at 4 to 8°C. The reaction was quenched with ethylmaleimide (1 mg, 8 μmol) and then diafiltered against PBS (pH 7.2, cellulose acetate membrane, 30-kDa cutoff).
TABLE 2.
Experiments under typical conditions for conjugation of sPRP to thiolated proteins
| Conjugate | Reaction conditions
|
Thiolated protein/protein ratio (mg) | Yield based on sPRP (%) | |
|---|---|---|---|---|
| Thiolated protein (mg) | sPRP (mg) | |||
| sPRP-HSA | 20.8 | 16.8 | 1.0:3.0 | 42.7 |
| sPRP-BSA | 13.0 | 7.0 | 1.0:2.9 | 63.0 |
| sPRP-TT | 14.0 | 13.3 | 1.0:2.2 | 40.9 |
| sPRP-OMP | 17.8 | 5.0 | 1.0:9.2 | 31.2 |
The protein and carbohydrate contents in the final conjugate were determined by the methods of Lowry et al. (11) and orcinol (4), respectively. The oligosaccharide-protein conjugates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10).
Immunization methods.
Four-week-old female Sprague Dawley rats were maintained at the animal facilities at the National Institute for Biological Standards and Control, London, United Kingdom. Groups of five rats were immunized subcutaneously at weeks 0 and 4, with 2 μg of immunogen (based on polyribosylribitolphosphate). Serum samples were collected at week 6.
Six- to eight-week-old female BALB/c mice were supplied by the Center for the Production of Laboratory Animals (Bejucal, Havana, Cuba) and maintained gn our animal facilities. Groups of 10 mice were immunized intraperitoneally with 2.5 μg of immunogen (based on polyribosylribitolphosphate). Immunizations were performed at weeks 0, 2, and 4, and serum samples were collected at week 6.
Four-week-old female New Zealand rabbits were supplied by the Center for the Production of Laboratory Animals (Bejucal, Havana, Cuba) and maintained in our animal facilities. Groups of three rabbits were immunized subcutaneously with 5 μg of immunogen (based on polyribosylribitolphosphate). The immunization was performed with either two doses at 0 and 2 weeks or three doses at 0, 2, and 4 weeks. Serum was collected at 0 and 3 weeks or 0, 1, 3, and 5 weeks, respectively.
ELISA.
Antibody titers against polyribosylribitolphosphate were determined by enzyme-linked immunosorbent assay (ELISA). The ELISA method used is one based on that described by Phipps et al. (16) adapted for the evaluation of animal serum. Plates were coated with one of the following conjugates for antigenicity studies: HbO-HA, sPRP-HSA, or sPRP-BSA. For immunogenicity studies only HbO-HA was used. The albumin conjugates were dissolved at 1 μg/ml (based on polyribosylribitolphosphate) in PBS and incubated overnight at 37°C. After that, plates were washed four times with 0.05% Tween 20 aqueous solution. Plates were blocked with 1% bovine serum albumin in PBS and incubated for 30 min at 37°C. The wells were washed four times and incubated with a serial twofold dilution of serum sample diluted in PBS solution containing 0.3% Tween 20, 10 mM EDTA, and 1% bovine serum albumin for 90 min at room temperature. The wells were then rinsed four times, and the corresponding anti-immunoglobulin G whole molecule horseradish peroxidase conjugate was added to each well. Plates were washed again after 90 min of incubation at room temperature, and the substrate solution was added: O-phenylendiamine, H2O2 in citrate buffer, pH 5. After 20 min in darkness, the reaction was stopped with 3 M HCl and read at 492 nm with an ELISA Sunrise reader.
Antibody titers were defined as the log of the highest dilution giving twice the absorbance value calculated against that of sera from control animals (immunized with buffer), with a minimum value of 0.2.
Inhibition studies were performed with the same procedure with the following modification. Serum dilution was selected in order to obtain an optical density of 1 and was incubated separately with a serial dilution of inhibitor. The procedure was continued as above.
