Abstract
Stroke is a major health issue in women. Our previous studies in male rats showed decreased myogenic tone in middle cerebral arteries (MCAs) after ischemia and reperfusion (I/R), while tone in parenchymal arterioles (PAs) was increased. This vascular response may aggravate stroke damage in males by limiting reperfusion, however, the effect in females is not known. The current study investigated the effect of I/R and tissue plasminogen activator (tPA) on myogenic tone and reactivity of MCAs and PAs in female rats. Nitrosative stress by peroxynitrite and recruitment of inflammatory neutrophils to the microvasculature were also studied. Female rats were subjected to 2 h MCA filament occlusion (n=16) or sham surgery (n=17) and given tPA (1 mg/kg, i.v) or vehicle followed by 30 min reperfusion. Myogenic tone and reactivity were measured in isolated and pressurized MCAs and PAs from the same animals. Cerebrovascular F-actin, 3-nitrotyrosine (3-NT, peroxynitrite marker), and intravascular neutrophils were quantified. Myogenic tone and constriction to the nitric oxide synthase inhibitor Nω-Nitro-L-arginine were decreased in MCAs but unchanged in PAs after I/R with no effect of tPA. F-actin and 3-NT expression were unaffected by I/R or tPA. Our study showed that MCAs from females, similar to what have been seen in males, are dilated after I/R and have decreased myogenic tone while tone in PAs was unchanged. Increased small vessel resistance may contribute to decreased reperfusion and worse outcome after stroke.
Keywords: Cerebral ischemia, reperfusion, myogenic tone, middle cerebral arteries, parenchymal arterioles, 3-Nitrotyrosine
Introduction
Stroke is the fifth leading cause of death in women and the seventh in men [1]. Women suffer from worse functional outcomes and are more likely to be institutionalized after stroke [2, 3]. Ischemic stroke is a severe neurological disorder that is associated with compromised cerebral blood flow [4]. While neurons are highly susceptible to ischemic damage, the cerebral vasculature is also negatively affected [4, 5]. Excessive vasodilation can result in hemorrhage and edema which may compress capillaries and restrict microvascular perfusion, while increased vasoconstriction further compromises cerebral blood flow and worsens the cerebral ischemia [4]. It therefore seems important to restore cerebrovascular function after an ischemic stroke to provide adequate perfusion and limit further brain damage [4]. Our previous studies in male rats showed that middle cerebral arteries (MCAs) had decreased myogenic tone and impaired myogenic reactivity after ischemia and reperfusion (I/R) [6–8]. In contrast, myogenic tone and reactivity were increased in parenchymal arterioles (PAs) during early post-ischemic reperfusion [9]. While cerebrovascular function after ischemic stroke has been extensively studied in males, less is known about the vascular response to I/R in females. Improving our knowledge on how I/R affects cerebrovascular function may contribute to the development of therapies to protect the cerebral vasculature and improve reperfusion after stroke, especially if there are sex-specific differences in the response to I/R.
Current available stroke treatments involve thrombolysis with tissue plasminogen activator (tPA) [10] and mechanical clot removal with endovascular treatments [11], both of which are reperfusion therapies. However, recanalization of the occluded artery does not always lead to reperfusion of the distal vascular bed [12, 13]. One suggested mechanism of the incomplete microcirculatory perfusion during I/R is capillary plugging by recruitment and adhesion of neutrophils to the vascular endothelium [14–18]. Recruitment and adhesion of neutrophils to the vascular wall are promoted by release of pro-inflammatory cytokines and chemokines after I/R [19, 20]. Interestingly, previous studies have showed that tPA in itself can promote vascular inflammation and recruitment of inflammatory cells after ischemic stroke in male mice [21, 22]. However, the effect of tPA on recruitment of intravascular neutrophils throughout the microcirculation of the ischemic hemisphere has not been studied.
Studies have shown higher recanalization rates and better early neurological improvement in women than in men after intravenous tPA treatment [23, 24]. Women may benefit more from treatment than men since the commonly seen sex differences in long-term functional outcome are nullified by tPA [25, 26]. However, a recent study showed that men in the oldest age group >80 years had better functional outcome than women after tPA treatment [27]. A recent study in humans showed beneficial effects from the combination therapy of tPA with the antioxidant uric acid after ischemic stroke, but the effect was only seen in female patients [28]. Although the free radical formation was not measured, the women in the study had lower endogenous levels of uric acid and the authors suggested that women may be in greater need of antioxidants than men. tPA in itself could induce harmful oxidative stress responses as demonstrated after cortical administration to healthy rat brains [29]. In the study, co-administration of an antioxidant diminished the oxidative stress responses. Our previous study in cerebral arteries from male rats, demonstrated that peroxynitrite, an oxidant formed during I/R, was associated with decreased myogenic tone and depolymerization of smooth muscle F-actin [30]. Perfusion of tPA in vitro results in decreased myogenic reactivity and tone of cerebral arteries from male rats [31]. However, the in vivo effects of tPA on myogenic tone and peroxynitrite-mediated reactions on F-actin have not been studied in females.
MCAs and PAs are important determinants of downstream capillary pressure and perfusion and play a major role in the brain hemodynamics and hence, the functional outcome after stroke. Understanding how cerebral arteries from females are affected by I/R and by tPA treatment may contribute to the development of stroke therapies to improve microvascular perfusion and functional outcome in women after stroke. Therefore, the present study investigated the early effects of I/R and treatment with tPA on myogenic tone and reactivity of MCAs and PAs in female rats. The effects of I/R with tPA on nitrosative stress by peroxynitrite and presence of neutrophils in microvessels were also studied.
Methods
The current study was performed according to the ARRIVE guidelines for reporting of animal research [32]. Female Wistar rats were ovariectomized (OVX) at 9 weeks by the vendor (Harlan Laboratories, Dublin, VA) and used for experiments at 13 to 17 weeks of age (14±0.2). All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont and were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. Rats were housed in the Animal Care Facility at the University of Vermont, an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility, and were allowed food and water ad libitum.
