Abstract
The role of σB in Listeria monocytogenes infection of human intestinal epithelial cells was investigated. Invasion defects associated with loss of σB paralleled those of a ΔinlA strain independently of the σB-dependent P2prfA promoter. Concomitantly, amounts of inlA transcript and InlA protein were significantly decreased in the ΔsigB strain.
The gram-positive facultative intracellular food-borne pathogen Listeria monocytogenes is associated with serious invasive infections in humans and animals (10). To establish a food-borne bacterial infection, a pathogen must survive rapidly changing environmental conditions encountered in the gastrointestinal tract, such as exposure to bile salts, organic acids, and changing osmolarity. Survival of extreme and rapidly changing conditions requires timely and appropriate alterations in bacterial gene expression and protein activity. At the transcriptional level, these alterations are often controlled by alternative sigma factors and the catalytic core of RNA polymerase (24). Alternative sigma factors differentially associate with the core polymerase and essentially reprogram promoter recognition specificities of the enzyme, thus allowing expression of new sets of target genes. For L. monocytogenes, the alternative sigma factor σB (encoded by sigB) contributes to bacterial resistance to environmental stress conditions, such as reduced temperature, oxidative stress, carbon starvation, and low pH (2, 3, 12, 13, 39), as well as to virulence in the mouse (29, 39).
L. monocytogenes is capable of survival and growth under widely varying environmental conditions (11). Further, this organism is able to invade and multiply in a wide range of professional and nonprofessional phagocytic mammalian cells (38). Two cell wall-anchored proteins, internalin A (InlA) and internalin B (InlB), are necessary for efficient invasion of human nonprofessional phagocytic cells (7, 16). The intracellular infectious cycle is characterized by escape from the primary vacuole, intracytosolic replication, actin-based motility, and direct spread to neighboring cells, where a new cycle initiates (17, 36). Several virulence factors responsible for these cellular events have been identified. A pore-forming hemolysin (listeriolysin O) (17), along with two phospholipases (PI-PLC and PC-PLC) (6, 37), is involved in bacterial escape from vacuoles, and the ActA surface protein is responsible for F-actin polymerization and intracellular bacterial motility (21). A DNA binding protein, PrfA, controls expression of these virulence genes (5, 8, 22, 23, 25, 33), but the precise mechanism of regulation is not well understood. prfA is transcribed from three independent promoters, PplcA, P1prfA, and P2prfA (15). PrfA has been suggested to exist in functionally active or inactive forms, depending on environmental conditions (5, 19, 27, 31, 32), and to interact differently at various target promoters (15, 40). The presence of σB-dependent promoters upstream of prfA (P2prfA [29]) and inlA (P4inlA [20]) suggests that σB contributes to L. monocytogenes virulence. Further, Milohanic et al. (28) have reported that a number of PrfA-regulated genes have putative σB-dependent promoters. In the present study, the role of σB in infection of human intestinal epithelial cells was investigated.
σB contributes to early stages of infection in human intestinal epithelial cells.
