Abstract
Background
Immune system dysfunction is implicated in the pathophysiology of major depression, and is hypothesized to normalize with successful treatment. We aimed to investigate immune dysfunction in melancholic depression and its response to ECT.
Methods
55 melancholic depressed patients and 26 controls participated. 33 patients (60%) were referred for ECT. Blood samples were taken at baseline, one hour after the first ECT session, and 48 hours after ECT series completion.
Results
At baseline, melancholic depressed patients had significantly higher levels of the pro-inflammatory cytokine IL-6, and lower levels of the regulatory cytokine TGF-β than controls. A significant surge in IL-6 levels was observed one hour after the first ECT session, but neither IL-6 nor TGF-β levels normalized after completion of ECT series. Seventy per cent (n=23) of ECT recipients showed clinical response and 42% (n=10) reached remission. Neither IL-6 nor TGF-β changes correlated with clinical improvement following ECT. No significant changes in IL-10, TNF-α and CRP levels were found in relation to melancholia or response to ECT.
Limitations
As a naturalistic study, some potential confounders could not be eliminated or controlled, including medication use.
Conclusions
Melancholic depressed patients demonstrated a peripheral increase in IL-6 and reduction in TGF-β, which did not normalize despite clinical response to ECT. These findings may be consistent with emerging hypotheses of the role of inflammation in mediating neurotropin expression. The implications of chronic inflammation in the melancholic depressed population for future medical health, particularly cardiovascular risk, are largely unknown and warrant further investigation.
Keywords: depression, melancholic subtype, psychoneuroimmunology, cytokines, electroconvulsive therapy
INTRODUCTION
The Cytokine Theory of Depression
The cytokine theory of depression postulates that major depressive disorder (MDD) results from enhanced production of peripheral proinflammatory cytokines which, acting as neuromodulators, represent the key factor in the (central) mediation of the behavioral, neuroendocrine and neurochemical features of depression (Schiepers et al., 2005).
Significant empirical support exists for a link between inflammation and depression, including: sickness behaviour, a syndrome that mimics several symptoms of depression following infection (Dantzer and Kelley, 2007; Dantzer et al., 2008; Goshen et al., 2008); development of a selective serotonin re-uptake inhibitor (SSRI)-responsive depression following cytokine immunotherapy (Capuron et al., 2002; Capuron et al., 2009); increased rates of depression in populations with inflammatory conditions (Gold and Irwin, 2009; Kurd et al., 2010; Lichtman et al., 2008; Uguz et al., 2009); the association of immune dysregulation with psychological stressors that often precede episodes of depression (Carroll et al., 2011; Cole et al., 2007; Danese et al., 2009; Miller and Cole, 2012); the interaction of cytokines with the pathophysiological processes of depression (Miller et al., 2009); and the observation that antidepressants can have immunomodulating properties (Guloksuz et al, 2014). The evidence that a significant subset of depressed persons demonstrate upregulation of inflammatory markers also supports the complex, and likely bidirectional, relationship between depression and inflammation (Howren et al., 2009).
The most consistently replicated findings are for interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and the acute phase protein C-reactive protein (CRP) (Dowlati et al., 2010; Howren et al., 2009; Raison et al., 2006). Studies have shown evidence for increased proinflammatory to anti-inflammatory ratios in depressed populations (Dhabhar et al., 2009; Song et al., 2007). Peripheral pro-inflammatory cytokines are known from animal models to affect glial cell function, neurotrophic systems, and result in decreased neurogenesis, via free radical and glucocorticoid production (Song and Wang, 2011). However, the emerging evidence base on the association between depression and inflammation is complicated by numerous contradictory and negative studies coupled with wide variability in the reported levels of inflammation (Glassman & Miller, 2007). These conflicting study findings may partly be accounted for by the heterogeneity of the patient populations fulfilling criteria for diagnosis of MDD. It has been noted that increased consistency and specificity may be achieved by differentiating between clinical subtypes of depression (Baune et al., 2012; Lamers et al., 2013).
Melancholic Depression as a distinct subtype of Major Depressive Disorder
In DSM-5, melancholic depression is a subtype characterized by profound anhedonia, a distinct quality of depressed mood, diurnal mood variation, insomnia with early morning wakening, excessive guilt, significant anorexia or weight loss, and psychomotor retardation or agitation (American Psychiatric Association (APA), 2013). The proposed concept of melancholia as a biologically distinct subtype is supported by studies showing that it is more frequently associated with higher plasma noradrenaline levels (Gold et al., 2005; Kelly and Cooper, 1997; 1998); hypercortisolaemia, as reflected in non-suppression of the dexamethasone suppression test (Shorter and Fink, 2010; Taylor and Fink, 2006); characteristic disturbances of the sleep architecture in electroencephalogram profiles (Armitage, 2007); and psychomotor disturbance, as measured by the CORE scale (Parker & Hadzi-Pavlovic, 1996). Melancholia has historically been considered to be predictive of a positive response to electroconvulsive therapy (ECT) and recent studies support this hypothesis (Fink et al., 2007; Hickie et al., 1996; Parker et al., 2001; Rasmussen, 2011).
Although some subtypes of depression have been linked with elevated inflammation (e.g., O’Donovan et al., 2013; Lanquillon et al., 2000; Raison et al., 2013), the field of psychoneuroimmunology has thus far offered only a few studies into differences in immune function in melancholic versus non-melancholic depression. One study found that cytokine production was reduced in acutely depressed melancholic patients, and normalized after treatment; whereas no change in cytokine production was observed in non-melancholic patients (Rothermundt et al., 2001b). Another study demonstrated activation of the hypothalamic-pituitary-adrenal (HPA) axis in melancholic patients, with partial normalization upon remission, but failed to demonstrate activation of the cytokine system. (Kaestner et al., 2005). A positive association between serum levels of IL-6 and brain-derived neurotrophic factor (BDNF) was recently found in depressed patients, with IL-6 being a robust predictor of BDNF only in the melancholic depressed subgroup (Patas et al, 2014).
Immunological effects of ECT
There is strong evidence that ECT is an effective treatment for severe depression (Kennedy et al., 2009; UK ECT Review Group 2003). However, since ECT was first introduced into psychiatric clinical practice in 1938 (Shorter and Healy, 2007), no consensus has yet emerged regarding its mechanism of action. The prevailing hypothesis is that ECT causes an alteration in the post-synaptic response to central nervous system (CNS) neurotransmitters; while promising animal research shows ECT can enhance hippocampal neurogenesis (Madsen et al., 2000; Perera et al., 2007). Successful ECT treatment has been shown to correlate with normalisation of HPA axis dysregulation (Bolwig, 2011). However, the immunological effects of ECT have received only limited research attention to date in small study samples. A recent review notes that ECT induces a transient immune activation, while repeated ECT may down-regulate immune activation, but this evidence is based on small studies (Guloksuz et al, 2014).