Animal model of transient focal ischemia
Transient proximal middle cerebral artery occlusion (MCAO) was induced by the intraluminal filament technique as described earlier [33, 34]. Briefly, animals were anesthetized with 3% isoflurane followed by intubation and mechanical ventilation with 1.5–2% isoflurane in oxygen to maintain blood gases at physiological levels (Table 1). Two laser Doppler flowmetry probes were placed on thinned areas of the skull to monitor cerebral blood flow over the right MCA (2 mm posterior to bregma and 4 mm lateral to the right of midline), as well as collateral flow by placing a second probe over the right anterior cerebral artery (2 mm anterior to bregma and 2 mm lateral to the right of midline). A skin incision was made to the midline of the neck and the right common carotid artery was exposed followed by insertion of a 5-O monofilament coated with silicon into the internal carotid artery until it occluded the MCA, verified by laser Doppler. The MCA was occluded for 2 hours of ischemia followed by filament removal to allow reperfusion for 30 minutes. Sham-operated animals underwent the same procedure except for no filament insertion. Treatment with vehicle (Vh, lactated Ringer’s solution) or tPA (Alteplase, Genentech, San Francisco, CA, USA) was given 10 min prior to reperfusion for 10 minutes (1 mg/kg, i.v., 10% as a bolus and 90% as continuous perfusion). Group assignments where randomized and blinded to the experimenters that performed all subsequent experiments except for the pressurized arteriography experiments on PAs randomization and surgical procedures was performed by the same experimenter. Un-blinding of group assignments were performed after the data was analyzed.
Table 1.
Physiological parameters of animals subjected to sham surgery or middle cerebral artery occlusion
| Sham + Vehicle (n=9) | Sham + tPA (n=8) | MCAO + Vehicle (n=8) | MCAO + tPA (n=8) | |
|---|---|---|---|---|
| Weight (g) | 339 ± 13 | 330 ± 8 | 326 ± 13 | 325 ± 11 |
| Age (wks) | 14.7 ± 0.4 | 13.8 ± 0.4 | 14.3 ± 0.3 | 14.9 ± 0.5 |
| CBF % Drop vs. Basala | N/A | N/A | −48.5 ± 4.5 | −59.0 ± 4.6 |
| CBF % Recovery vs. Basala | N/A | N/A | 12.5 ± 13.1 | −10.7 ± 7.3 |
| Blood Gases Start | ||||
| pH | 7.45 ± 0.01 | 7.45 ± 0.01 | 7.47 ± 0.02 | 7.46 ± 0.01 |
| pCO2 (mmHg) | 39.5 ± 1.5 | 39.1 ± 0.9 | 39.1 ± 1.6 | 40.5 ± 0.5 |
| pO2 (mmHg) | 129 ± 4 | 131 ± 5 | 129 ± 7 | 113 ± 7 |
| Blood Gases Middle | ||||
| pH | 7.46 ± 0.01 | 7.46 ± 0.01 | 7.47 ± 0.01 | 7.46 ± 0.01 |
| pCO2 (mmHg) | 38.8 ± 1.7 | 41.6 ± 0.9 | 40.0 ± 1.2 | 40.1 ± 0.4 |
| pO2 (mmHg) | 125 ± 5 | 119 ± 6 | 124 ± 6 | 110 ± 6 |
| Blood Gases End | ||||
| pH | 7.46 ± 0.00 | 7.47 ± 0.01 | 7.48 ± 0.01 | 7.47 ± 0.01 |
| pCO2 (mmHg) | 41.1 ± 0.7 | 40.8 ± 0.9 | 38.8 ± 0.7 | 40.1 ± 0.4 |
| pO2 (mmHg) | 121 ± 4 | 118 ± 6 | 121 ± 6 | 116 ± 6 |
The values represents % changes in cerebral blood flow measured by laser Doppler over the MCA territory.
Preparation of isolated middle cerebral arteries and pressurized arteriograph
Animals were quickly decapitated while under anesthesia, and the brains were carefully removed and placed in cold, oxygenated physiological salt solution. Ipsilateral PAs that branched off MCA between the M1 and M2 regions, and ipsilateral MCAs between the M2 and M3 region, were dissected and mounted on glass cannulas in separate arteriograph chambers, as described previously [31, 35]. MCAs and PAs were equilibrated for 1 hour at 75 mmHg and 20 mmHg, respectively, during which the vessels developed myogenic tone. Myogenic reactivity was studied within the pressure range of 75 to 150 mmHg for MCAs, whereas PAs were studied in the pressure range of 20 to 100 mmHg. Subsequent experiments were performed at intravascular pressures of 75 mmHg and 40 mmHg for MCAs and PAs, respectively, approximately corresponding to the pressures in vivo [36]. To determine the contribution of nitric oxide to myogenic tone, the nitric oxide synthase inhibitor Nω-nitro-L-arginine (L-NNA, 0.1 mM) was added to the arteriograph chamber that will elicit a vasoconstriction and the inner diameter was recorded once stable, approximately after 20 to 25 min. Subsequently, while L-NNA was still present in the arteriograph chamber, dilation to the Rho-associated kinase (ROCK) inhibitor Y27632 (10 nM to 10 μM) was studied.
The inner diameters of MCAs were measured at the beginning of equilibration (approx. pH 7.2 and 22°C) and used as the passive inner diameters for calculations because these vessels were fixed pressurized with tone. Thus, passive measurements in calcium-free PSS were not obtained for MCAs. PAs were superfused with diltiazem (10 μmol/L) in calcium-free PSS at the end of the experiment to obtain fully passive diameter measurements. Myogenic tone was calculated as a percent decrease from the initial passive inner diameter by the following equation: 1 – (IDActive / IDPassive) · 100; where IDActive diameter of arteries with tone and IDPassive = passive diameter of arteries. Constriction to L-NNA was calculated as percent decrease in diameter from baseline. The reactivity for Y27632 was calculated by the equation: (IDDrug – IDStart) / (IDPassive – IDStart) · 100; where IDDrug is the inner diameter at a specific concentration of Y27632 and IDStart which here was set to the inner diameter at the first concentration of Y27632 (10 nM). Percent sensitivity was calculated by the equation: (IDStart – IDDrug) / (IDStart – IDMax. drug) · 100; where IDMax drug is the inner diameter at the highest concentration of Y27632. The effective concentrations that elicited half the maximal response (EC50) for Y27632 were calculated from the sensitivity curves.
