Abstract
We constructed highly transformable and infectious Borrelia burgdorferi B31 by inactivating BBE02, a putative restriction-modification gene on the linear plasmid lp25. The low-passage-number B31 clones 5A4 (containing all plasmids) and 5A18 (lp28-4− lp56−) were used for this study, and BBE02 was disrupted by homologous recombination. The transformation efficiency with the shuttle vector pBSV2C03::gntΔkan was increased from <1 to ∼10 colonies per μg of DNA for 5A4 and 5A4 BBE02::Kanr and from 14 to approximately 600 colonies per μg of DNA for 5A18 and 5A18 BBE02::Kanr. lp25, which is required for infectivity in mice, was retained in BBE02 mutants transformed with pBSV2C03::gntΔkan, but lp25 was not detected in transformants of the parental clones 5A4 and 5A18. BBE02 disruptants and pBSV2C03::gntΔkan transformants of these clones remained infectious in C3H/HeN mice, and the 50% infective doses of the BBE02 disruptants were <102 organisms per mouse. The inactivation of BBE02 thus eliminates a transformation barrier for infectious B. burgdorferi B31 and will provide a valuable tool for studying the virulence factors of Lyme disease.
Lyme disease is a multistage systemic disease caused by members of the spirochete genus Borrelia and is transmitted to humans by Ixodes ticks. Borrelia burgdorferi is the principal causative agent of Lyme disease in the United States, whereas B. burgdorferi, B. afzelii, and B. garinii have each been shown to cause human disease in regions of Europe. The genome of B. burgdorferi B31 has been sequenced (9) and is composed of a linear chromosome, 9 circular plasmids (cp), and 12 linear plasmids (lp) (3, 9). These plasmids range in size from 5 kb (lp5) to 56 kb (lp56). The continuous in vitro passage of organisms leads to a spontaneous loss of plasmids. The loss of the linear plasmid lp25, lp28-1, or lp28-4 has been correlated with a reduced infectivity of B. burgdorferi B31 in needle-inoculated mice (13, 14, 19), indicating that factors important for the virulence of Lyme disease-causing Borrelia organisms are encoded by these plasmids. The nicotinamidase gene pncA (BBE22) carried on lp25 was identified as an essential factor in the experimental infection of mice (20), but the virulence-associated genes of lp28-1 and lp28-4 have not been determined.
The study of virulence determinants of Lyme disease-causing Borrelia organisms has been limited due to the lack of efficient methods for genetic manipulation. During the past decade, several groups have contributed to the development of genetic tools for these bacteria. The first successful transformation and genetic exchange in B. burgdorferi utilized resistance to coumermycin resulting from mutations of the native gyrB gene (23). Since that time, four additional selective antibiotic markers have been developed: (i) a kanamycin resistance gene from the Tn903 transposon connected with the flaB or flgB promoter (2); (ii) ermC, originally isolated from Lactococcus lactis, which confers resistance to erythromycin (25); (iii) aadA, which confers streptomycin resistance (8); and (iv) aacC1, which provides resistance to gentamicin (6). These selectable markers have been used to develop DNA vectors for B. burgdorferi. The shuttle vector pBSV2, constructed by Stewart et al. (26), contains the origin of replication and associated genes from the 9-kb circular plasmid cp9 of B. burgdorferi and the ColE1 origin of replication from an Escherichia coli plasmid. Eggers et al. (5) established the shuttle plasmid pCE320, which includes the replication locus of the 32-kb plasmid cp32-3. Sartakova et al. (25) demonstrated that the broad-range host vector pGK12 can also transform B. burgdorferi; however, it does not appear to be as stable as the vectors containing origins of replication from Borrelia plasmids (5, 26).
Transformation studies with B. burgdorferi B31 have been attempted in low-passage-number and high-passage-number strains (4, 12), and low transformation efficiencies have been repeatedly observed for infectious, low-passage-number strain B31 (7, 15, 26). The presence of the linear plasmids lp25 and lp56 of B. burgdorferi B31 was found to be strongly associated with decreased transformation efficiencies; also, lp25 was lost in low-passage-number organisms transformed with pBSV2, indicating that variants lacking lp25 are transformed preferentially (15). These properties were tentatively attributed to the putative restriction-modification genes BBE02 and BBQ67, carried on lp25 and lp56, respectively (15). The purpose of the present study was to determine whether inactivation of the putative restriction-modification gene BBE02 in Lyme disease-causing Borrelia results in an increased transformation rate in infectious, low-passage-number clones of B. burgdorferi B31.
