Abstract
Live mycobacteria have been reported to signal through both Toll-like receptor 2 (TLR2) and TLR4 in vitro. Here, we investigated the role of TLR2 in the long-term control of the infection by the attenuated Mycobacterium, Mycobacterium bovis BCG, in vivo. We sought to determine whether the reported initial defect of bacterial control (K. A. Heldwein et al., J. Leukoc. Biol. 74:277-286, 2003) resolved in the chronic phase of BCG infection. Here we show that TLR2-deficient mice survived a 6-month infection period with M. bovis BCG and were able to control bacterial growth. Granuloma formation, T-cell and macrophage recruitment, and activation were normal. Furthermore, the TLR2 coreceptor, TLR6, is also not required since TLR6-deficient mice were able to control chronic BCG infection. Finally, TLR2-TLR4-deficient mice infected with BCG survived the 8-month observation period. Interestingly, the adaptive response of TLR2- and/or TLR4-deficient mice seemed essentially normal on day 14 or 56 after infection, since T cells responded normally to soluble BCG antigens. In conclusion, our data demonstrate that TLR2, TLR4, or TLR6 are redundant for the control of M. bovis BCG mycobacterial infection.
The pathogen pattern recognition receptors (PRRs) involved in sensing Mycobacterium tuberculosis antigens include mannose receptor, complement receptors, and Toll-like receptors (TLRs; reviewed in references 14 and 32). Both TLR2 and TLR4 have been implicated in the recognition of mycobacteria such as Mycobacterium bovis BCG or M. tuberculosis (24, 25; reviewed in reference 14), leading to dendritic cell (DC) maturation (38), reduced major histocompatibility complex (MHC) class II expression and antigen processing (29), and direct intracellular killing by macrophages (37). Inactivation of both TLR2 and TLR4 prevented most activation of murine macrophages infected with live M. bovis (8) or of human DCs with BCG cell wall skeleton (38). TLR1 and TLR6 that heterodimerize with TLR2 have also been implicated in the recognition of mycobacterial antigens (4, 36).
Most purified mycobacterial antigens tested thus far signal through TLR2. Lipoarabinomannan from rapidly growing mycobacteria (Ara-LAM) (20, 22, 25), lipomannan from slow-growing mycobacteria M. tuberculosis or M. bovis BCG (30), and their membrane anchors PIM2 and PIM6 (11, 20) activate macrophages and DCs via TLR2, resulting in high expression of tumor necrosis factor (TNF), interleukin-12 (IL-12), and costimulatory molecules CD40 and CD86 and the production of nitric oxide. Mycobacterial 19-kDa lipoprotein signals through TLR2 to induce cell activation, apoptosis, and mycobacterial killing (2, 3, 37) but also reduction in MHC class II antigen expression, antigen processing, and presentation (29) that may play a role in the poor recognition of infected macrophages by T cells and thus help to establish and maintain chronic mycobacterial infection (2, 3, 29, 37). Based on the in vitro data, it was hypothesized that TLR2 contributes significantly to the initiation and maintenance of the innate and adaptive immune responses involved in the host response to mycobacterial infection.
In vivo, we showed that bacterial growth was uncontrolled in the lungs of TLR2-deficient mice infected with 500 M. tuberculosis CFU per lung and that the mice could not control the chronic phase of the infection, which was fatal within 6 months (6), a finding in line with the mortality seen after high-dose M. tuberculosis infection (31) and with the transient defect in cytokine expression and uncontrolled bacteria in TLR2-deficient mice after low-dose infection with M. tuberculosis Kurono strain (33).
In a short-term, acute model of M. bovis BCG infection, TLR2-deficient mice showed a 10-fold increase of pulmonary bacterial load 2 weeks postinfection (106 CFU given intraperitoneally), with a reduced adaptive response (15).
Here, we focused on the chronic infection with the nonvirulent M. bovis BCG strain to see whether the initial defect in bacterial control did resolve in the chronic phase of the infection. We show that not only TLR2-deficient mice but also TLR2-TLR4-deficient mice survived a 6- to 8-month infection with M. bovis BCG, controlled bacterial growth, and developed an adaptive response. In addition, we demonstrate that the TLR2 coreceptor, TLR6, is redundant for the control of chronic M. bovis BCG infection. Therefore, the absence of TLR2, TLR4, and/or TLR6 did not result in a relevant defect of immune response to BCG infection, as is seen for TNF-deficient mice in the same model (19; unpublished observations).
MATERIALS AND METHODS
Mice and infection.
Six- to twelve-week-old TLR2-deficient mice (obtained from C. Kirschning [26]), TLR6-deficient mice (from S. Akira (35), or intercross of TLR2- and TLR4-deficient mice (from C. Kirschning [26] and S. Akira [16], respectively) and their control littermates were bred under specific-pathogen-free conditions in the Transgenose Institute animal breeding facility (Orleans, France). All mice were backcrossed on a C57BL/6 background (N6 for TLR2 and TLR6 and N2 for TLR2/TLR4) and littermate controls from heterozygous breeding used. Mice were injected intravenously (i.v.) with a dose of 2 × 106 CFU of M. bovis BCG (Pasteur strain #1173P2; Pasteur Institute, Paris, France). All animal experiments complied with the French government's ethical and animal experiment regulations. Groups of 12 to 16 mice were infected, weighed weekly, and monitored regularly for clinical status.
Quantification of viable mycobacteria in organs.
At specific time points, mice were sacrificed, and their lungs, livers, and spleens were aseptically removed and weighed. One-half of each organ was homogenized in 0.9% NaCl solution containing 0.04% Tween 20 (Sigma). Serial dilutions of the homogenates were plated in duplicate onto 7H11 Middlebrook agar supplemented with 10% OADC (Difco Laboratories, Detroit, Mich.) and 0.5% glycerol, followed by incubation at 37°C, and CFU were enumerated after 21 days.
Histopathology.