Measurement of relative avidity by ELISA.
The measurement of relative avidity by ELISA was followed as described above, with slight modifications. The serum dilution was chosen to obtain an optical density of 1. After the plates were washed following the incubation of the serial serum dilution, ammonium thiocyanate in PBS was added to the appropriate wells in concentrations ranging from 0.1 to 1 M. The plates were then allowed to stand for 15 min at room temperature before washing and continuing the assay as described above. The avidity index was calculated by the formula avidity index = C × f, where C is the concentration of ammonium thiocyanate destabilizing 50% of antigen-antibody interaction and f is the dilution factor.
Serum bactericidal assay.
The functional activity of the antibodies obtained was measured by a serum bactericidal assay (14). Serum samples, heat inactivated at 56°C for 30 min, were tested in twofold dilutions in cold Hanks' balanced salt solution with 0.15 mM CaCl2 and 0.5 mM MgCl2. The reaction mixtures contained 12.5 μl of serum sample, 12.5 μl of human serum lacking bactericidal activity as a complement source, and 12.5 μl of an 8 × 103/ml log-phase H. influenzae type b cell suspension (strain Eagan). The final volume was completed to 60 μl with Hanks' balanced salt solution. All reactions were performed in duplicate, and the mixtures were incubated for 30 min at 37°C. After that, the reactions were plated onto brain heart infusion plates supplemented with NAD and hemin. Colonies were counted the next day, and bactericidal titer was determined as the reciprocal of the highest dilution of serum capable of killing more than 50% of the initial bacterial inoculum. For measurement of the specific inhibition of bactericidal activity, 100 μl of serum was incubated with 100 μl of native polyribosylribitolphosphate (0.05 mg of polyribosylribitolphosphate/ml, final concentration) at 37°C for 1 h before the serum bactericidal assay was performed.
RESULTS
Conjugation of synthetic oligosaccharides to protein.
Synthetic oligosaccharides are small molecules differing from the capsular polysaccharide by their low absorption on ELISA plates and by the presence (usually) of a single epitope. To better understand their antigenic and immunogenic properties, they were conjugated to several proteins. The first step in this conjugation process is the thiolation of the carrier protein, which was performed initially with N-hydroxysuccinimide dithiopropionate followed by reduction with dithiothreitol. The procedure is a general one and can be applied to different proteins. The results of derivatization are shown in Table 1.
TABLE 1.
Conditions and results obtained during the thiolation of proteins (step 1 in our conjugation process)
| Protein | Wt (mg) | Reaction conditionsa
|
SH/protein ratio (μmol/μmol) | |||
|---|---|---|---|---|---|---|
| Mass (Da) | Reaction vol (ml) | DSP (mg) | DTT (mg) | |||
| HSA | 24.5 | 68,000 | 5.0 | 2.9 | 19.3 | 25 |
| BSA | 13.3 | 67,000 | 2.8 | 2.4 | 10.8 | 20 |
| TT | 18.5 | 150,000 | 4.0 | 1.5 | 15.4 | 32 |
| OMPc | 30.0 | 6.0 | 5.4 | 23.1 | 0.24 | |
DSP, N-hydroxysuccinide dithiopropionate. DTT, dithiothreitol.
SH, thiol.
OMP, for which the SH/protein ratio is expressed in micromoles per milligram.
The maleimido group from the spacer in the synthetic polyribosylribitolphosphate has a strong thiophilic character that facilitated conjugation to proteins. The conjugation reaction proceeds smoothly, giving yields ranging from 30 to 60% based on synthetic polyribosylribitolphosphate. The results are summarized in Table 2. All conjugates were characterized by polyacrylamide gel electrophoresis (Fig. 2). These show an increase in the molecular mass as well as a broadening in the band typical for this random introduction of several carbohydrate chains.
FIG. 2.