The first study exploring the effect of ECT on plasma cytokines demonstrated a dramatic increase in IL-6 ten minutes after seizure induction (Kronfol et al., 1990). This was replicated by a later study, which also showed an acute increase in IL-1β for up to 6 hours after ECT (Lehtimaki et al., 2008). Other acute effects of ECT include an increase in NK cell activity (Albrecht et al, 1985), IL-6 activity (Kronfol et al, 1990), leucocytes, IL-6, IL-10, and TNF-α (Fluitman et al, 2011).
Immunological effects following a course of ECT include increased numbers of activated lymphocytes (Fischler et al, 1992), reductions in pro-inflammatory cytokines IL-5 and eotaxin-3 (Rotter et al, 2013); and reductions in TNF-α, levels of which were shown to decrease in severely depressed patients along with clinical improvement with ECT, to become comparable to healthy controls by the end of the study (Hestad et al., 2003). Acute decreases in the absolute number of total blood lymphocytes and T8+ and Leu11+ cell subsets one hour after a single ECT session have also been shown in ten MDD patients (Fischler et al., 1992). In a separate study of ten MDD patients, baseline low levels, hypofunctionality and poor immunoreactivity of leukocyte G proteins normalized with ECT, and this normalization of G protein measures preceded, and thus predicted, clinical improvement (Avissar et al., 1998). The existing study findings, although limited, indicate that ECT can act as an immunomodulatory agent by altering levels of cytokines and other immune markers most likely in the CNS, which are measurable in peripheral plasma.
Animal studies of electroconvulsive stimuli have shown an increase in brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brains of rats, notably in hippocampal and frontal areas (Angelucci et al, 2002); although other researchers have not demonstrated an increase in serum BDNF following electroconvulsive series in rats (Kyeremanteng et al, 2012). Monocyte secretion of BDNF has been demonstrated to be enhanced by IL-6 and TNF-α (Schulte-Herbrüggen et al, 2005); and positive association between IL-6 and BDNF has been found in clinical patients (Patas et al, 2014). Van Buel et al (2015) in a recent review of the literature, note the bidirectional influence of immune system activation and neurotrophins; and postulate that the acute increases in cytokines following ECT (such as IL-6), stimulate neurotrophin release, thus leading to hippocampal neurogenesis and clinical response.
Study Aims and Hypotheses
The aim of our study was to investigate differences in inflammatory markers between patients with melancholic major depression and healthy controls, and further to investigate the effect of ECT on the cytokine profiles of melancholic patients. Levels of the proinflammatory cytokines IL-6 and TNF-α, the anti-inflammatory cytokine IL-10, the regulatory cytokine TGF-β, and the acute phase protein CRP were measured in hospital in-patients with melancholic MDD and healthy controls. We hypothesized that cytokine markers would differentiate melancholic from healthy populations, with higher levels of proinflammatory markers and lower anti-inflammatory markers in the melancholic group. We also hypothesized that melancholic patients treated with ECT would show acute changes in cytokine variables, particularly IL-6, within 1-hour of their first ECT session and at 48-hours after completion of the ECT treatment series. Lastly, we predicted that melancholic patients’ clinical presentation would correlate with cytokine levels after ECT series completion, in keeping with the cytokine theory of depression, which relates a reduction in proinflammatory cytokines to improvement in mood.
METHODS
PARTICIPANTS
The study sample consisted of 55 hospital in-patients with a primary diagnosis of melancholic depression and 26 healthy age- and sex-matched controls. In-patients with melancholic depression were recruited from two large urban hospitals, St. Patrick’s University Hospital and St. Vincent’s University Hospital, in Dublin, Ireland. The control sample was recruited through local advertisements. All participants underwent a comprehensive medical history, psychiatric assessment (MINI and HAM-D), physical examination and routine blood testing. Neither patients nor controls had any active alcohol or substance misuse disorders, and patients were not taking regular anti-inflammatory medications. Exclusion criteria included the presence of a chronic illness, an acute infection or allergy, however obesity was not an exclusion criterion. Patients without a primary diagnosis of the melancholic subtype of MDD, patients with an involuntary status, or patients who had received ECT in the three months preceding recruitment were also excluded. All controls lacked any psychiatric history and did not take any psychotropic medications. All patients were prescribed psychotropic medications. The institutional committees at each of the hospitals where data were collected approved the study, and written informed consent was obtained from all participants.
MEASURES
Psychiatric Diagnosis
The diagnosis of the melancholic subtype was confirmed using the Mini International Neuropsychiatric Instrument 5.0.0 (MINI). This is a structured interview for the assessment of psychiatric symptoms and disorders based on the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV-TR). A validation study comparing the MINI with the Structured Clinical Interview for DSM-IV yielded a high diagnostic concordance for MDD (Sheehan, 1998). Either feature 1 or 2 in the DSM-IV Criteria A and three (or more) features from Criteria B must be present to qualify for melancholic features (APA, 2000). Two trained interviewers conducted all interviews.
Depression Severity, Response, Remission
Severity of depression was assessed with the Hamilton Rating Scale for Depression (HAM-D), a structured interview that assesses the severity of 17 depressive symptoms during the past week and can be used as a guide to evaluate recovery over time (Hamilton, 1960). Response was defined as a decrease from baseline of at least 50% on the HAM-D17 total score. Remission was defined as a HAM-D17 total score of ≤ 7. The same trained and calibrated interviewers who conducted the MINI administered the HAM-D.
Demographic and Clinical Information
Demographic and clinical information was collected at the time of the initial structured interview through self-report and was validated by medical chart review when necessary and possible. Patients reported on the duration of their present episode, number of prior episodes of depression, number of past episodes of deliberate self-harm, and the presence or absence of a family history of depression.