MATERIALS AND METHODS
Bacteria.
The low-passage-number B. burgdorferi B31 clones 5A1 (lp56−), 5A4 (containing all plasmids), 5A13 (lp25−), 5A14 (lp25− lp56−), and 5A18 (lp56− lp28-4−) were obtained by subsurface colony formation of passage 5 organisms (17). The plasmid profiles of these clones and the transformants were determined by PCRs using plasmid-specific primers (19). E. coli Top10F′ (Invitrogen) was used for the preparation of plasmids for electroporation into B. burgdorferi.
Plasmid construction.
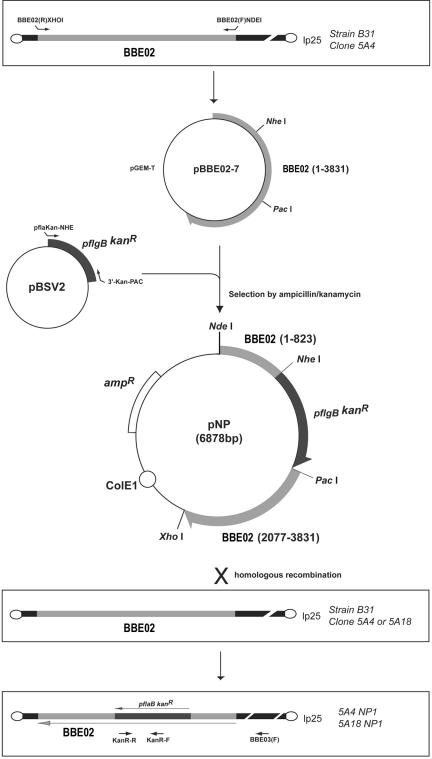
Plasmids pBSV2 and pBSV2C03::gnt were graciously provided by P. E. Stewart and P. A. Rosa (National Institute of Allergy and Infectious Diseases, Rocky Mountain Laboratories, Hamilton, Mont.). The suicide vector pNP was constructed for the disruption of BBE02 (Fig. 1). A 3,831-bp fragment of lp25 (coordinates 326 to 4,156; accession number AE000785) (9) containing BBE02 was amplified by use of the primers BBE02(R)XHOI (5′-TTT ATT CTC GAG TTT ATG ATA AAA AAT TTT ATT ATT TAG T-3′) and BBE02(F)NDEI (5′-AAG CAT AGC ATA TGA AAA CTA ATG ATA TCG TAA AAA C-3′). The PCR product was cloned into the pGEM-T vector (Promega), and the resulting construct was named pBBE02-7. The kanamycin resistance gene with the flgB promoter (pflgB Kanr) carried on plasmid pBSV2 was amplified by use of the primers pflaKan-NHE (5′-TGT CGC TAG CTA ATA CCC GAG CTT CAA GGA AGA T-3′) and 3′-Kan-PAC (5′-GAA TTT AAT TAA GCC GTC CCG TCA AGT CAG CG-3′), which include NheI and PacI sites (underlined). The resulting product was purified by use of a HighPure PCR product purification kit (Roche) according to the manufacturer's instructions. The pBBE02-7 plasmid and the PCR product containing pflgB Kanr were digested with NheI and PacI and then ligated, resulting in plasmid pNP (Fig. 1). The plasmid pBSV2C03::gntΔkan was constructed by the deletion of a PvuI DNA fragment from pBSV2C03::gnt in this study.
FIG. 1.
Strategy of inactivation of BBE02 in B. burgdorferi B31 infectious clones 5A4 and 5A18.
Electroporation of B. burgdorferi.
The electroporation of B. burgdorferi clones was performed as described previously (15) and as originally reported by Samuels (24). Up to 10 μg of pNP DNA was used for suicide vector transformations. An amount from 0.5 to 1.0 μg of DNA was used for shuttle vector transformations. For suicide vector transformation studies, Borrelia was incubated for 6 days with kanamycin (0.1 mg/ml) following incubation for a day without kanamycin (2). For antibiotic selection of suicide vector transformants, kanamycin (0.2 mg/ml) was used. For shuttle vector transformations, Borrelia was incubated for 24 h without any selectable antibiotics after transformation (24). The cultures were then plated on a soft agar overlay (17) on Barbour-Stoenner-Kelly II (BSK-II) medium with or without selectable antibiotics. For antibiotic selection of shuttle vector transformants, kanamycin (0.2 mg/ml) and gentamicin (0.2 mg/ml) were used.