Quantification of F-actin and nitrotyrosine in middle cerebral arteries
Subsequent to the reactivity experiments, while still pressurized at 75 mmHg, MCAs were fixed for 30 min in the arteriograph chamber with 4% methanol-free paraformaldehyde solution made fresh daily followed by an additional 30 minutes after careful removal from the cannulas. The arteries were stored in 0.1 M PBS pH 7.4 at 4°C until staining of F-actin with Alexa Fluor 488-conjugated phalloidin (1:200, Life technologies, Carlsbad, CA, USA) and 3-nitrotyrosine (3-NT) with a mouse monoclonal anti-3-NT antibody (1:50, #05-233, Millipore, Billerica, MA, USA). Following blocking with 10% normal goat serum in 0.1 M PBS/1% Triton X-100/1% BSA for 30 min, primary antibodies were applied overnight at room temperature. Secondary antibody used for 3-NT was goat anti-mouse IgG-conjugated Alexa Fluor 555 (1:500, Life technologies). A negative control that omitted the primary antibody was included to test for unspecific binding from the goat anti-mouse IgG-conjugated Alexa Fluor 555 secondary antibody. Additionally, a mouse IgG2bK isotype control was included to test for non-specific binding of the 3-NT primary antibody.
Images were acquired with a Zeiss LSM 510 laser scanning confocal microscope at 40X. Optimal gain for the F-actin staining was based on MCAs from sham-operated animals treated with Vh, and the 3-NT gain was set from MCAs of MCAO animals treated with tPA. Images were focused on the vascular smooth muscle cells and acquired during the same day using the same settings for gain and amplifier offset to minimize the introduction of non-biological variations in fluorescence intensity between samples. F-actin and 3-NT fluorescence pixel intensity per square micrometer (units/μm2) in five regions of interest per artery were quantified using MetaMorph Imaging systems (Molecular Devices) and averaged for each individual artery. Background staining was eliminated by setting the threshold value for each channel using the negative control as well as the isotype control.
Quantification of 3-nitrotyrosine in brain microvessels
Brains were cut anterior to the region where PAs to be used in the reactivity experiments were dissected from, and fixed in 4% paraformaldehyde for 24 hours. Two coronal sections (20 μm thick) per animal were mounted on glass slides (TruBond 380, Tru Scientific, Bellingham, WA, USA) coated with an adhesive protein solution (Cell-Tak, Corning, NY, USA), and subjected to heat-induced antigen retrieval in pH 6.1 citrate buffer (DAKO, Carpinteria, CA, USA). Autofluorescence from aldehyde fixation and hemoglobin was reduced by sequential treatment with 0.1% sodium borohydride in PBS for 30 min and 0.1% Sudan black in 70% ethanol for 20 min [37]. Sections were blocked with 10% normal goat serum with 0.1% Triton-X, 5% BSA in PBS, incubated overnight with primary mouse monoclonal anti-3-NT antibody (1:100, #05-233, Millipore) followed by application of secondary goat anti-mouse IgG-conjugated Alexa Fluor 555 antibody (Life technologies). Cell nuclei were visualized using 4′,6-diamidino-2-phenylindole (DAPI, Life Technologies). Isotype control using mouse IgG2bK and omission of the primary antibody were included as controls. Images from four random areas within the ipsilateral MCA territory were acquired per brain section using a Zeiss LSM 510 laser scanning confocal microscope at 25X. The 3-NT fluorescence pixel intensity was analyzed with MetaMorph Imaging systems (Molecular Devices) and threshold was set using the negative and isotype controls. 3-NT staining was almost exclusively present in the vasculature and was normalized to the microvessel area within each image by using a lower threshold that allowed for detection and quantification of microvessel area (μm2).
Quantification of intravascular polymorphonuclear neutrophils in microvessels
For the quantification of intravascular neutrophils, brain sections were prepared as above, subjected to heat-induced epitope retrieval and immunostained using mouse monoclonal anti-collagen IV (Col-IV, 1:500, #SAB4200500, Sigma-Aldrich, St Louis, MO, USA) and polyclonal rabbit anti-myeloperoxidase (MPO, 1:200, #A0398, DAKO). Secondary antibodies used were goat anti-mouse IgM-conjugated Alexa Fluor 488 (1:500, Life technologies) and goat anti-rabbit IgG-conjugate Alexa Fluor 555 (Life technologies, 1:500). Isotype control using rabbit IgG, a negative control omitting primary antibodies and a positive control for MPO using rat spleen tissue were included the experiment. Images from four random areas within the ipsilateral MCA territory were acquired per brain section using a Zeiss LSM 510 laser scanning confocal microscope at 25X. Intravascular neutrophils were identified by an operator blinded to the groups, counted and presented as the number of neutrophils per square millimeter. Capillary densities were calculated based on Col-IV positive staining.
Statistics
All data are presented as mean ± SEM. Two-way ANOVA with intervention (MCAO or sham) and treatment (Vh or tPA) as independent factors was used followed by Bonferroni test for multiple comparisons. Unpaired t test was used to compare the basal tone in MCAs and PAs of Sham Vh animals. One-way ANOVA was used to compare the reactivity to Y27632 in MCAs and PAs from MCAO Vh and MCAO tPA. P<0.05 was considered significant.
Results
A total of 34 animals were used for this study. One animal was excluded due to hemorrhage during surgery. No significant differences in the physiological parameters were detected between the groups (Table 1). The drop in cerebral blood flow over the MCA territory was similar between MCAO Vh and MCAO tPA, −49±5% and −59±5%, respectively; P>0.05 (Table 1). Collateral flow, as measured by laser Doppler over the right anterior cerebral artery territory, was similar between groups. During the first 15 min of ischemia, the change in cerebral blood flow compared to baseline was: MCAO Vh: −15±9% and MCAO tPA: −18±7%, followed by a return to baseline at 1 h of ischemia (MCAO Vh: −1±6%, MCAO tPA: −1±7%). At these time points, cerebral blood flow over the MCA territory was maintained and did not change markedly (MCAO Vh: −57±5% vs −51±5%, MCAO tPA: −69±4% vs. −60±4%). Thus, the return of cerebral blood flow to baseline measured over the right anterior cerebral artery suggests collateral flow.