Inflammatory Markers
Samples were collected in 5 ml EDTA coated tubes (BD Vacutainer, Franklin Lakes, NJ). High-sensitivity enzyme-linked immunosorbent assays (ELISAs) were used to quantify plasma levels of IL-6, IL-10, TNF-α (R&D Systems, Minneapolis, MN) and TFG-β (BD Biosciences, Franklin Lakes, NJ). High sensitivity CRP analyses were conducted by means of a particle-enhanced immunonephlebotometry assay using CardioPhase hsCRP reagents on the BN System (Dade Behring, Deerfield, IL). The detection range for IL-6 was 0.016–0.110 pg/ml. The intra-assay coefficient of variation was 7.8% at the 2.45 pg/ml level and the inter-assay coefficient of variation was 7.2% at the 2.78 pg/ml level. The lower limit of detection for IL-10 was 0.5 pg/ml. The intra-assay coefficient was 8.5% at 17.7 pg/ml and the inter-assay coefficient was 10.2% at 6.28 pg/ml. The detection range for TNF-α was 0.038–0.191 pg/ml with an intra-assay coefficient of 4.3% at 11.5 pg/ml and an inter-assay coefficient of 7.3% at 10.5 pg/ml. The lower limit of detection for TGF-β was <15.6 pg/ml. The intra-assay coefficient was 5.7% at 577.66 pg/ml and the inter-assay coefficient was 6.6% at 564.1 pg/ml. The lower limit of detection for hsCRP was 0.16 mg/l. All samples were assayed in duplicate.
PROCEDURES
Participant Progress through Study
Of the 55 melancholic depressed patients, 33 were referred for ECT, 22 were not. The decision to refer for ECT was made by the patients’ treating psychiatrists, who had no involvement with the present study. The 33 ECT recipients were re-administered the HAM-D within one week of ECT series completion.
Blood Collection Protocol
Baseline fasting blood samples for inflammatory markers were collected from the control group at 8am, on the morning of the interviews. Blood was collected from the 33 ECT treated patients at three separate time points: (1) 8am on the morning of the first ECT session (fasting baseline sample); (2) 1 hour after the first session of ECT; and (3) 48 hours after ECT series completion at 8am (fasting).
ECT Procedure
Electroconvulsive therapy was performed according to the standardized policy at each of the hospitals. All patients received bilateral treatment twice weekly, as per department protocol, using the MECTA spECTrum 5000Q® machine. The patients’ treating consultant psychiatrists decided the number of sessions of ECT.
Details of other electroconvulsive therapy parameters (e.g. electrical current, stimulus duration and frequency, number of sessions of ECT, etc.) were not recorded in the present study.
DATA ANALYSIS
Statistical power was calculated using G*Power 3 (Erdfelder et al., 1996). A sample size of 26 was generated for each arm of the study to compare the healthy and depressed populations, based on a previous study in the literature investigating the effects of a course of ECT on TNF-α (Hestad et al, 2003). Tests of normality of the distribution of the data were conducted. Independent samples t-tests were used to compare means between samples for all demographic variables and HAM-D scores. Due to non-normal distribution for all inflammatory markers under study (IL-6, IL-10, TNF-α, TGF-β, and CRP), a nonparametric test procedure was employed. The Mann-Whitney U test for unpaired data was used for comparisons between the medians of groups of individuals in relation to each inflammatory marker. In the paired situation, Friedman’s test was used a priori, followed by Wilcoxon Signed Ranks test to compare the levels of inflammatory markers (i.e. 1 hour post-session 1 of ECT and 48 hours post-ECT series completion) with the baseline levels (i.e. before the first ECT session) over the three time points. The Pearson product-moment correlation coefficient was performed to examine the relationship between HAM-D scores and each inflammatory marker level before and after ECT. The SPSS software (version 18) was used for all statistical analysis (SPSS, Inc.) and the significance level was set at P < .05.
RESULTS
Sample Characteristics
Control vs Melancholic groups
The control (N = 26) and melancholic major depression (N = 55) groups were compared on a number of demographic variables. There were no significant differences between the two groups in relation to age (t = .135, P = .893), gender (t = .064, P = .949) or the presence of a positive family history of psychiatric illness (t = −1.042, P = .301). There was a significant difference in HAM-D scores between controls (M = 2.4, SD = 2.4) and the melancholic (M = 31, SD = 9) groups (t = −22.114, P = .000*).
Comparison of Cytokine Profiles in Melancholic and Healthy Samples
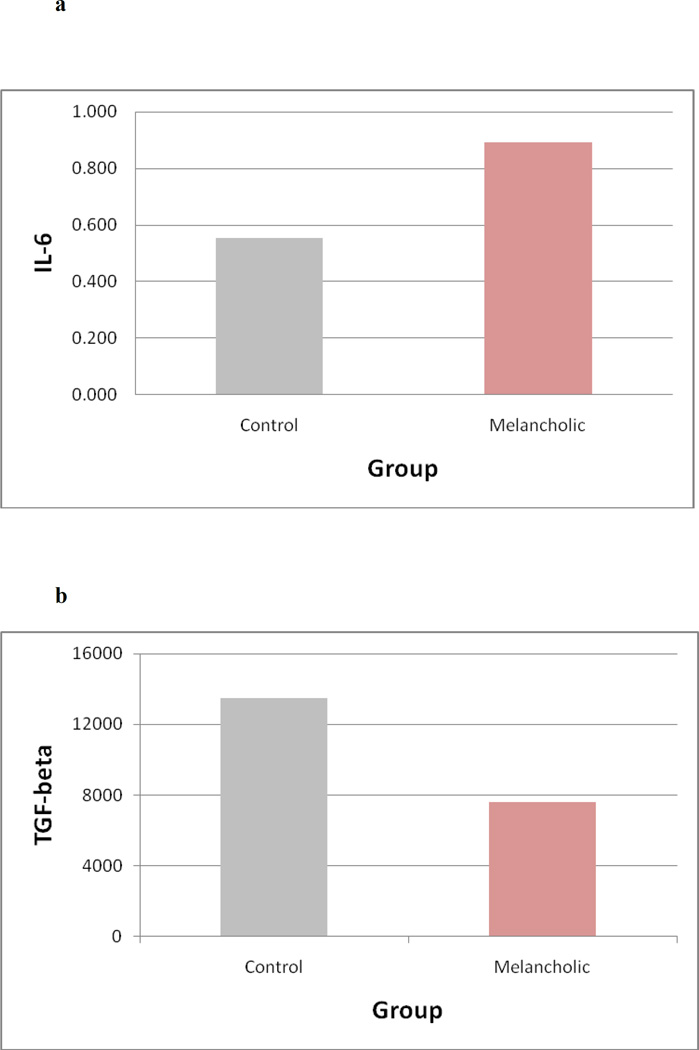
Basal levels of cytokines were compared between the control and melancholic groups. IL-6 levels were significantly higher (U = 452, Z = −2.661, P = 0.008) in patients with melancholic MDD compared to healthy controls (Fig. 1a). Levels of the regulatory cytokine TGF-b were significantly lower (U=324, Z= −2.064, P=0.039) in melancholic patients compared to healthy controls at baseline (Fig. 1b). No significant differences were detected between the groups for the cytokines IL-10 (U=245, Z=−1.448, P=0.148), TNF-α (U=310, Z= −0.00, P=1.00) or the acute phase protein CRP (U=209, Z= −.163, P=0.87).