Coomassie brilliant blue-stained sodium dodecyl sulfate-polyacrylamide gel of conjugates. A, B, and C, gels at 10% polyacrylamide; D, gel at 7.5% polyacrylamide. Lanes: 1, BSA; 2, sPRP-BSA; 3, human serum albumin; 4, sPRP-HSA; 5, OMP; 6, sPRP-OMP; 7, TT; and 8, sPRP-TT.
Antigenicity of synthetic polyribosylribitolphosphate conjugates.
To determine if synthetic oligosaccharides mimic the capsular polysaccharide epitopes and are recognized to the same extent, synthetic polyribosylribitolphosphate-albumin conjugates were prepared and used as the coating reagent for ELISA plates. In the first experiment, the ability of sPRP-HSA, prepared from synthetic antigen, and HbO-HA (National Institute for Biological Standard and Control, United Kingdom) to bind anti-H. influenzae type b antibodies was compared. The serum of rabbits immunized with two types of vaccines were used: PRP-TT, having a native capsular polysaccharide (full size) conjugated to tetanus toxoid, and PRP-CRM197, having oligosaccharides obtained by partial hydrolysis of the capsular polysaccharide conjugated to CRM197 protein.
Table 3 shows comparative titers for the two coating antigens calculated for the same serum. A general correlation of 0.972 was obtained between synthetic and native oligosaccharides conjugated to the same carrier (Table 3). A similar value, 0.978, was obtained for the synthetic antigen conjugated with two different carriers (Table 3). Furthermore, the reaction between the anti-H. influenzae type b antibodies (pools from rabbit serum PRP-CRM197 and PRP-TT) and sPRP-HSA or sPRP-BSA was inhibited by the native capsular polysaccharide, thereby demonstrating the reaction is specific for polyribosylribitolphosphate (Fig. 3).
TABLE 3.
Comparative recognition of synthetic and natural PRP oligosaccharide conjugatesa
| Rabbit no. | Vaccine | Polysaccharide | sPRP-HSA | HbO-HA | sPRP-BSA |
|---|---|---|---|---|---|
| 1 | PRP-CRM197 | Oligosaccharide | 1.98 | 2.14 | |
| 2 | PRP-CRM197 | Oligosaccharide | 3.51 | 3.66 | |
| 3 | PRP-CRM197 | Oligosacchide | 2.93 | 3.00 | |
| 4 | PRP-TT | Polysaccharide | 2.63 | 2.73 | |
| 5 | PRP-TT | Polysaccharide | 1.54 | 1.13 | |
| 6 | PRP-TT | Polysaccharide | 3.25 | 3.10 | |
| 7 | PRP-TT | Polysaccharide | 2.08 | 1.73 | |
| 8 | PRP-TT | Polysaccharide | 2.72 | 2.60 | |
| 11 | PRP-CRM197 | Oligosacchide | 3.76 | 3.73 | |
| 12 | PRP-CRM197 | Oligosacchide | 3.23 | 3.23 | |
| 13 | PRP-CRM197 | Oligosacchide | 2.91 | 2.94 | |
| 14 | PRP-TT | Polysaccharide | 2.62 | 2.46 | |
| 15 | PRP-TT | Polysaccharide | 2.27 | 2.42 | |
| 16 | PRP-TT | Polysaccharide | 2.72 | 2.83 | |
| 17 | PRP-TT | Polysaccharide | 3.29 | 3.37 | |
| 18 | PRP-TT | Polysaccharide | 2.76 | 2.84 |
Rabbits were immunized with PRP-TT or PRP-CRM197. For rabbits 1 to 8, sPRP- and PRP-HSA conjugates were used as coating reagent; for rabbits 11 to 18, the coating antigen used was sPRP with two different protein carriers, HSA and BSA. Values are for individual sera.
FIG. 3.
Inhibition of the ELISA shown in Table 3 with the capsular H. influenzae type b polysaccharide. The serum samples used are a pool of sera from rabbits immunized with PRP-CRM197 and PRP-TT.