Samples of lung, liver, and spleen were fixed in 10% buffered formalin (Shandon, Pittsburgh, Pa.) before paraffin embedding. Tissue sections (3 μm) were stained with hematoxylin and eosin or Ziehl-Neelsen acid-fast stain for evaluation of pathological changes and bacillary load, respectively. Granuloma were quantified by counting 10 microscopic fields at ×200 magnification. Typical clusters of more than 10 mononuclear cells were counted as a granuloma.
Immunohistochemistry.
Samples of lung, liver, and spleen were embedded in Tissue-Tek (Sakura, Zoeterwoude, The Netherlands) in cryomolds, immediately frozen on dry ice, and stored at −80°C as described before (19). The frozen tissues were cut at 6-μm thickness on a cryostat (Leica, Nussloch, Germany), air dried, and stored at −80°C. Fixation in acetone (10 min at 4°C) or paraformaldehyde 4% was performed just before immunolabeling. After a rinse in phosphate-buffered saline (PBS), the sections were saturated with normal rabbit serum before incubation for 16 h at 4°C with the primary antibody. Antibodies to CD3, F4/80, and MHC class II were from BD Pharmingen (San Diego, Calif.), and rabbit antiserum to iNOS was from J. Pfeilschifter, University of Frankfurt, Frankfurt, Germany. The sections were then washed in PBS and incubated for 30 min at room temperature with the appropriate biotinylated secondary antibody. Avidin-biotin complex was added to the sections for 30 min (ABC Vector kit; Vector, Burlingame, Calif.), washed, and revealed with DAB substrate (Dako, Glostrup, Denmark). After a rinse in PBS, the sections were mounted in Eukitt (Kindler & Co., Freiburg, Germany).
Cutaneous DTH reaction.
Delayed-type hypersensitivity (DTH) analysis was performed as described previously (19). TLR2/TLR4-deficient and control mice were infected i.v. with 2 × 106 live M. bovis BCG as described above. Eight months later, mice were injected with 20 IU of tuberculin-purified protein derivative (PPD; Bovituber, Merial, Lyon, France) in 50 μl subcutaneously in the left footpad. Swelling was measured 48 h later with a micrometer (Mitotuyo; Bruetsch, Wallisellen, Switzerland) and compared to the saline-injected right footpad.
Primary macrophage and DC cultures.
Murine bone marrow cells were isolated from femurs and differentiated into macrophages after culturing at 106 cells/ml for 7 days in Dulbecco minimal essential medium (DMEM; Sigma) supplemented with 20% horse serum and 30% L929 cell-conditioned medium as a source of granulocyte-macrophage colony-stimulating factor (as described in reference 27). Three days after washing and reculturing in fresh medium, the cell preparation contained a homogenous population of macrophages. Alternatively, murine bone marrow cells were differentiated into myeloid dendritic cells after culturing at 2.105 cells/ml for 10 days (change on day 3, 6 and 8) in RPMI supplemented with 10% fetal calf serum and 4% J558L cell-conditioned medium as a source of granulocyte-macrophage colony-stimulating factor (as described in reference 23).
Stimulation of macrophages and DCs.
Bone marrow-derived macrophages and DCs were plated in 96-well microculture plates (at 105 cells/well) and stimulated with lipopolysaccharide (LPS; Escherichia coli serotype O111:B4 [Sigma, St. Louis, Mo.] at 100 ng/ml), or a synthetic bacterial lipopeptide (BLP), Pam3CSK4 ({S-[2,3-bis-(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-Lys4-OH} trihydrochloride [EMC Microcollections, Tuebingen, Germany] at 0.5 μg/ml), or infected with M. bovis BCG (Pasteur strain 1173P2 [Pasteur Institute] at a multiplicity of infection [MOI] of two bacteria per cell). The BCG preparations used for stimulation were a lyophilized preparation of BCG culture supernatant, heat-killed M. bovis BCG, or extended freeze-dried BCG (17)—all kind gifts of G. Marchal (Pasteur Institute). Cell supernatants were harvested after 24 h of stimulation in the presence of gamma interferon (IFN-γ; 100 U/ml) for cytokines and nitrite measurements and analyzed immediately or stored at −20°C.
Antigen-specific IFN-γ production.
T-cell priming was assessed by the production of IFN-γ upon antigen restimulation ex vivo. Single cell suspensions of splenocytes were prepared from wild-type and TLR2-TLR4-deficient mice 14 and 56 days after BCG infection with 2 × 106 live M. bovis BCG. Splenocytes were cultured at 5 × 105 cells/ml in RPMI 1640 with glutamine, 5% fetal calf serum, and antibiotics and stimulated with either 2.5 μg of concanavalin A/ml (Sigma), a lyophilized soluble fraction from BCG culture (10 μg/ml), BCG (two bacteria per cell), or heat-killed Listeria monocytogenes (100 bacteria per cell) for 2 days at 37°C. IFN-γ production in the supernatant was quantified by enzyme-linked immunosorbent assay (ELISA).
Cytokine measurements.
Commercially available ELISA reagents for TNF, IL-12p40, and IFN-γ were used (Duoset R&D Systems, Abingdon, United Kingdom). Alternatively, a cytokine profile analysis was performed by using the Perbio SearchLight Proteome Arrays system (Perbio Science France, Bezons, France) with pools of supernatants from three independent cultures of TLR2- and/or TLR4-deficient macrophages.
Nitrite measurements.
Nitrite concentrations in cell supernatants were determined by using the Griess reagent in presence of 1% p-aminobenzene-sulfonamide and 0.1% n-1-napthylethylene-diamide in 2.5% phosphoric acid as described previously (13). The absorbance at 540 nm was measured after 30 min of incubation.
Flow cytometry analysis.