Figure 1.
(A – B). Figures show median levels of the inflammatory markers interleukin-6 (IL-6) (1A) and transforming growth factor-beta (TGF-β) (1B) in controls compared to patients with melancholic major depression.
Patients with melancholic major depression had significantly higher levels of IL-6 than controls (U = 452, Z = −2.661, P = 0.008) and significantly lower levels of TGF-β than controls (U=324, Z= −2.064, P=0.039).
Characteristics of Melancholic patients referred to ECT vs Melancholic patients not referred to ECT
Of the 55 melancholic patients, 33 were referred to ECT and 22 were not. The two groups did not differ significantly in any of the cytokine variables tested. Those referred on for ECT did have higher HAM-D scores (34.2 vs 26.7 mean scores; p=0.002) and a longer duration of depression (M=352.23 days, SD=507.22; vs M=43.83, SD=88.17; p=0.004).
The Effect of ECT on Cytokine Levels
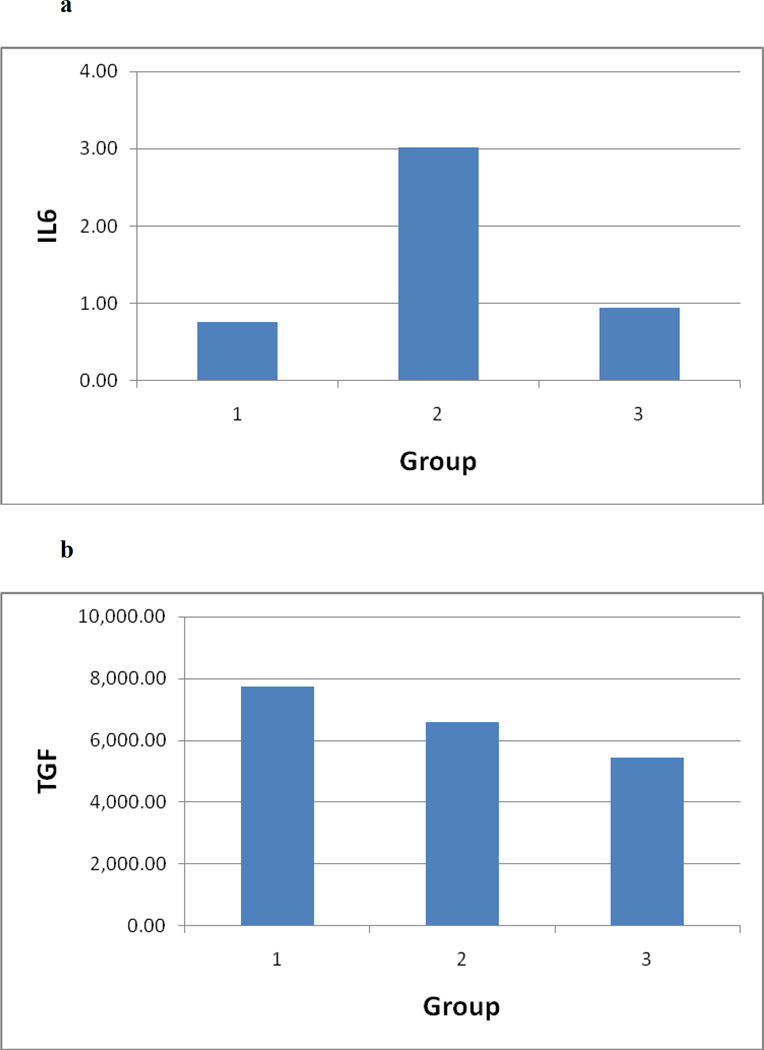
A significant acute surge in IL-6 levels occurred between baseline and one hour after the first ECT session (Z = −4.107, assym sig = .000). A significant decline in IL-6 ensued between 1 hour after the first ECT session and 48 hours after ECT series completion (Z = −3.632, assym sig = .000). However, no significant difference was found between IL-6 levels at baseline and after ECT series completion (Z = −.545, asymp sig = .586) (Fig. 2a). TGF-β levels did not normalize between baseline and ECT series completion and remained lowered (Z = −.229, P = .819). Across the three time points, there were no significant changes in TGF-β levels (χ2(2) = .118, P = 0.94, Friedman test) (Fig. 2b).
Figure 2.
(A – B). Figures show levels of IL-6 (2A) and TGF-β (2B) over three time periods: Time 1 = baseline (pre-ECT commencement); Time 2 = 1 hour following seizure induction of session 1 of ECT; Time 3 = 48 hours following ECT series completion.
A significant rise in IL-6 occurred between time 1 and 2 (Z = −4.107, assym sig = .000) and a significant decrease occurred between time 2 and 3 (Z = −3.632, assym sig = .000), but no change occurred between time 1 and 3 (Z = −.545, assym sig = .586). No significant changes occurred in TGF-β over the three time points (χ2(2) = .118, P = .0943, Friedman test).
No significant difference was found between baseline and post-ECT series completion in the plasma levels of IL-10 (Z = −.019, P = .985), TNF-α (Z = −.545, p=.586) and CRP (Z = −.307, P = .759). Furthermore, across the three time points under study, no significant changes occurred in the levels of IL-10 (χ2(2) = .23, P = .891, Friedman test), TNF-α (χ2(2) = 3.583 P = .167) and CRP (χ2(2) = .562, P = .755).
Inflammatory Variables and HAM-D Scores
In the ECT treatment group, 70% (N = 23) responded and, of these, 42% (N = 10) achieved remission. Changes in levels of IL-6 and TGF- β were compared to changes in HAM-D scores pre and post ECT series completion. No statistically significant correlation was found between the changes in levels of IL-6 (Spearman’s r=0.15, n=33, P=0.42) or TGF–β (r=0.252, n=33, P=0.16) and change in the HAM-D scores. The lack of correlation held true for the all other inflammatory variables: TNF-α (r=0.605, n=33, P=0.093), IL-10 (r=0.137, n=33, P=0.447) and CRP (r= −0.124, n=33, P=0.491).