Immunogenicity of synthetic oligosaccharide-protein conjugates.
To study the ability of synthetic oligosaccharide to induce an immune response useful in vaccine development, we selected two different bacterial proteins successfully used as carriers in conjugate vaccines, Neisseria meningitidis outer membrane protein and tetanus toxoid. Our conjugates were studied initially in BALB/c mice and Sprague Dawley rats. Two sPRP-OMP conjugates with different carbohydrate/protein ratios (1:4 and 1:12) were used in this preliminary study. The response to both conjugates was strong (Fig. 4) and comparable to the commercially available PRP-CRM197 vaccine. The response to sPRP-TT in a wide carbohydrate/protein ratio (1:2 to 1:12) was weak and inconsistent (not shown). sPRP-OMP with a ratio of 1:12 was selected for further studies in rats. This serum was completely inhibited by the native capsular polysaccharide (Fig. 5), showing that the synthetic polyribosylribitolphosphate conjugate with a suitable carrier was also immunogenic.
FIG. 4.
Comparative immunogenicity in Sprague Dawley rats and BALB/c mice of synthetic polyribosylribitolphosphate conjugated to Neisseria meningitidis outer membrane protein and a commercial vaccine, PRP-CRM197.
FIG. 5.
Inhibition of anti-polyribosylribitolphosphate serum elicited in rats by sPRP-OMP (1:12). HbO-HA was used as the coating antigen. Sera 1, 2, and 3 are from individual rats. The capsular polysaccharide was used at increasing concentrations.
In other experiments we sought to find a suitable animal model for developing both types of conjugates as vaccine candidates and for comparing their immunogenic properties. New Zealand rabbits consistently responded to both sPRP-TT (1:2) and sPRP-OMP (1:9), in the presence or even in the absence of aluminum hydroxide (Fig. 6). Despite the strong differences reported for such conjugates in the literature (5), the immune response was very similar. In another experiment, a group of rabbits were immunized with both types of conjugates containing the synthetic polyribosylribitolphosphate. No major differences were found in the kinetics or in the responses compared to conjugate containing the capsular antigen PRP-TT (Fig. 7).
FIG. 6.
Titers of anti-H. influenzae type b serum elicited in New Zealand rabbits (pooled serum) by sPRP-TT and sPRP-OMP alone or with aluminum hydroxide. PRP-CRM197 was used as a control.
FIG. 7.
Kinetics of anti-H. influenzae type b antibody formation in New Zealand rabbits (pooled sera) by sPRP-OMP and sPRP-TT. PRP-TT was used as a positive control.
The rabbit sera were further selected for a more detailed study including relative avidity for the second and third doses (Table 4). The behavior of sPRP-TT resembled that of PRP-TT with a small increase in avidity, sPRP-OMP inducing a stronger increase in avidity after the third dose.
TABLE 4.