Effect of I/R and tPA on myogenic tone of MCAs and PAs
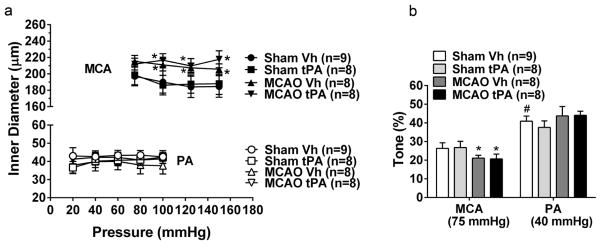
MCAs and PAs developed spontaneous myogenic tone and demonstrated decreased diameter during equilibration (data not shown). MCAs and PAs in all groups maintained inner diameter in response to increased pressure, demonstrating that the vessels were myogenically active and constricted to increases in intravascular pressure (Fig. 1a). However, the active inner diameter of MCAs at intravascular pressures of 100 to 150 mmHg was significantly larger in the I/R groups compared to sham (P<0.05, Two-way ANOVA), independent of treatment with Vh or tPA. Concomitantly, decreased myogenic tone at 75 mmHg was observed in the MCAs after I/R compared to sham (P<0.05, Two-way ANOVA, Fig. 1b), similar to previous observations in MCAs of male rats [6, 8, 38, 39]. Myogenic tone in PAs was significantly higher than tone in MCAs (Sham Vh, 26±3% vs 41±3%, P<0.01), but was not affected by I/R (Fig. 1b). tPA did not affect myogenic tone or reactivity in MCAs or PAs.
Fig. 1.
Effect of early post-ischemic reperfusion and tissue plasminogen activator (tPA) on myogenic reactivity and tone of middle cerebral arteries (MCAs) and parenchymal arterioles (PAs). Early post-ischemic reperfusion decreased myogenic tone in MCAs but not in PAs. (a) Inner diameter of MCAs and PAs in response to pressure. MCAs and PAs showed myogenic reactivity to increased pressure in all groups. The active diameters of MCAs were significantly larger after middle cerebral artery occlusion (MCAO). (b) Percent tone of MCAs at 75 mmHg and PAs at 40 mmHg. MCAs had decreased tone after MCAO independent on treatment with vehicle (Vh) or tPA. No effect of MCAO and tPA on tone was seen in PAs. *P<0.05, Two-way ANOVA main effect of MCAO. #P<0.05, unpaired t test vs MCA: Sham Vh
Effect of I/R and tPA on constriction to nitric oxide synthase inhibition in MCAs and PAs
To investigate possible mechanisms for the decreased myogenic tone in MCAs, we studied the contribution of nitric oxide to basal tone in MCAs and PAs [40]. Addition of the nitric oxide synthase inhibitor L-NNA caused constriction of MCAs and PAs in all groups, but the constriction was decreased after I/R in MCAs compared to sham (P<0.01, Two-way ANOVA, Fig. 2). Constriction to L-NNA was not different between MCAs and PAs in Sham Vh and Sham tPA, but was higher in PAs of MCAO Vh (P<0.01) and MCAO tPA (P<0.05, Fig. 2).
Fig. 2.
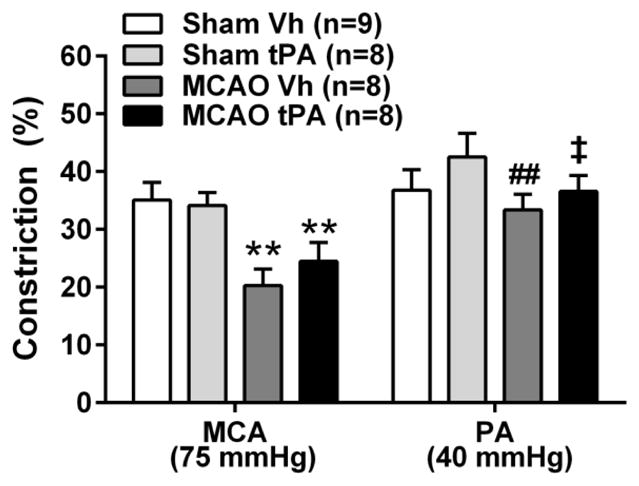
Constriction to the nitric oxide synthase inhibitor L-NNA in middle cerebral arteries (MCAs) and parenchymal arterioles (PAs) after early post-ischemic reperfusion and tissue plasminogen activator (tPA). MCAs had decreased constriction to the nitric oxide synthase inhibitor L-NNA after ischemia and reperfusion. Administration of tPA (1.0 mg/kg) did not affect the constriction to L-NNA in MCAs or PAs. Intravascular pressures were 75 mmHg for MCAs and 40 mmHg for PAs, corresponding to the approximate pressures in vivo [52]. **P<0.01, Two-way ANOVA, main effect of MCAO. ##P<0.01, Unpaired t test vs MCA: MCAO Vh, ‡P<0.05 Unpaired t test vs MCA: MCAO tPA
Effect of I/R and tPA on Rho kinase inhibition
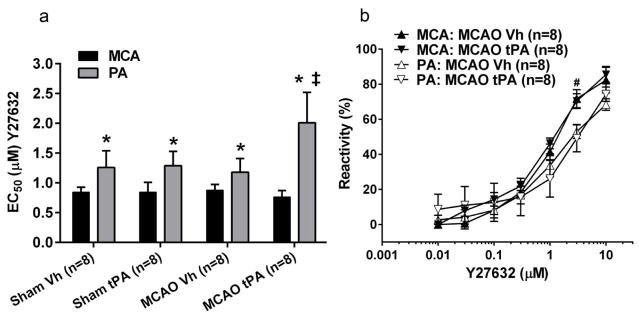
Rho kinase has been suggested to play an important role in myogenic tone regulation [41–43]. Therefore, we studied the effect of I/R and tPA on the sensitivity to inhibition of Rho kinase in MCAs and PAs. Y27632 caused a concentration-dependent vasodilation in MCAs and PAs of all groups. Sensitivity to Y27632 was not significantly affected by I/R with tPA in MCAs or PAs, but PAs were less sensitive than MCAs (P<0.05, Two-way ANOVA, Fig. 3a). Interestingly, PAs were less sensitive to inhibition by Y27632 than MCAs after I/R in presence of tPA (P<0.05). As demonstrated in Fig. 3b, the reactivity to Y27632 was less in PAs compared to MCAs and statistically significant at 3 μM of Y27632 (P<0.05).