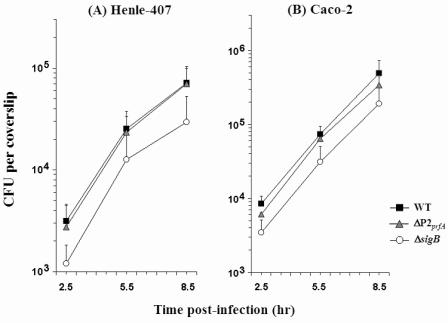
Intracellular growth of the wild-type L. monocytogenes strain 10403S (4) as well as ΔsigB (FSL A1-254) (39) and ΔP2prfA (DP-L1957) (14) isogenic mutant strains was examined in two intestinal epithelial cell lines, Henle-407 and Caco-2. The assay was performed as previously described (30). Briefly, bacteria were grown at 30°C overnight in brain heart infusion (BHI) broth (with shaking at 250 rpm) and washed in phosphate-buffered saline (PBS) before infection. Host cell infections were performed at 37°C in a 5% CO2 atmosphere. Monolayers of host cells on glass coverslips were infected with a multiplicity of infection (MOI) of approximately 30 and 3 for Henle-407 and Caco-2 cells, respectively. Cells were washed at 1 h postinfection, and gentamicin was added at 1.5 h postinfection. Gentamicin concentrations were experimentally optimized to 150 μg/ml (Henle-407) or 50 μg/ml (Caco-2) to achieve effective killing of extracellular bacteria within 30 min. The number of intracellular bacteria per coverslip was determined at various time points during infection by plating appropriate serial dilutions of infected host cell lysates onto Luria-Bertani (LB) agar plates. Intracellular doubling times were evaluated by one-way analysis of variance (ANOVA), and individual comparisons were made with Bonferroni's multiple comparison test to determine whether a mean doubling time for one strain was significantly different from those for other strains. Intracellular growth rates were not significantly different among the various strains tested (Fig. 1; Table 1). However, at 2.5 h postinfection intracellular ΔsigB numbers were consistently 2.0- to 2.5-fold lower than those of the wild-type strain in both cell lines. Intracellular ΔP2prfA numbers were not different than those of the wild-type strain in either cell line. These results suggest that σB contributes to infection independently of its activity at P2prfA, at a stage prior to intracellular bacterial multiplication in human intestinal epithelial cells.
FIG. 1.
Intracellular growth of L. monocytogenes in human epithelial cells. Viable bacterial counts were determined as CFU per coverslip at specific time points postinfection in (A) Henle-407 and (B) Caco-2 cells. Data represent mean CFU from three independent experiments. Error bars reflect standard deviations from each mean. WT, wild type.
TABLE 1.
Intracellular doubling timesa
| Strain | Doubling time (min) for Henle-407 at:
|
Doubling time (min) for Caco-2 at:
|
||
|---|---|---|---|---|
| 2.5-5.5 h | 5.5-8.5 h | 2.5-5.5 h | 5.5-8.5 h | |
| Wild type | 59 ± 5 | 119 ± 61 | 58 ± 3 | 67 ± 5 |
| ΔsigB | 59 ± 11 | 163 ± 87 | 59 ± 7 | 70 ± 8 |
| ΔP2prfA | 59 ± 8 | 116 ± 35 | 54 ± 5 | 77 ± 12 |
The means and standard deviations from three independent experiments are shown for each strain. At 2.5 to 5.5 h and 5.5 to 8.5 h, P values were 0.9934 and 0.6299 for Henle-407, respectively, and they were 0.5380 and 0.8503, respectively, for Caco-2.
σB promotes invasion of human epithelial cells.
To define the role of σB in early infection, we evaluated the relative abilities of the wild-type, ΔsigB, and ΔP2prfA strains to adhere to Henle-407 and Caco-2 human epithelial cells. At 30 min prior to infection, cytochalasin D (1 μg/ml) was added to host cell monolayers to disrupt actin polymerization and prevent bacterial internalization. Bacteria were grown as described above, and cells were infected to an MOI of approximately 5. After either 1 h (Henle-407) or 30 min (Caco-2) of incubation, cells were washed six times with PBS and the number of adherent bacteria per coverslip was determined as described above. The fraction of inoculum recovered was calculated for each individual sample. To control for inhibition of bacterial internalization, a sample was treated with gentamicin after the wash and bacterial counts were determined after 1 h. No bacteria were recovered under these conditions. Results were evaluated by one-sample t test to determine if a given mutant strain phenotype was significantly different from that of the wild-type strain. The three bacterial strains tested did not differ in their abilities to adhere to either host cell line (Table 2).
TABLE 2.