Macrophages cultured on non-tissue-culture-treated petri dishes were harvested with cold PBS and gentle pipetting. Cells were stained for cell surface markers by using fluorescence-labeled antibodies to CD11b-PercP-Cy5.5 (clone H1/70), CD11c-APC (clone HL3), CD40-phycoerythrin (clone 3/23), and CD86-fluorescein isothiocyanate (clone GL1). All antibodies were used at 0.2 μg/106 cells and were obtained from BD Pharmingen (San Diego, Calif.). All staining procedures were performed in PBS containing 0.5% bovine serum albumin for 20 min at 4°C. Cells were analyzed by flow cytometry using CellQuest software (BD Immunocytometry Systems, San Jose, Calif.) on an LSR analyzer. Positive cells for CD11b or CD11c were gated and analyzed.
Confocal microscopy.
Macrophage monolayers were established by plating 105 cells in 0.2 ml of DMEM supplemented with 10% fetal calf serum onto sterile glass coverslips. After overnight attachment at 37°C in humidified air containing 5% CO2, coverslips were transferred into 24-well microtiter plates containing DMEM for 4 h. Fluorescent M. bovis BCG expressing green fluorescent protein (GFP; a kind gift from V. Snewin, London, United Kingdom) stored at −80°C was rapidly thawed, passed 30-fold through a 25G needle and then 10-fold through a 30G needle, sonicated six times for 15 s, and immediately added to the cultures at an MOI of 1. After 2 h at 37°C under a humidified atmosphere containing 5% CO2, the medium was removed, and the cultures were washed once with warm phosphate-buffered saline (PBS, pH 7.2) and fixed with paraformaldehyde 4% in PBS. After overnight fixation, macrophages on coverslips were washed once in PBS for 10 min. Cells were then permeabilized for 3 min with 0.1% Triton X-100 in PBS, washed twice 5 min with PBS, quenched with 50 mM NH4Cl in PBS for 30 min, preincubated for 30 min with 1% bovine serum albumin in PBS. To stain F-actin, macrophages were incubated for 20 min with β-phalloidin conjugated to rhodamine at 5 U/ml (Molecular Probes, Eugene, Oreg.), followed by two 5-min washes in PBS. Coverslips were mounted by using Dako mounting medium.
BCG-GFP internalization was assessed with a fluorescence Leica DM IRBE microscope (Leica, Reuil Malmaison, France; ×40 oil immersion objective lens) by counting the macrophages containing one or two isolated intracellular bacteria and the noninfected macrophages over 10 different observation fields per slide (three slides per experiment and four experiments in total). The slides were counted blindly.
Statistical analysis.
The significance (P) was assessed by using Student t test or the Fisher-Yates-Terry C1 test when indicated.
RESULTS
Impaired cytokine and nitric oxide secretion of TLR2-deficient macrophages in response to mycobacterial antigens.
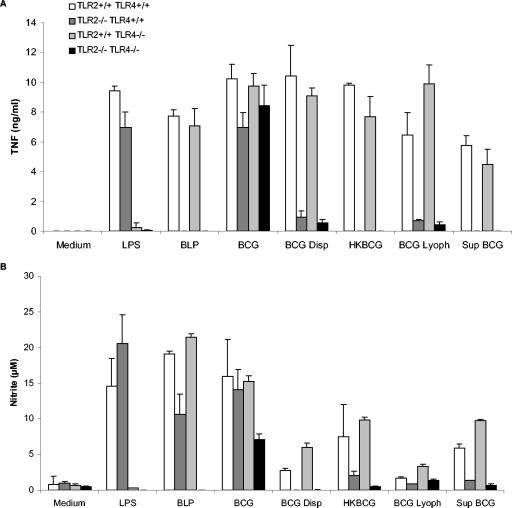
Mycobacteria and mycobacterial antigens were reported to activate macrophages and DCs through TLR2 and TLR4 (30). We first verified how macrophages deficient for TLR2 and/or TLR4 responded to live BCG or BCG antigens. Wild-type macrophages produced abundant TNF, IL-12p40 (not shown), and nitric oxide after infection with live BCG or stimulation by heat-killed BCG, BCG killed by lyophilization, or soluble antigens released in BCG culture supernatant (Fig. 1). The levels of cytokines and nitrite produced in response to BCG fractions were comparable to those obtained after LPS or Pam3CSK4 (BLP) stimulation. We showed previously that in the absence of functional TLR4 in C3H/HeJ bone marrow-derived macrophages decreased by 30 to 50% the TNF response to M. bovis BCG infection at an MOI of 0.2- to 2-fold, and this effect was further accentuated in the absence of TLR2 in C3H/HeJ TLR2−/− macrophages (8). We show here that macrophages from mice inactivated for TLR2 and/or TLR4 are still strongly activated by the same BCG preparation, whereas a preparation of well-dispersed BCG grown in the presence of detergent seemed to be strongly TLR2, but not TLR4, dependent.
FIG. 1.
Reduced TNF and nitrite production in TLR2-deficient macrophages stimulated with BCG or mycobacterial preparations. Bone marrow-derived macrophages from TLR2- and/or TLR4-deficient mice and controls were incubated with LPS (0.1 μg/ml), BLP (0.5 μg/ml), heat-killed BCG (HKBCG; 10 μg/ml), BCG killed by lyophilization (BCG Lyoph; 10 μg/ml), and a lyophilized preparation of BCG culture supernatant (Sup BCG; 10 μg/ml) or were infected with BCG (two bacteria per cell) or dispersed BCG (two bacteria per cell) for 24 h. TNF (A) and nitrite (B) were measured in the supernatants by ELISA and Griess reaction, respectively. The results are means ± the standard deviations (SD) from n = two mice and are from one representative experiment out of three independent experiments.
TLR2-deficient macrophages were unable to respond to stimulation by any of the mycobacterial antigens tested, BCG killed by heat or lyophilization, or a fraction of BCG soluble antigens. TNF and nitric oxide were undetectable (Fig. 1), and IL-12p40 production was also strongly reduced in the absence of TLR2 (data not shown). Similarly, the stimulation of macrophages by purified BCG antigens such as PIM2, PIM6, or LM was inhibited in the absence of TLR2 (11, 30). Further cytokine secretion profiles realized by SearchLight proteome array analysis showed a reduction (ca.50%) of IL-1β, IL-6, TNF, and IL-10 in TLR2−/− macrophages compared to wild-type cells after stimulation with live BCG (data not shown). Therefore, the response to BCG antigens, either soluble or particulate, was greatly reduced in TLR2-deficient macrophages and DCs (data not shown).