Average avidity index of antibodies after ammonium thiocyanate dissociation
| Inmunogen | Avidity index (log)
|
|
|---|---|---|
| Dose 2 | Dose 3 | |
| sPRP-OMP | 1.53 ± 0.09 | 2.48 ± 0.40 |
| sPRP-TT | 1.76 ± 0.45 | 2.01 ± 0.36 |
| PRP-TT | 1.65 ± 0.05 | 1.82 ± 0.14 |
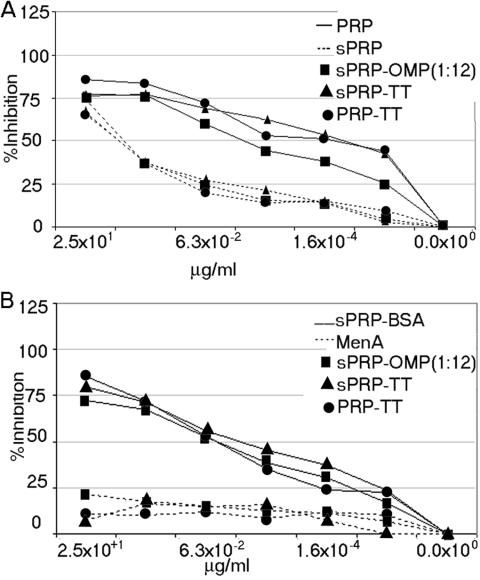
The specificity of the antibodies in a serum pool was studied by determining their inhibition with four different compounds: capsular polyribosylribitolphosphate, sPRP-BSA, synthetic polyribosylribitolphosphate, and unrelated capsular polysaccharide from Neisseria meningitidis group A (MenA). For simplicity, results are shown in two different panels (Fig 8A and B). Inhibition was independent of the source of polyribosylribitolphosphate in the vaccine or in the inhibitor. All three sera display similar results. The best inhibitors were a capsular polysaccharide and sPRP-BSA. Synthetic polyribosylribitolphosphate required a higher concentration for the same inhibition, as expected because of its monovalent nature. The low inhibition even at a high concentration of MenA capsular polysaccharide demonstrated once again the specificity of the interaction.
FIG. 8.
(a) Comparative inhibition of anti-H. influenzae type b serum elicited in rabbits against sPRP-OMP, sPRP-TT, and control vaccine PRP-TT. HbO-HA was used in all cases as the coating reagent. The inhibitors used were capsular polysaccharide (polyribosylribitolphosphate) and synthetic polyribosylribitolphosphate (sPRP). (b) Comparative inhibition of anti-H. influenzae type b serum elicited in rabbits against sPRP-OMP, sPRP-TT, and control vaccine PRP-TT. HbO-HA was used in all cases as the coating reagent. The inhibitors used were sPRP-BSA conjugate and nonrelated MenA capsular polysaccharide.
The bactericidal power of rabbit serum in the presence of complement was also studied. As can be seen from Fig. 9, the sera of rabbits immunized with sPRP-OMP, sPRP-TT, or control PRP-CRM197 display strong bactericidal activity inhibited by the capsular polysaccharide. Rabbit serum displayed a nonspecific residual bactericidal activity against H. influenzae type b that was not inhibited by the capsular polysaccharide. The results displayed in Fig. 9 showed the overall and the nonspecific bactericidal activity after inhibition with the capsular polysaccharide. The difference corresponds to specific bactericidal activity.
FIG. 9.
Overall and residual nonspecific bactericidal activity of serum against the Eagan strain of Haemophilus influenzae type b expressed as the last serum dilution killing >50% of bacteria. The differences correspond to specific anti-H. influenzae type b bactericidal activity.
DISCUSSION
Capsular polysaccharides and their oligosaccharide fragments have been used for the preparation of conjugate vaccines against H. influenzae type b (8, 12). In parallel, several attempts to reproduce the fragment of the capsular polysaccharide by chemical synthesis have been made successfully (2, 9, 13). The synthetic fragments are then conjugated to protein carriers and tested in laboratory animals for their potential as vaccine candidates (2, 14). None of these synthetic antigens were previously tested in humans. This was probably because the efficiency of the synthetic process is too low for competing with conventional, well-established antigen production processes from native sources.
We therefore developed a new improved procedure for the synthesis of H. influenzae type b oligosaccharide fragment (17). This synthetic antigen could be produced and conjugated to carrier proteins as efficiently as happens with the use of the capsular polysaccharide (Tables 1 and 2).
The first experiments were designed to determine if the synthetic polyribosylribitolphosphate displayed the same epitopes as the fragment of capsular polysaccharides. The results obtained in those experiments showed an excellent correlation between the synthetic and capsular oligosaccharides, demonstrating that the epitopes exposed in synthetic polyribosylribitolphosphate are very similar to those of fragments from native polysaccharide. Furthermore, the reaction was inhibited by a very low polysaccharide concentration, 3 × 10−3 to 1.6 × 10−4 μg/ml for 50% inhibition (Fig. 3). This confirmed that the synthetic polyribosylribitolphosphate reproduces the epitopes recognized by anti-H. influenzae type b antibodies.