DISCUSSION
This study provides evidence for a peripheral increase in the levels of the proinflammatory cytokine IL-6, and a reduction in the regulatory cytokine TGF-β, in patients with melancholic major depression compared to healthy controls. Although plasma levels of IL-6 increased markedly in response to the first application of ECT; plasma levels of IL-6 and TGF-β did not normalize by ECT series completion. The ECT response rate of 70% in our study compares favorably with reported response rates of 50–60% in a treatment-resistant population (Prudic et al., 1996), while higher rates of 80–90% have been reported with ECT as a first-line treatment (Petrides et al., 2001). The present study showed clinical response to ECT did not correlate with changes in IL-6 or TGF-β levels, indicating that normalization of cytokine levels is not necessary for a positive treatment response. The study failed to detect any significant differences between melancholic and control groups in the basal levels of the cytokine variables IL-10 and TNF-α, or the acute-phase protein CRP. Similarly, no changes in these three variables, nor in TGF-β, were observed following the first seizure induction or completion of the ECT series.
Acute increases in IL-6
ECT resulted in an acute rise in IL-6 following the first seizure induction, in line with previous studies (Kronfol et al., 1990; Lehtimaki et al., 2008). A short-term rise in pro-inflammatory cytokines has been demonstrated after every ECT application in a series of ECT treatment (Fluitman, 2011). Lehtimaki et al. (2008) followed 9 patients for up to 24 hours after each ECT treatment and found that plasma levels of IL-6 were significantly increased at the 3- to 6-hour time points compared with basal levels, and this was highly correlated with the stimulus dose used, implying that neuronal depolarization was the mechanism inducing cytokine release. As IL-1 and IL-6 stimulate the production of neurotropic factors, it has been postulated that increases in both may initiate a cascade of growth factors, resulting in plasticity in neuronal circuits related to depression, thereby potentially explaining the therapeutic effects of ECT (Lehtimaki et al., 2008). This hypothesis was developed further by van Buel et al (2015) in a review of the evidence for bidirectional interaction between the immunological and neurotropic systems. As peripheral BDNF release is stimulated by IL-6 and TNF-α, and ECT is linked to central release of BDNF; van Buel et al (2015) suggest that a causative link may exist between immunological activation and eventual clinical response, mediated by BDNF.
Non-normalisation of IL-6 despite Clinical Response
Increased plasma levels of IL-6 in depressed patients have been demonstrated in several studies (Lehtimaki et al., 2008; Maes et al., 1997; Penninx et al., 2003; Sluzewska et al., 1996), including a robust meta-analysis (Dowlati et al., 2010). Although study results are conflicting, normalization has generally been considered necessary for a treatment response to depression. Previous studies of antidepressant therapy in MDD found that elevated levels of IL-6 normalized or significantly reduced following successful antidepressant treatment (Basterzi et al., 2005; Frommberger et al., 1997; Hiles et al., 2012; Lanquillon et al., 2000; Sluzewska et al., 1995). In contrast, IL-6 levels remained significantly elevated in partial and non-responder subgroups in some studies (Sluzewska et al., 1995; Lanquillon et al., 2000). The hypothesis that ECT would result in normalization of IL-6 levels, or at least a significant reduction, was not supported in our study despite a high ECT response rate. It is possible that repeated measurements one week post ECT series completion may have shown more of a trend towards normalisation (in keeping with the findings of Hestad et al, 2003); however the fact that clinical response had occurred by this point implies that any further change in cytokine levels would not have been causative of clinical effect.
One interpretation of this finding is that abnormalities of proinflammatory cytokines may not be central to the pathophysiological processes of melancholic depression, but that they may constitute secondary epiphenomena; and may account for medical co-morbidities in depressed populations. Elevated IL-6 levels are associated with an increased risk of cardiovascular events, with stronger evidence existing for elevated CRP levels (Ridker et al., 2000; Frasure-Smith et al, 2007; Haverkate et al, 1997; Whooley et al, 2007; Danesh et al., 2004; 2008). A prospective study showed that elevated levels of CRP and IL-6 predict the development of type 2 diabetes mellitus (Pradhan et al., 2001). These data support a possible role for inflammation in atherogenesis and diabetogenesis. Given that the highly efficacious treatment of ECT failed to significantly reduce IL-6 levels, this may suggest that depression is associated with systemic pathology and that the mood component is but one manifestation of a broader, more complex psychoneuroimmunological disturbance.
Another interpretation is that normalisation of cytokine levels may not be necessary for clinical response to ECT. Emerging evidence of the relationship between immunoactivation and neurotropins, has led to a hypothesis that raised inflammatory markers in depressed patients may be secondary to low levels of neurotropins; and that activation of the innate immune system after each ECT session stimulates release of neurotropins, leading to eventual clinical response and then resolution of inflammation (van Buel et al, 2015). If this hypothesis is correct, clinical response would be predicted to occur prior to normalisation of cytokine levels, as occurred in the present study. However, as our study was not designed to repeat cytokine levels beyond 48 hours post ECT series completion, we cannot determine if normalisation of IL-6 did indeed occur at a later point.
Implications of low levels of TGF-β
TGF-β is a regulatory cytokine (Th3) that is thought to maintain the Th1 (proinflammatory) and Th2 (anti-inflammatory) balance, and plays an important role in oral tolerance (Blobe et al., 2000; Weiner, 2000). In our study, basal TGF-β levels were lowered in melancholic depression and did not change in response to ECT treatment. Other studies also found lowered basal TGF-β levels in depressed patients, but the levels increased significantly after 8 weeks of treatment with an antidepressant (Myint et al., 2005; Sutcigil et al., 2007). Myint et al. (2005) showed that the Th1/Th3 ratio was higher in depressed patients and that TGF-β levels were inversely correlated with HAM-D scores, proposing that antidepressants react through the regulatory effect of TGF-β on the Th1 and Th2 balance. In comparison, Kim et al. (2007) demonstrated an increase in basal TGF-β levels that reduced with antidepressant treatment, while Lee et al. (2006) uncovered no difference between levels in control and depressed samples, but did detect a significant increase in TGF-β levels with antidepressant treatment. Clearly, the precise role of TGF-β in depression and treatment response warrants further clarification. Increased TGF-β production correlates with the resolution of inflammatory responses, particularly in organ specific autoimmune diseases (Prud’homme and Piccirillo, 2000). TGF-β also has a crucial regulatory function in cell cycles (Blobe et al., 2000), and is thought to be neuroprotective in ischaemic lesions (Ruocco, 1999; Vivien and Ali, 2006;) and multiple other processes through its anti-inflammatory, -apoptotic, and –excitotoxic actions (Dobolyi et al., 2012). In the present study, patients demonstrated reduced TGF-β levels pre- as well as post-ECT, which could potentially lead to medical co-morbidity.