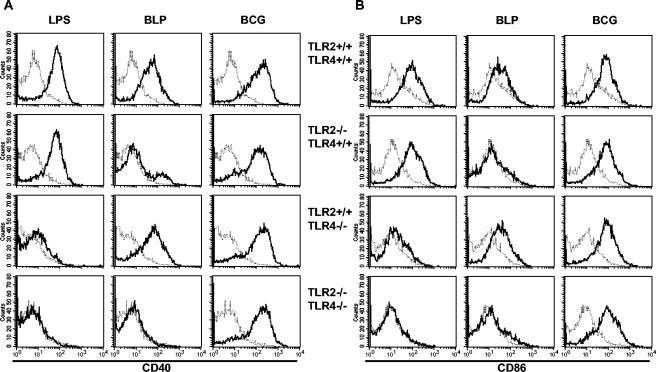
TLR2-independent CD40 and CD86 upregulation by BCG.
We then sought to determine whether the expression of costimulatory molecules by antigen-presenting cells is induced in the absence of TLR2. As shown in Fig. 2, LPS, BLP, and BCG induced similar CD40 and CD86 expression in wild-type macrophages. As expected, CD40 and CD86 expression in response to BLP was inhibited in TLR2−/− macrophages, and CD40 and CD86 expression in response to LPS was absent in TLR4−/− macrophages. However, TLR2- and/or TLR4-deficient macrophages expressed CD40 and CD86 in response to BCG infection (Fig. 2). Therefore, the upregulation of the costimulatory molecules in response to BCG is TLR2 and TLR4 independent.
FIG. 2.
Expression of CD40 and CD86 in BCG-infected TLR2- and/or TLR4-deficient macrophages. CD40 (A) and CD86 (B) expression in TLR2- and/or TLR4-deficient macrophages after 18 h stimulation with LPS or BLP or after infection with BCG was evaluated. Unstimulated (thin line) and stimulated (thick line) cells were analyzed. The results are from cells pooled from two mice and are representative of three independent experiments.
Reduced internalization of BCG-GFP in TLR2-TLR4 doubly deficient mice.
To verify whether the different cytokine response of TLR2- and/or TLR4-deficient macrophages to BCG may be due to a defect of internalization of the bacteria, macrophages lacking TLR2 and/or TLR4 were incubated with BCG-GFP for 2 h, and the proportion of infected macrophages was analyzed by confocal microscopy. The absence of either TLR2 or TLR4 alone did not lead to a significant difference in the internalization of isolated BCG-GFP (Table 1). However, in the absence of both TLR2 and TLR4 there was a 36% decrease of the internalization of isolated BCG-GFP (Table 1).
TABLE 1.
Reduced internalization of GFP-BCG by TLR2-TLR4-deficient macrophagesa
| Mouse genotype | Infection index | Uptake inhibition (%) | P |
|---|---|---|---|
| TLR2+/+ TLR4+/+ | 4.91 ± 1.67 | 0 | |
| TLR2+/+ TLR4−/− | 4.46 ± 2.15 | 9.3 | NS |
| TLR2−/− TLR4+/+ | 4.87 ± 2.70 | 1.0 | NS |
| TLR2−/− TLR4−/− | 3.13 ± 1.29 | 36.4 | 0.01-0.02 |
Results are expressed as the infection index (percentage of cells containing one or two isolated bacilli) ± the standard deviation determined from 10 different observation fields for 1 slide (12 slides) that represents 5,200 macrophages observed per genotype. The percentage of inhibition of uptake is indicated. P = significance (C1 test of Fisher-Yates-Terry). P values of < 0.05 were considered to be statistically significant.
Therefore, TLRs are not only sensing pathogens but seem to convey a signal for the intracellular uptake of mycobacteria, although they might not be the major actor of internalization.
Based on the in vitro data, we hypothesized that a lack of cytokine production could induce a delay or default in BCG infection control. To verify this hypothesis, TLR2-deficient, TLR6-deficient, and TLR2-TLR4-deficient mice were infected with BCG.
Control of BCG infection in TLR2-deficient mice.
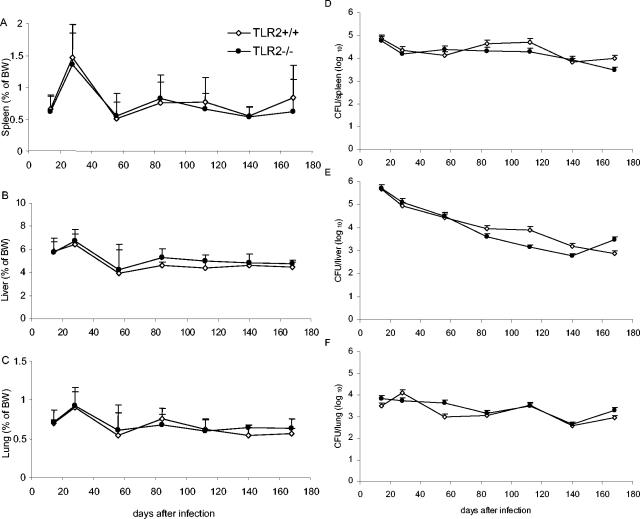
TLR2-deficient and wild-type control mice infected with live BCG (2 × 106 CFU per mouse, given i.v.) showed neither clinical signs nor body weight loss during the 6 months of the infection (not shown). The relative organ weights, recorded as an indicator of inflammation, followed the same trend in TLR2-deficient and wild-type mice, with a transient increase in spleen, liver, and lung weights 28 days postinfection, followed by resolution of the inflammation thereafter (Fig. 3A to C). Both TLR2-deficient and wild-type mice controlled the infection, as measured by the number of CFU recovered per organ at different points of the infection (Fig. 3D to F). In particular, the liver mycobacterial burden decreased with similar kinetics in both mice strains.