Fig. 3.
Effect of early post-ischemic reperfusion and tissue plasminogen activator (tPA) on the sensitivity and reactivity to Rho kinase inhibition with Y27632. Parenchymal arterioles (PAs) were less sensitive to Rho kinase inhibition than middle cerebral arteries (MCAs). (a) Comparison of half maximal effective concentration (EC50) values in MCAs and PAs. A higher EC50 value, equal to less sensitivity to inhibition with Y27632, was observed in PAs after middle cerebral artery occlusion (MCAO) in the presence of tissue plasminogen activator (tPA) compared to MCAs. (b) Percent reactivity to Y27632 in MCAs and PAs after MCAO and treatment with vehicle (Vh) or tPA. The reactivity to Y27632 was less in PAs compared to MCAs. Intravascular pressures were 75 mmHg for MCAs and 40 mmHg for PAs, corresponding to the approximate pressure in vivo [52]. *P<0.05, Two-way ANOVA, main effect arterial segment. ‡P<0.05, Bonferroni multiple comparisons test vs MCA: MCAO tPA. #P<0.05, One-way ANOVA
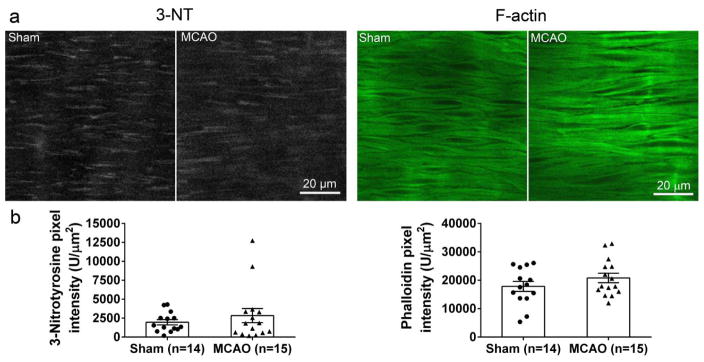
Effect of I/R on smooth muscle F-actin and 3-NT in MCAs
Decreased myogenic tone in MCAs after I/R in male rats has previously been associated with decreased F-actin content in vascular smooth muscle cells [6]. In addition, depolymerization of F-actin in smooth muscle of cerebral arteries has been demonstrated after application of peroxynitrite [30, 44]. We therefore measured the levels of F-actin and 3-NT, a marker of peroxynitrite, in pressurized and fixed MCAs. Since we did not observe an effect of tPA on tone of MCAs, data for Vh and tPA were combined within sham and MCAO groups. Representative images of the 3-NT staining and F-actin staining with phalloidin are shown in Fig. 4a. Quantified fluorescence pixel intensity (U/μm2) for 3-NT and phalloidin were not significantly affected by I/R (Fig. 4b).
Fig. 4.
Effect of early post-ischemic reperfusion and tissue plasminogen activator (tPA) on 3-nitrotyrosine levels (3-NT) and smooth muscle F-actin in middle cerebral arteries (MCAs). Smooth muscle 3-NT and filamentous (F)-actin were not affected by early post-ischemic reperfusion. (a) Representative images of MCAs from all groups of animals and (b) quantification of smooth muscle 3-NT and F-actin in all groups of animals. 3-NT and F-actin content of MCAs were not significantly affected by MCAO
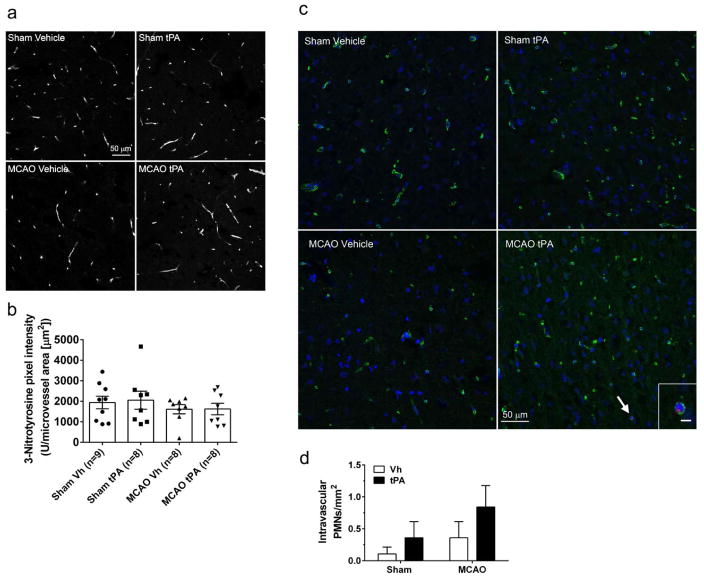
To begin to understand the effects of I/R with tPA on the microcirculation downstream of MCAs and PAs, we also quantified 3-NT in the microvasculature of the ipsilateral MCA territory using immunohistochemistry. 3-NT staining was present in all groups and almost exclusively found within the vasculature (Fig. 5a). We did not measure any significant difference in microvascular 3-NT expression after I/R with tPA (Fig. 5b).
Fig. 5.