Characterization of BBE02 disruptants.
The suicide vector pNP was electroporated into clone 5A4 or 5A18, and BBE02 was disrupted by double-crossover homologous recombination (Fig. 1). Transformants were selected by subsurface plating on BSK-II medium with 0.2 mg of kanamycin/ml according to the method reported by Bono et al. (2). Colonies were selected from the BSK-II plates and were cultured until mid-log phase. BBE02 disruption was confirmed by PCRs using primers BBE03(F) (5′-TTC TAG ACC AAA TAA GGC TTC CGG-3′) and KanR-R (5′-AAA CTC ACC GAG GCA GTT CC-3′), by Southern hybridization, and by sequencing of the BBE03(F)/KanR-R PCR products by standard procedures (22). The primers KanR-F (5′-TCA GAC TAA ACT GGC TGA CG-3′) and KanR-R, which amplify a 509-bp region of the kanamycin resistance cassette from the shuttle vector pBSV2 (accession number AY187276), were used to prepare a DNA probe for Southern analysis.
Mouse infection studies.
Five-week-old female C3H/HeN mice were purchased from Charles River Japan, Inc. (Yokohama, Japan) and were used for all mouse experiments. Cloned B31 strains, as described above, were cultured in BSK-II medium from frozen stocks just before use. The spirochetes were cultured until early or mid-log phase and then diluted to 2 × 106 cells/ml in sterile BSK-II medium. A 50-μl volume (105 cells) was inoculated subcutaneously (s.c.) by use of a needle into the backs or right hind footpads of the mice. For the initial analysis of infectivity (see Table 3), three mice were inoculated with 105 cells of each of the B. burgdorferi clones examined. The mice were sacrificed on day 14 or 28 after inoculation. Samples from the ear, bladder, and right tibiotarsal joint were collected aseptically from each mouse for cultivation in BSK-II medium. The cultures were examined for spirochetes by dark field microscopy after 2 and 3 weeks of culture. This experiment was repeated, and the results were combined. The median infectious dose (ID50) was determined by the inoculation of groups of six mice with serial 10-fold dilutions of organisms (105, 104, 103, 102, and 101) s.c. into the back. All mice were sacrificed on day 28 after inoculation, and samples from the ear, bladder, and tibiotarsal joint were collected aseptically from each mouse for cultivation. ID50 values were calculated by the method of Reed and Muench (21).
TABLE 3.
Infectivity of BBE02 disruptant B31 clones for C3H/HeN mice
| B31 clone or expt no. | Genotype | Additional shuttle vector | No. of culture-positive samples/total no. of samplesa
|
|||
|---|---|---|---|---|---|---|
| Ear | Bladder | Joint | All sites | |||
| Expt 1 | ||||||
| 5A4 | Contains all plasmids | 3/3 | 3/3 | 3/3 | 9/9 | |
| 5A4 NP1 | cp9− BBE02::Kanr | 3/3 | 3/3 | 3/3 | 9/9 | |
| 5A4 NP1 cl1 | cp9− BBE02::Kanr | pBSV2C03::gntΔkan | 3/3 | 3/3 | 3/3 | 9/9 |
| 5A4 NP1 cl2 | cp9− BBE02::Kanr | pBSV2C03::gntΔkan | 3/3 | 3/3 | 3/3 | 9/9 |
| 5A13 | lp25− | 0/3 | 0/3 | 0/3 | 0/9 | |
| Expt 2 | ||||||
| 5A4 | Contains all plasmids | 3/3 | 3/3 | 3/3 | 9/9 | |
| 5A4 NP1 | cp9− BBE02::Kanr | 3/3 | 3/3 | 3/3 | 9/9 | |
| 5A4 NP1 cl1 | cp9− BBE02::Kanr | pBSV2C03::gntΔkan | 3/3 | 3/3 | 3/3 | 9/9 |
| 5A4 NP1 cl2 | cp9− BBE02::Kanr | pBSV2C03::gntΔkan | 3/3 | 3/3 | 3/3 | 9/9 |
| 5A18 | lp56− lp28-4− | 3/3 | 3/3 | 3/3 | 9/9 | |
| 5A18 NP1 | lp56− lp28-4− BBE02::Kanr | 3/3 | 3/3 | 3/3 | 9/9 | |
| 5A18 NP1 cl1 | lp56− lp28-4− BBE02::Kanr | pBSV2C03::gntΔkan | 3/3 | 3/3 | 3/3 | 9/9 |
| 5A18 NP1 cl2 | lp56− lp28-4− BBE02::Kanr | pBSV2C03::gntΔkan | 3/3 | 3/3 | 3/3 | 9/9 |
| 5A13 | lp25− | 0/3 | 0/3 | 0/3 | 0/9 | |
Mice were sacrificed for bacterial isolation 2 weeks after s.c. inoculation with 105 B. burgdorferi spirochetes, except for the 5A13 group in experiment 1 (sacrificed at 4 weeks postinoculation).