Roles of IL-10, TNF-α and CRP
The present study did not find a role for the anti-inflammatory and immunoregulatory cytokine IL-10 in relation to melancholia or response to ECT. The data on IL-10 changes in MDD is varied: studies have found both increased (Simon et al., 2008) and decreased (Dhabhar et al., 2009) levels compared to controls, while others have found no group differences (Huang and Lee, 2007; O’Brien et al., 2007). Consistent with the cytokine theory of depression, studies report increased levels of TNF-α in MDD, with a reduction upon recovery (Raison et al., 2006; Miller et al., 2009; Dowlati et al., 2010). Our study failed to find any differences in TNF-α levels between healthy and depressed subjects in keeping with a small study of ten elderly depressed females (Brambilla and Maggioni, 2007). Furthermore, our finding that TNF-α levels did not change in response to ECT contrasted to the findings of Hestad et al (2003), where TNF-α was markedly raised in depressed patients compared to controls, and that clinical improvement with ECT correlated with a decline in TNF-α levels, with levels comparable to controls by the study’s end (Hestad et al., 2003). Finally, the present study did not detect any significant changes in CRP related to MDD or ECT, in keeping with one previous study (Giltay et al., 2008), but in contrast with several other reports of increased positive acute phase proteins such as CRP in patients with active and remitted MDD (Howren et al., 2009; Kling et al., 2007; O’Brien et al., 2006).
LIMITATIONS
The present study has several limitations, mainly relating to its naturalistic design. The effect of the various ECT parameters - including the number of sessions, electrical dosage, electrode placement, and the duration of the seizure - was not explored in relation to cytokine change. The study sample was relatively small, and cytokine measurements were only performed after the first and last ECT treatments. Patients were not taken off antidepressants or other psychotropic medications prior to ECT, which may have influenced levels of cytokine variables (Hiles et al., 2012). Additionally, the differentiated effects of ECT treatment versus medication cannot be determined, as there was no medication free sample receiving ECT. The naturalistic study design, coupled with the small sample size, limits causal inferences regarding the relationship between cytokine abnormalities and melancholic major depression or the mechanism of action of ECT. Our study could not control for lifetime stress exposure or acute stress precipitated by the ECT procedure; nor could our study control for the effects of adverse lifestyle factors known to alter immune function, such as smoking, obesity, exercise and sleep (Hamer et al., 2009). Finally, our study was not designed to repeat cytokine levels beyond 48 hours after ECT series completion, and further reduction in levels cannot be ruled out, as discussed above.
CONCLUSIONS
The present data provide evidence for immune dysfunction in a well-defined sample of melancholic major depressed patients that is not correlated with clinical recovery despite the prominent antidepressant effect of ECT. Hence, our results do not support the supposition that remission of a depressive episode is associated with normalization of circulating levels of inflammatory markers. Elucidating the mechanisms for the increase in the proinflammatory IL-6 and the decrease in the regulatory cytokine TGF-β may lead to new insights into the pathophysiology and pharmacological treatment of MDD. The relevance of the identified immune dysfunction for the mental and physical health of the melancholic depressed population remains unclear and warrants investigation in future longitudinal studies. The acute increases in cytokines following ECT, and clinical response in the absence of normalisation of cytokine levels, may be consistent with a hypothesis that clinical effect of ECT is mediated through activation of immune system and hence stimulation of neurotropins, however further studies are needed to investigate this directly. In sum, our data supports the conceptualization of depression as a psychoneuroimmunological disturbance.
Supplementary Material
Table 1.
Demographics of the control healthy population and the melancholic group are shown, along with the results of t-tests comparing the healthy and melancholic populations in terms of demographic variables.
| Control Population |
Melancholic Population |
t | p value | |
|---|---|---|---|---|
| N | 26 | 55 | ||
|
Age (mean) years (SD) |
52.04 (13.1) | 51.54 (16.3) | 0.135 | 0.893 |
| Male:Female | 1:2.25 | 1:2.06 | 0.064 | 0.949 |
|
% with positive family history |
38.5% | 51% | −1.042 | 0.301 |
|
HAM-D score: mean (SD) |
2.4 (2.4) | 31 (9) | −22.114 | <0.001* |
= statistically significant
Table 2.
Comparing non-parametric biological variables in the control group and melancholic group using Mann-Whitney tests
| Biological Variable |
Group | N | Median | IQR | Mann- Whitney U Statistic |
Z value |
P value |
|---|---|---|---|---|---|---|---|
| IL-6 | 1. Control 2. Melancholic |
26 55 |
0.555 0.892 |
0.46 0.926 |
452 |
−2.661 |
0.008* |
| IL-10 | 1. Control 2. Melancholic |
25 51 |
1.03 1.23 |
1.0 0.54 |
245 |
−1.448 |
0.148 |
|
TNF- alpha |
1. Control 2. Melancholic |
26 50 |
2.605 2.881 |
1.73 2.64 |
311 |
−0.00 |
1.0 |
| TGF-beta | 1. Control 2. Melancholic |
23 41 |
13467 7610 |
14017 11112 |
324 |
−2.064 |
0.039* |
| CRP | 1. Control 2. Melancholic |
23 43 |
0.98 1.87 |
3 4 |
210 |
−0.163 |
0.87 |
= statistically significant
Highlights.
We tested inflammatory markers in 55 melancholic depressed patients and 26 controls.
Inflammatory markers were deranged in melancholic depressed patients.
33 patients went on to treatment with electroconvulsive therapy (ECT).
Despite clinical response to ECT, patients’ cytokine levels did not normalise.
Acknowledgments
Dr. Gavin Rush would like to acknowledge receipt of a scholarship funded by Lundbeck which partly funded this study. Dr. Aoife O’Donovan would like to acknowledge support from the Craig Dobbin Newman Scholarship in Mental Health Research, a Society in Science – Branco Weiss Fellowship, and a National Center for Advancing Translational Sciences Career Development Award (KL2 TR000143).
Role of the Funding Source:
The funding sources had no influence on the design of the study, the collection, analysis or interpretation of data, the writing of the paper, or the decision to submit the paper for publication.
Footnotes
Conflicts of Interest:
The authors declare that they have no conflicts of interest.