Adhesion and invasion characteristics of L. monocytogenes wild-type and mutant strainsa
| Strain | Adhesion
|
Invasion
|
||
|---|---|---|---|---|
| Henle-407 | Caco-2 | Henle-407 | Caco-2 | |
| Wild type | 1.00 | 1.00 | 1.00 | 1.00 |
| ΔsigB | 0.81 ± 0.17 | 0.68 ± 0.20 | 0.54 ± 0.11***b | 0.23 ± 0.10*** |
| ΔP2prfA | 0.95 ± 0.18 | 1.28 ± 0.33 | 1.08 ± 0.11 | 1.07 ± 0.11 |
| ΔinlA | NDc | ND | 0.52 ± 0.20** | 0.21 ± 0.10** |
| ΔinlB | ND | ND | 0.79 ± 0.27 | 0.93 ± 0.35 |
Results are reported as ratios of mutant strain numbers relative to wild-type strain numbers, with wild-type numbers arbitrarily set to 1. The means and standard deviations from at least three independent experiments are shown for each strain.
P values (*, <0.05; **, <0.01; ***, <0.001) for comparison between respective mutant strain and wild-type invasion by one-sample t test are indicated by asterisks.
ND, not determined.
Next we evaluated σB's contribution to invasion of human epithelial cells. L. monocytogenes invasion of nonphagocytic cells is predominantly mediated by two cell surface-anchored proteins, InlA and InlB (7, 16). The inlA and inlB genes comprise an operon. Transcriptional analysis has identified four promoters upstream of inlA and one upstream of inlB (23). P3inlA is a PrfA-regulated promoter (23), whereas P4inlA is σB dependent (20). A sequence resembling a σB-dependent promoter also has been reported upstream of inlB, but promoter activity at this site has not been confirmed (20). Therefore, we hypothesized that σB's contribution to inlA expression, and possibly to inlB expression, would influence the ability of L. monocytogenes to invade nonphagocytic cells. To assess the respective contributions of σB, P2prfA, InlA, and InlB to host cell invasion, invasion capabilities were compared among wild-type 10403S, ΔsigB (FSL A1-254) (39), ΔP2prfA (DP-L1957) (14), ΔinlA (EJL12) (provided by Jeff Miller) (1), and ΔinlB (HEL-137; from this study). For this purpose, we created an inlB internal in-frame deletion mutant. The deletion was generated by site-directed mutagenesis by overlap extension (18), using primers Marq 37 (5′-GCG GAT CCT ACG GCA CCA ACA AAA G-3′), Marq 38 (5′-GTG GAA CTA GTC CTT ATT CGC TTC CTT CTT GGG TTG TGC-3′), Marq 39 (5′-GCA CAA CCC AAG AAG GAA GCG AAT AAG GAC TAG TTC CAC-3′), and Marq 41 (5′-GCT AGC ATG CAG TGA AAT TAT TGC TGG T-3′) (7). The inlB deletion was introduced into the chromosome of L. monocytogenes strain 10403S by allelic exchange as previously described (6), creating HEL-137. As for the intracellular growth assay, bacteria were grown at 30°C overnight (with shaking at 250 rpm) and host cell infections were performed at 37°C in a 5% CO2 atmosphere. Host cells were infected to an MOI of approximately 30, and infection times were optimized for each cell type. For invasion of Henle-407 cells, cells were washed at 1 h postinfection and were treated with gentamicin at 1.5 h postinfection. For invasion of Caco-2 cells, cells were washed at 30 min postinfection and were treated with gentamicin at 45 min postinfection. Numbers of internalized bacteria per coverslip were determined 30 min after gentamicin treatment as described above. The fraction of inoculum recovered was calculated for each individual sample. Results were statistically evaluated by one-sample t test. The ΔsigB strain was less effective at invading Henle-407 (P < 0.001) and Caco-2 cells (P < 0.001) than the wild-type strain (Table 2). The invasion defect of the ΔsigB strain was comparable to that of the ΔinlA strain (P = 0.791 for Henle-407, P = 0.784 for Caco-2), but it was significantly more pronounced than that of the ΔinlB strain (P = 0.029 for Henle-407, P = 0.001 for Caco-2). Specifically, relative invasion capabilities of the ΔsigB, ΔinlA, and ΔinlB strains were reduced by 1.9-, 1.9-, and 1.3-fold, respectively, in Henle-407 cells and by 4.3-, 4.8-, and 1.1-fold, respectively, in Caco-2 cells. Invasion phenotypes of ΔP2prfA and wild-type strains did not differ. Overall, these results show that σB significantly increases the efficacy of L. monocytogenes to invade human epithelial cells, independent of P2prfA transcriptional activity and of InlB. It is possible that σB influences InlB-dependent invasion in other cell types, such as hepatocytes or fibroblasts cells (7).