Measurements of footpad or ankle diameters were performed as previously described (16). Briefly, footpad swelling was determined by measurement with a dial thickness gauge on days 0, 3, 5, 7, 10, 14, and 21 after footpad inoculation with 105 organisms. Ankle diameter increases were determined by measurement with a thickness gauge on days 0 and 21 after inoculation. Tibiotarsal joints from mice inoculated with B. burgdorferi were examined for histopathological changes. Mice inoculated with clones 5A4, 5A4NP1, 5A18NP1, and 5A13 were sacrificed 28 days after inoculation, and the tibiotarsal joint tissue was collected. Tissues were fixed in formalin, decalcified, sectioned, and stained with hematoxylin and eosin by standard methods.
RESULTS
BBE02 inactivation in infectious low-passage-number B31.
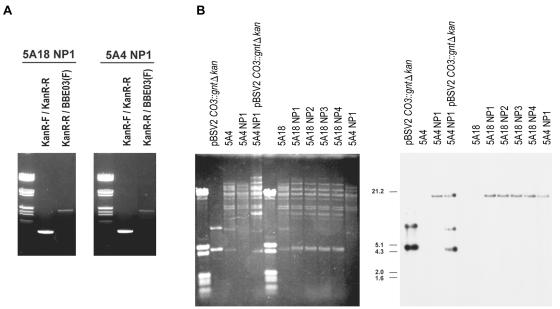
BBE02, a putative restriction-modification gene, was successfully disrupted by homologous double-crossover events in the B. burgdorferi B31 clones 5A4 and 5A18 (Fig. 1). Seven colonies of 5A4 and >1,000 colonies of 5A18 were observed on BSK-II plates containing kanamycin when the suicide vector pNP was used for transformation. Representative colonies were selected and named 5A4NP1 through 5A4NP3 or 5A18NP1 through 5A18NP5. BBE02 disruption was confirmed by PCR (Fig. 2A), Southern hybridization (Fig. 2B), and sequencing of the homologous recombination site (data not shown) for clones 5A4NP1 and 5A18NP1 to 5A18NP3. DNA bands of the expected sizes (0.5 and 2.2 kb) were obtained by PCRs using the primer pairs KanR-F-KanR-R and BBE03(F)-KanR-R, respectively (Fig. 2A). A DNA band that migrated at an apparent size of ∼20 kb hybridized with the Kanr probe by Southern hybridization. The aberrant migration of this band (which corresponds to the 25-kb plasmid lp25) may be due to the low G+C content (23.2%) of this plasmid. The plasmid profiles of BBE02 disruptants were assessed by PCR and compared with that of the relevant parental clone (data not shown). The plasmid profile of 5A18 (which lacks lp56 and lp28-4) was conserved in all BBE02-disrupted daughter clones. 5A4NP1 contained all of the 19 B. burgdorferi plasmids examined except for cp9. Previous studies indicated that cp9 is not essential for infection of mice (13, 19). Therefore, 5A4NP1 and 5A18NP1 to 5A18NP3 were selected for further analysis. When clone 5A1 (which lacks lp56) (19) was transformed with pNP, a total of 86 colonies were cultured on BSK-II plates containing kanamycin. However, none of the transformants were positive by PCRs using primers specific for the Kanr cassette, and no signal was detected by Southern hybridization analysis with the Kanr probe (data not shown). Therefore, these kanamycin-resistant clones did not result from integration of the kanamycin resistance gene but appear to have arisen from spontaneous mutations leading to resistance.