Effect of early post-ischemic reperfusion and tissue plasminogen activator (tPA) on microvascular 3-nitrotyrosine (3-NT) content and intravascular polymorphonuclear neutrophils. (a) Representative images of brain 3-NT content in the ipsilateral MCA territory. 3-NT was almost exclusively found in the vasculature with no effect of ischemia and reperfusion or tPA found. (b) Quantification of 3-NT fluorescence pixel intensity (U) normalized to microvessel area (μm2). No significant differences were observed between the groups. (c) Representative image of collagen IV and myeloperoxidase staining in the ipsilateral MCA territory after MCAO and treatment with tPA demonstrating the presence of an intravascular neutrophil (arrow). Inset: scale bar 5 μm. (d) Intravascular neutrophils per square millimeter (mm2). A trend towards increased number of neutrophils was seen after MCAO (P=0.145, Two-way ANOVA) and after treatment with tPA (P=0.145, Two-way ANOVA). Sham Vh n=9; Sham tPA: n=8; MCAO Vh: n=8; MCAO tPA: n=8
Effect of I/R and tPA on intravascular neutrophils
Increased intravascular neutrophils and obstruction of capillary flow is one of the suggested mechanisms for incomplete microcirculatory perfusion after I/R [14, 17, 18, 45]. We therefore studied the effect of I/R with tPA on the number of intravascular neutrophils in microvessels of the ipsilateral MCA territory using collagen IV as a marker of the vasculature and myeloperoxidase as a marker of neutrophils (Fig. 5c). The total number of intravascular neutrophils was low in all groups, being <1/mm2. There was a trend towards increasing number of neutrophils after I/R with tPA was seen (Fig. 5d, Two-way ANOVA effect of tPA and MCAO, P=0.15).
Discussion
In the present study, we showed that MCAs had decreased myogenic tone and constriction to the nitric oxide synthase inhibitor L-NNA after I/R in female rats, while vasodilation to Rho kinase inhibition was unaffected. In contrast, myogenic tone, constriction to L-NNA and sensitivity to Rho kinase inhibition were not altered in PAs after I/R. Our study suggests that during early post-ischemic reperfusion, vascular impairment is present in the MCAs while PAs, on the parameters studied here, have maintained cerebrovascular function. The finding of differential effects of I/R on MCAs and PAs are in agreement with previous studies in male rats [6, 8, 9, 33, 46], but this effect has not been demonstrated in females. These findings suggest that small vessel resistance was increased while upstream large vessel resistance decreased, an effect that may be protective of the microcirculation, but could also contribute to decreased reperfusion and worse outcome after stroke.
Myogenic reactivity to pressure appeared to be maintained in MCAs after I/R in female rats in the present study while myogenic tone was decreased. This was demonstrated by the capacity of MCAs to maintain a fairly constant inner diameter to increases in pressure, although the overall inner diameter was larger and hence, myogenic tone was lower, after I/R with vehicle or tPA compared to sham. In a previous study we investigated the threshold duration of reperfusion for myogenic tone and reactivity in MCAs from male rats [6]. In that study, decreased myogenic tone was seen after 30 min ischemia and 30 min reperfusion, while myogenic reactivity was not impaired until after 6 h of reperfusion [6]. The previous data demonstrate that decreased myogenic tone precedes impaired myogenic reactivity, which is in agreement with the current findings.
In an attempt to uncover underlying mechanisms for the decreased myogenic tone in MCAs after I/R, we studied the contribution of nitric oxide and Rho kinase to myogenic tone. A decreased constriction to the nitric oxide synthase inhibitor L-NNA was seen in MCAs after I/R. This finding suggests that nitric oxide-mediated pathways contributes less to tone after I/R and is likely not the responsible mechanism for reduced tone in these arteries. Rho kinase inhibition with Y27632 caused a dose-dependent vasodilation that was different in MCAs and PAs, with less sensitivity in PAs. However, no effect of I/R or tPA was seen in MCAs that could explain the decreased myogenic tone. Decreased myogenic tone in MCAs after I/R is not suggested to be due to nitric oxide or Rho kinase pathways may involve effects smooth muscle cell constriction and ion channels, which yet remains to be investigated.
The effects of I/R on myogenic tone and reactivity of cerebral arteries and arterioles has until now not been investigated in conjunction with in vivo treatment using tPA, currently the only approved drug for stroke treatment. Besides its potential to provide recanalization after ischemia, tPA has also been associated with adverse effects such as impaired vascular function, oxidative stress and cerebrovascular inflammation [21, 22, 29, 31]. Uncovering potential contributing effects of tPA to the reperfusion injury after ischemia might lead to development of adjuvants to improve outcome after thrombolysis with tPA. In the present study, we investigated the effects of early post-ischemic reperfusion with tPA on myogenic tone and reactivity of MCAs and PAs, 3-NT in MCAs and microvessels, thereby different segments of the cerebrovasculature were studied. Our results showed that tPA did not affect myogenic tone and reactivity in MCAs and PAs during early post-ischemic reperfusion. Furthermore, I/R with tPA was not associated with increased nitrosative stress in MCAs or in the microvasculature, suggesting no adverse effects of tPA on the vasculature in this model of female stroke. Results from clinical studies suggest that women may benefit more from tPA treatment and have higher rates of recanalization than men [23, 24]. A recent study that investigated the effect of combining tPA treatment with the antioxidant uric acid showed a benefit in women but not in men [28]. Endogenous levels of uric acid were lower in women than in men, but the levels of reactive oxygen species were not measured in the study. It is possible that women have less adverse effects of tPA than men and benefit more from its use.
While tPA did not affect myogenic tone and nitrosative stress in cerebral arteries after I/R, we did note a trend (P=0.15) in increased number of intravascular neutrophils in the MCA territory of the ischemic hemisphere. Enhanced recruitment and adhesion of neutrophils to the vascular endothelium have been suggested as an underlying mechanism for the “no-reflow” phenomena seen after I/R [14, 17, 18, 45]. The total number of intravascular neutrophils measured in the present study was low, likely due to the early time point of reperfusion (30 minutes) that was assessed. A previous study in male mice demonstrated significantly enhanced leukocyte adhesion in the superficial middle cerebral artery veins at 3 hours but not at 1 hour after administration of tPA in photothrombotic stroke in mice [22]. It is possible that neutrophil accumulation would be significant either alone or with tPA at longer durations of reperfusion.