σB contributes to inlA expression.
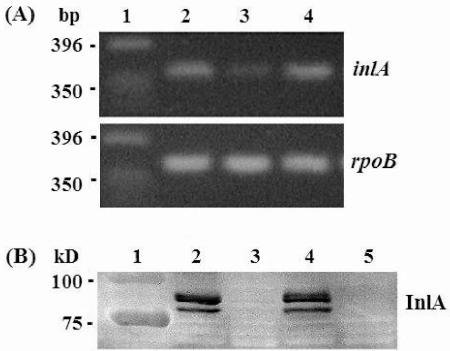
The above observations led us to focus on InlA as an important factor affecting L. monocytogenes virulence in a σB-dependent manner. One of the key early events in human listeriosis is bacterial entry into the intestinal epithelium. A number of studies have shown that InlA is a major bacterial ligand involved in this process (9, 16, 23, 26). Therefore, levels of inlA expression in wild-type, ΔsigB, and ΔP2prfA strains were determined by semiquantitative reverse transcription-PCR (RT-PCR). Bacteria were grown with shaking (250 rpm) in BHI broth overnight at 30°C. Total RNA was isolated with the RNeasy Midi kit (Qiagen, Valencia, Calif.) according to the manufacturer's protocol. Purified RNA was quantified by absorbance at 260 nm immediately before use, and RT was performed with the Superscript First-Strand Synthesis RT-PCR system (Invitrogen, Carlsbad, Calif.) with 50 ng of total RNA as template (35). The rpoB gene was used as an internal control. Primers used in this assay (InlATqMnF1 [5′-GGT CTC ACA AAC AGA TCT AGA CCA AGT-3′], InlATqMnR1 [5′-TCA AGT ATT CCA CTC CAT CGA TAG ATT-3′], RpoBTqMnF1, and RpoBTqMnR1 [35]) were designed with PrimerExpress software (Applied Biosystems, Foster City, Calif.). Briefly, cDNA was synthesized with oligonucleotides specific for inlA (InlATqMnR1) and rpoB (RpoBTqMnR1) and then amplified by PCR using the AmpliTaq Gold DNA polymerase system (Applied Biosystems). Thirty PCR cycles were selected for cDNA amplification, as no visible products were observed after either 15 or 22 cycles. RT-PCR products were run on 3% agarose gels and quantified with LabImage software (Kapelan, Halle, Germany). Results were statistically analyzed by one-sample t test. RT-PCR results clearly showed decreased levels of inlA transcript in the ΔsigB strain relative to that in the wild-type strain (Fig. 2A). In the absence of σB, inlA expression was decreased by 3.8-fold (P = 0.001), which is consistent with the relative invasion defect observed for the ΔsigB strain in human epithelial cells. Quantities of the inlA transcript in the ΔP2prfA strain were essentially identical to those of the wild-type strain (P = 0.212), which is also consistent with the invasion phenotype of this strain in human epithelial cells.
FIG. 2.