FIG. 2.
PCR and Southern analyses of BBE02 disruption. (A) The presence of Kanr was indicated by PCRs with primers KanR-F and KanR-R (expected amplification fragment size, 0.5 kb), which specifically amplified the kanamycin resistance gene. The generation of a PCR product (KanR-R/BBE03[F]) of the expected size (2.2 kb) between Kanr and BBE03 indicated integration of the Kanr marker within BBE02. Sample DNAs were electrophoresed in a 0.8% agarose gel and were stained with ethidium bromide. (B) Southern blot hybridization with a fragment of the kanamycin resistance gene. pBSV2C03::gntΔkan, which contains a portion of the Kanr gene, was used as a positive control. Clones 5A4 and 5A18 were the parental clones of BBE02 disruptants. Plasmid DNAs were electrophoresed in a 0.4% agarose gel, stained with ethidium bromide (left), blotted, and hybridized with the Kanr probe (right). The sizes of molecular standards are indicated in kilobases.
Transformation efficiencies in BBE02 disruptants.
The B. burgdorferi clones and BBE02 disruption mutants were transformed with pBSV2C03::gntΔkan for an evaluation of their transformation efficiencies. This plasmid contains a gentamicin resistance cassette in the cp9 gene BBC03, but a part of the kanamycin resistance cassette has been deleted. Gentamicin could thus be used to select transformants in the Kanr background of the BBE02 disruptants. As in previous studies (15), three different transformation phenotypes (high, intermediate, and low) were observed (Table 1). The low-passage-number clones 5A1 (lp56−) and 5A14 (lp25− lp56−) (19) were used as controls and exhibited intermediate and high transformation phenotypes, respectively. The transformation efficiency with the shuttle vector pBSV2C03::gntΔkan increased from <1 to ∼10 colonies per μg of DNA when we compared 5A4 and 5A4NP1, representing a change in transformation phenotype from low to intermediate. In the evaluation of 5A18 and the three 5A18NP clones examined, the number of gentamicin-resistant colonies isolated increased from 14 colonies to 551 to 628 colonies per μg of DNA. As predicted, the BBE02 mutation in 5A18 resulted in a high transformation rate phenotype, increasing the transformation efficiency ∼40- to 46-fold.
TABLE 1.
Transformation efficiency of B. burgdorferi B31 clones with pBSV2C03::gntΔkan
| B31 clone | Plasmid content
|
No. of gentamicin-resistant colonies/μg of DNA for expta
|
Transformability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ip25 | lp56 | 1 | 2 | 3 | 4 | 5 | Mean | ||
| 5A4 | + | + | 0 | 0 | NT | NT | 0 | 0 | Low |
| 5A4 NP1 (BBE02::Kanr) | + | + | 20 | 5 | 5 | NT | 10 | 10 | Intermediate |
| 5A18 | + | − | NT | NT | 10 | 15 | 16 | 14 | Intermediate |
| 5A18 NP1 (BBE02::Kanr) | + | − | NT | NT | 672 | 317 | 664 | 551 | High |
| 5A18 NP2 (BBE02::Kanr) | + | − | NT | NT | 438 | NT | 716 | 577 | High |
| 5A18 NP3 (BBE02::Kanr) | + | − | NT | NT | 615 | NT | 640 | 628 | High |
| 5A1 | + | − | 10 | 15 | 24 | 21 | 4 | 15 | Intermediate |
| 5A14 | − | − | 225 | 250 | 316 | 507 | 490 | 358 | High |
NT, not tested.
Retention of lp25 by B. burgdorferi transformants.
Previously, it was reported that both lp25 and lp56 tended to be lost when B. burgdorferi B31 was transformed with the shuttle vector pBSV2 (15). We suspected that BBE02 and BBQ67 were DNA restriction-modification genes and that they caused decreased transformation efficiencies in Borrelia (15). In this scheme, if BBE02 is the factor in lp25 that decreases the electroporation efficiency in Borrelia, lp25 should be retained in BBE02 disruptants after transformation. To determine whether lp25 was still present in transformants, we tested the plasmid content of pBSV2C03::gntΔkan transformants by PCR (Table 2). No loss of lp25 was observed in transformants when BBE02::Kanr clones were used. In contrast, the transformants of parental clones 5A1 and 5A18 were all negative for lp25 by PCR (Table 2). Additional experiments demonstrated that the presence of kanamycin was not required for the retention of lp25 in BBE02::Kanr clones transformed with pBSV2C03::gntΔkan; in the absence of kanamycin selection, 20 of 20 colonies contained both Kanr and lp25 markers, as determined by PCR (data not shown).