Decreased myogenic tone in MCAs was not associated with increased smooth muscle 3-NT expression. Two previous studies that investigated vascular oxidative stress by superoxide and 3-NT, as well as MCA function after I/R also did not report an association of myogenic tone and oxidative stress [47, 48]. In those studies, treatment with CR-6, a structural derivative of vitamin E or uric acid prevented the I/R-induced vascular oxidative stress in male rats but did not restore the myogenic tone and prevent a larger inner diameter of MCAs [47, 48].
The 1 mg/kg dose of t-PA used in the present study is similar to 0.9 mg/kg that is used in humans. Some studies with tPA use a higher dose (10 mg/kg), based on previous in vitro studies showing that the fibrinolytic system in rats would be 10-fold less sensitive to tPA than in humans [49]. However, two studies that compared the dose of 0.9 mg/kg to 10 mg/kg in two different thrombotic stroke models in rat demonstrate similar or close to similar recanalization rates with the two doses [50, 51]. In addition, studies of the thrombolytic effect of tPA was not the scope in the present study, but rather its potential adverse effects on the cerebrovasculature. Therefore we used a dose similar to the one used in humans to better mimic the concentration of tPA that the vasculature would be exposed to.
There are some limitations with the current study. The use of the intraluminal middle cerebral artery occlusion model followed by treatment with tPA allowed us to study the potential deleterious effects of tPA independently of its thrombolytic activity. However, thrombolysis products from a dissolved blood clot could affect cerebrovascular function and inflammation and cannot be ruled out. The effect from tPA on the infarct size after I/R was not measured in the current study since it was not the focus of the study. Furthermore, our experiments were performed on ovariectomized female rats to eliminate hormonal fluctuations by “surgical induction” of menopause. Effects of I/R and tPA in a naturally senescent aged female rat remain to be studied. Cerebral blood flow values after MCAO were somewhat lower in these animals, e.g. −65% in a previous study using male rats [9]. Direct comparisons between age-matched males and females in the same within the same study would be needed to investigate if this is a sex-specific effect, but it is possible that a greater depth of ischemia might have had a larger effect on the vascular reactivity of the MCAs and PAs. In addition, it would be interesting to measure parenchymal blood flow to compare with the findings from the in vitro reactivity studies.
In conclusion, the present study demonstrates differential effects on cerebrovascular function of MCAs and PAs after I/R in female rats. MCAs exhibited decreased myogenic tone and constriction to the nitric oxide synthase inhibitor L-NNA, while no effects were seen on PAs. Early post-ischemic reperfusion with tPA was not associated with adverse effects on the cerebrovascular function or increased oxidative stress by peroxynitrite, but might enhance recruitment of intravascular neutrophils. Treatments to protect from vascular dysfunction to improve microvascular perfusion may improve stroke outcome in both males and females.
Acknowledgments
This study was funded by National Institute of Neurological Disorders and Stroke Grants R01 NS045940 and R01 NS093289 and National Heart, Lung, and Blood Institute Grant P01 HL095488, and the Totman Medical Research Trust.
Footnotes
Compliance with ethical standards
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–7. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullberg T, Zia E, Petersson J, Norrving B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke. 2015;46:389–94. doi: 10.1161/STROKEAHA.114.006538. [DOI] [PubMed] [Google Scholar]
- 4.Kunz A, Iadecola C. Cerebral vascular dysregulation in the ischemic brain. Handb Clin Neurol. 2009;92:283–305. doi: 10.1016/S0072-9752(08)01914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 6.Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke. 2002;33:2094–9. doi: 10.1161/01.str.0000020712.84444.8d. [DOI] [PubMed] [Google Scholar]
- 7.Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke. 2001;32:1658–64. doi: 10.1161/01.str.32.7.1658. [DOI] [PubMed] [Google Scholar]
- 8.Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–80. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla MJ, Chan SL, Sweet J, Tavares MJ, Gokina N, Brayden JE. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke. 2014;45:2425–30. doi: 10.1161/STROKEAHA.114.005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 11.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 12.De Silva DA, Fink JN, Christensen S, Ebinger M, Bladin C, Levi CR, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET) Stroke. 2009;40:2872–4. doi: 10.1161/STROKEAHA.108.543595. [DOI] [PubMed] [Google Scholar]
- 13.Soares BP, Tong E, Hom J, Cheng SC, Bredno J, Boussel L, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. 2010;41:e34–40. doi: 10.1161/STROKEAHA.109.568766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–83. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 15.Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) Am J Pathol. 1994;144:188–99. [PMC free article] [PubMed] [Google Scholar]
- 16.Grogaard B, Schurer L, Gerdin B, Arfors KE. Delayed hypoperfusion after incomplete forebrain ischemia in the rat. The role of polymorphonuclear leukocytes. J Cereb Blood Flow Metab. 1989;9:500–5. doi: 10.1038/jcbfm.1989.73. [DOI] [PubMed] [Google Scholar]
- 17.Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17:246–53. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- 18.Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–8. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho-Tavares J, Hickey MJ, Hutchison J, Michaud J, Sutcliffe IT, Kubes P. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–8. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- 20.Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35:888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenglet S, Montecucco F, Denes A, Coutts G, Pinteaux E, Mach F, et al. Recombinant tissue plasminogen activator enhances microglial cell recruitment after stroke in mice. J Cereb Blood Flow Metab. 2014;34:802–12. doi: 10.1038/jcbfm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki T, Kimura Y, Ohata H, Hashimoto T, Shibata K, Hasumi K, et al. Distinct effects of tissue-type plasminogen activator and SMTP-7 on cerebrovascular inflammation following thrombolytic reperfusion. Stroke. 2011;42:1097–104. doi: 10.1161/STROKEAHA.110.598359. [DOI] [PubMed] [Google Scholar]