FIG. 3.
Control of BCG infection in TLR2-deficient mice. Spleen (A), liver (B), and lung (C) relative weights of TLR2-deficient (•) and control (⋄) mice infected with BCG (2 × 106 CFU/mouse, given i.v.) are expressed as the percentage of body weight (BW; mean + the SD). Viable bacteria were assessed by counting CFU in spleen (D), liver (E), and lung (F) homogenates. The data are expressed as mean CFU values per whole organ + the SD. (n = four to six mice per point; P > 0.05).
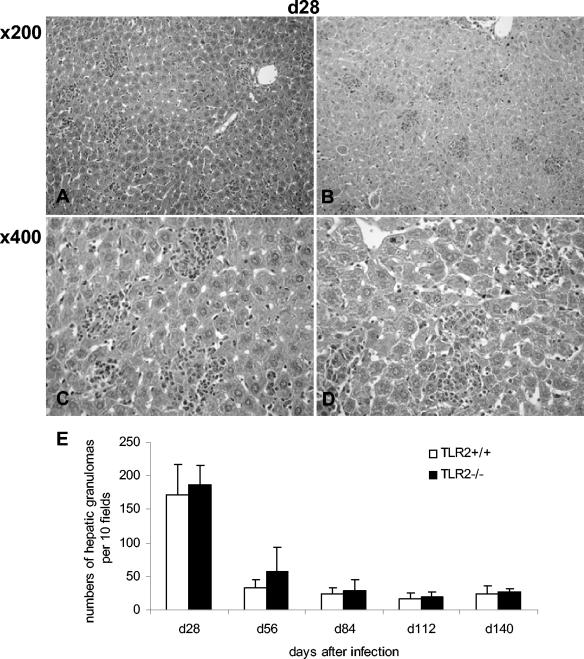
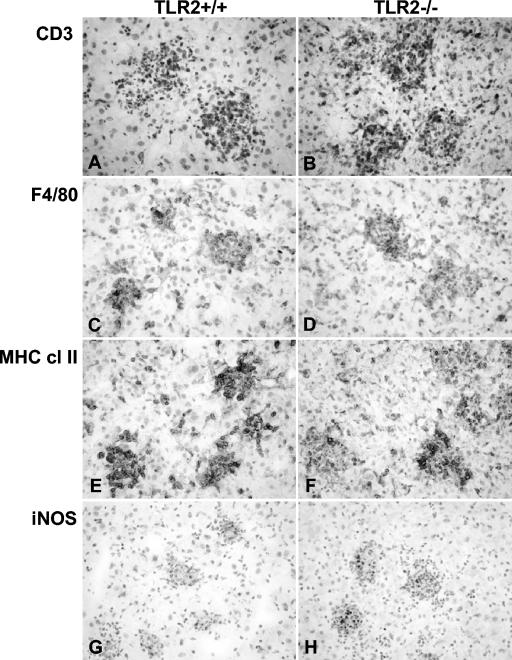
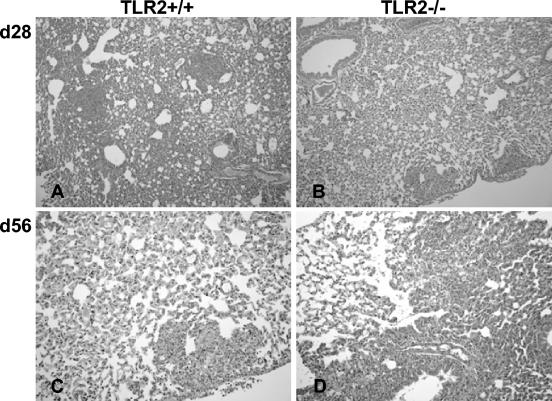
Histologically, the sizes, morphology, and numbers of liver granulomas were comparable in both groups (Fig. 4). The immunohistochemistry analysis revealed a similar recruitment of macrophages and granulocytic and lymphocytic cells to the liver granuloma, with equivalent expression of MHC class II antigens and iNOS (Fig. 5 and data not shown). However, there was a small delay in the peak of inflammation from days 28 to 56 in TLR2-deficient lungs (Fig. 6).
FIG. 4.
Normal hepatic granuloma formation in BCG-infected TLR2-deficient mice. Hepatic granulomas from control (A and C) and TLR2-deficient (B and D) mice 28 days after BCG infection (2.106 CFU/mouse, given i.v.) were examined at magnifications of ×200 and ×400. (E) A histogram compilation of the hepatic granuloma counts on day 28 to 140 postinfection is shown (mean + the SD, n = four to six mice; P > 0.05).
FIG. 5.
Cellular recruitment and activation in BCG-infected TLR2-deficient mice. Liver immunostaining was done for CD3 (A and B), F4/80 (C and D), MHC class II (E and F), and iNOS (G and H) samples at 28 days after BCG infection in control (A, C, E, and G) and TLR2-deficient (B, D, F and H) mice. Four mice per group were examined, and representative sections are shown. Magnification, ×400.
FIG. 6.
Delayed lung inflammation in BCG-infected TLR2-deficient mice. Lung sections from control (A and C) and TLR2-deficient (B and D) mice infected with BCG (2 × 106 CFU/mouse, given i.v.) were examined at 28 days (A and B; magnification, ×100) and 56 days (C and D; magnification, ×200) postinfection.
Therefore, in the absence of functional TLR2, BCG growth was controlled, and granuloma formation, T-cell and macrophage recruitment and activation, and iNOS expression were normal, although a small delay in lung inflammation was apparent in TLR2-deficient mice.
Controlled BCG infection in TLR6-deficient mice.