TABLE 2.
Retention of lp25 in pBSV2C03::gntΔkan transformants
| Recipient B31 clone | lp25 content prior to electroporation | lp25 content after electroporation (no. of positive clones/total no. of clones examined) |
|---|---|---|
| 5A1 | + | 0/10 |
| 5A18 | + | 0/10 |
| 5A4NP1 | + | 10/10 |
| 5A18NP1 | + | 10/10 |
Infectivity of BBE02 disruptants and pBSV2C03::gntΔkan transformants.
Both 5A4NP and 5A18NP clones retained lp25 after transformation. However, it was not known whether BBE02 inactivation affected Borrelia infectivity. To determine whether the insertion of pflgB Kanr in BBE02 had an effect on infectivity, we inoculated mice with 105 organisms. The BBE02-disrupted clones 5A4NP1 and 5A18NP1 were consistently isolated from all tissues (Table 3), indicating that BBE02 was not essential for infection. The pBSV2C03::gntΔkan transformants examined (5A4NP1 cl1, 5A4NP1 cl2, 5A18NP1 cl1, and 5A18NP1 cl2) were also infectious when mice were inoculated with 105 organisms (Table 3).
ID50 determination for BBE02 disruptants.
To further examine the infectivities of 5A4NP1 and 5A18NP1 relative to those of 5A4 and 5A18, we determined their ID50s (Table 4). In this experiment, the ID50 determined for 5A4NP1 was 83 organisms, whereas that for the 5A4 clone was 25 organisms. Similarly, the ID50 for 5A18 NP1 was 83 organisms, whereas that for 5A18 was 46 organisms. These results indicated that BBE02 disruptants are infectious in mice at low dosages. The lp25− clone 5A13 was noninfectious at a dose of 105 organisms, as demonstrated previously (20).
TABLE 4.
ID50s of BBE02 disruptant B31 clones in C3H/HeN mice
| B31 clone (ID50) | Challenge dose (no. of organisms) | No. of culture-positive samples/total no. of samples
|
No. of infected total no. of mice | ||
|---|---|---|---|---|---|
| Ear | Bladder | Joint | |||
| 5A4 (25) | 105 | 6/6 | 6/6 | 6/6 | 6/6 |
| 104 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 103 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 102 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 101 | 1/6 | 1/6 | 1/6 | 1/6 | |
| 5A18 (46) | 105 | 6/6 | 6/6 | 6/6 | 6/6 |
| 104 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 103 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 102 | 4/6 | 4/6 | 4/6 | 4/6 | |
| 101 | 2/6 | 2/6 | 2/6 | 2/6 | |
| 5A4 NP1 (83) | 105 | 6/6 | 6/6 | 6/6 | 6/6 |
| 104 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 103 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 102 | 3/6 | 3/6 | 3/6 | 3/6 | |
| 101 | 1/6 | 1/6 | 1/6 | 1/6 | |
| 5A18 NP1 (83) | 105 | 6/6 | 6/6 | 6/6 | 6/6 |
| 104 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 103 | 6/6 | 6/6 | 6/6 | 6/6 | |
| 102 | 3/6 | 3/6 | 3/6 | 3/6 | |
| 101 | 1/6 | 1/6 | 1/6 | 1/6 | |
| 5A13 (≥105) | 105 | 0/6 | 0/6 | 0/6 | 0/6 |
Pathological changes.