Contributors:
Authors G.R. and K.M. conceived and designed the study. Authors K.M. and J.L. supervised the study. Authors G.R. and A.O.D collected the data. Author A.M.McC conducted and analysed biochemicial assays. Authors G.R. and C.F. undertook the statistical analysis. Authors C.C. and L.N. conducted the literature searches, interpreted the data, and drafted the manuscript. All authors contributed to and have approved the final manuscript.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Angelucci F, Aloe L, Jiménez-Vasquez P, Mathé AA. Electroconvulsive stimuli alter the regional concentrations of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in adult rat brain. J ECT. 2002;18(3):138–143. doi: 10.1097/00124509-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol. Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Armitage R. Sleep and circadian rhythyms in mood disorders. Acta. Psychiatr. Scand. 2007;115(Suppl. 433):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Avissar S, Nechamkin Y, Roitman G, Schreiber G. Dynamics of ECT normalization of low G protein function and immunoreactivity in mononucear leukocytes of patients with major depression. Am. J. Psychiatry. 1998;155:666–671. doi: 10.1176/ajp.155.5.666. [DOI] [PubMed] [Google Scholar]
- Basterzi AD, Aydemir C, Kisa C, Aksaray S, Tuzer V, Yazici K, Göka E. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum. Psychopharmacol. Clin. Exp. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- Baune BT, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, Berger K. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Transl. Psychiatry. 2012;2:e92. doi: 10.1038/tp.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Bolwig T. How does electroconvulsive therapy work? Theories on its mechanism. Can. J. Psychiatry. 2011;56:13–18. doi: 10.1177/070674371105600104. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta. Psychiatr. Scand. 1998;97:309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatr. Scand. 2007;97:309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- Caird H, Worrall A, Lelliott P. The Electroconvulsive Therapy Accreditation Service. The Psychiatrist. 2004;28:257–259. [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J. Affect. Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav. Immun. 2011;25:1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two prospective studies and a systematic review. Plos Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, Wolkowitz OM. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J. Psychiatr. Res. 2009;43:962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Vincze C, Pál G, Lovas G. The neuroprotective functions of transforming growth factor Beta proteins. Int. J. Mol. Sci. 2012;13:8219–8258. doi: 10.3390/ijms13078219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Bouchner A. A general power analysis program. Behavior Research Methods, Instruments and Computers. 1996;28:1–11. [Google Scholar]
- Fink M, Rush AJ, Knapp R, Rasmussen K, Mueller M, Rummans TA, O’Connor K, Husain M, Biggs M, Bailine S, Kellner CH. Corsortium for Research in ECT (CORE) Study Group. J. ECT. 23:139–146. doi: 10.1097/yct.0b013e3180337344. [DOI] [PubMed] [Google Scholar]
- Fischler B, Bocken R, Schneider I, De Waele M, Thielemans K, Derde MP. Immunce changes induced by electroconvulsive therapy (ECT) Ann. N.Y. Acad. Sci. 1992;650:326–330. doi: 10.1111/j.1749-6632.1992.tb49146.x. [DOI] [PubMed] [Google Scholar]
- Fluitman SB, Heijnen CJ, Denys DA, Nolen WA, Balk FJ, Westenberg HG. Electroconvulsive therapy has acute immunological and neuroendocrine effects in patients with major depressive disorder. J. Affect. Disord. 2011;131:388–392. doi: 10.1016/j.jad.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F, Irwin MR, Sauvé C, Lespérance J, Théroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol. Psychiatry. 2007;62:302–308. doi: 10.1016/j.biopsych.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Eur. Arch. Psychiatry Clin. Neurosci. 247, 228–233. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: a comparison between the acute state and after remission. Eur. Arch. Psychiatry Clin. Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Kho KH, Blansjaar BA. Serum markers of brain-cell damage and C-reactive protein are unaffected by electroconvulsive therapy. World J. Biol. Psychiatry. 2008;9:231–235. doi: 10.1080/15622970701310989. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Miller GE. Where there is depression, there is inflammation…sometimes! Biol. Psychiatry. 2007;62:280–281. doi: 10.1016/j.biopsych.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Gold SM, Irwin MR. Depression and Immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol. Allergy Clin. North Am. 2009;29:309–320. doi: 10.1016/j.iac.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Guloksuz S, Rutten BP, Arts B, van Os J, Kenis G. The immune system and electroconvulsive therapy for depression. Journal of ECT. 2014;30(2):132–137. doi: 10.1097/YCT.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, de Oliveira C, Demakakos P. Persistent depressive symptomatology and inflammation: to what extent do health behaviours and weight control mediate this relationship? Brain Behav. Immun. 2009;23:413–418. doi: 10.1016/j.bbi.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- Hestad KA, Tønseth S, Støen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J. ECT. 2003;19:183–188. doi: 10.1097/00124509-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Hickie I, Mason C, Parker G, Brodaty H. Prediction of ECT response: validation of a refined sign-based (CORE) system for diagnosing melancholia. Br. J. Psychiatry. 1996;169:68–74. doi: 10.1192/bjp.169.1.68. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol. Med. 2012;42:2015–2026. doi: 10.1017/S0033291712000128. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lee CT. T-helper 1/T-helper 2 cytokine imbalance and clinical phenotypes of acute-phase major depression. Psychiatry Clin. Neurosci. 2007;61:415–420. doi: 10.1111/j.1440-1819.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, Arolt V, Cassens U, Rothermundt M. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J. Affect. Disord. 2005;87:305–311. doi: 10.1016/j.jad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Kagaya A, Kugaya A, Takebayashi M, Fukue-Saeki M, Saeki T, Yamawaki S. Plasma concentrations of interleukin-1beta, interleukin-6, soluble interleukin-2 receptor and tumor necrosis factor alpha of depressed patients in Japan. Neuropsychobiology. 2001;43:59–62. doi: 10.1159/000054867. [DOI] [PubMed] [Google Scholar]
- Kelly CB, Cooper SJ. Plasma noradrenaline response to electroconvulsive therapy in depressive illness. Br. J. Psychiatry. 1997;171:182–186. doi: 10.1192/bjp.171.2.182. [DOI] [PubMed] [Google Scholar]
- Kelly CB, Cooper SJ. Differences and variability in plasma noradrenaline between depressive and anxiety disorders. J. Psychopharmacol. 1998;12:161–167. doi: 10.1177/026988119801200208. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Miley R, Giacobbe P, Ramasubbu R, Lam RW, Parikh SV, Patten SB, Ravindran AV. Canadian Network for Mood and Anxiety Treatments (CANMAT) Clinical guidelines for the management of major depressive disorder in adults. IV. Neurostimulation therapies. J. Affect. Disord. 2009;117:S44–S53. doi: 10.1016/j.jad.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Kyeremanteng C, James J, Mackay J, Merali Z. A study of brain and serum brain-derived neurotrophic factor protein in Wistar and Wistar-Kyoto rat strains after electroconvulsive stimulus. Pharmacopsychiatry. 2012;45(6):244–249. doi: 10.1055/s-0032-1306278. [DOI] [PubMed] [Google Scholar]