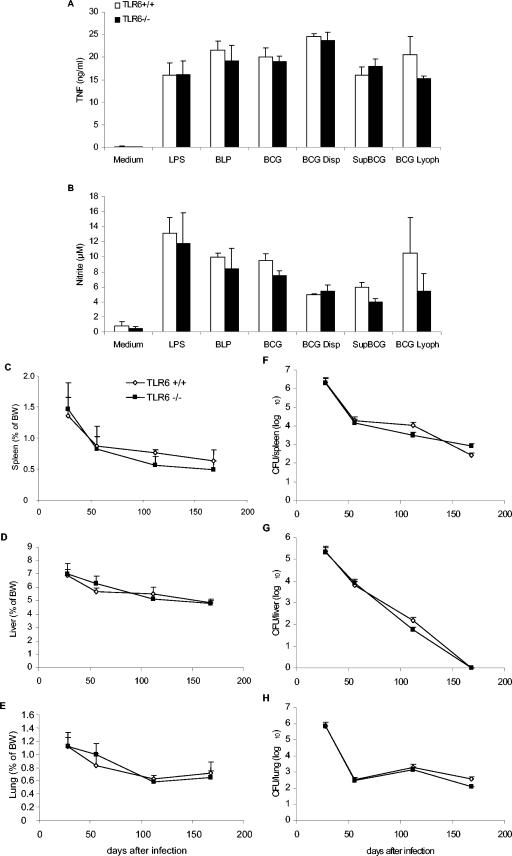
TLR6 has been reported to heterodimerize with TLR2 and to contribute to soluble tuberculosis factor recognition, as shown by inhibition with a dominant-negative TLR6 construct (4). We thus investigated the role of TLR6 in the recognition and response to BCG. TLR6-deficient macrophages expressed CD40 upon BCG infection (data not shown) and responded to stimulation by live BCG, lyophilized BCG, or BCG soluble antigens, with TNF and nitric oxide levels similar to those of wild-type macrophages (Fig. 7A and B). In vivo, TLR6-deficient mice controlled chronic M. bovis BCG infection (2 × 106 CFU per mouse, given i.v.) for the 8-month observation period. The relative organ weights of spleens, livers, and lungs as an indication of inflammation (Fig. 7C to E) and bacterial load (Fig. 7F to H) were similar in TLR6-deficient and wild-type mice. Histologically, there were no differences in the sizes, morphology, and numbers of liver granulomas or in the mycobacterial load of the granuloma, as revealed by Ziehl-Neelsen coloration (data not shown). Thus, the absence of TLR6 did not impair the responses to BCG either in vitro or in vivo.
FIG. 7.
Control of BCG infection in TLR6-deficient mice. Unimpaired TNF (A) and nitrite (B) production in TLR6-deficient macrophages stimulated with BCG or mycobacterial preparations as in Fig. 1. The results are means ± the SD from n = two mice and are from one representative experiment out of three independent experiments. Spleen (C), liver (D), and lung (E) relative weights of TLR6-deficient (▪) and control (⋄) mice infected with BCG (2 × 106 CFU/mouse, given i.v.) are expressed as the percentage of body weight (BW; mean ± the SD, n = four mice per point; P > 0.05). Viable bacteria were assessed by counting CFU in spleen (F), liver (G), and lung (H) homogenates. The data are expressed as mean CFU values per whole organ ± the SD (n = four mice per point; P > 0.05).
Normal T-cell priming and control of BCG infection in TLR2-TLR4-deficient mice.
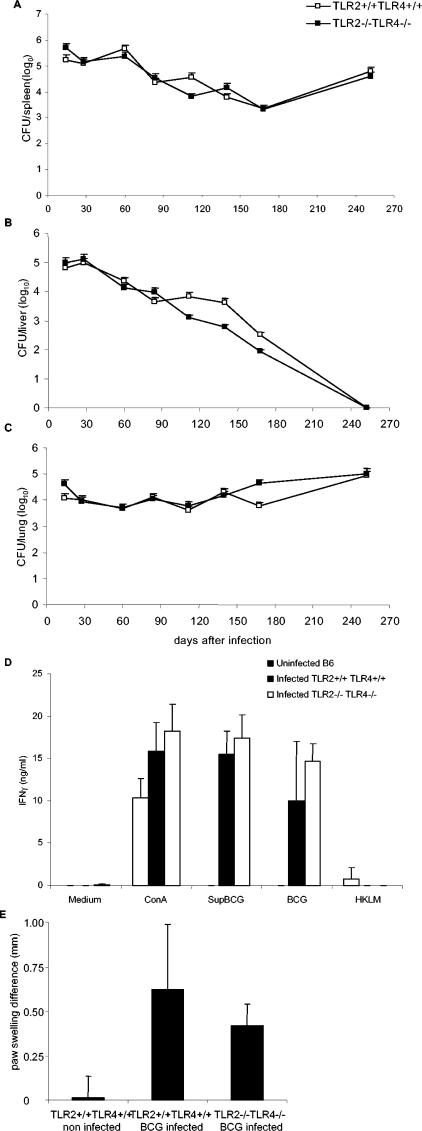
To assess the potential compensation between TLR2- and TLR4-mediated responses to mycobacterial infection, we next infected TLR2 and TLR4 doubly deficient mice with BCG (2 × 106 CFU per mouse, given i.v.). TLR2-TLR4-deficient mice survived the 8-month observation period with no apparent clinical signs, and they controlled the infection, as well as did the wild-type mice (Fig. 8A to C). The adaptive response of TLR2-TLR4-deficient mice was essentially normal on day 14 or 56 of infection, as seen with ex vivo restimulation of spleen cells with BCG or soluble BCG antigens (Fig. 8D). This response was specific since there was no response to unrelated heat-killed L. monocytogenes antigens. In addition, the DTH response of TLR2-TLR4-deficient mice was also normal 8 months after BCG infection, as measured by paw swelling after local injection of PPD (Fig. 8E). Therefore, there was no apparent defect in mounting and maintaining a specific adaptive response to BCG antigens in the absence of TLR2 and TLR4.