C3H inbred mice develop severe arthritis within 2 weeks after s.c. or intraperitoneal inoculation with wild-type B. burgdorferi (1). Furthermore, Masuzawa et al. (16) demonstrated that these mice exhibit footpad swelling and neutrophil infiltration when Borrelia organisms are inoculated directly into the hind footpad. In the present study, mice inoculated with parental clones or the BBE02-inactivated clones 5A4NP1 and 5A18NP1 showed severe footpad swelling from days 7 to 14 after inoculation, whereas mice inoculated with the B31 5A13 clone did not show any detectable swelling (Fig. 3A). Furthermore, mice inoculated with BBE02-inactivated clones exhibited gross joint swelling (Fig. 3B). A histologic examination of the tibiotarsal joints of animals infected with 105 organisms of the 5A4NP1 or 5A18NP1 clone for 4 weeks demonstrated that pathological changes (tendonitis and periostitis) were present in all mice tested, which was comparable with the histology of mice infected with the 5A4 clone (Fig. 4). No histopathological abnormalities were observed when mice were inoculated with clone 5A13.
FIG. 3.
Footpad swelling (A) and ankle diameter increase (B) after inoculation of C3H/HeN mice with BBE02 disruptants. Mice were 5 weeks old at the time of infection and were inoculated in the right hind footpad with 105 Borrelia organisms in 50 μl of BSK-II medium.
FIG. 4.
Histopathology of tibiotarsal joints from mice inoculated with B. burgdorferi BBE02 disruptants 5A4NP1 and 5A18NP1. C3H/HeN mice were inoculated in the right hind footpad with 105 Borrelia organisms and were sacrificed on day 28 after inoculation. (A) 5A4 (containing all plasmids) (positive control); (B and E) 5A4NP1 (BBE02 disrupted, cp9−); (C) 5A18NP1 (BBE02 disrupted, lp56− lp28-4−); (D and F) 5A13 (missing lp25) (negative control). Areas of inflammation are indicated with arrows. The tissues were stained with hematoxylin and eosin. Magnification, ×100.
DISCUSSION
The low transformation rate of infectious B. burgdorferi has limited the usefulness of genetic techniques for studying virulence factors of B. burgdorferi. For this study, we successfully established phenotypes of high and intermediate transformation efficiencies in an infectious B31 background by BBE02 inactivation. The transformable clones 5A4NP1 and 5A18NP1 were found to be infectious and to give rise to pathological changes in mice. The recovery of infectious B. burgdorferi after transformation has only been reported four times previously. In the first instance, the noninfectious B31 clone 5A13 was restored to infectivity by transformation with a shuttle vector containing a segment of lp25 with the gene BBE22 (pncA) and the small open reading frame BBE23 (20). The inactivation of BBE22 by point mutagenesis led to the conclusion that a functional copy of this gene is required for infectivity. In other studies, Grimm et al. (11) showed that ospC disruption in the B31 A3 clone eliminated its infectivity in mice; the infectivity was restored by introducing an intact copy of ospC into the ospC disruption site (11). Pal et al. (18) found that the ability of B. burgdorferi N40 to invade tick salivary glands was reduced by ospC disruption and restored by complementation. Lastly, inactivation of the ospAB operon in B. burgdorferi 297 had no apparent effect on its infection of mice but reduced its ability to colonize the tick midgut (27). In an earlier report by Elias et al. (6), a serologic conversion of mice inoculated with the transformed B31 A3 clone was documented, but organisms could not be cultured from the inoculated mice.
It was shown previously that the presence of either lp25 or lp56 decreased the transformation efficiency of B. burgdorferi B31 clones with pBSV2 ∼50-fold; the presence of both completely inhibited transformation (15). In addition, the few transformants that were isolated from lp56− lp25+ clones were all lp25−, indicating that lp25− variants were transformed preferentially. The results reported herein demonstrate that BBE02 inactivation increased the transformation rate in clones containing lp25, providing evidence that BBE02 encodes a restriction-modification enzyme (15). The absence of lp56 in 5A18 BBE02 disruptants further increased the rate of transformation, validating the hypothesis that lp56 (and potentially the predicted restriction-modification gene BBQ67) also represents a transformation barrier. We expect that BBE02 inactivation will provide one approach for greatly increasing the efficiency of genetic studies of Borrelia infectivity.
It should be noted, however, that the present report and a previous study (15) only examined transformation with the shuttle vector pBSV2 and its derivatives. Certain low-passage-number, infectious B. burgdorferi clones (e.g., B31 A3 and a 297 clone) can be transformed at a low frequency (≤10−7) by the integration of suicide vectors or by double-crossover allelic exchange and still retain infectivity (10, 27). However, this is a variable property, as some clones of the B. burgdorferi strain are transformable while others are not (7, 10). Indeed, in the present study, the disruption of BBE02 was achieved at a low frequency in one clone (B31-5A4, containing all of the B. burgdorferi native plasmids), but not in another (B31-5A1, which is lp56−), i.e., opposite of the expected outcome. These results suggest that unknown minor genetic differences in otherwise isogenic clones of B. burgdorferi affect the acquisition, stability, and integration of foreign DNA.