Among the proteins that are currently in use as carriers for anti-H. influenzae type b vaccine are Neisseria meningitidis outer membrane protein, tetanus toxoid, diphtheria toxoid, and CRM197. We selected the first two for their ready availability and also for the sharp differences found in clinical trials with these two conjugates. The conjugation proceeded in both cases with similar good yields, and although tetanus toxoid is more readily available, we were interested in obtaining a general immunological profile for both conjugates.
The first and the most important response differences were found in rodents. Usually the response to sPRP-OMP was stronger in titer and also in number of animals responding. Our experience in using sPRP-OMP as an immunogen in mice indicates that animals rarely failed to respond. This may be associated with the ability of outer membrane proteins to induce a Th1-type response, especially in BALB/c mice. The same was not true for sPRP-TT; many animals failed to respond, and the titers were usually very weak. This initial experiment with rodents demonstrated that the synthetic antigen conjugated to a suitable carrier was able to induce anti-H. influenzae type b antibodies.
In a search for a more suitable animal model independent of the protein carrier, we found that New Zealand rabbits respond equally well to both types of conjugates.
The anti-H. influenzae type b immune response in rabbits was very good with or without aluminum hydroxide, regardless of the protein carrier used (Fig. 6). The antibody titers always increased after the second dose, and an additional increment occurred after the third dose. Both conjugates were able to induce a strong anti-H. influenzae type b response with a small increase in the avidity index for both vaccines and control. This is associated with the vaccine's ability to induce maturation in the anti-H. influenzae type b antibody response. The low capsular polysaccharide concentration needed for 50% inhibition indicated that a specific reaction was induced by synthetic polyribosylribitolphosphate conjugates. On the other hand the very low sPRP-BSA concentration, similar to capsular polysaccharide needed for 50% inhibition of the reaction between anti-PRP-TT serum and HbO-HA, indicated that synthetic antigen as a BSA conjugate with multiple copies interacts in a similar fashion to the capsular polysaccharide. The nonrelated MenA capsular polysaccharide showed only a low, nonspecific inhibition even at high concentrations. Finally, in both cases, sPRP-TT and sPRP-OMP, the antibodies displayed bactericidal activity similar to that induced by the commercially available anti-H. influenzae type b vaccine, demonstrating that despite the synthetic origin, the antigen possess all the relevant properties of their native counterparts.
In conclusion, our data show that these synthetic polyribosylribitolphosphate fragments display both antigenicity and immunogenicity patterns in laboratory animals similar to those of their native analogues currently used in other commercial vaccine preparations. This finding supports further pharmaceutical development and clinical evaluations of our conjugates as alternatives to conventional vaccines against H. influenzae type b.
Acknowledgments
This work was supported by grants from the Cuban State Council and the Ministry of Science, Technology and Environment and also from the Pan-American Health Organization (PAHO).
We gratefully recognize J. L. DiFabio (PAHO) for many helpful discussions and support, M. Beurret (RIVM, Biltjoven, The Netherlands) for providing the native polyribosylribitolphosphate, and J. B. Robbins (NIH) for providing the H. influenzae type b Eagan strain. C. H. Fox is gratefully acknowledged for critical reading of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Anderson, P. 1983. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect. Immun. 39:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong, P., N. Chan, A. Kandil, B. Triplet, O. James, Y. P. Yang, S. P. Shi, and M. Klein. 1997. A strategy for rational design of fully synthetic glycopeptide conjugate vaccines. Infect. Immun. 65:4918-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu, C., R. Schneerson, J. B. Robins, and S. C. Rastorgi. 1983. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect. Immun. 40:245-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dische, Z. 1962. Color reactions of pentoses, p. 484-488. In R. L. Whistler, M. L. Wolfrom, J. N. BeMiller, and F. Shafizadeh (ed.), Methods in carbohydrate chemistry, vol. 1. Academic Press, New York, N.Y.