FIG. 8.
Control of BCG infection and T-cell priming in TLR2-TLR4-deficient mice. Viable bacterial load was assessed in spleen (A), liver (B), and lung (C) homogenates of TLR2-TLR4-deficient (▪) and control (□) mice infected with BCG (2 × 106 CFU/mouse, given i.v.). The data are expressed as mean CFU values per whole organ ± the SD (n = four mice per point; P > 0.05). (D) Splenocytes isolated from BCG-infected control and TLR2-TLR4-deficient mice 14 days after infection (2 × 106 CFU/mouse, given i.v.) or from uninfected control mice were restimulated in vitro in the presence of concanavalin A (ConA; 2.5 μg/ml), soluble BCG antigens (Sup BCG, 100 μg/ml), live BCG (two bacteria/cell), or an unrelated antigen, HKLM (100 bacteria/cell). IFN-γ production was quantified in the supernatants after 48 h of incubation. The results are means ± the SD (from n = four mice per genotype). (E) Cutaneous DTH response was performed 8 months after infection of TLR2-TLR4-deficient and control mice with BCG (2 × 106 CFU, given i.v.) by assessing the footpad swelling in response to PPD injection. Paw swelling is defined as the difference in thickness between the left paw injected with PPD and the right paw injected with saline. DTH responses of noninfected TLR2-TLR4 wild-type mice are shown as controls. The data are expressed as means ± the SD (n = 3).
DISCUSSION
TLRs are thought to play a critical role in the innate and adaptive response to mycobacteria (21). We first confirmed that TLR2 is essential in macrophage proinflammatory cytokine and nitrite production in response to M. bovis BCG particulate or soluble fractions, as shown for isolated antigens such as PIMs and LM (11, 30). LM turned out to be anti-inflammatory in the absence of TLR2 (30). However, the proinflammatory response to live M. bovis BCG and the expression of costimulatory molecules such as CD40 and CD86 was largely independent of TLR2 and TLR4.
Based on the in vitro data, we hypothesized that the inflammatory response to BCG antigens might be reduced in TLR2-deficient mice, and we investigated the net effect of the absence of TLR2 on the control of M. bovis BCG infection in vivo in TLR2-deficient mice. TLR2-deficient mice survived a 6-month infection period with M. bovis BCG, with controlled bacterial growth and normal granuloma formation, T-cell and macrophage recruitment and activation, and iNOS expression. Since TLR2 heterodimerizes with TLR6 and since TLR6 was reported to be involved in the recognition of soluble tuberculosis factor (4), we also investigated the role of TLR6 in the control of M. bovis infection in vivo. TLR6-deficient mice controlled chronic M. bovis infection for the 6-month observation period, in line with their capacity to control an acute aerosol infection by M. tuberculosis Kurono strain (33).
TLR2 has recently been reported to promote E. coli phagocytosis upon stimulation with specific ligands (5). We studied the role of TLR2 and TLR4 in BCG-GFP internalization and reported little effect from the absence of TLR2 or TLR4 separately. TLR2 and TLR4 might be redundant, and we show that the absence of both TLR2 and TLR4 yielded a significant reduction of internalization. Thus, TLRs seem to contribute to BCG internalization, although it is not clear whether this effect is direct or indirect. The physiological consequences of reduced mycobacterial phagocytosis are difficult to anticipate, especially in an environment where triggering of proinflammatory cytokines by mycobacterial antigens is impaired. We thus infected TLR2 and TLR4 doubly deficient mice with M. bovis BCG and report that they controlled the infection and survived the 8-month observation period. Therefore, the absence of either TLR2 or TLR6 alone or both TLR2 and TLR4 is dispensable to control a low-virulence M. bovis BCG infection which, however, kills TNF-deficient mice.
Interestingly, the adaptive response of TLR2- and/or TLR4-deficient mice was normal on day 56 of infection (data not shown). Since there seemed to be discrepancies with the reduced adaptive response found after 2 weeks of i.p. BCG infection (15), the early response of TLR2-TLR4-deficient mice on day 14 of infection was verified and found to be normal in terms of spleen cells restimulation with live BCG or soluble BCG antigens. Further, T-cell priming was efficient in TLR2-TLR4-deficient mice, as evidenced by positive cutaneous DTH reaction 8 months after i.v. BCG infection. Therefore, we document that recognition by TLR2 and TLR4 is not essential to mount a mycobacterial antigen-specific T-cell response.
The in vitro data indicate that soluble mycobacterial antigens, as well as killed BCG, signal mainly through TLR2, whereas live BCG triggers other pathways, which may be linked to the phagocytosis process. Mycobacteria such as M. tuberculosis or M. bovis BCG can bind to and enter macrophages through complement receptors, mannose receptors, surfactant protein A, and scavenger receptors (e.g., Sp-A) (reviewed in reference 7). In addition, DC-SIGN has recently been identified as the major M. tuberculosis receptor on DCs (34). Besides contributing to the entry of the bacilli, these receptors may influence the innate immune response and contribute to compensatory mechanisms during altered homeostasis. For instance, DC-SIGN and mannose receptor were reported to interfere with TLR signaling and to downmodulate the LPS-induced proinflammatory response after binding LAM (10, 28).
Expression of CD40 and CD86 after BCG stimulation was independent of TLR2 and TLR4, pointing to another receptor pathway other than TLRs being involved in costimulatory molecule expression. Likely candidates for alternative host defense mechanisms are cytosolic receptors of the Nod family involved in bacterial detection (12, 18). NOD1 and NOD2 stimulate inflammatory responses through peptidoglycan recognition, independently of the TLR pathways, although the two pathways might cooperate. In particular, Nod2 senses muramyl dipeptide MurNAc-l-Ala-d-isoGln, a well-known adjuvant for antigen-specific T-cell responses that induces both proinflammatory cytokines and costimulatory molecules expression. Alternatively, a collaborative induction of inflammatory responses has recently been reported for TLR2 and dectin-1, a C-type lectin expressed by DCs as a phagocytic receptor for β-glucan-containing particles such as zymosan (9). However, the implication of NODs or dectin-1 in mycobacterial responses or in compensatory mechanisms in a TLR-deficient environment remains still to be characterized.