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1044–1053. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kim YK, Suh IB, Kim H, Han CS, Lim CS, Choi SH, Licinio J. The plasma levels of interleukin-12 in schizophrenia, major depression and bipolar mania: effects of psychotropic drugs. Mol. Psychiatry. 2002;7:1107–1114. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, Duncko R, Drevets WC, Manji HK, Charneys DS, Gold PW, Neumeister A. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol. Psychiatry. 2007;62:309–313. doi: 10.1016/j.biopsych.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol Z, Lemay L, Nair M, Kluger M. Electroconvulsive therapy increases plasma levels of interleukin-6. Ann. N.Y. Acad. Sci. 1990;594:463–465. [Google Scholar]
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch. Dermatol. 2010;146:891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry. 2013;18:692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lee KM, Kim YK. The role of IL-12 and TGF- β1 in the pathophysiology of major depressive disorder. Int. J. Immunopharmacol. 2006;6:1298–1304. doi: 10.1016/j.intimp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Lehtimäki K, Keränen T, Huuhka M, Palmio J, Hurme M, Leinonen E, Peltola J. Increase in plasma proinflammatory cytokines after electroconvulsive therapy in patients with depressive disorder. J. ECT. 2008;24:88–91. doi: 10.1097/YCT.0b013e3181571abb. [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GR, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol. Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HWM, Steinbusch, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J. Affect. Disord. 2005;88:167–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- O’Brien S, Scott LV, Dinan TG. Antidepressant therapy and C-reactive protein levels. Br. J. Psychiatry. 2006;188:449–452. doi: 10.1192/bjp.bp.105.011015. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatr. Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, O’Farrelly C, Malone KM. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress. Anxiety. 2013;30:307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- Parker G, Hadzi-Pavlovic D, editors. Melancholia: A Disorder of Movement and Mood. New York, NY: Cambridge University Press; 1996. [Google Scholar]
- Parker G, Roy K, Wilhelm K, Mitchell P. Assessing the comparative effectiveness of antidepressant therapies: a prospective clinical practice study. J. Clin. Psychiatry. 2001;62:117–125. doi: 10.4088/jcp.v62n0209. [DOI] [PubMed] [Google Scholar]
- Patas K, Penninx BW, Bus BA, Vogelzangs N, Molendijk ML, Elzinga BM, Bosker FJ, Oude Voshaar RC. Association between serum brain-derived neurotrophic factor and plasma interleukin-6 in major depressive disorder with melancholic features. Brain Behav Immun. 2014;36:71–79. doi: 10.1016/j.bbi.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol. Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides G, Fink M, Husain MM, Knapp RG, Rush AJ, Mueller M, Rummans TA, O’Connor KM, Rasmussen KG, Jr, Bernstein HJ, Biggs M, Bailine SH, Kellner CH. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J. ECT. 2001;17:244–253. doi: 10.1097/00124509-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6 and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Prud’homme GJ, Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF- β1) in autoimmune diseases. J. Autoimmun. 2000;14:23–42. doi: 10.1006/jaut.1999.0339. [DOI] [PubMed] [Google Scholar]
- Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, Greenberg R, Rifat SL, Sackeim HA. Resistance to antidepressant medications and short-term clinical response to ECT. Am. J. Psychiatry. 1996;153:985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KG. Electroconvulsive therapy and melancholia: review of the literature and suggestions for further study. J. ECT. 2011;27:315–322. doi: 10.1097/YCT.0b013e31820a9482. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Peters M, Gutbrodt H, Fenker J, Kersting A, Kirchner H. Inflammatory markers in major depression and melancholia. J. Affect. Disord. 2001a;63:93–102. doi: 10.1016/s0165-0327(00)00157-9. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Fenker J, Gutbrodt H, Peters M, Kirchner H. Different immune patterns in melancholic and non-melancholic major depression. Eur. Arch. Psychiatry Clin. Neurosci. 2001;251:90–97. doi: 10.1007/s004060170058. [DOI] [PubMed] [Google Scholar]
- Rotter A, Biermann T, Stark C, Decker A, Demling J, Zimmermann R, Sperling W, Kornhuber J, Henkel A. Changes of cytokine profiles during electroconvulsive therapy in patients with major depression. J. ECT. 2013;29(3):162–169. doi: 10.1097/YCT.0b013e3182843942. [DOI] [PubMed] [Google Scholar]
- Ruocco A, Nicole O, Docagne F, Ali C, Chazalviel L, Komesli S, Yablonsky F, Roussel S, MacKenzie ET, Vivien D, Buisson A. A transforming growth factor-beta antagonist unmasks the neuroprotective role of this endogenous cytokine in excitotoxic and ischaemic brain injury. J. Cereb. Blood Flow Metab. 1999;19:1345–1353. doi: 10.1097/00004647-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schlatter J, Ortuño F, Cervera-Enguix S. Monocytic paramters in patients with dysthymia versus major depression. J. Affect. Disord. 2004;78:243–247. doi: 10.1016/S0165-0327(02)00316-6. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbrüggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol. 2005;160(1–2):204–209. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-international Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Shorter E, Fink M. Endocrine Psychiatry: Solving the Riddle of Melancholia. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Shorter E, Healy D. Shock Therapy: A History of Electroconvulsive Treatment in Mental Illness. New Brunswick, NJ: Rutgers University Press; 2007. [Google Scholar]
- Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, Wiktorowicz K. Indicators of immune activation in major depression. Psychiatry Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann. N.Y. Sci. 2005;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med. Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42:182–188. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(3):760–768. doi: 10.1016/j.pnpbp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin. Dev. Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MA, Fink M. Melancholia: The Diagnosis, Pathophysiology and Treatment of Depressive Illness. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT. Increase in interleukin-1beta in late-life depression. Am. J. Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- Uguz F, Akman C, Kucuksarac S, Tufekci O. Anti-tumor necrosis factor-alpha therapy is associated with less frequent mood and anxiety disorders in patients with rheumatoid arthritis. Psychiatry Clin. Neurosci. 2009;63:50–55. doi: 10.1111/j.1440-1819.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- van Buel EM, Patas K, Peters M, Bosker FJ, Eisel ULM, Klein HC. Immune and neurotrophin stimulation by electroconvulsive therapy: is some inflammation needed after all? Translational Psychiatry. 2015;5:e609. doi: 10.1038/tp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivien D, Ali C. Transforming growth factor-beta signalling in brain disorders. Cytokine Growth Factor Rev. 2006;17:121–128. doi: 10.1016/j.cytogfr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengarda P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc. Nat. Acad. Sci. U.S.A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol. Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.