Most other bacteria possess DNA restriction-modification systems that present barriers to transformation through the restriction site cleavage of unmodified DNAs. BBE02, with >1,200 amino acid residues, has both a consensus endonuclease site and methyltransferase signature sequences that are conserved in methyltransferases of other bacteria; homologs of BBE02 that contain a similar set of motifs (Eco57I, LlaGI, HaeIV, etc.) are known to have both endonuclease and methylase activities (15). We have not yet demonstrated that BBE02 has endonuclease activity. However, preliminary data indicated that the transformation frequency was increased in the infectious strain B31 with pBSV2 that was coincubated with BBE02 in E. coli (unpublished data). These results suggest that the premodification of donor DNA may be an effective procedure for enhanced transformation of wild-type Lyme disease-causing Borrelia.
We expect that BBE02 inactivation will provide one approach for greatly increasing the efficiency of genetic studies of Borrelia infectivity. The availability of highly transformable infectious strains of B. burgdorferi may permit the wide application of techniques such as site-directed mutagenesis, plasmid incompatibility studies (5, 10), transposon mutagenesis, and even in vivo expression tag or signature-tagged mutagenesis studies for the assessment of genes that are important for Borrelia infectivity.
Acknowledgments
We thank Patricia A. Rosa, Philip E. Stewart, and Jonathan Krum (National Institute of Allergy and Infectious Diseases, National Institutes of Health) for generously providing the vectors used for this study. We also thank Douglas J. Botkin for helpful advice.
This work was supported by a grant from Emerging and Reemerging Infectious Diseases H12-Shinkou-29 and H15-Shinkou-14, Ministry of Health, Labour and Welfare, Japan (H.K.), and by a grant-in-aid for scientific research (16790266) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (H.K.).
Editor: J. T. Barbieri
REFERENCES
- 1.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 2.Bono, J. L., A. F. Elias, J. J. Kupko III, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient target mutagenesis in Borrelia burgdorferi. J. Bacteriol. 182:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 4.Chaconas, G., P. E. Stewart, K. Tilly, J. L. Bono, and P. Rosa. 2001. Telomere resolution in the Lyme disease spirochete. EMBO J. 20:3229-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 6.Elias, A. F., J. L. Bono, J. J. Kupko III, P. E. Stewart, J. G. Krum, and P. Rosa. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 6:29-40. [DOI] [PubMed] [Google Scholar]
- 7.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank, K. L., S. F. Bundle, M. E. Kresge, C. H. Eggers, and D. S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, C. M., S. Casjens, W. H. Huang, G. G. Sutton, R. Clayton, et al. 1997. Genomic sequence of Lyme disease spirochete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 10.Grimm, D., A. F. Elias, K. Tilly, and P. A. Rosa. 2003. Plasmid stability during in vitro propagation of Borrelia burgdorferi assessed at a clonal level. Infect. Immun. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hübner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by an RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labandeira-Rey, M., E. Baker, and J. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4609-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmid lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuzawa, T., T. Kurita, H. Kawabata, H. Suzuki, and Y. Yanagihara. 1994. Experimental induction of Lyme arthritis in outbred mice. Microbiol. Immunol. 38:263-268. [DOI] [PubMed] [Google Scholar]
- 17.Norris, S. J., J. K. Howell, S. A. Garza, M. S. Ferdows, and A. G. Barbour. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 21.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoint. Am. J. Hygiene 27:493-497. [Google Scholar]
- 22.Sambrook, J., and D. W. Russell. 2000. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Samuels, D. S., R. T. Marconi, W. M. Huang, and C. F. Garon. 1994. gyrB mutation in coumermycin A1-resistant Borrelia burgdorferi. J. Bacteriol. 176:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartakova, M., E. Dobrikova, and F. C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart, P. E., R. Thalken, J. L. Bono, and P. Rosa. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714-721. [DOI] [PubMed] [Google Scholar]
- 27.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]