- 5.Donelly, J. J., R. Randall Deck, and M. A. Liu. 1990. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein complex conjugate vaccine. Immunology 145:3071. [PubMed] [Google Scholar]
- 6.Ellman G. L. 1958. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 74:443.. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Santana, V., R. Gonzalez-Lio, J. Sarracent-Perez, and V. Verez Bencomo. 1998. Synthesis of inmunogens containing lewisa and lewisb haptens by the use of glycosides of 5-azido-3-oxapentanol. Glycoconj. J. 306:163-170. [DOI] [PubMed] [Google Scholar]
- 8.Gordon, L. K. 1984. Characterization of a hapten-carrier conjugate vaccine: H. influenzae diphtheria conjugate vaccine, p. 393-396. In R. M. Chanock and R. A. Lerner (ed.), Modern approaches to vaccines. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 9.Hoogerhout P., D. Evenberg, C. A. A. van Boeckel, J. T. Poolman, E. C. Beuvery, C. A. van der Marel, and J. H. van Boom. 1987. Synthesis of fragments of the capsular polysaccharide of Haemophilus influenzae type b, comprising two or three repeating units. Tetrahedron Lett. 28:1553. [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 12.Marburg, S. D. Jorn, R. L. Tolman, B. Arison, J. McCauley, P. J. Kniskern, A. Hagopian, and P. P. Vella. 1986. Bimolecular chemistry of macromolecules-synthesis of bacterial polysaccharide conjugates with Neisseria meningitidis membrane protein. J. Am. Chem. Soc. 108:5282-5297. [Google Scholar]
- 13.Nilsson, S., M. T. Bengtsson, and O. Norberg. 1992. Solid-phase synthesis of a fragment of the capsular polysaccharide of Haemophilus influenzae using H-phosphonate intermediates. J. Carbohydr. Chem. 11:265-285. [Google Scholar]
- 14.Peeters, C. C. A. M., D. Evenberg, P. Hoogerhout, H. Kayhty, L. Saarinen, C. A. A. van Boeckel, G. A. van der Marel, J. H. van Boom, and J. T. Poolman. 1992. Synthetic trimer and tetramer of 3-β-d-ribose-(1-1)-d-ribitol-5-phosphate conjugated to protein induce antibody responses to Haemophilus influenzae type b capsular polysaccharide in mice and monkeys. Infect. Immun. 60:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltola, H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phipps, D. C., J. West, R. Eby, M. Koster, D. V. Madore, and S. A. Quataert. 1990. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J. Immunol. Methods 135:121-128. [DOI] [PubMed] [Google Scholar]
- 17.Verez-Bencomo, V., V. Fernández-Santana, E. Hardy, M. E. Toledo, M. C. Rodríguez, L. Heynngnezz, A. Rodriguez, A. Baly, L. Herrera, M. Izquierdo, A. Villar, Y. Valdés, K. Cosme, M. L. Deler, M. Montane, E. Garcia, A. Ramos, A. Aguilar, E. Medina, G. Toraño, I. Sosa, I. Hernandez, R. Martínez, A. Muzachio, A. Carmenates, L. Costa, F. Cardoso, C. Campa, M. Diaz, and R. Roy. 2004. A synthetic capsular polysaccharide vaccine against Haemophilus influenzae type b polysaccharide. Science 305:522-524. [DOI] [PubMed] [Google Scholar]
- 18.Verez-Bencomo, V., and R. Roy. August1999. Oligosaccharides derived from ribose-ribitol-phosphate, and vaccines containing them. Patent Community Treaty WO 01/16146. U.S. patent 6,765,091.