- 23.Saposnik G, Di Legge S, Webster F, Hachinski V. Predictors of major neurologic improvement after thrombolysis in acute stroke. Neurology. 2005;65:1169–74. doi: 10.1212/01.wnl.0000180687.75907.4b. [DOI] [PubMed] [Google Scholar]
- 24.Savitz SI, Schlaug G, Caplan L, Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke. 2005;36:1447–51. doi: 10.1161/01.STR.0000170647.42126.a8. [DOI] [PubMed] [Google Scholar]
- 25.Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke. 2005;36:62–5. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- 26.Hill MD, Kent DM, Hinchey J, Rowley H, Buchan AM, Wechsler LR, et al. Sex-based differences in the effect of intra-arterial treatment of stroke: analysis of the PROACT-2 study. Stroke. 2006;37:2322–5. doi: 10.1161/01.STR.0000237060.21472.47. [DOI] [PubMed] [Google Scholar]
- 27.Buijs JE, Uyttenboogaart M, Brouns R, de Keyser J, Kamphuisen PW, Luijckx GJ. The Effect of Age and Sex on Clinical Outcome after Intravenous Recombinant Tissue Plasminogen Activator Treatment in Patients with Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2016;25:312–6. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Llull L, Laredo C, Renu A, Perez B, Vila E, Obach V, et al. Uric Acid Therapy Improves Clinical Outcome in Women With Acute Ischemic Stroke. Stroke. 2015 doi: 10.1161/STROKEAHA.115.009960. [DOI] [PubMed] [Google Scholar]
- 29.Lukic-Panin V, Deguchi K, Yamashita T, Shang J, Zhang X, Tian F, et al. Free radical scavenger edaravone administration protects against tissue plasminogen activator induced oxidative stress and blood brain barrier damage. Curr Neurovasc Res. 2010;7:319–29. doi: 10.2174/156720210793180747. [DOI] [PubMed] [Google Scholar]
- 30.Maneen MJ, Cipolla MJ. Peroxynitrite diminishes myogenic tone in cerebral arteries: role of nitrotyrosine and F-actin. Am J Physiol Heart Circ Physiol. 2007;292:H1042–50. doi: 10.1152/ajpheart.00800.2006. [DOI] [PubMed] [Google Scholar]
- 31.Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–5. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- 32.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab. 2013;33:1486–92. doi: 10.1038/jcbfm.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koizumi J, Yoshida Y, Nakasawa T, Ooneda G. Experimental studies of ischemic brain edema, I: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 35.Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Ann Biomed Eng. 1984;12:463–79. doi: 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- 36.Faraci FM, Baumbach GL, Heistad DD. Cerebral circulation: humoral regulation and effects of chronic hypertension. J Am Soc Nephrol. 1990;1:53–7. doi: 10.1681/ASN.V1153. [DOI] [PubMed] [Google Scholar]
- 37.Casella GT, Bunge MB, Wood PM. Improved immunocytochemical identification of neural, endothelial, and inflammatory cell types in paraffin-embedded injured adult rat spinal cord. J Neurosci Methods. 2004;139:1–11. doi: 10.1016/j.jneumeth.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34:1269–75. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 39.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40:1451–7. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol (1985) 2006;100:318–27. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- 41.Gokina NI, Park KM, McElroy-Yaggy K, Osol G. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol (1985) 2005;98:1940–8. doi: 10.1152/japplphysiol.01104.2004. [DOI] [PubMed] [Google Scholar]
- 42.Maeda Y, Hirano K, Nishimura J, Sasaki T, Kanaide H. Rho-kinase inhibitor inhibits both myosin phosphorylation-dependent and -independent enhancement of myofilament Ca2+ sensitivity in the bovine middle cerebral artery. Br J Pharmacol. 2003;140:871–80. doi: 10.1038/sj.bjp.0705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno-Dominguez A, El-Yazbi AF, Zhu HL, Colinas O, Zhong XZ, Walsh EJ, et al. Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries. J Biol Chem. 2014;289:20939–52. doi: 10.1074/jbc.M114.553743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maneen MJ, Hannah R, Vitullo L, DeLance N, Cipolla MJ. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke. 2006;37:894–9. doi: 10.1161/01.STR.0000204043.18592.0d. [DOI] [PubMed] [Google Scholar]
- 45.Ritter LS, Orozco JA, Coull BM, McDonagh PF, Rosenblum WI. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke. 2000;31:1153–61. doi: 10.1161/01.str.31.5.1153. [DOI] [PubMed] [Google Scholar]
- 46.Jimenez-Altayo F, Martin A, Rojas S, Justicia C, Briones AM, Giraldo J, et al. Transient middle cerebral artery occlusion causes different structural, mechanical, and myogenic alterations in normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H628–35. doi: 10.1152/ajpheart.00165.2007. [DOI] [PubMed] [Google Scholar]
- 47.Jimenez-Altayo F, Caracuel L, Perez-Asensio FJ, Martinez-Revelles S, Messeguer A, Planas AM, et al. Participation of oxidative stress on rat middle cerebral artery changes induced by focal cerebral ischemia: beneficial effects of 3,4-dihydro-6-hydroxy-7-methoxy-2,2-dimethyl-1(2H)-benzopyran (CR-6) J Pharmacol Exp Ther. 2009;331:429–36. doi: 10.1124/jpet.109.157131. [DOI] [PubMed] [Google Scholar]
- 48.Onetti Y, Dantas AP, Perez B, Cugota R, Chamorro A, Planas AM, et al. Middle cerebral artery remodeling following transient brain ischemia is linked to early postischemic hyperemia: a target of uric acid treatment. Am J Physiol Heart Circ Physiol. 2015;308:H862–74. doi: 10.1152/ajpheart.00001.2015. [DOI] [PubMed] [Google Scholar]
- 49.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–5. [PubMed] [Google Scholar]
- 50.El Amki M, Lerouet D, Coqueran B, Curis E, Orset C, Vivien D, et al. Experimental modeling of recombinant tissue plasminogen activator effects after ischemic stroke. Exp Neurol. 2012;238:138–44. doi: 10.1016/j.expneurol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Haelewyn B, Risso JJ, Abraini JH. Human recombinant tissue-plasminogen activator (alteplase): why not use the ‘human’ dose for stroke studies in rats? J Cereb Blood Flow Metab. 2010;30:900–3. doi: 10.1038/jcbfm.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]