Detection of inlA transcript and InlA protein. (A) Total RNA was isolated from wild-type L. monocytogenes as well as from ΔsigB and ΔP2prfA strains, and inlA transcript levels were assayed by RT-PCR. rpoB, encoding the β subunit of the DNA-dependent RNA polymerase, was used as an internal control. No PCR products were obtained in the absence of RNA (data not shown). Lane 1, DNA markers in base pairs (bp); lane 2, wild type; lane 3, ΔsigB; lane 4, ΔP2prfA. (B) Cell wall proteins were isolated from wild-type L. monocytogenes as well as from ΔsigB, ΔP2prfA, and ΔinlA strains, and InlA was detected by Western immunoblotting. Samples were normalized for equal CFU (7.5 × 108) from each original culture. Lane 1, prestained protein markers; lane 2, wild type; lane 3, ΔsigB; lane 4, ΔP2prfA; lane 5, ΔinlA. kD, kilodaltons.
Levels of cell wall-associated InlA in wild-type, ΔsigB, and ΔP2prfA strains were estimated by Western immunoblotting. For isolation of L. monocytogenes cell wall proteins, bacterial cells in protoplast buffer (0.5 M sucrose, 50 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 0.02% sodium azide) were treated with mutanolysin to a final concentration of 100 U/ml of original bacterial culture at an OD600 of 1.0. Protoplast formation was monitored by microscopy. Cell wall extracts were processed for detection of InlA by Western immunoblotting as previously described (34). InlA was virtually undetectable in the ΔsigB strain (Fig. 2B). On the other hand, equivalent amounts of InlA were present in the wild-type and ΔP2prfA strains (Fig. 2B). Based on the observations that inlA transcription is regulated in a growth-phase-dependent manner and that inlA expression peaks upon entry into stationary phase in the absence of PrfA, Sheehan et al. (33) suggested the existence of a PrfA-independent mechanism that contributes to regulation of inlA expression in stationary-phase L. monocytogenes. Our data suggest that this proposed stationary-phase mechanism is mediated by σB. σB activity is also regulated in a growth-phase-dependent manner, with maximal activity upon entry into stationary phase (13, 35). Most importantly, we have demonstrated that σB is a major contributor to stationary-phase inlA expression. Taken together, our observations highlight the critical contribution of σB to expression of inlA and consequently to invasion of human intestinal epithelial cells.
In conclusion, we have identified a specific mechanism linking the general stress-responsive sigma factor, σB, with the ability of L. monocytogenes to invade human epithelial cells. Specifically, we have demonstrated that the loss of σB significantly impairs the ability of L. monocytogenes to invade human epithelial cells in a manner that is independent of the σB-dependent promoter P2prfA. Moreover, our data clearly demonstrate that the importance of σB in bacterial invasion is related, at least in part, to its role in the regulation of inlA expression, presumably by controlling activity at P4inlA.
Acknowledgments
We thank Jeff Miller for the ΔinlA strain. We also thank Terry Potter for mouse antiserum to L. monocytogenes InlA.
This work was supported in part by National Institutes of Health award no. RO1-AI052151-01A1 (to K.J.B.).