In conclusion, although the immediate innate cytokine response to mycobacterial antigens by macrophages and DCs is strongly reduced in the absence of TLR2 alone or in the absence of both TLR2 and TLR4, antigen-presenting cells express costimulatory molecules and are competent to mount an adaptive response to BCG in vivo. This response, which is not sufficient to contain a long-term virulent infection by M. tuberculosis (1, 6, 31), suffices to control long-term infection by attenuated M. bovis BCG.
Acknowledgments
We thank S. Akira (Osaka, Japan) and C. Kirschning (Munich, Germany) for kindly providing the TLR-deficient mice used in this study.
Editor: F. C. Fang
REFERENCES
- 1.Abel, B., N. Thieblemont, V. J. Quesniaux, N. Brown, J. Mpagi, K. Miyake, F. Bihl, and B. Ryffel. 2002. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J. Immunol. 169:3155-3162. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 4.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167:987-994. [DOI] [PubMed] [Google Scholar]
- 5.Doyle, S. E., R. M. O'Connell, G. A. Miranda, S. A. Vaidya, E. K. Chow, P. T. Liu, S. Suzuki, N. Suzuki, R. L. Modlin, W. C. Yeh, T. F. Lane, and G. Cheng. 2004. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drennan, M. B., D. Nicolle, V. J. Quesniaux, M. Jacobs, N. Allie, J. Mpagi, C. Fremond, H. Wagner, C. Kirschning, and B. Ryffel. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 164:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, J. D. 1998. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fremond, C. M. C., D. M. M. Nicolle, D. S. Torres, V. F. J. Quesniaux. 2003. Control of Mycobacterium bovis BCG infection with increased inflammation in TLR4-deficient mice. Microbes Infect. 5:1070-1081. [DOI] [PubMed] [Google Scholar]
- 9.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilleron, M., V. F. Quesniaux, and G. Puzo. 2003. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette-Guerin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 278:29880-29889. [DOI] [PubMed] [Google Scholar]
- 12.Girardin, S. E., L. H. Travassos, M. Herve, D. Blanot, I. G. Boneca, D. J. Philpott, P. J. Sansonetti, and D. Mengin-Lecreulx. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:41702-41708. [DOI] [PubMed] [Google Scholar]
- 13.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 14.Heldwein, K. A., and M. J. Fenton. 2002. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 4:937-944. [DOI] [PubMed] [Google Scholar]
- 15.Heldwein, K. A., M. D. Liang, T. K. Andresen, K. E. Thomas, A. M. Marty, N. Cuesta, S. N. Vogel, and M. J. Fenton. 2003. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J. Leukoc. Biol. 74:277-286. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 17.Hubeau, C., M. Singer, M. Lagranderie, G. Marchal, and B. Vargaftig. 2003. Extended freeze-dried Mycobacterium bovis Bacillus Calmette-Guerin induces the release of interleukin-12 but not tumour necrosis factor-alpha by alveolar macrophages, both in vitro and in vivo. Clin. Exp. Allergy 33:386-393. [DOI] [PubMed] [Google Scholar]
- 18.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs, M., N. Brown, N. Allie, and B. Ryffel. 2000. Fatal Mycobacterium bovis BCG infection in TNF-LT-alpha-deficient mice. Clin. Immunol. 94:192-199. [DOI] [PubMed] [Google Scholar]
- 20.Jones, B. W., T. K. Means, K. A. Heldwein, M. A. Keen, P. J. Hill, J. T. Belisle, and M. J. Fenton. 2001. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 69:1036-1044. [PubMed] [Google Scholar]
- 21.Krutzik, S. R., and R. L. Modlin. 2004. The role of Toll-like receptors in combating mycobacteria. Semin. Immunol. 16:35-41. [DOI] [PubMed] [Google Scholar]
- 22.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 23.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 24.Means, T. K., B. W. Jones, A. B. Schromm, B. A. Shurtleff, J. A. Smith, J. Keane, D. T. Golenbock, S. N. Vogel, and M. J. Fenton. 2001. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J. Immunol. 166:4074-4082. [DOI] [PubMed] [Google Scholar]
- 25.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 26.Michelsen, K. S., A. Aicher, M. Mohaupt, T. Hartung, S. Dimmeler, C. J. Kirschning, and R. R. Schumann. 2001. The role of Toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCs): peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 27.Muller, M., H. P. Eugster, M. Le Hir, A. Shakhov, F. Di Padova, C. Maurer, V. F. Quesniaux, and B. Ryffel. 1996. Correction or transfer of immunodeficiency due to TNF-LT alpha deletion by bone marrow transplantation. Mol. Med. 2:247-255. [PMC free article] [PubMed] [Google Scholar]
- 28.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477-7485. [DOI] [PubMed] [Google Scholar]
- 29.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 30.Quesniaux, V. J., D. M. Nicolle, D. Torres, L. Kremer, Y. Guerardel, J. Nigou, G. Puzo, F. Erard, and B. Ryffel. 2004. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172:4425-4434. [DOI] [PubMed] [Google Scholar]
- 31.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 32.Stenger, S., and R. L. Modlin. 2002. Control of Mycobacterium tuberculosis through mammalian Toll-like receptors. Curr. Opin. Immunol. 14:452-457. [DOI] [PubMed] [Google Scholar]
- 33.Sugawara, I., H. Yamada, C. Li, S. Mizuno, O. Takeuchi, and S. Akira. 2003. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol. Immunol. 47:327-336. [DOI] [PubMed] [Google Scholar]
- 34.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 37.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of Toll-like receptors. Infect. Immun. 68:6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]