Editor: V. J. DiRita
REFERENCES
- 1.Bakardjiev, A. I., B. A. Stacy, S. J. Fisher, and D. A. Portnoy. 2004. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect. Immun. 72:489-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, L. A., M. S. Cetin, R. W. Hutkins, and A. K. Benson. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, L. A., S. N. Evans, R. W. Hutkins, and A. K. Benson. 2000. Role of σB in adaptation of Listeria monocytogenes to growth at low temperature. J. Bacteriol. 182:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 5.Bohne, J., H. Kestler, C. Uebele, Z. Sokolovic, and W. Goebel. 1996. Differential regulation of the virulence genes of Listeria monocytogenes by the transcriptional activator PrfA. Mol. Microbiol. 20:1189-1198. [DOI] [PubMed] [Google Scholar]
- 6.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 8.Dramsi, S., C. Kocks, C. Forestier, and P. Cossart. 1993. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator prfA. Mol. Microbiol. 9:931-941. [DOI] [PubMed] [Google Scholar]
- 9.Drevets, D. A., R. T. Sawyer, T. A. Potter, and P. A. Campbell. 1995. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect. Immun. 63:4268-4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenlon, D. R. 1999. Listeria monocytogenes in the natural environment, p. 21-37. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis, and food safety, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 12.Ferreira, A., C. P. O'Byrne, and K. J. Boor. 2001. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 67:4454-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira, A., D. Sue, C. P. O'Byrne, and K. J. Boor. 2003. Role of Listeria monocytogenes σB in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl. Environ. Microbiol. 69:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freitag, N. E., and D. A. Portnoy. 1994. Dual promoters of the Listeria monocytogenes prfA transcriptional activator appear essential in vitro but are redundant in vivo. Mol. Microbiol. 12:845-853. [DOI] [PubMed] [Google Scholar]
- 15.Freitag, N. E., L. Rong, and D. A. Portnoy. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 17.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 19.Johansson, J., P. Mandin, A. Renzoni, C. Chiaruttini, M. Springer, and P. Cossart. 2002. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell 110:551-561. [DOI] [PubMed] [Google Scholar]
- 20.Kazmierczak, M. J., S. C. Mithoe, K. J. Boor, and M. Wiedmann. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 185:5722-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 22.Leimeister-Wachter, M., C. Haffner, E. Domann, W. Goebel, and T. Chakraborty. 1990. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 87:8336-8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lingnau, A., E. Domann, M. Hudel, M. Bock, T. Nichterlein, J. Wehland, and T. Chakraborty. 1995. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun. 63:3896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loewen, P. C., and R. Hengge-Aronis. 1994. The role of the sigma factor σS (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 48:53-80. [DOI] [PubMed] [Google Scholar]
- 25.Mengaud, J., S. Dramsi, E. Gouin, J. A. Vazquez-Boland, G. Milon, and P. Cossart. 1991. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol. Microbiol. 5:2273-2283. [DOI] [PubMed] [Google Scholar]
- 26.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 27.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 23:1075-1085. [DOI] [PubMed] [Google Scholar]
- 28.Milohanic, E., P. Glaser, J. Y. Coppee, L. Frangeul, Y. Vega, J. A. Vazquez-Boland, F. Kunst, P. Cossart, and C. Buchrieser. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 47:1613-1625. [DOI] [PubMed] [Google Scholar]
- 29.Nadon, C. A., B. M. Bowen, M. Wiedmann, and K. J. Boor. 2002. Sigma B contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect. Immun. 70:3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renzoni, A., A. Klarsfeld, S. Dramsi, and P. Cossart. 1997. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect. Immun. 65:1515-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ripio, M. T., K. Brehm, M. Lara, M. Suarez, and J. A. Vazquez-Boland. 1997. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J. Bacteriol. 179:7174-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheehan, B., A. Klarsfeld, T. Msadek, and P. Cossart. 1995. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 177:6469-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder, A., and H. Marquis. 2003. Restricted translocation across the cell wall regulates secretion of the broad-range phospholipase C of Listeria monocytogenes. J. Bacteriol. 185:5953-5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sue, D., K. J. Boor, and M. Wiedmann. 2003. σB-dependent expression patterns of compatible solute transporter genes opuCA and lmo1421 and the conjugated bile salt hydrolase gene bsh in Listeria monocytogenes. Microbiology 149:3247-3256. [DOI] [PubMed] [Google Scholar]
- 36.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez-Boland, J. A., C. Kocks, S. Dramsi, H. Ohayon, C. Geoffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60:219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiedmann, M., T. J. Arvik, R. J. Hurley, and K. J. Boor. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams, J. R., C. Thayyullathil, and N. E. Freitag. 2000. Sequence variations within PrfA DNA binding sites and effects on Listeria monocytogenes virulence gene expression. J. Bacteriol. 182:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]